Highlights
-
•
Anti-NMDA-receptor encephalitis is a paraneoplastic syndrome caused by teratomas.
-
•
The syndrome includes psychiatric symptoms followed by autonomic dysregulation.
-
•
Rapid diagnosis and removal of the tumor is essential for optimizing outcomes.
-
•
Following recovery, counsel on contraceptive options, particularly LARC methods.
-
•
If no tumor is identified initially, monitor for development of ovarian teratoma.
Keywords: Teratoma, Encephalitis, Paraneoplastic syndrome
1. Introduction
Anti- N-methyl-D-aspartate (NMDA)-receptor encephalitis is a recently described syndrome of psychiatric symptoms and neurologic sequelae associated with ovarian teratomas. Due to the rarity of anti-NMDA-receptor encephalitis, diagnosis may be delayed while more common conditions, such as primary psychiatric disorders and infectious encephalitis, are ruled out. With correct diagnosis, and treatment including resection of the teratoma and adjunctive immunotherapy, favorable outcomes may be achieved. Patients may require prolonged monitoring for development of teratoma, and must be counseled on appropriate contraceptive use if remaining on long-term immunosuppression.
2. Case
A 12 year-old previously healthy pre-menarchal female presented to the emergency department with her parents with a one-day history of abnormal behavior. They reported that she started experiencing auditory hallucinations then became increasingly emotionally labile and confused, all within a 24-h period. On presentation to the emergency department, she was crying, speaking incoherently, and complaining of auditory hallucinations. She had not had any trauma, recent travel, sick contacts, or ingestions of new foods or medications; however she had reported mild intermittent headaches over the week prior to admission. She was afebrile with stable vital signs. Computed tomography scan of the head, complete blood count, comprehensive metabolic profile, thyroid stimulating hormone, beta human chorionic gonadotropin, acetaminophen level, alcohol level, salicylate level, urinalysis and urine toxicology were all unrevealing. She was determined to be medically cleared and was admitted to inpatient psychiatry service for acute psychosis.
During three days of psychiatric hospitalization, her condition declined despite multiple anti-psychotic medications. She became nonverbal, unable to swallow, and incontinent. She was brought to the Inova Fairfax Hospital emergency department where she was found to have constricted and minimally reactive pupils. She was obtunded and unable to respond to commands. The remainder of her exam, including abdominal exam, was within normal limits. Brain magnetic resonance imaging (MRI) showed no abnormalities. She was admitted for further evaluation. Lumbar puncture demonstrated elevated lymphocytes consistent with autoimmune encephalitis or viral meningitis. Cerebrospinal fluid (CSF) and serum viral polymerase chain reaction and autoimmune encephalitis studies were obtained. While this workup was being completed, she became febrile, tachycardic, and required intubation. Due to a high index of suspicion for autoimmune encephalitis, including anti-NMDA-receptor encephalitis, an abdominal ultrasound was ordered to evaluate for ovarian teratoma. Ultrasound demonstrated an 11.1 × 6.6 × 8.1 cm complex cystic and solid mass arising from the left ovary (Fig. 1).
Fig. 1.
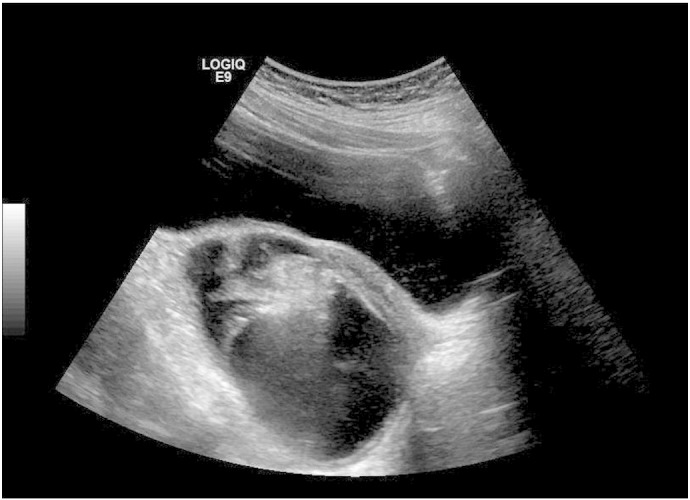
Ultrasound image of left adnexa showing 11 cm complex cystic and solid mass replacing left ovary.
Although results were not yet available from anti-NMDA-receptor antibody studies of the patient's CSF, her failure to respond to anti-psychotics and development of progressive neurological signs and symptoms, CSF lymphocytosis, and the finding of a mass consistent with teratoma were highly concerning for anti-NMDA-receptor encephalitis. Given the rapid deterioration in the patient's condition despite supportive care, the decision was made to remove her adnexal mass in hopes of preventing further disease progression. She underwent exploratory laparotomy, left salpingo-oophorectomy, small bowel mesenteric lymph node biopsy, staging peritoneal biopsies, and partial omentectomy. The patient's left ovary had been fully replaced by the complex mass seen intact in Fig. 2 and in cross-section in Fig. 3. Final pathology of surgical specimens revealed a mature teratoma without any evidence of malignancy. CSF studies later demonstrated anti-NMDA-receptor antibodies, confirming her diagnosis.
Fig. 2.
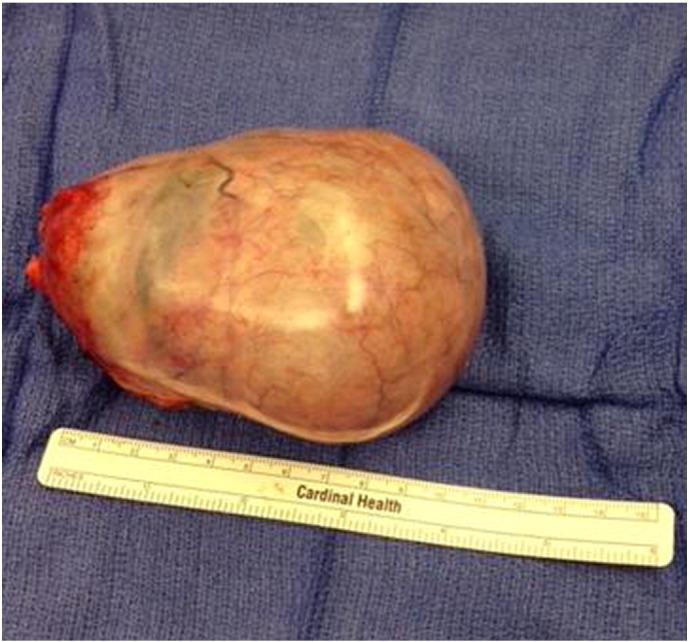
Intact left ovarian mass.
Fig. 3.
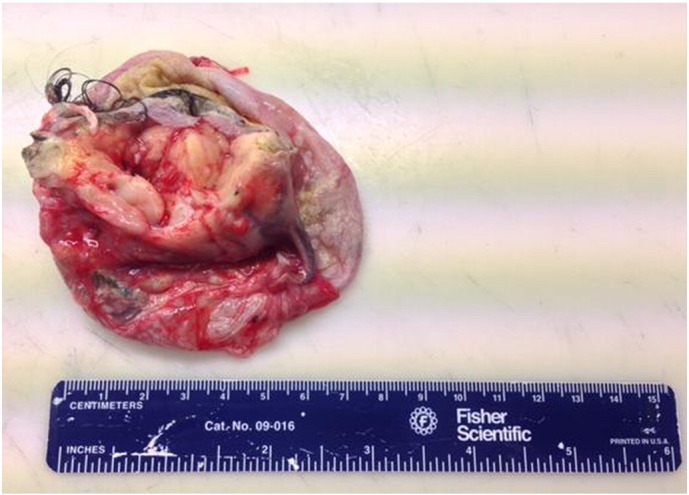
Cross-section of left ovarian mass, demonstrating multiple tissue types consistent with final diagnosis of mature teratoma.
Following removal of the teratoma, she was treated with a modified version of the BrainWorks protocol for severe antibody-mediated inflammatory brain disease. Treatment included intravenous methylprednisolone, intravenous immunoglobulins, and rituximab, in addition to routine supportive care. By post-operative day six, she was extubated. She had substantial autonomic instability characterized by tachycardia and fevers, but mental status slowly improved. Two weeks post-operatively, she started opening her eyes. She began tracking faces and to making an effort to smile when asked to do so. By three weeks post-operatively, she was following basic commands, responding to yes/no questions and attempting to speak. By one month post-operatively, she was oriented, able to converse clearly without difficulty, follow all commands and answer questions appropriately, though she was mildly impulsive and required prompting to complete tasks. She was transferred to inpatient rehabilitation exactly one month post-operatively to continue her recovery. At six months post-operatively, the patient had resumed all of her normal activities and was achieving straight-As in school.
3. Comment
Anti-NMDA-receptor encephalitis was initially described in 1997, in two separate reports of young women. These women presented with an ovarian teratoma and symptoms that included psychiatric manifestations and altered level of consciousness. In both patients, there was a gradual significant improvement in symptoms after tumor removal (Nokura et al., 1997, Okamura et al., 1997). In 2005, a series of four women with ovarian teratoma, psychiatric symptoms, altered level of consciousness and central hypoventilation was described. Authors hypothesized that the syndrome was a paraneoplastic process due to an antibody to an unknown antigen expressed in the hippocampus (Vitaliani et al., 2005). The associated antibody was discovered to be anti-NMDA-receptor in 2007 (Dalmau and Tüzün, 2007). In subsequent years, hundreds of cases have been reported in the neurology literature in both men and women, with approximately 80% of cases in females (Mann et al., 2014). The median age at onset of symptoms is 21 years old, although cases have been reported in patients ranging from 8 months to 85 years (Titulaer et al., 2013, Dalmau et al., 2011). Teratomas are found in large numbers of patients, most commonly in women between age 12 and 45 and in patients of Asian or African American descent (Titulaer et al., 2013, Dalmau et al., 2011). Most commonly, these are ovarian teratomas, though other germ-cell and rarely non-germ cell tumors have also been described in association with anti-NMDA-receptor encephalitis (Dalmau and Tüzün, 2007, Titulaer et al., 2013, Dalmau et al., 2011). Three cases have been described in pregnant women, all of whom recovered after removal of their ovarian teratoma and immunosuppression. Two of these women went on to have healthy infants, while one underwent termination of pregnancy (Kumar et al., 2010).
The syndrome often begins with viral-like symptoms including headache, nausea, vomiting, fever, and fatigue (Dalmau et al., 2011). The non-specific nature of these symptoms generally precludes diagnosis at this stage and is recognized as a prodrome only after the illness progresses with a spectrum of neuropsychiatric symptoms. These symptoms have been divided into early and late stage symptoms. Early stage symptoms generally present with two weeks of prodromal symptoms and include confusion, memory loss, paranoia, hallucinations, mood disturbances, anxiety, self-harming behaviors, seizures and movement disorders such as facial twitching and choreoathetosis (Dalmau et al., 2011). As the psychiatric symptoms are often the most prominent, 77% of patients are initially seen by psychiatrists (Mann et al., 2014), and many patients are diagnosed with new-onset psychiatric disorders. However, these patients do not respond to anti-psychotics and progress to late stage symptoms, such as decreased responsiveness, hypoventilation, and autonomic instability including hypotension or hypertension, bradycardia or tachycardia, hyperthermia, and urinary incontinence (Mann et al., 2014).
In patients with acute onset of psychiatric symptoms with any neurologic findings, or symptoms unresponsive to anti-psychotic medications, the diagnosis of encephalitis should be considered. In particular, serum and CSF studies for markers of viral and autoimmune causes of encephalitis, MRI, and electroencephalogram (EEG) may be useful in obtaining a diagnosis. Of note, while MRI may be normal in two-thirds of patients with anti-NMDA-receptor encephalitis, EEG abnormalities are seen in more than 90% of these patients (Titulaer et al., 2013). Definitive diagnosis is made when anti-NMDA-receptor antibodies are detected in the blood or CSF (Titulaer et al., 2013, Dalmau et al., 2011).
Once definitive diagnosis has been obtained, imaging studies such as pelvic ultrasound, MRI, computed tomography, and positron emission tomography may be used to evaluate for an underlying teratoma. In rapidly-deteriorating patients in whom anti-NMDA-receptor encephalitis is highly suspected, but not yet confirmed, providers may consider imaging and removal of any detected neoplasms, as in this case. Delayed treatment may result in progression of the autoimmune process with associated clinical deterioration to autonomic instability, catatonia, status epilepticus, or coma. In one series of patients with anti-NMDA-receptor encephalitis, five out of six patients with an ovarian teratoma who did not undergo surgery died (Titulaer et al., 2013). In contrast, removal of the tumor may be curative. In approximately 80% of patients who undergo tumor removal and immunosuppressive treatment, substantial neurological improvement occurs (Dalmau et al., 2011). In the case above and in previously reported cases, symptoms of anti-NMDA-receptor encephalitis markedly improved within one month of tumor removal and immunosuppressive treatment, though recovery can continue for up to 24 months (Titulaer et al., 2013, Dalmau et al., 2011).
Multiple regimens of immunosuppressive treatments have been described, including first line treatment with intravenous steroids and intravenous immunoglobulins (IVIG) or plasmapheresis and second line treatment with rituximab and cyclophosphamide (Titulaer et al., 2013, Dalmau et al., 2011). For the patient described above, a modified version of the BrainWorks protocol for severe antibody-mediated inflammatory brain disease was used (BrainWorks). This protocol consists of 1 g prednisone daily for three to seven days, followed by 60 mg daily for one month, followed by a six-month taper. Simultaneously, patients receive seven plasmapheresis treatments over 14 days, 70 g of IVIG every two weeks for five doses, rituximab 500 mg/m2 in two doses two weeks apart, and daily calcium and vitamin D supplementation. This protocol was developed by the BrainWorks Network based on experience from a web-based, international prospective cohort of pediatric patients with inflammatory brain diseases. It differs from previously described protocols (Titulaer et al., 2013, Dalmau et al., 2011) in that it is based solely on experience in the pediatric population, and includes rituximab in the initial treatment, rather than using it as a second line agent.
In patients with no detectable underlying neoplasms, it has been hypothesized that the syndrome may be caused by microscopic germ cell tumors undetectable by imaging (Mann et al., 2014). This hypothesis is supported by the findings that recovery is more common and relapse less likely in patients with a detectable tumor (Dalmau et al., 2011). In addition, there have been reported cases of ovarian teratomas being detected years after presentation of anti-NMDA-receptor encephalitis symptoms (Mann et al., 2014). Therefore, for patients without detectable tumors, it is recommended to continue immunosuppression with azathioprine or mycophenolate for a minimum of 1 year after symptom relapse, and in female patients over age 12 without detectable tumors, to screen for ovarian teratomas with pelvic MRI or ultrasound every 6 months for 4 years (Mann et al., 2014, Titulaer et al., 2013, Dalmau et al., 2011).
Following neurologic recovery, women of childbearing potential should receive counseling on contraception, particularly for those remaining on azathioprine and mycophenolate. These medications are both classified as Pregnancy Category D. These women should have a pregnancy test prior to treatment initiation and as indicated at follow up visits. For mycophenolate in particular, it is recommended to avoid pregnancy for an additional 6 weeks following discontinuation of treatment. Particular consideration should be given to use of long-acting reversible contraceptives in these patients given that many may have residual cognitive deficits including difficulties with memory and impulsivity that may make consistent contraceptive use more challenging.
With appropriate diagnosis and rapid treatment, including resection of any underlying teratoma and immunosuppression, patients with anti-NMDA-receptor encephalitis have a high likelihood of a positive outcome. The gynecologist has an important role to play within the multidisciplinary team caring for these patients as early tumor detection and removal results in an improved prognosis, and those without tumors require frequent monitoring for tumor development.
Conflict of interest statement
The authors report no conflicts of interest. We received no funding for this research.
References
- BrainWorks. The International Inflammatory Brain Disease Outcome Study. 2014. Protocol for antibody-mediated inflammatory brain disease: Severe disease. Retrieved from: < http://www.sickkids.ca/pdfs/Research/BrainWorks/62062-Antibody%20IBrainD.pdf>. [Google Scholar]
- Dalmau J., Tüzün E. Wu H-y, Masjuan J, Rossi JE, Voloschin A., et al. Paraneoplastic anti–N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J., Lancaster E., Martinez-Hernandez E., Rosenfeld M., Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Jain A., Dechant V.E., Saito T., Rafael T., Aizawaet H. Anti-N-methyl-D-aspartate receptor encephalitis during pregnancy. Arch. Neurol. 2010;67(7):884–887. doi: 10.1001/archneurol.2010.133. [DOI] [PubMed] [Google Scholar]
- Mann A., Grebenciucova E., Lukas R. Anti- N-methyl-D-aspartate receptor encephalitis: diagnosis, optimal management, and challenges. Ther. Clin. Risk Manag. 2014;10:517–525. doi: 10.2147/TCRM.S61967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokura K., Yamamoto H., Okawara Y., Koga H., Osawa H., Sakai K. Reversible limbic encephalitis caused by ovarian teratoma. Acta Neurol. Scand. 1997;95:367–373. doi: 10.1111/j.1600-0404.1997.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Okamura H., Oomori N., Uchitomi Y. An acutely confused 15-year-old girl. Lancet. 1997;350:488. doi: 10.1016/S0140-6736(97)06208-9. [DOI] [PubMed] [Google Scholar]
- Titulaer M. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliani R., Mason W., Ances B., Zwerdling T., Jiang Z., Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann. Neurol. 2005;58(4):594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]


