1. Background
The Cancer Intervention and Surveillance Modeling Network (CISNET) (http://cisnet.cancer.gov) is a consortium of National Cancer Institute (NCI) – funded investigators that uses mathematical modeling to improve understanding of the impact of cancer control interventions (e.g., prevention, screening, and treatment) on population trends in incidence and mortality for a variety of cancer sites, including lung cancer. Modeling actual and hypothetical scenarios over time can yield insight into the key factors influencing cancer mortality. The resulting models can be used to project future trends, to help identify optimal cancer control strategies, and to help policy makers decide how best to allocate scarce public health resources.
The CISNET-lung modeling effort was conducted by the five groups funded directly through CISNET (Fred Hutchinson Cancer Research Center, Erasmus Medical Center, Pacific Institute for Research and Evaluation, Rice University/MD Anderson Cancer Center, Yale University), and one affiliate group (Massachusetts General Hospital). CISNET affiliates are funded through other mechanisms but were granted membership in the CISNET collaboration because of shared interests. Although each of the principal investigators has pursued research questions of individual interest, they agreed from the outset to be part of a consortium committed to meeting semi-annually to share and compare their respective models of the impact of tobacco smoking on lung cancer deaths in the U.S. between 1975 and 2000. A comparative modeling approach was employed, to examine differences between models in a systematic way. In this joint collaboration, a set of common population inputs was applied across all models and a common set of outputs was then compared across models.
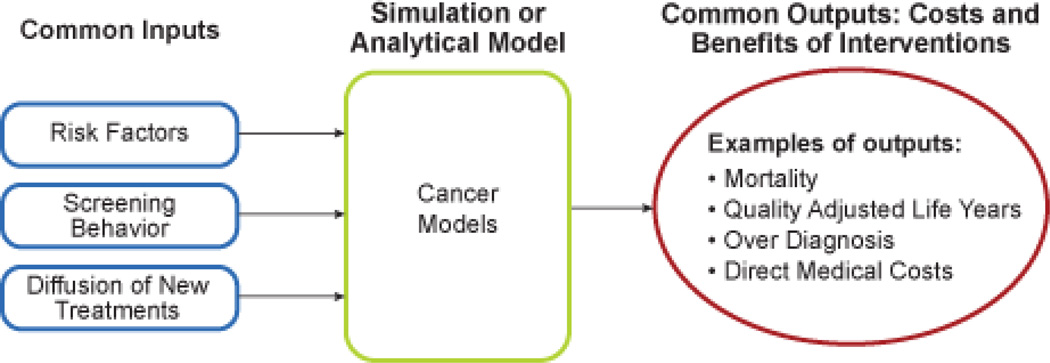
The principal goal for each individual CISNET investigator was to develop a comprehensive model of the cancer process, which, depending on the cancer and the specific model, could be modified by a range of cancer control interventions (i.e., changing risk factor profiles of the population through modification of cancer-causing behaviors, new early detection modalities, and treatment regimens). Models are either biologic (including the preclinical natural history of cancer), or epidemiologic (modeling only observed quantities as a person transitions from being the general population to being diagnosed with cancer to death from cancer or other causes). While both types of models predict epidemiologic outcomes, the biological models utilize latent biologic variables in a mechanistic model to predict incidence and mortality. Risk factors can modify the onset and progression rates of tumors (for biologic models) or the incidence rates of cancer (for epidemiologic models); screening can detect (and potentially remove) precancerous lesions and pre-clinical cancer (for biologic models) or identify the stage, size or grade of disease at diagnosis (for epidemiologic models); and treatment can alter post-diagnosis survival rates. Outputs range from incidence and mortality, to variations in cost, to unobservable quantities such as overdiagnosis and estimated lead time (although these latter quantities are only available in biologic models (Figure 1)). The collaborative work in CISNET was intended to develop important common applications of the model, the common inputs necessary to evaluate the reliability of these applications, to collate and interpret the results, and to determine the public health policy implications of the work and suggest future model refinements
Figure 1.
General formulation of CISNET models that can handle a wide range of cancer control intervention inputs.
Of the two major causes of death for those over the age of 30, deaths due to heart disease have been falling quite rapidly in the last 30 years, while cancer deaths have been falling more slowly.(1) Lung cancer now represents 15% of all incident cancers annually in the United States and 28% of all cancer deaths.(1–3) More U.S. men die from lung cancer than from any other cancer and, since 1987, more U.S. women die each year from lung cancer than from breast cancer--previously the most common cause of cancer death in women.(4) US lung cancer death rates have changed dramatically in the last 35 years. After increasing through the 1980s, the age-adjusted mortality rate for men has been declining steadily from a high of 90 per 100,000 in 1984 to about 70 per 100,000 in 2005.(5) After rising steeply in previous decades, the age-adjusted mortality rate for women leveled off in the 1990s, and has remained at about 40 per 100,000 since 1998.(5) Early detection and treatment of cancer has been critical to efforts to reduce deaths from such major cancers as breast cancer, colorectal cancer and melanoma.(6–8) Although promising new approaches are being tested(9), the impact to date of lung cancer early detection and treatment on a population level has been limited. The American Cancer Society’s annual report on cancer incidence and mortality(4) estimated that 222,520 Americans would be diagnosed with lung cancer in 2010, and more than three quarters of patients diagnosed with lung cancer will die within three years of diagnosis. Changes in behavioral factors, in particular the decline in cigarette smoking, have been identified as the major reason for the observed decline in the number of U.S. men being diagnosed with lung cancer.(5)
Before 1930 lung cancer was a rare disease in the US and in other countries.(10) The advent of the mass produced cigarettes at the turn of the century with attendant reduced cost made cigarettes more accessible to the public.(11) With aggressive marketing practices, cigarette consumption increased first among men, and then among young women and youth.(12) A number of innovations in the composition of cigarettes were introduced, including blended cigarettes, which were first successfully marketed in the US starting in 1913. These new tobacco products, which quickly became popular, were associated with deeper inhalation.(13) The availability of cigarettes among soldiers during World War I boosted usage among men, and by 1930, a majority of males were regular cigarettes smokers.(11) While prevalence was initially low, aggressive tobacco marketing targeted to women was associated with a steadily increasing female smoking prevalence during the next three decades.(14–15) Urbanization, the changing role of women, the changing composition of cigarettes, and other factors also contributed to the increased uptake of smoking before 1960.(11, 16)
After a series of retrospective studies were published in the early 1950s,(17–19) showing a strong relationship between smoking and lung cancer, prospective cohort studies (i.e. studies which identified a cohort of patients that characterized their smoking habits, following them over time recording diagnoses of cancer and/or their date and cause of death) were initiated as the next phase of research.(20–23) The relationship of smoking to lung cancer has subsequently been established by consistently corroborative biological and epidemiological studies.(24)
At a population level, lung cancer has been strongly linked to the smoking dose, as measured both in terms of the number of cigarettes smoked per day and especially the number of years smoked.(25–31) These studies are based primarily on large cohort studies, such as the Cancer Prevention Study (CPS)-I, the CPS-II, the Nurses Health /Health Professionals Follow-up Study and the British Doctors Study. However, none of these data adequately represent the US, because they over-represent middle class, married, White, and more educated Americans.(32–33) In addition, the relative risks of smoking appear to be changing over time. Comparing the CPS-I (covering the years 1959 through 1981) to the CPS-II (covering the years 1982 through 1988), the relative risks of lung cancer in smokers increased from 11.9 to 23.2 for men and from 2.7 to 12.8 for women.(34) While much of that change can be explained by the changes in cohort-related smoking behaviors, such as duration and intensity, a large portion of the difference is still unexplained.34–35 Changes in the design and composition of cigarettes have been proposed as an explanation, but the evidence about this hypothesis is mixed.(24) Another possible explanation is that the demographic characteristics of the average smoker have changed, with increasing disparities in educational attainment between the average smoker and nonsmoker.(36) Increasing disparities in education, in turn, are associated with increasing disparities in mortality.(37) While the risks from smoking may be changing over time, cigarette smoking has been established as the leading cause of lung cancer deaths, with almost 90% of lung cancer deaths attributable to smoking.(24) U.S. smoking prevalence has declined from a high of 53% for males and 32% for females in 196438 to the 2008 rates of 23% for males and 18% for females.(39)
Much of the reduction in smoking rates can be attributed to the adoption of tobacco control policies that increasingly restrict where people can smoke, youth’s ability to purchase tobacco products, and how much tax tobacco products must bear.(40–45) Beginning with the publication of the first Surgeon General’s Report on Smoking and Health in 1964,(23) efforts have been aimed at reducing smoking; bans have been placed on certain types of advertising, clean indoor air (“smoke-free”) laws have been implemented, media campaigns and other educational campaign have been implemented, cessation treatments have been developed and made more readily available, and higher taxes have been imposed on cigarettes and other tobacco products. Studies indicate that these policies have been successful at reducing smoking rates.(40–45) Applying a straightforward trend model to lung cancer deaths, Thun and Jemal(46) found that reductions in smoking were responsible for preventing over 146,000 male lung cancer deaths between 1991 and 2003, suggesting the importance of tobacco control policies. However, their study represented a straightforward projection of lung cancer deaths, and did not directly model the relationship between U.S. mortality trends and trends in smoking rates and the risk reduction ex-smokers gain as more years pass since they last smoked. Increased understanding in the second half of the 20th century of the relationship of smoking and lung cancer, and efforts to discourage the initiation of smoking among youths and encourage cessation have yielded public health success and lower lung cancer rates.(47) However, this success needs to be extended to the approximately 20% of U.S. adults who still smoke.
2. Purpose
To better understand the contribution of cigarette smoking and its changing role in lung cancer, this special issue of Risk Analysis considers the relationship between smoking and lung cancer death rates during the period 1975–2000 for U.S. men and women aged 30–84 years. While the basic relationships between smoking and lung cancer are well understood, the relationships between population trends in smoking, mortality from other causes, and mortality from lung cancer are more complex. To date there has been limited attention to how changes in current and former smoking prevalence explain these trends either at an aggregate level or by age group or cohort.
To provide the context for our efforts to characterize the impact of changing smoking rates on lung cancer mortality between 1975 and 2000, three types of model scenarios were considered. The first, labeled Actual Tobacco Control (ATC), models the impact of the observed trends in smoking on lung cancer mortality, and thus reflects the impact of the tobacco control policies that were actually implemented. A second scenario, called Complete Tobacco Control (CTC), represents a lower bound on the lung cancer mortality rates that could have been achieved if, just after the seminal first Surgeon General’s report in 1964,(23) all current smokers had quit their tobacco use for good and there was no further initiation of new smokers. This represents a hypothetical “best case” scenario, since in the early days of the tobacco control efforts, it was unclear what strategies would be most effective. A final scenario, No Tobacco Control (NTC) scenario represents a continuation of the smoking patterns that prevailed prior to the first studies in the 1950’s began to inform the public about the health consequences of smoking and prior to the subsequent tobacco control efforts that occurred in the second half of the 20th century. For this final scenario smoking patterns are held fixed based on the pattern of initiation and cessation that existed prior to 1955. This frozen “worst case” scenario provides a baseline against which the actual and potential effects could be measured, although it cannot be ascertained if in the absence of tobacco control efforts smoking rates may have climbed even higher.
The difference between the estimates from the ATC and NTC scenarios represents a more mechanistic extension of the work by Thun and Jemal(46) characterizing how many avoided lung cancer death rates in the United States can be attributed to reductions in tobacco smoking? The difference in the number of lung cancer deaths between the NTC and CTC scenarios represents the potential lung cancer deaths that could have been averted if tobacco control efforts had been immediately and completely effective. Using these three scenarios, each group is applying their respective model to estimating 1) the levels of smoking and associated lung cancer deaths that would have occurred if the tobacco control efforts starting mid-century had never been initiated, and 2) the number of lung cancer deaths that could have been avoided had tobacco control efforts been completely effective. The models also specifically consider how risks of former smokers decline with years quit.
3. The Models
The central component of each model is a dose-response module that provides a quantitative description of the age-specific mortality of lung cancer among never smokers, continuing smokers and former smokers by the duration and intensity of their smoking history. Five of the six models use the Two Stage Clonal Expansion Model (TSCE),(30–31, 48) although fit to different cohorts. The TSCE model, based on a mathematical formulation of the biological paradigm of initiation, promotion, and progression in carcinogenesis, recognizes that carcinogenesis is a process of mutation accumulation and clonal expansion of partially altered cells on the pathway to malignancy. The model may be used to generate biological hypotheses regarding the mechanism of tobacco-induced lung cancer and to explore the extent to which projected risks depend on the mechanism of smoking-induced lung cancer. These models have generally shown clonal expansion (promotion) of partially altered (initiated) cells by cigarette smoke is the dominant model mechanism, and have confirmed the disproportionate importance of smoking duration for predicting lung cancer risk.(30–31) The sixth and final model (Mass General Hospital) estimates the risk of lung cancer for 5 histologic types (adenocarcinoma, large cell, small cell, squamous, other) using 5 logistic regression equations that predict the development of the first cancer cell as a function of current age, years of smoking, cigarettes per day, and years since quitting. Growth of the tumor is estimated using a continuous Gompertz growth model specific to the histologic type.
Each of the models incorporate different types of stochastic extensions (beyond the TSCE model in 5 of the 6 models) to represent the various stochastic events that occur between the first malignant cell and death from lung cancer or other causes. For analyzing the impact of smoking on trends in lung cancer mortality, this extension could be as simple as a lag time distribution that measures the amount of time from the first malignant cell to death from lung cancer (in the absence of death from other causes). To model the benefits and harms of screening, the extension could assign stochastic events such as a cell type, a tumor growth rate, a stage and size at clinical detection, and post diagnostic survival. Details of the stochastic extension for each model are described in the model specific chapters in this monograph.
Four of the six models (Fred Hutchinson, Erasmus Medical Center, Massachusetts General Hospital, Rice-MD Anderson) can be characterized as individual cohort models, meaning that they utilize as inputs individual smoking histories from birth (including age at initiation, levels of smoking, age at cessation). They incorporate this information as part of a biologic model to determine when a lung cancer starts to grow, and when it becomes symptomatic. The remaining two of the six models (Yale and PIRE) can be characterized as group cross-sectional models, with inputs comprised of a cross-sectional picture defined by calendar year-specific, gender-specific, and age-specific proportions of the population stratified by smoking status (i.e., never smokers, current smokers, former smokers). In some instances these categories are further stratified (e.g., years since quitting for former smokers). These aggregated data are then used as part of an epidemiologic model to estimate the incident number of symptomatic lung cancers for each smoking status category.
The CISNET models estimated the relationship between smoking and lung cancer based on cohorts that are admittedly not perfectly representative of the US population, nor are they able to exhaustively model all smoking-related factors (e.g. changes in the content of cigarettes that have putatively changed the lethality of cigarettes), or other non-smoking-related factors (e.g. increasing remediation of household radon exposure and decreasing exposure to radon from the increasing proportion of people living on the second floor or above) that changed over time. To appropriately scale both trends and levels of lung cancer mortality rates it is therefore necessary to calibrate the ATC estimates to observed mortality. Once the ATC estimates are calibrated, these calibration factors can be used to adjust the NTC and CTC estimates. Based on these calibrated estimates (conducted in three models), actual numbers of lung cancer deaths and lung cancer deaths averted can be estimated.
Absolute measures (such as the actual count of lung cancer deaths averted) from independently formulated models are dependent on the cohort the model is fit to, and whether or not the model is calibrated to observed mortality. Therefore relative measures of the effect of policies have generally been more robust across models and are arguably more relevant.(49) The primary outcome metric used to compare the different models is the percentage of lung cancer deaths averted in the last quarter of the 20th century among the deaths that could have been averted if tobacco control efforts had been completely effective perfect. It is computed as:
[D(NTC) – D(ATC)] / [D(NTC) – D(CTC)] * 100
where D(x) represents the lung cancer deaths from 1975–2000 (ages 30–84) for a specific scenario. This metric summarizes not only how far we have come, but how far we have yet to go in our tobacco control efforts with respect to lung cancer. Of course, tobacco smoking causes premature death from other cancers besides lung cancer (e.g. head and neck cancers, bladder cancer, squamous cell cancer of the esophagus), and other diseases (e.g., heart disease, emphysema) besides cancer, but quantifying this is beyond the scope of this monograph. In addition to computing the cumulative results for 1975–2000, results are also computed for two more recent periods, i.e. 1991–2000 and for just the year 2000. The inclusion of 1991–2000 allows an approximate comparison to the results of Thun and Jemal(46) for the period 1991–2003., with the results shown here resulting in a much larger number of lung cancer deaths averted than the straight line projection of Thun and Jemal.
The focus of the models in the following chapters will be their ability to predict actual lung cancer rates and reflect the impact of tobacco control policies. While the epidemiologic relationships between smoking and lung cancer have been well characterized by the models of different groups, the distinct approaches employed here allow flexible extensions to other applications. The PIRE model has been used extensively to study the relationship between various tobacco control policies (e.g. taxation, clean in-door air policies, mass media campaigns, advertising bans, youth access policies, cessation treatment and coverage), and lung cancer mortality for states and various countries.(42–43, 50) The more biologically-based models that include a natural history component have been applied to evaluate the likely impact of policies advocating increased use of CT screening for lung cancer early detection on lung cancer mortality.(51)
4. Model Inputs
While detailed data by cohort are generally available on lung cancer mortality rates for decades, reliable data on cigarette smoking behaviors are not as readily available over a long time period. The studies in this volume utilize a unique, detailed data set developed specifically for the purpose of considering the relationship between variations in smoking prevalence and trends in lung cancer deaths. These data, which represent an update and extension of the work in Chapter 2: Cigarette Smoking Behavior in the United States from Monograph 8: Changes in Cigarette-Related Disease Risks and Their Implication for Prevention and Control, of the Smoking and Tobacco Control Monographs of the National Cancer Institute,(35) are a compilation of cross-sectional smoking histories from National Health Interview Surveys since 1965. These cross-sectional data have been smoothed and converted into birth cohort-specific sets of initiation and cessation rates after adjusting for the differential mortality among smokers who might not be alive to respond to a survey. To characterize individuals aged 30–84 years between 1975 and 2000, information on birth cohorts from 1890 to 1970 were required. Accurate smoking initiation data for cohorts born in the 19th century were not readily available from NHIS in part due to the age of this population when the surveys were conducted beginning in the mid-1960s and due to the relatively low frequency of cigarette smoking in the 19th century. Since we do not have exact information, we made a somewhat arbitrary assumption that initiation rates for birth cohorts 1890–1894 and 1895–1899 were 25% and 50% lower, respectively, than the 1900–1904 birth cohort. The impact of this arbitrary choice was considered to be small because (a) cigarette smoking rates are very low in these cohorts, especially in women, and (b) although these are the oldest individuals with the highest mortality rates, they only affect the earliest years of this analysis (ages 75–84 in 1975, 76–84 in 1976, …, 83–84 in 1983 and 84 in 1984), and (c) tobacco control efforts and the benefits of tobacco control on lung cancer were only beginning to be realized for these earliest birth cohorts. These smoking histories, when combined, represent the US experience in smoking in the very last part of the 19th century and most of the twentieth century.
Because the cancer models only simulate lung cancer initiation, diagnosis and death as a function of smoking histories (or a subset of these in the group cross-sectional models), it was necessary to develop birth cohort-specific other cause (i.e. non-lung cancer) mortality life tables as a function of smoking history. These life tables were developed starting with the Berkeley birth cohort life tables (http://www.demog.berkeley.edu/~bmd/index.html), and incorporated relative risks for all causes of death other than lung cancer from the CPS I and II studies conducted by the American Cancer Society,32 and smoking prevalence estimates from the National Health Interview Surveys. (http://www.cdc.gov/nchs/nhis.htm) The risk of death from lung cancer versus other causes is incorporated into the models using an assumption of conditional independence, i.e. the risk of death from other causes and lung cancer are assumed to be independent conditional on the person’s smoking history.
These two inputs (smoking histories and other cause mortality), are combined into a “Smoking History Generator”, which for a specific year of birth and specified gender randomly generates age at smoking initiation, a level of smoking (in quintiles of age and birth cohort distributions), age at cessation, and an age at non-lung cancer death (conditional on the smoking history). Smoking history generators were also developed for the complete tobacco control (CTC) and no tobacco control (NTC) scenarios, thus making a total of six versions of the generator (ATC, CTC, NTC for each gender). The output from the smoking history generator is directly used as inputs into the individual cohort models. For the group cross-sectional models, the smoking history generator is run repeatedly, and cross-sectional age-specific smoking prevalence estimates are cumulated. These smoking prevalence estimates (cross-tabulated to fit the needs of each model using factors such as number of years smoked and years elapsed since quit date), are used as inputs into these models.
5. Organization of the Monograph
This monograph is divided into three major sections. Section I (The Inputs) has chapters summarizing the smoking histories of the US population (Chapter 2), life tables for competing causes of mortality(Chapter 3), counterfactual smoking histories of the US population (i.e. the CTC and NTC counterfactuals) (Chapter 4), and lung cancer incidence and mortality among never smokers (Chapter 5). Section II (The Models) summarizes the results for each of the 6 models participating in this work: Massachusetts General Hospital (Chapter 7), Fred Hutchinson Cancer Research Center (Chapter 8), Erasmus Medical Center (Chapter 9), Yale (Chapter 10), Pacific Institute for Research and Evaluation (the SIMSMOKE model) (Chapter 11), and Rice-MD Anderson (Chapter 12). These model descriptions are supplemented by online Model Profiles (http://cisnet.cancer.gov/profiles/), in the form of structured documentation conforming to a common template, allowing for easy cross-model comparisons. Section III (Model Comparisons) first compares model assumptions and structure (Chapter 13). Because all but one of the six models described in this monograph use the TSCE model (albeit calibrated to different cohorts), we consider how well two other commonly used models of the relationship between smoking and lung cancer(28–29) compare to the TSCE model in explaining historical trends in lung cancer (Chapter 14). Chapter 15 presents a summary of results across all of six models and a summary and discussion of the results.
Acknowledgments
This work was supported by the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) lung cancer group, under grant numbers U01CA097450, U01CA097432, U01CA097415, U01CA097431, U01CA097416, and U01CA152956.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA-Cancer J. Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA-Cancer J. Clin. 2004 Jan-Feb;54(1):8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Wingo PA, Ries LAG, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J. Natl. Cancer Inst. 1999 Apr;91(8):675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA-Cancer J. Clin. 2010 Jul 7; doi: 10.3322/caac.20073. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Thun MJ, Ries LAG, et al. Annual Report to the Nation on the Status of Cancer, 1975–2005, Featuring Trends in Lung Cancer, Tobacco Use, and Tobacco Control. J. Natl. Cancer Inst. 2008 Dec 3;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for Breast Cancer: Recommendation statement. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [Google Scholar]
- 7.U.S. Preventive Services Task Force. Screening for Colorectal Cancer: Recommendation statement. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 8.U.S. Preventive Services Task Force. Screening for Skin Cancer: Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [Google Scholar]
- 9.Bach PB, Niewoehner DE, Black WC. Screening for lung cancer - The guidelines. Chest. 2003 Jan;123(1):83S–88S. doi: 10.1378/chest.123.1_suppl.83s. [DOI] [PubMed] [Google Scholar]
- 10.Wynder EL, Hoffmann D. Smoking and lung cancer - scientific challenges and opportunities. Cancer Res. 1994 Oct;54(20):5284–5295. [PubMed] [Google Scholar]
- 11.Kluger R. Ashes to Ashes. New York: Alfred Knopf; 1996. [Google Scholar]
- 12.Pierce JP, Gilpin EA. A historical analysis of tobacco marketing and the uptake of smoking by youth in the United States - 1890–1977. Health Psychol. 1995 Nov;14(6):500–508. doi: 10.1037//0278-6133.14.6.500. [DOI] [PubMed] [Google Scholar]
- 13.Slade J. The tobacco epidemic - lessons from history. J. Psychoact. Drugs. 1989 Jul-Sep;21(3):281–291. doi: 10.1080/02791072.1989.10472169. [DOI] [PubMed] [Google Scholar]
- 14.Ernster V. Mixed messages for women, A social history of cigarette smoking and advertising. New York State Journal of Medicine. 1985;85(7):335–340. [PubMed] [Google Scholar]
- 15.Brandt AM. Recruiting women smokers: the engineering of consent. Journal of the American Medical Women's Association. 1996;51(1–2):63–66. [PubMed] [Google Scholar]
- 16.Giovino GA. The tobacco epidemic in the United States. Am. J. Prev. Med. 2007 Dec;33(6):S318–S326. doi: 10.1016/j.amepre.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Wynder EL, Graham EA. Tobacco Smoking As A Possible Etiologic Factor In Bronchiogenic Carcinoma - A Study Of 684 Proved Cases. JAMA-J. Am. Med. Assoc. 1950;143(4):329–336. doi: 10.1001/jama.1950.02910390001001. [DOI] [PubMed] [Google Scholar]
- 18.Doll R, Hill AB. Smoking And Carcinoma Of The Lung - Preliminary Report. Br. Med. J. 1950;2(4682):739–748. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doll R, Hill AB. A Study Of The Aetiology Of Carcinoma Of The Lung. Br. Med. J. 1952;2(4797):1271–1286. doi: 10.1136/bmj.2.4797.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thun MJ. When truth is unwelcome: the first reports on smoking and lung cancer. Bulletin of the World Health Organization. 2005;83:144–145. [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond EC. Smoking in relation to lung cancer. Connecticut State Medical Journal. 1954;18:3–9. [PubMed] [Google Scholar]
- 22.Doll R, Hill AB. Mortality in relation to smoking-- 10 years observations of British doctors. Br. Med. J. 1964;1(539) doi: 10.1136/bmj.1.5395.1399. 1399-&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health Education and Welfare. "Smoking and Health". In: Surgeon General's Advisory Committee on Smoking and Health aUSPHSOotSG, editor. United States: Public Health Service. Office of the Surgeon General; 1964. p. 386. [Google Scholar]
- 24.U.S. Department of Health and Human Services. The Health Consequences of Smoking. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 25.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. Br. Med. J. 2004 Jun;328(7455):1519–1528. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. MORTALITY IN RELATION TO SMOKING-- 40 YEARS OBSERVATIONS ON MALE BRITISH DOCTORS. Br. Med. J. 1994 Oct;309(6959):901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoke JD, Burns DM, Thun MJ. The change in excess risk of lung cancer attributable to smoking following smoking cessation: an examination of different analytic approaches using CPS-I data. Cancer Causes Control. 2008 Mar;19(2):207–219. doi: 10.1007/s10552-007-9086-5. [DOI] [PubMed] [Google Scholar]
- 28.Knoke JD, Shanks TG, Vaughn JW, Thun MJ, Burns DM. Lung cancer mortality is related to age in addition to duration and intensity of cigarette smoking: An analysis of CPS-I data. Cancer Epidemiol. Biomarkers Prev. 2004 Jun;13(6):949–957. [PubMed] [Google Scholar]
- 29.Flanders WD, Lally CA, Zhu BP, Henley SJ, Thun MJ. Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: Results from Cancer Prevention Study II. Cancer Res. 2003 Oct;63(19):6556–6562. [PubMed] [Google Scholar]
- 30.Hazelton WD, Clements MS, Moolgavkar SH. Multistage carcinogenesis and lung cancer mortality in three cohorts. Cancer Epidemiol. Biomarkers Prev. 2005 May;14(5):1171–1181. doi: 10.1158/1055-9965.EPI-04-0756. [DOI] [PubMed] [Google Scholar]
- 31.Meza R, Hazelton WD, Colditz GA, Moolgavkar SH. Analysis of lung cancer incidence in the nurses' health and the health professionals' follow-up studies using a multistage carcinogenesis model. Cancer Causes Control. 2008 Apr;19(3):317–328. doi: 10.1007/s10552-007-9094-5. [DOI] [PubMed] [Google Scholar]
- 32.Garfinkel L. Selection, follow-up, and analysis in the American Cancer Society prospective studies. National Cancer Institute Monographs. 1985;67:49–52. [PubMed] [Google Scholar]
- 33.Stellman SD, Garfinkel L. SMOKING-HABITS AND TAR LEVELS IN A NEW AMERICAN-CANCER-SOCIETY PROSPECTIVE-STUDY OF 1.2 MILLION MEN AND WOMEN. J. Natl. Cancer Inst. 1986 Jun;76(6):1057–1063. [PubMed] [Google Scholar]
- 34.Thun MJ, Heath CW. Changes in mortality from smoking in two American Cancer Society prospective studies since 1959. Prev. Med. 1997 Jul-Aug;26(4):422–426. doi: 10.1006/pmed.1997.0182. [DOI] [PubMed] [Google Scholar]
- 35.Thun M, Day-Lally C, Myers DG, et al. Trends in tobacco smoking and mortality from cigarette use in Cancer Prevention Study I (1959–1965) and II (1982–1988) In: National Cancer Institute, editor. Tobacco Control Monograph #8. 1997. pp. 305–382. [Google Scholar]
- 36.Meara ER, Richards S, Cutiew DM. The gap gets bigger: Changes on mortality and life expectancy, by education, 1981–2000. Health Aff. 2008 Mar-Apr;27(2):350–360. doi: 10.1377/hlthaff.27.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zajacova A. Education, gender, and mortality: Does schooling have the same effect on mortality for men and women in the US? Soc. Sci. Med. 2006 Oct;63(8):2176–2190. doi: 10.1016/j.socscimed.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Department of Health Education and Welfare (USDHEW) Smoking and Health - A report of the Surgeon General. Washington DC: U.S. Department of Health Education, and Welfare. Public Health Service; 1979. [Google Scholar]
- 39.Centers for Disease Control (CDC) Cigarette Smoking Among Adults and Trends in Smoking Cessation — United States, 2008. Morbidity Mortality Weekly Report. 2009;58(44):1227–1232. [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services. Reducing tobacco use: a report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2000. [Google Scholar]
- 41.Levy DT, Nikolayev L, Mumford E. Recent trends in smoking and the role of public policies: results from the SimSmoke tobacco control policy simulation model. Addiction. 2005 Oct;100(10):1526–1536. doi: 10.1111/j.1360-0443.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 42.Levy DT, Chaloupka F, Gitchell J. The effects of tobacco control policies on smoking rates: a tobacco control scorecard. J Public Health Manag Pract. 2004 Jul-Aug;10(4):338–353. doi: 10.1097/00124784-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Levy DT, Ross H, Powell L, Bauer JE, Lee HR. The role of public policies in reducing smoking prevalence and deaths caused by smoking in Arizona: results from the Arizona tobacco policy simulation model. J Public Health Manag Pract. 2007 Jan-Feb;13(1):59–67. doi: 10.1097/00124784-200701000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Fichtenberg CM, Glantz SA. Association of the california tobacco control program with declines in cigarette consumption and mortality from heart disease. N. Engl. J. Med. 2000 Dec;343(24):1772–1777. doi: 10.1056/NEJM200012143432406. [DOI] [PubMed] [Google Scholar]
- 45.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. Br. Med. J. 2002 Jul;325(7357):188–191. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thun MJ, Jemal A. How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking? Tob. Control. 2006 Oct;15(5):345–347. doi: 10.1136/tc.2006.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cowling DW, Kwong SL, Schlag R, Lloyd JC, Bal DG. Declines in Lung Cancer Rates --- California, 1988–1997. Morbidity Mortality Weekly Report. 2000;49:1066–1069. [PubMed] [Google Scholar]
- 48.Moolgavkar SH, Knudson AG. Mutation and cancer - a model for human carcinogenesis. J. Natl. Cancer Inst. 1981;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- 49.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of Mammography Screening Under Different Screening Schedules: Model Estimates of Potential Benefits and Harms. Ann. Intern. Med. 2009 Nov;151(10) doi: 10.1059/0003-4819-151-10-200911170-00010. 738-W247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levy DT, Bales S, Lam NT, Nikolayev L. The role of public policies in reducing smoking and deaths caused by smoking in Vietnam: Results from the Vietnam tobacco policy simulation model. Soc. Sci. Med. 2006 Apr;62(7):1819–1830. doi: 10.1016/j.socscimed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 51.McMahon PM, Kong CY, Weinstein MC, et al. Adopting Helical CT Screening for Lung Cancer Potential Health Consequences During a 15-Year Period. Cancer. 2008 Dec;113(12):3440–3449. doi: 10.1002/cncr.23962. [DOI] [PMC free article] [PubMed] [Google Scholar]