Abstract
Purpose of review
A major goal in repopulating hematopoietic stem cell (HSC) gene therapies is achieving high-efficacy gene transfer, while maintaining robust HSC engraftment and differentiation in vivo. Recent studies have documented that rapamycin treatment of HSC during lentiviral vector transduction enhances gene transfer to human and mouse HSCs and maintains engraftment capacity. In this review, we place into context the role of mammalian target of rapamycin (mTOR) pathways in HSC quiescence and function, endocytic regulation, and lentiviral gene delivery.
Recent findings
Lentiviral vector transduction of human and mouse HSCs is considerably enhanced by rapamycin treatment. Furthermore, rapamycin preserves long-term engraftment of human and mouse HSCs. Investigations of cellular mechanisms that contribute to increased transduction in HSCs uncovered a role for mTOR inhibition-dependent activation of endocytosis.
Summary
Rapamycin enhances lentiviral vector transduction of HSCs through regulation of endocytic activity via mTOR inhibition. An important attribute of rapamycin treatment during transduction is the preservation of HSC function, allowing reconstitution of long-term hematopoiesis in vivo in murine models.
Keywords: gene therapy, hematopoietic stem cells, hematopoietic stem cell engraftment, mTOR, rapamycin
INTRODUCTION
A long sought-after holy grail in the field of hematopoietic stem cell (HSC) gene therapy is a method for highly efficient lentiviral vector transduction that does not impair in-vivo reconstitution potential of HSCs following human transplantation. Although modest lentiviral vector transduction efficiency of 15–25% has been reported in HSCs in the absence of cytokine activation, this level may not be sufficient for clinical use [1,2]. Various strategies have been demonstrated or proposed for improving transduction efficiency in HSCs, including small-molecule approaches such as proteasome inhibitors, PGE2, SR1, and pyrimidoindole derivatives, and genetic approaches such as knockdown of the p21 protein [3–7]. However, most of these methods are still in early-stage preclinical investigation, and none has yet been translated to clinical usage, due to treatment-associated cytotoxicity or cumbersome genetic manipulations of HSCs. Recently, we and others made the intriguing discovery that the clinically used drug rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR) kinase, greatly enhanced lentiviral transduction efficiency in human and mouse primitive HSCs, with very little detrimental, or even beneficial, effects on the engraftment and reconstitution potential of HSCs [8▪▪,9,10▪,11]. We uncovered the involvement of an unconventional and little-studied aspect of mTOR signaling – regulation of the endocytic pathway – in the enhancement of lentiviral transduction by rapamycin. Here, we review recent literature on the role of mTOR signaling in HSC function, mTOR regulation by rapamycin in hematopoietic cells, and the connection between mTOR signaling and the endocytic pathway that may inform the mechanistic basis of improved lentiviral vector transduction.
MAMMALIAN TARGET OF RAPAMYCIN SIGNALING PATHWAY
The mTOR kinase is a central molecule that coordinates cellular energy sensing pathways and metabolic flux. Studies in yeast, drosophila, and mammalian cell lines have allowed extensive mapping of the mTOR signaling pathway (reviewed in [12]). mTOR is regulated upstream by the phosphotidylinositol-3-kinase (PI3K) pathway, which plays a major role in promoting cell growth and proliferation. PI3K activity results in the phosphorylation and activation of protein kinase B (PKB or AKT). AKT in turn phosphorylates many cellular targets that are important in cell growth and survival, including the tuberous sclerosis complex (TSC). TSC is a negative regulator of mTOR; thus, inhibition of TSC by an active PI3K/AKT leads to activation of mTOR. The phosphatase and tensin homolog (PTEN) negates the action of PI3K and is a negative regulator of mTOR (Fig. 1).
FIGURE 1.
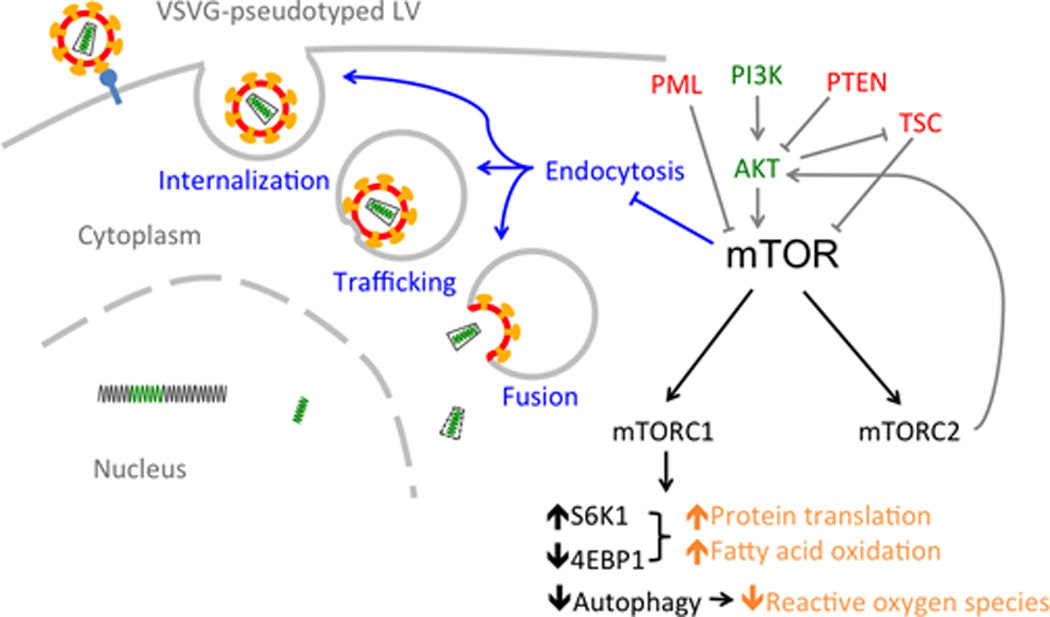
The role of mTOR in HSC maintenance and transduction. Gray arrows denote upstream regulators of mTOR that are important for HSC maintenance (green: mTOR activators; red: mTOR inhibitors). Black arrows denote downstream effectors regulated by mTOR. mTOR is the active kinase subunit of two complexes: mTORC1 and mTORC2. mTORC1 regulates three processes implicated in HSC maintenance, shown in orange [26,29,30,31▪]. In addition to promoting HSC maintenance, mTOR inhibition also enhances lentiviral vector transduction of HSCs by enhancing one or more early steps of vector endocytosis, shown in blue, potentially via an mTORC2-mediated mechanism [8▪▪]. HSC, hematopoietic stem cell; mTOR, mammalian target of rapamycin.
Mammalian target of rapamycin is the active kinase subunit of two complexes, mTORC1 and mTORC2, which carry out nonredundant functions. The functions of mTORC1, which is conventionally thought to be rapamycin-sensitive, are much better mapped out. The mTORC1 complex is characterized by the presence of the protein Raptor. The most well studied downstream actions of mTORC1 include activation of protein translation through phosphorylation of ribosomal S6 kinase (S6K1) and eukaryotic initiation factor 4E binding protein (4E-BP1), and inhibition of autophagy. The mTORC2 complex is characterized by the presence of Rictor, and is traditionally thought to be rapamycin-insensitive. A well defined mTORC2-specific downstream effect is phosphorylation of AKT at Ser473 [13,14]. Thus, mTORC2 positively regulates signaling of the AKT/mTOR axis.
REGULATION OF MTORC1 AND MTORC2 BY RAPAMYCIN
Although rapamycin is extensively used as a canonical mTORC1 inhibitor, its use comes with a few important caveats. Firstly, rapamycin does not completely inhibit mTORC1, its canonical target, in mammalian cells, with a number of rapamycin-insensitive mTOR downstream processes such as 4E-BP1 phosphorylation and autophagy [15–18]. Rapamycin allosterically inhibits mTORC1 through binding to the adaptor protein FKBP12. Rapamycin– FKBP12 does not bind mTORC2, which is traditionally believed to be insensitive to inhibition by rapamycin. However, since rapamycin–FKBP12 does bind free mTOR, prolonged rapamycin treatment (>24 h) impairs mTORC2 turnover by sequestering free mTOR [19]. In addition, different concentrations of rapamycin have been reported to differentially modulate mTORC1 and mTORC2, with high concentrations in the micromolar range inhibiting mTORC2 assembly [20]. Thus, the interpretations of studies reporting autophagy induction and function in hematopoietic cells in which micromolar concentrations of rapamycin were utilized may be confounded by the effects of mTORC2 induction [21,22].
For lentiviral vector transduction enhancement in HSCs, the inhibition of mTOR by rapamycin or other active site inhibitors is required, but it is unknown which mTOR complex is involved, or the downstream molecular events that regulate function following inhibition [8▪▪]. The effective rapamycin concentration for HSC transduction enhancement is in the micromolar range, concentrations at which mTORC2 inhibition may be invoked [20]. Supportive of a potential mTORC2-dependent mechanism, we found no evidence for the involvement of autophagy, an mTORC1-specific cytoprotective pathway. Although autophagy may contribute to certain aspects of lentiviral transduction of HSCs, such as the kinetics of intracellular trafficking of vector particles, it does not appear to affect the stable gain of transduction efficiency that results from rapamycin treatment.
ROLE OF MAMMALIAN TARGET OF RAPAMYCIN IN HEMATOPOIETIC STEM CELL QUIESCENCE
Mammalian target of rapamycin complexes play a central role in the regulation of cellular energy sensing and response pathways, which are particularly important for the function of primitive stem cells. Consistent with observations in other cell types, inhibition of mTOR exhibits a general effect in promoting HSC quiescence, and inappropriate activation leads to HSC proliferation, depletion, and oncogenic transformation, discussed in detail below.
EFFECT OF MAMMALIAN TARGET OF RAPAMYCIN ACTIVATION
The PI3K/AKT pathway, through regulation of mTOR, plays a key role in HSC quiescence. Over-activation of mTOR, either through increased AKT signaling [23], or depletion of negative regulators of mTOR, including PTEN, TSC, and PML, leads to HSC proliferation and depletion, as well as myeloproliferative disease [24–27]. Phenotypes associated with activation of AKT, depletion of PTEN, TSC, or PML, are reversed by rapamycin treatment, demonstrating involvement of mTOR [23,24,26,27]. Furthermore, HSC aging is associated with mTOR activation, and activating mTOR through TSC depletion in young mouse HSCs mimics an aged phenotype, which is corrected by rapamycin [28].
Mechanistically, mTOR activation may impact HSC quiescence by three potential mechanisms: increasing the amount of reactive oxygen species, as in the case of TSC depletion [26,29]; limiting fatty acid oxidation [30]; or deregulating the rate of protein synthesis, as in the case of PTEN depletion [31▪]; all three are detrimental for HSC maintenance (Fig. 1). Furthermore, the effect of mTOR activation on production of reactive oxygen species may be mediated by repression of autophagy, as depleting Atg7 also leads to accumulation of mitochondria and generation of reactive oxygen species, and consequently proliferation and exhaustion of HSCs [32].
EFFECT OF MAMMALIAN TARGET OF RAPAMYCIN INHIBITION
The effect of mTOR inhibition on HSC function has been studied through pharmacological or genetic means. Rapamycin specifically reduced expansion of common myeloid progenitors (Lin+CD34+), but not primitive HSCs (Lin−CD34+) or lineage committed progenitors (Lin+CD34−) [33]. The different effects of rapamycin on primitive and progenitor cells may be due to different propensities for autophagy induction, as mouse HSCs are more prone to autophagy induction relative to myeloid progeny, due to the action of the transcription factor FOXO3A [34]. A similar mechanism may also underlie different rapamycin sensitivities of HSCs from different sources, as HSCs show augmented autophagy following G-CSF-mediated mobilization [35].
In recent years, generation of mouse models with conditional ablation of Raptor and Rictor has allowed specific examination of the functions of mTORC1 and mTORC2 complexes in hematopoiesis. Neither complex is essential for normal HSC maintenance under homeostatic conditions [36,37], but does affect differentiation of various downstream progenitors. While not essential in homeostatic hematopoiesis, mTORC1 is required for HSC regeneration following transplantation [36]. Inhibition of mTORC1 through Raptor depletion causes pancytopenia and skewing of hematopoiesis toward myelomonocytic lineages through impaired granulocyte and B-cell development, but does not affect proliferation of hematopoietic and myeloid progenitors [36,38,39]. Both mTORC1 and mTORC2 contribute to T-cell lymphopoiesis, but at different stages. Depletion of Raptor inhibits early T-cell development at the DN1 stage, whereas depletion of Rictor impairs development at the DN3 stage [39,40]. Interestingly, rapamycin blocks T-cell development at the DN3 stage, similar to the effect of Rictor depletion and distinct from the effect of Raptor depletion, suggesting that rapamycin may act to modulate mTORC2 [39]. The action of mTORC2 in hematopoiesis seems to specifically affect T-cell lymphopoiesis, as Rictor depletion does not affect the function of HSCs or development of B cell, erythroid, or myeloid lineages [37,40]. Depletion of either Raptor or Rictor protects from PTEN loss evoked leukemogenesis, indicating the involvement of both mTORC1 and mTORC2 in abnormal hematopoiesis in response to a hyperactive AKT pathway [36,37]. Of note, PTEN inhibits mTORC2 signaling in adult, but not neonatal HSCs, highlighting important differences in mTOR pathway regulation in HSCs of different origins that may be relevant to HSC transduction [37].
CONNECTION BETWEEN HEMATOPOIETIC STEM CELL QUIESCENCE AND TRANSDUCTION
Regulation of HSC quiescence is a key aspect in HSC transduction protocols, due to the need to maintain stem cell functions such as homing, engraftment, and reconstitution for subsequent transplantation following lentiviral vector transduction. However, in addition to its intrinsic importance, regulation of quiescence may be mechanistically linked to lentiviral vector transduction efficiency. It has long been shown that the ability to transduce HSCs with lentiviral vectors is dependent on the cell activation state, as HSCs and progenitor cells need to exit G0 and progress to G1 to allow efficient transduction [41]. Accordingly, several strategies that improve lentiviral vector transduction in HSCs also affect cell cycling status. Rapamycin causes cell cycle delay in HSCs, consistent with previous studies [8▪▪]. Fibronectin, commonly used to enhance HSC lentiviral vector transduction, may also be implicated in HSC maintenance [42–44]. Silencing of the p21 protein leads to ex-vivo expansion of HSCs and decreases the fraction of HSCs in G0, and also increases lentiviral vector transduction efficiency [7,45]. However, we showed that rapamycin-mediated lentiviral vector transduction enhancement occurred via a rapidly dissipating effect on endocytosis of lentiviral vectors, which was likely distinct from rapamycin-mediated inhibition of cell cycle progression [8▪▪]. Nevertheless, cell activation status plays a key role in regulating HSC lentiviral vector transducibility through regulation of low-density lipoprotein receptor (LDLR) levels, the cellular receptor for vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped lentiviral vectors [46▪]. It is unclear whether mTOR is involved in the up-regulation of LDLR upon cellular activation; our findings indicated that rapamycin did not affect LDLR levels in quiescent or activated HSCs [8▪▪].
CONNECTION BETWEEN MAMMALIAN TARGET OF RAPAMYCIN AND ENDOCYTOSIS
Apart from its well studied role in regulating HSC quiescence, the mTOR pathway plays additional roles that are yet to be elucidated. Although we recently demonstrated a role for mTOR inhibition in enhancing endocytic entry of lentiviral vectors, resulting in increased transduction efficiency (Fig. 1), a relationship between mTOR and endocytosis has not been conclusively established [8▪▪]. It is evident that the endocytic machinery is involved in mTOR signaling. In yeast, TOR localizes to vesicular and endosomal membranes [47,48]. In mammalian cells, localization of mTOR to late endosomal and lysosomal compartments is involved in amino acid-induced mTORC1 signaling [49]. Furthermore, integrity of the late endosome is crucial for mTORC1 signaling, which is inhibited by blocking the conversion between early and late endosomes [50]. Rab5 GTPases, which regulate endosome formation and maturation, also mediate mTORC1 localization to the lysosomal compartment in both yeast and mammalian cells [51]. Therefore, several lines of evidence link mTOR signaling to functional endocytic machinery.
On the contrary, whether mTOR signaling in turn affects endocytic function has been more elusive. In drosophila, TOR interacts with clathrin-uncoating ATPase, and TOR signaling in the drosophila fat body differentially promotes bulk endocytosis while preventing endocytic degradation of amino acid transporter to increase nutrient availability. In this case, TOR is both regulated by endocytosis, as signaling is disrupted by disruption of endocytosis, and in turn regulates the endocytic process [52]. TOR may also have an indirect effect on protein endocytosis at the apical epithelium in drosophila pupal wing and mouse kidney proximal tubules, by inhibiting the transcription of the multiligand receptor Megalin [53].
While initially aiming to target postentry lentiviral restriction in HSCs, we instead showed that rapamycin-mediated transduction enhancement was solely attributed to enhanced vector entry, demonstrating cytoplasmic entry to be a major restrictive step in lentiviral transduction of HSCs that can be regulated by mTOR (Fig. 1). The regulation of endocytic pathways in HSC by mTOR is a novel finding. Since VSV-G, which is commonly employed for lentiviral vector pseudotyping, mediates entry via the LDLR [54], this may be a byproduct of mTOR regulation of lipid metabolic pathways. Lipid metabolic pathways play a role in HSC function, as LDL promotes mouse HSC proliferation in vitro and in vivo [55]. Furthermore, mTOR functions in the regulation of lipid metabolic pathways and rapamycin have been reported to down-regulate the LDLR at both messenger and protein levels in hepatic cells [56,57]. However, we found in CD34+ HSCs that rapamycin did not affect the cell surface levels of LDLR or bound lentiviral vectors, but rather increased the amount of vector cores in the cytoplasm. This suggests that rapamycin acts at the stages of vesicle internalization, trafficking, or lentiviral– endosomal fusion. The VSV-G pseudotype mediates cell entry in clathrin-coated vesicles that are dependent on actin for internalization [58]. Mechanistically, mTOR could potentially modulate lentiviral entry by acting on the cytoskeleton, as both yeast TOR2 and mammalian mTORC2 modulate organization of the actin cytoskeleton [59–62]. Actin cytoskeleton plays an important role in cargo sorting and maturation of early endosomes – the site of fusion for VSV-G pseudotyped vectors [63,64]. It is unclear whether mTOR inhibition affects other entry pathways in addition to clathrinmediated endocytosis, but there is evidence that drosophila TOR may be selective in promotion of clathrin vs. caveolin and raft-mediated endocytosis [52]. A genome-wide analysis of kinases in HeLa cells identified the mTOR pathway in the specific maintenance of clathrin-mediated endocytosis, as silencing of mTOR and signaling pathway members blocked clathrin-dependent endocytic events such as VSV infection, and transferrin internalization and trafficking [65]. This is in contrast to our finding that mTOR inhibition stimulates clathrin-dependent endocytosis of VSV-G pseudotyped lentiviral vectors in HSCs, and may reflect differences in cell type-specific metabolic programming.
CONCLUSION
The mTOR pathway is a key consideration in HSC gene therapy, both due to its well studied role in HSC maintenance, and our recent demonstration of its novel role in lentiviral vector endocytosis. The regulation of HSC endocytic machinery by mTOR reveals a novel aspect of mTOR signaling that is relevant to HSC gene therapy, and brings to light a number of further mechanistic and therapeutic questions. For example, the molecular events that bridge mTOR inhibition and endocytic enhancement need to be elucidated. Even though our findings suggest a non-mTORC1 mechanism in endocytic enhancement, certain aspects of mTORC1 signaling, such as autophagy, may be involved at additional stages of lentiviral vector entry. The contributions of mTORC1 and mTORC2 to the transduction process should be assessed, and may inform upon the choice of small molecule mTOR inhibitors with more specificity for therapeutic use. Additionally, it is unknown whether the regulation of endocytosis by mTOR exists as a physiological signaling pathway to bring in nonlentiviral vector ligands during metabolic stress, and whether this phenomenon is restricted to stem cells due to their particular metabolic requirements. Therapeutically, the efficacy of mTOR inhibitors in improving transduction by non-VSV-G-pseudotyped vectors that use alternative entry pathways remains to be evaluated. Finally, the dosage of mTOR inhibitors needs to be optimized in animal and human studies to maximize both transduction efficiency and preservation of HSC function for clinical purposes.
KEY POINTS.
Rapamycin significantly augments lentiviral gene delivery to HSCs while preserving engraftment potential.
Rapamycin-mediated transduction in hematopoietic stem cells uncovered a role for mTOR inhibition-dependent activation of endocytosis.
mTOR signaling regulates HSC quiescence and maintenance.
Acknowledgements
The authors wish to thank Ellie for her help in supporting the writing of the manuscript, as well David Rawlings and Blythe Sather for their contributions in understanding the role of rapamycin in lentiviral vector hematopoietic stem cell transduction. B.E.T. would like to thank Gabor Veres and Olivier Negre at Bluebird Bio for their support and hematopoietic stem cell gene therapy insights during the past year.
Financial support and sponsorship
Our research was partially funded by P50GM103368 (B.E.T.), R01HL116221 (B.E.T.), R01HL091219 (B.E.T.), with doctoral research awards from the Canadian Institutes of Health Research 237503 (C.X.W.) and CHRP D12-SRI-355 (C.X.W.). This is publication MEM #29045 from The Scripps Research Institute.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Miyoshi H, Smith KA, Mosier DE, et al. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science (New York, NY) 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 2.Guenechea G, Gan OI, Inamitsu T, et al. Transduction of human CD34+ CD38− bone marrow and cord blood-derived SCID-repopulating cells with third-generation lentiviral vectors. Mol Ther. 2000;1:566–573. doi: 10.1006/mthe.2000.0077. [DOI] [PubMed] [Google Scholar]
- 3.Santoni de Sio FR, Cascio P, Zingale A, et al. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood. 2006;107:4257–4265. doi: 10.1182/blood-2005-10-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science (New York, NY) 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science (New York, NY) 2014;345:1509–1512. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Attar E, Cohen K, et al. Silencing p21(Waf1/Cip1/Sdi1) expression increases gene transduction efficiency in primitive human hematopoietic cells. Gene Ther. 2005;12:1444–1452. doi: 10.1038/sj.gt.3302544. [DOI] [PubMed] [Google Scholar]
- 8. Wang CX, Sather BD, Wang X, et al. Rapamycin relieves lentiviral vector transduction resistance in human and mouse hematopoietic stem cells. Blood. 2014;124:913–923. doi: 10.1182/blood-2013-12-546218. Suppression of mTOR by rapamycin was shown to enhance HSC lentiviral vector gene delivery through alterations in endocytic activity.
- 9.Johnston JM, Denning G, Moot R, et al. High-throughput screening identifies compounds that enhance lentiviral transduction. Gene Ther. 2014;21:1008–1020. doi: 10.1038/gt.2014.80. [DOI] [PubMed] [Google Scholar]
- 10. Petrillo C, Cesana D, Piras F, et al. Cyclosporin A and rapamycin relieve distinct lentiviral restriction blocks in hematopoietic stem and progenitor cells. Mol Ther. 2014 doi: 10.1038/mt.2014.193. The demonstration of distinct HSC pathways that restrict lentiviral vector gene delivery.
- 11.Rohrabaugh SL, Campbell TB, Hangoc G, Broxmeyer HE. Ex vivo rapamycin treatment of human cord blood CD34+ cells enhances their engraftment of NSG mice. Blood Cells Mol Dis. 2011;46:318–320. doi: 10.1016/j.bcmd.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 14.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY) 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 15.Choo AY, Yoon SO, Kim SG, et al. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle (Georgetown, Tex) 2009;8:567–572. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 17.Hsu PP, Kang SA, Rameseder J, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science (New York, NY) 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Foster DA, Toschi A. Targeting mTOR with rapamycin: one dose does not fit all. Cell cycle (Georgetown, TX) 2009;8:1026–1029. doi: 10.4161/cc.8.7.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyei GB, Dinkins C, Davis AS, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol. 2009;186:255–268. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanchet FP, Moris A, Nikolic DS, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharas MG, Okabe R, Ganis JJ, et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Liu Y, Liu R, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito K, Bernardi R, Morotti A, et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gan B, Sahin E, Jiang S, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito K, Carracedo A, Weiss D, et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Signer RA, Magee JA, Salic A, Morrison SJ. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature. 2014;509:49–54. doi: 10.1038/nature13035. Increased rate of protein synthesis as a result of PTEN depletion impaires HSC function. Along with refs. [26] and [30], it suggests three potential mechanisms for mTOR-mediated HSC maintenance.
- 32.Mortensen M, Soilleux EJ, Djordjevic G, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208:455–467. doi: 10.1084/jem.20101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geest CR, Zwartkruis FJ, Vellenga E, et al. Mammalian target of rapamycin activity is required for expansion of CD34+ hematopoietic progenitor cells. Haematologica. 2009;94:901–910. doi: 10.3324/haematol.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warr MR, Binnewies M, Flach J, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leveque L, Vu T, Kuns RD, et al. Autophagy is required for long-term hematopoietic stem cell (HSC) function and G-CSF-induced HSC mobilization. Blood. 2013;122 [Google Scholar]
- 36.Kalaitzidis D, Sykes SM, Wang Z, et al. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11:429–439. doi: 10.1016/j.stem.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magee JA, Ikenoue T, Nakada D, et al. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 2012;11:415–428. doi: 10.1016/j.stem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoshii T, Tadokoro Y, Naka K, et al. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J Clin Investig. 2012;122:2114–2129. doi: 10.1172/JCI62279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoshii T, Kasada A, Hatakeyama T, et al. Loss of mTOR complex 1 induces developmental blockage in early T-lymphopoiesis and eradicates T-cell acute lymphoblastic leukemia cells. Proc Natl Acad Sci U S A. 2014;111:3805–3810. doi: 10.1073/pnas.1320265111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang F, Wu Q, Ikenoue T, et al. A critical role for Rictor in T lymphopoiesis. J Immunol (Baltimore, MD) 2012;189:1850–1857. doi: 10.4049/jimmunol.1201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton RE, Reitsma MJ, Uchida N, Brown PO. Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent. J Virol. 1999;73:3649–3660. doi: 10.1128/jvi.73.5.3649-3660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokota T, Oritani K, Mitsui H, et al. Growth-supporting activities of fibronectin on hematopoietic stem/progenitor cells in vitro and in vivo: structural requirement for fibronectin activities of CS1 and cell-binding domains. Blood. 1998;91:3263–3272. [PubMed] [Google Scholar]
- 43.Dao MA, Hashino K, Kato I, Nolta JA. Adhesion to fibronectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells. Blood. 1998;92:4612–4621. [PubMed] [Google Scholar]
- 44.Sagar BM, Rentala S, Gopal PN, et al. Fibronectin and laminin enhance engraftibility of cultured hematopoietic stem cells. Biochem Biophys Res Commun. 2006;350:1000–1005. doi: 10.1016/j.bbrc.2006.09.140. [DOI] [PubMed] [Google Scholar]
- 45.Stier S, Cheng T, Forkert R, et al. Ex vivo targeting of p21Cip1/Waf1 permits relative expansion of human hematopoietic stem cells. Blood. 2003;102:1260–1266. doi: 10.1182/blood-2002-10-3053. [DOI] [PubMed] [Google Scholar]
- 46. Amirache F, Levy C, Costa C, et al. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood. 2014;123:1422–1424. doi: 10.1182/blood-2013-11-540641. Different levels of LDL receptor account for differences in transducibility between quiescent and stimulated HSCs.
- 47.Kunz J, Schneider U, Howald I, et al. HEAT repeats mediate plasma membrane localization of Tor2p in yeast. J Biol Chem. 2000;275:37011–37020. doi: 10.1074/jbc.M007296200. [DOI] [PubMed] [Google Scholar]
- 48.Chen EJ, Kaiser CA. LST8 negatively regulates amino acid biosynthesis as a component of the TOR pathway. J Cell Biol. 2003;161:333–347. doi: 10.1083/jcb.200210141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science (New York, NY) 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flinn RJ, Yan Y, Goswami S, et al. The late endosome is essential for mTORC1 signaling. Mol Biol Cell. 2010;21:833–841. doi: 10.1091/mbc.E09-09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bridges D, Fisher K, Zolov SN, et al. Rab5 proteins regulate activation and localization of target of rapamycin complex 1. J Biol Chem. 2012;287:20913–20921. doi: 10.1074/jbc.M111.334060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennig KM, Colombani J, Neufeld TP. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J Cell Biol. 2006;173:963–974. doi: 10.1083/jcb.200511140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gleixner EM, Canaud G, Hermle T, et al. V-ATPase/mTOR signaling regulates megalin-mediated apical endocytosis. Cell Rep. 2014;8:10–19. doi: 10.1016/j.celrep.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 54.Finkelshtein D, Werman A, Novick D, et al. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng Y, Schouteden S, Geenens R, et al. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. PloS One. 2012;7:e47286. doi: 10.1371/journal.pone.0047286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharpe LJ, Brown AJ. Rapamycin down-regulates LDL-receptor expression independently of SREBP-2. Biochem Biophys Res Commun. 2008;373:670–674. doi: 10.1016/j.bbrc.2008.06.108. [DOI] [PubMed] [Google Scholar]
- 57.Ai D, Chen C, Han S, et al. Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. J Clin Investig. 2012;122:1262–1270. doi: 10.1172/JCI61919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cureton DK, Massol RH, Saffarian S, et al. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 2009;5:e1000394. doi: 10.1371/journal.ppat.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helliwell SB, Howald I, Barbet N, Hall MN. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics. 1998;148:99–112. doi: 10.1093/genetics/148.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt A, Kunz J, Hall MN. TOR2 is required for organization of the actin cytoskeleton in yeast. Proc Natl Acad Sci U S A. 1996;93:13780–13785. doi: 10.1073/pnas.93.24.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 62.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 63.Ohashi E, Tanabe K, Henmi Y, et al. Receptor sorting within endosomal trafficking pathway is facilitated by dynamic actin filaments. PloS One. 2011;6:e19942. doi: 10.1371/journal.pone.0019942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johannsdottir HK, Mancini R, Kartenbeck J, et al. Host cell factors and functions involved in vesicular stomatitis virus entry. J Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pelkmans L, Fava E, Grabner H, et al. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]


