1 INTRODUCTION
1.1 Progress and challenges in the new age of engineering immunity
The immune system plays a critical role in our health. No other component of human physiology plays a decisive role in as diverse an array of maladies, from deadly diseases with which we are all familiar to equally terrible esoteric conditions: HIV, malaria, pneumococcal and influenza infections; cancer; atherosclerosis; autoimmune diseases such as lupus, diabetes, and multiple sclerosis. The importance of understanding the function of the immune system and learning how to modulate immunity to protect against or treat disease thus cannot be overstated. Fortunately, we are entering an exciting era where the science of immunology is defining pathways for the rational manipulation of the immune system at the cellular and molecular level, and this understanding is leading to dramatic advances in the clinic that are transforming the future of medicine.1,2 These initial advances are being made primarily through biologic drugs– recombinant proteins (especially antibodies) or patient-derived cell therapies– but exciting data from preclinical studies suggest that a marriage of approaches based in biotechnology with the materials science and chemistry of nanomaterials, especially nanoparticles, could enable more effective and safer immune engineering strategies. This review will examine these nanoparticle-based strategies to immune modulation in detail, and discuss the promise and outstanding challenges facing the field of immune engineering from a chemical biology/materials engineering perspective.
1.1.1 Key cellular actors in the immune system
A brief summary of the cellular players in the immune response is worthwhile to preface the many immunomodulatory approaches described in this review. The immune system can be viewed at a high level as a collection of mobile cells that include members that traffic throughout the body in search of invading pathogens as well as cells that reside as sentinels at portals of entry (i.e. the airways, skin, gastrointestinal tract, etc.).3 These cells belong to one of two major arms, the innate immune system and adaptive immune system. Innate immune cells such as neutrophils and macrophages are poised to rapidly respond to pathogen invasion, expressing receptors that recognize conserved molecular motifs characteristic of bacteria, viruses, and fungi, to quickly phagocytose (internalize) microbes and secrete reactive oxygen species or cytokines that provide an immediate response to invading pathogens. The adaptive immune system is comprised of T-cells and B-cells, including CD4+ helper T-cells that secrete cytokines to direct the functions of innate cells, killer cells, and B-cells; and CD8+ killer T-cells that recognize and destroy infected or transformed cells. B-cells play a canonical role in vaccine responses by producing antibodies that bind to and neutralize the ability of microbes to invade host cells and/or promote their phagocytosis. The adaptive immune system is so named because of the clonal nature of T and B lymphocytes– each T-cell and B-cell expresses a unique T-cell receptor or B-cell receptor, respectively, which is generated in part by a process of stochastic DNA recombination, enabling the pool of lymphocytes the potential to recognize any microbial antigen they may encounter.4 When a T- or B-cell binds an antigen (essentially, any biological molecule from a microbe that is recognized by a T-cell receptor (TCR) or B-cell receptor (BCR)), this triggers massive proliferation of the antigen-specific cell, generating a pool of effectors within ~7 days following exposure. These effector T-cells and B-cells play an important role in backing up innate immune defenses to clear the invading pathogen. Following pathogen clearance, the majority of these cells (~90%) undergo programmed cell death, leaving a small pool of differentiated memory cells that can remain for the lifetime of the individual, to provide rapid recall protection if the same microbe is ever encountered again.5 A final key group of immune cells are the antigen presenting cells (APCs), and particularly a critical APC known as the dendritic cell, which is responsible for activating naïve T-cells (and in some cases B-cells).6,7 Dendritic cells (DCs) are innate-like cells that reside in all peripheral tissues, and which act as sentinels, collecting antigens from the surrounding fluid and staying on constant alert for “danger signals”- molecular motifs signifying tissue damage or pathogen invasion. DCs and other immune cells express a host of receptors that specifically recognize danger signals to trigger their activation; the most studied among these receptors are the Toll-like receptors.8 If activated by danger signals, DCs migrate from their home tissue through the lymphatic vessels to local draining lymph nodes, where they physically present antigen to T-cells and B-cells. For T-cell activation, this is through the loading of short (8–15 amino acids) peptide fragments of antigens into the cleft of major histocompatibility complex (MHC) molecules displayed on the DC surface. These peptides are surveyed by the TCRs of T-cells, and on finding a cognate peptide, T-cells become activated by the DC to proliferate and carry out their effector functions.
The vastly complex set of cellular interactions summarized above (greatly oversimplified) is the network of interest to those interested in immune engineering, and in this review we aim to summarize the myriad ways in which materials scientists, chemical engineers, bioengineers, chemists, and physicists (often in collaboration with immunologists) are using nanomaterials as powerful tools to probe or manipulate immune responses for therapeutic ends. To set the stage for the rest of the review, we will briefly discuss two of the areas where synthetic nanoparticles have the prospect to play a significant role in the ongoing revolution of immunology in medicine– vaccines and active immunotherapy.
1.1.2 Designing new vaccines
Vaccines are pharmaceutical preparations of antigens– macromolecules derived from pathogens– which when administered to the body elicit an immune response and establish immunologic memory of the antigen, usually in the form of long-lived production of antibodies against the antigen.9 A strong case can be made that vaccines are the single most impactful medical technology to be developed in history– as vaccines have saved hundreds of millions of lives in the past century.10,11 The introduction of an effective new vaccine has been repeatedly shown to have an immediate, dramatic impact on the frequency of disease in a given population, as illustrated by the annual cases of polio and measles in the United States before and after the introduction of their respective vaccines (Fig. 1).12,13 Prophylactic vaccines (administered to healthy individuals to protect against future exposures) have successfully eradicated or greatly reduced the frequency of infectious diseases that, at the beginning of the 20th century, exacted a serious toll in deaths and morbidity, including diphtheria, tetanus, measles, polio, smallpox, mumps, rubella, and typhoid, to name a few. These successes make the value of an effective vaccine very clear. However, successful vaccines remain elusive for a number of important diseases, including HIV, tuberculosis, and malaria.10 These diseases share a number of features that are distinct from those for which existing vaccine strategies have been successful.14 Successful vaccines have been generated primarily for diseases that cause acute infections, and for which the natural immune response in a fraction of persons will be protective and establish life-long protective immunity, mediated in the vast majority of cases by neutralizing antibodies (produced by B-cells and their progeny, plasma cells). By contrast, diseases for which we do not yet have an effective vaccine often establish chronic infections, which the immune system of unvaccinated individuals is unable to eradicate, and which do not induce protective immune memory against re-exposure.15 Further, T-cells, in addition to B-cells, are thought to potentially be important in attacking these pathogens.16 This problem highlights the challenge facing modern vaccine design– we must develop vaccines that can achieve what the natural immune system cannot, potentially employing both arms of adaptive immunity.
Figure 1.
Examples of the impact of vaccination on disease burdens in the United States. (A) Poliomyelitis before and after introduction of the polio vaccines.12 Reprinted form reference 12. (B) Measles cases before and after introduction of the measles vaccine.13 Reprinted from Center for Disease Control and Prevention: Vaccines and Immunizations Publications. Parents guide to childhood immunization http://www.cdc.gov/vaccines/pubs/parents-guide/downloads/parents-guide-part3.pdf#page=10 (accessed Feb 1, 2015).
A second major challenge for current vaccine efforts is the development of effective therapeutic vaccines that can treat established disease. Therapeutic vaccines could impact not only infectious diseases such as HIV and tuberculosis, but also non-infectious diseases such as cancer.17,18 In addition, the possibility of vaccines that shut off immune responses to target antigens instead of turning them on has been proposed, as a means to eliminate unwanted immune reactions in autoimmune disease and allergies.10 Therapeutic vaccines for cancer and autoimmune disease introduce the additional complexity that they aim to employ the immune system, which has evolved to deal with infectious disease, to instead treat non-infectious disorders.
1.1.3 Capitalizing on the promise of immunotherapy
Vaccines represent an intervention with long-established benefits to public health, based on inoculation of the immune system with a target antigen in a way that induces immunological memory to protect against future exposures to the source pathogen. However, in the presence of existing disease, there are other routes to instructing the immune system to attack malfunctioning or tumorigenic cells, broadly termed immunotherapies. Immunotherapies have been pursued for a broad range of diseases, but arguably the greatest effort has been invested in the development of therapeutics that prime the immune system to attack cancer;2,19,20 for the purpose of setting the stage for later discussions in this review, we will focus on this specific case as an example of current treatment strategies in the clinic.
A diverse set of approaches has been taken to develop cancer immunotherapies, including the administration of therapeutic vaccines, recombinant cytokines, immunomodulatory antibodies, small molecule drugs, and adoptively transferred immune cells. Among these various approaches, two strategies have recently been proven to be capable of dramatic impacts in advanced cancer patients: treatment of patients with so-called checkpoint blockade antibodies, and adoptive transfer of chimeric antigen receptor T-cells. Checkpoint blockade refers to the use of antibodies that block negative regulatory receptors on T-cells, in effect “taking the brakes off” the immune system and allowing endogenous natural immune responses against tumors to be unveiled. Two different checkpoint blockade treatments targeting different receptors (CTLA-4 and PD-1) have recently been approved by the FDA, on the basis of striking clinical trial results in melanoma, renal cell carcinoma, and lung cancer (and several additional similar therapeutics seem likely to be approved soon on the basis of ongoing trials).5,11,21,22Anti-PD-1 antibodies in particular have shown striking tumor regression in a subset of patients, even against traditionally difficult-to-treat tumors like non-small cell lung cancer.22 While checkpoint blockade therapies energize the native immune response against cancer, adoptive transfer of chimeric antigen receptor (CAR) T-cells represents true engineering of immune responses: Here, T-cells isolated from the blood of patients are genetically engineered to express synthetic receptors (CARs) based on a fusion of an antibody binding domain with T-cell signaling domains, replacing the natural TCR with a CAR that binds to surface-expressed proteins on target tumor cells. In clinical trials of leukemia, CAR therapy has resulted in remarkable tumor regressions, enabling complete tumor eradication in ~80% of treated patients.23 These advances provide crucial proof of principle that the immune system is capable of safely eliminating massive tumor burdens in patients that have no other option, and have signaled the beginning of an immunotherapy-led revolution in cancer treatment.
1.2 Guiding principles motivating synthetic nanomaterials in immune engineering
The purpose of this review is to highlight the many ways in which nanoparticle chemistry and engineering are being applied to tackle challenges in vaccine development and to build on recent successes in immunotherapy. With the exception of cell-based therapies being pursued in cancer immunotherapy, all of the immune engineering strategies currently in the clinic are based on traditional drug development approaches- antibodies, recombinant proteins, small molecule drugs. So what is the inspiration for novel engineered materials for this purpose? There are a number of over-arching themes that drive this work, which recur throughout this review. Some of these motivating concepts are common to other therapeutic areas where nanoparticles are of significant interest, such as cancer nanomedicine or nucleic acid delivery,24 while others are unique to the distributed cellular network that makes up the immune system. First is the principle that immunomodulatory compounds must reach their target cell types to exert their effects, and nanoparticle carriers can greatly increase the localization of these drugs in target lymphoid tissues or within specific immune cells, and thereby dramatically increase their potency.25 With this enhanced tissue and/or cellular targeting, these engineered vaccines and immunotherapies can also exhibit greatly enhanced safety.14,26–28 While safety is almost always considered secondary to efficacy in animal models of immunotherapy, it is the primary driver in clinical prophylactic vaccine development. In addition, because many immunomodulatory drugs such as recombinant cytokines act on diverse immune cell types, many of these agents have failed as systemically-administered treatments even in oncology, due to the severe toxicity of “on-target but off-tumor” side effects.29–31 Nanoparticle formulations offer the potential of making these powerful signaling agents effective for modulating the right target cells at the right location, and ablating the severe toxicity often associated with immunomodulators. Nanoparticle forms of antigens and immunomodulatory compounds can also change the function of these agents– by promoting multivalent receptor crosslinking, by altering intracellular processing, by promoting cytosolic delivery, or by physically co-localizing synergistic cues within the same intracellular compartment or cell surface site.1 Finally, nanomaterials can themselves have intrinsic immunomodulatory function, acting as adjuvants or immune potentiators. This last area is just beginning to be explored and offers the potential not only for new therapeutics but also may lead to new levels of understanding how the immune system defines “danger signals”. A broad range of synthetic nanomaterials are being studied as platforms to achieve these goals, and will be discussed throughout the review (a non-exhaustive summary of some of the most-studied materials is provided in Table 1). Promising preclinical data in these many different strategies for shaping immune responses via synthetic materials suggest that nanomaterials will have an important role to play in the future of vaccines and immunotherapy.
Table 1.
Synthetic nanoparticle characteristics for immunological applications
| Nanomaterial | Advantages | Challenges | Size range |
Refs |
|---|---|---|---|---|
| Gold nanoparticles |
|
|
10–100nm | a, b, 335 |
| Carbon nanotubes |
|
|
50–400nm | c, 230 |
| Dendrimers |
|
|
10–30nm | d, 131 |
| Solid polymer particles |
|
|
Varies | e, 139, 254, 257 |
| Polymer micelles |
|
|
10–100nm | e, 256 |
| Liposomes |
|
|
30–200nm | f, g, 110 |
Additional References:
Dykman, L. A.; Khlebtsov, N. G. Gold Nanoparticles in Biology and Medicine: Recent Advances and Prospects. Acta Naturae 2011, 3, 34–55.
Lin, A. Y.; Almeida, J. P. M.; Bear, A.; Liu, N.; Luo, L.; Foster, A. E.; Drezek, R. A. Gold Nanoparticle Delivery of Modified CpG Stimulates Macrophages and Inhibits Tumor Growth for Enhanced Immunotherapy. PLoS One 2013, 8, e63550.
Gottardi, R.; Douradinha, B. Carbon Nanotubes as a Novel Tool for Vaccination against Infectious Diseases and Cancer. J. Nanobiotechnology 2013, 11, 30.
Heegaard, P. M. H.; Boas, U.; Sorensen, N. S. Dendrimers for Vaccine and Immunostimulatory Uses. A Review. Bioconjug. Chem. 2010, 21, 405–418.
Sah, H.; Thoma, L. A.; Desu, H. R.; Sah, E.; Wood, G. C. Concepts and Practices Used to Develop Functional PLGA-Based Nanoparticulate Systems. Int. J. Nanomedicine 2013, 8, 747–765.
Kelly, C.; Jefferies, C.; Cryan, S.-A. Targeted Liposomal Drug Delivery to Monocytes and Macrophages. J. Drug Deliv. 2011, 2011, 727241.
Giddam, A. K.; Zamman, M.; Skwarczynski, M.;Toth, I. Liposome-Based Delivery System for Vaccine Candidates: Constructing an Effective Formulation. Nanomedicine 2012, 7(12), 1877–1893.
2 Nanoparticles regulating immunity at the single-cell level
Through years of research, many of the immunological mechanisms through which vaccines and immunotherapies interact with the innate and adaptive immune systems at the single cell level have been thoroughly characterized. In parallel several advances in chemistry and material science have made it possible to engineer synthetic materials to manipulate biological functions of cells32. There are three broad strategies that have been pursued to date in using nanoparticles to modulate immune responses at the single-cell level: In the first approach, nanoparticles are directly attached to or allowed to be internalized by immune cells ex vivo, thereby arming these cells for subsequent injection in vivo, where the nanoparticle cargo can release drugs that direct immune cell function or impart new functionalities to therapeutic cells. A second strategy is to exploit the “natural targeting” of nanoparticles to phagocytic cells in vivo, injecting free particles that are scavenged by monocytes, macrophages, dendritic cells, or neutrophils in the blood, spleen, liver, bone marrow, or other target tissue sites. Finally, so-called “active” targeting has been explored, whereby specific ligands or antibodies on the surface of nanoparticles are used to direct binding to specific cell targets in vivo. In this section, we discuss examples of these three approaches and various promising nanoparticle-based strategies to target different innate immune and adaptive immune populations.
2.1 Targeting the innate immune system
2.1.1 Modulating macrophages and monocytes
Macrophages are a highly heterogeneous class of phagocytic cells distributed in all tissues throughout the body such as the lungs (alveolar macrophages), liver (Kupffer cells), spleen, and bone marrow. These cells differentiate from immature monocytes that are in the bone marrow and blood circulation. Macrophages can engulf pathogens and apoptotic cells, process and present antigens and release cytokines to initiate and regulate the adaptive immune response. Macrophages are amongst the first immune cells to be recruited to sites of tissue injury or infection33 and play a central role in mediating inflammation which can either be host protective in the short term, or over prolonged periods result in inflammatory pathologies like atherosclerosis, inflammatory bowel disease, chronic obstructive pulmonary disorder (COPD) and tumor growth and metastasis. As macrophages differentiate, they can acquire a spectrum of different phenotypes depending on the factors present in their microenvironment. The ends of this spectrum of phenotypes are defined by ‘M1’ or classically activated macrophages that mediate host defense against pathogens and anti-tumor immunity, and ‘M2’ or alternatively activated macrophages that function during wound healing and promote tumor growth34. Both M1 and M2 macrophages actively endocytose and phagocytose material from their surroundings35. Due to their phagocytic nature, passive targeting of macrophages with synthetic nanoparticles is easily achieved.36 As different subsets and phenotypes of macrophages were identified and macrophage receptor expression characterized, targeting strategies have also been developed through surface engineering of particles with ligands that can bind to specific macrophage receptors.
A first approach to dose phagocytic cells with nanoparticles carrying immunoregulatory drugs has been to “feed” macrophages or monocytes with particulate cargo ex vivo, followed by injection of the particle-laden cells to allow their homing to tissue sites in vivo. In this way, phagocytes can be used as delivery vehicles to transport therapeutics to various disease sites since monocytes and macrophages migrate to sites of inflammation.33 The anti-retroviral (ARV) protease inhibitor idinavir (IDV) was delivered using this strategy with promising results in a humanized mouse model of HIV infection (Fig. 2)37 and HIV-induced encephalitis.38 To load bone marrow derived macrophages (BMDM) with IDV nanoparticles, they were incubated with IDV loaded in a phospholipid nanosuspension. Injection of these IDV-loaded BMDMs resulted in higher concentrations of IDV maintained in tissues and serum, and led to reduced HIV viral loads and improved CD4:CD8 T-cell ratios compared to systemic administration of the free drug.37 While results with nanoparticle-loaded macrophages for ARV drug delivery are promising, the clinical complexities of cell therapies make it likely that the first clinical testing of nanoparticulate ARVs will be done using injection of free nanoparticle forms of these drugs. Commercial formulations of polymer-stabilized solid drug nanoparticles of the ARV rilpivarine have recently been tested for biodistribution and pharmacokinetic behavior in rats and dogs and show lymphoid tissue deposition that suggests uptake by macrophages in vivo following i.v. injection.39 These results set the stage for clinical testing of such formulations as candidate long-acting ARV treatments.
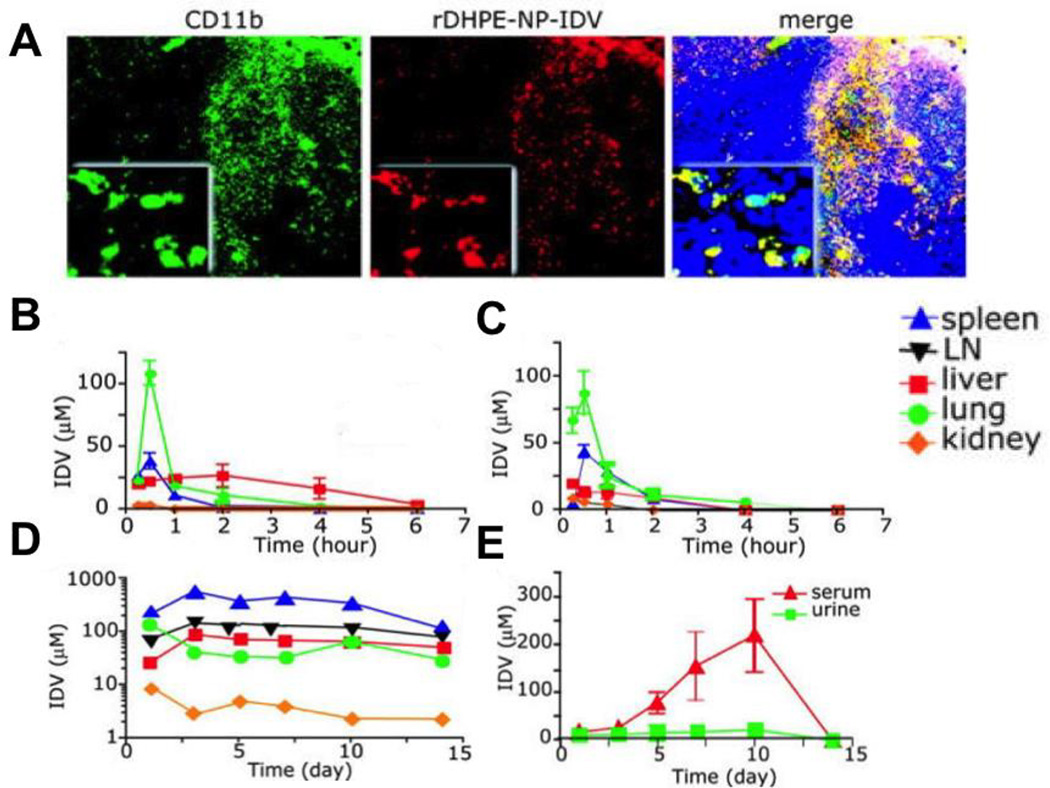
Figure 2.
Delivery of idinavir (IDV) nanoparticles loaded into bone marrow derived macrophages (BMDMs) results in increased serum and tissue drug concentrations. (A) Sections of spleen from mice at day 5 after the transfer of rhodamine-labeled idinavir nanoparticle loaded bone marrow derived macrophages (IDV-loaded BMDMs). Sections were stained for CD11b and examined by fluorescence microscopy. Higher magnification inserts demonstrate the co-localization of BMDM-IDVs (Red) with the cytoplasm of CD11b+ cells (Green). BMDMs appear yellow in the merged panel and nuclei are stained in blue. (B–E) IDV distribution in targeted tissues and body fluids was assessed in mice treated with a single dose of (B) IDV free drug solution (C) Cell- free IDV nanoparticles (D–E) BMDM-IDVs. IDV concentrations in mice treated with BMDM-IDVs were increased and prolonged over 14 days. Data represent mean ± SEM for n = 4 mice per group per time point. Magnifications are (originals) × 100 and (insets) × 400.37 Reprinted with permission from reference 37. Copyright 2006 American Society of Hematology.
Macrophages and monocytes also home to hypoxic regions of tumors.40 Macrophages loaded with liposome-encapsulated chemotherapeutics resulted in increased drug delivery to tumors compared to systemic administration of liposomes.41 In a model of glioblastoma, intra-cranial injection of cyclodextrin-based NPs resulted in their accumulation at tumor sites through transport by tumor-associated macrophages (TAMs).42 While it is clear that macrophages can be used to enhance the delivery of NPs to tumors, further work is required to optimize the in vivo therapeutic efficacy of this strategy.
Passive targeting of macrophages involves injecting particles with optimal shape,43 size and surface charge44 systemically to access monocytes and macrophages in specific tissue compartments. For optimal phagocytosis, particles require a shape with high length normalized curvature43 and a negative surface charge,45 however this can differ with the material being used. Depending on the material composition of the nanoparticle and the kind of macrophage it interacts with, the size range for particles being phagocytosed can vary from 85 nm to 10 µm.46–48 Within this size range, smaller nanoparticles (100–300 nm) are internalized less efficiently than larger nanoparticles (2–3 µm).45
The surface charge of nanoparticles also influences their uptake by phagocytic cells. While phagocytic cells can take up both cationic and highly anionic particles44, cationic particles can cause cytotoxicity due to plasma membrane disruption, production of reactive oxygen species (ROS) and inflammatory responses.45,47,49 However, this generalization is not universal, since certain anionic nanoparticles can also cause cytotoxicity when internalized by macrophage cell lines.50 Ultimately, it is the combination of multiple physical parameters including size, surface charge, hydrophobicity and material composition that influence cellular uptake and cytotoxicity of nanoparticles.
Following intravenous injection, particulate matter is typically removed from systemic circulation by specialized macrophages of the spleen and liver (Kupffer cells) due to the open fenestrations of endothelial cells and sinusoids in the spleen and liver resulting in greater access of macrophages to circulating blood.51 For example, this strategy was recently used to deliver siRNA to silence the regulatory receptor programmed death ligand-1 (PD-L1) expressed by Kupffer cells and sinusoidal endothelial cells in the liver.52 PD-L1 is a ligand for the inhibitory receptor Programmed death-1 (PD-1) expressed on activated T-cells53 and NK-cells;54,55 binding of PD-1 on lymphocytes to Kupffer cell PD-L1 normally restrains T-cell activation/inflammation and maintains a tolerogenic environment in the liver.56 Dolina et al. observed that intravenously-injected lipidoid nanoparticles (LNPs) carrying PD-L1 siRNA were taken up primarily by Kupffer cells in the liver and accumulated around the cell nuclei. This resulted in downregulation of PD-L1 expression and increased activation of NK cell and T-cells in the liver that enabled treated animals to clear hepatic viral infections (Fig. 3).52 Macrophages in the spleen can also be targeted by systemic nanoparticle administration: intravenous administration of CCR2-siRNA encapsulated in LNPs resulted in maximal accumulation in the Ly6Chi monocyte populations of the spleen and bone marrow. CCR2 is a chemokine receptor for the MCP-1 chemokine and signaling through the CCR2/MCP-1 axis is required for the recruitment of inflammatory monocytes to the site of tissue damage. Targeting of monocytes with CCR2-siRNA LNPs reduced the infiltration of inflammatory monocytes to the site of tissue injury in murine models of ischemia/reperfusion-induced myocardial infarction and reduced the size of atherosclerotic lesions in mice with established atherosclerosis. These LNPs also prolonged the survival of pancreatic islet grafts and reduced the size of lymphoma growth.57
Figure 3.
siRNA LNP is primarily engulfed in small vesicles by Kuppfer cells within the mononuclear cell pool. Representative images of cell stained with a surface lineage marker (Yellow), DAPI (Blue), and siRNA LNP (Red) are depicted (n = 3 per group). DAPI: 4’,6-diamidno-2phenylindole; DC: Dendritic Cell; IU: Infectious Unit; KC: Kuppfer Cell; LNP: Lipidoid nanoparticle; LSEC: Liver Sinusoidal Endothelial Cell.52 Reprinted with permission from reference 52. Copyright 2013 American Society of Gene & Cell Therapy.
Passive targeting of nanoparticles to phagocytic cells has also been used to reduce macrophage-mediated inflammation at sites of atherosclerotic plaques. Duivenvoorden et al. intravenously administered statin-loaded re-constituted high-density lipoprotein nanoparticles (S-rHDL NPs) and observed that they accumulated in the heart, aorta, spleen, liver and kidneys. More detailed analyses revealed that these S-rHDL NPs could accumulate in atherosclerotic lesions in an Apoe−/− mouse model of atherosclerosis. Vessel wall thickness and macrophage infiltration of plaques were decreased in groups that received S-rHDL NPs compared to groups that received empty particles or unencapsulated statin. S-rHDL NPs caused reduced viability and secretion of inflammatory cytokines from macrophages compared to empty rHDL particles in vitro. Statins have no effect on cholesterol levels in the Apoe−/− mouse model, and so their impact in these studies was ascribed to their anti-inflammatory effect on macrophages in atherosclerotic lesions.58
Active targeting through conjugation of ligands for cell-surface receptors can often increase the uptake of nanoparticles by phagocytic cells relative to non-targeted particles, and this has thus motivated exploration of this approach for delivery of immunoregulatory drugs to macrophages and monocytes. A number of receptors allow macrophage-specific targeting, including the Fc receptor, scavenger receptors and mannose receptors (MMR or CD206). Targeting the macrophage Fc receptor is achieved by coating nanoparticles with IgG, which accelerates uptake of particles and enhances their retention within macrophages.59 Nanoparticles can also be targeted to the F4/80 receptor expressed on the macrophage surface. Laroui et al. designed anti-inflammatory therapeutic by encapsulating TNF-α siRNA in nanoparticles synthesized using poly(lactic acid)/PEG block co-polymers (PLA-PEG NPs). PLA-PEG NPs were coated with anti-F4/80 antibody fragments and packaged into chitosan/alginate hydrogels. It is important to note that both empty and TNF-α siRNA encapsulated PLA-PEG NPs did not affect macrophage cell viability. Upon oral administration, these antibody-coated PLA-PEG NP-loaded hydrogels preferentially interacted with intestinal macrophages and attenuated the effects of dextran sodium sulphate mediated colitis.60
The macrophage mannose receptor (MMR) is expressed on mature macrophages and dendritic cells but not on monocytes in the blood circulation. Intratracheal delivery of mannosylated cationic liposomes has been used to specifically target alveolar macrophages.61 MMR expression is also increased on TAMs that are polarized toward the M2 phenotype.62 Locke et al. used mannosylated phospholipids to synthesize liposomes that encapsulated radionuclide64Cu to facilitate imaging of urethane-induced lung tumors. Fluorescently-labeled mannosylated liposomes (Man-Lipos) co-localized with lung tumors and had lower background signal than non-mannosylated liposomes demonstrating that Man-lipos can be used to specifically target TAMs and deliver cargoes to them (Fig. 4).63
Figure 4.
Mannosylated liposomes are targeted to macrophages in the tumor microenvironement. (A) Schematic diagram of DOTA containing plain liposomes (B) Schematic diagram of DOTA containing mannosylated liposomes (Man-Lipos) (C and D) Strong fluorescence signal associated with PEG liposomes and Man-Lipos is localized to lung tumors. However, compared to Man-lipos, PEG liposomes exhibit a higher background signal and poor tumor contrast.63 Reprinted with permission from reference 63. Copyright 2012 Elsevier Ltd.
Single-walled carbon nanotubes (SWNTs) have also been used to target phagocytic cells trafficking to tumors. SWNTs coated with lipid-tailed poly(ethylene glycol) terminated by an integrin-targeting peptide RGD (arginine-glycine-aspartic acid) were shown to accumulate at tumor sites in mouse models of cancer.64 This was attributed to the preferential accumulation of SWNTs in tumors via the enhanced permeability retention (EPR) effect65 and the ability of the RGD peptide to bind to integrins expressed on tumor vasculature and on the surface of tumor cells.66 However, upon closer examination using intravital microscopy, Smith et al. showed that in addition to accumulating in the tumor due to EPR, 25% of intravenously injected SWNTs were taken up preferentially by Ly6Chi monocytes in the circulation, which were then recruited to the site of the tumor in response to inflammation. Conjugating RGD to SWNTs increased the recruitment of Ly6Chi monocytes into the tumor interstitium and resulted in increased accumulation of SWNTs at the tumor site.67 The mechanisms underlying preferential uptake of SWNTs by Ly6Chi monocytes are still unknown.
2.1.2 Neutrophils
Neutrophils are the “first responders” at sites of inflammation and play an important role in providing the initial defense against invading pathogens through phagocytosis of microbes and secretion of cytokines and reactive oxygen species.68 However, prolonged neutrophil-mediated inflammation can lead to tissue damage and the pathogenesis of diseases such as arthritis, cancer and COPD.69 Targeting neutrophils in contexts where they mediate inflammation has been explored as a strategy to limit chronic inflammatory responses and minimize tissue damage. In many cases, completely inhibiting neutrophil entry into the site of tissue injury may also abrogate the beneficial aspect of the inflammatory response. Using nanomaterials to specifically control the delivery of agents that reduce neutrophil recruitment to different extents may be able to ameliorate an excessive inflammatory response without interfering with the beneficial role of inflammation. When neutrophils respond to inflammation-inducers like bacteria, they trans-migrate through activated endothelial cells to reach the site of tissue injury or infection. Wang et al. synthesized denatured bovine serum albumin (BSA) nanoparticles that are specifically endocytosed by activated neutrophils that adhere to inflamed blood vessels. These nanoparticles were synthesized through a desolvation process using ethanol and stabilized through glutaraldehyde-induced cross-linking and could be loaded with drugs that modulate neutrophil function such as Syk inhibitors. Upon intravenous delivery, these particles reduced the accumulation of neutrophils adherent to activated lung endothelium in response to a systemic LPS injection.70 In a related strategy, polymer nanoparticles carrying the anti-inflammatory peptide Ac2-26 were targeted to sites of tissue injury by using collagen IV targeting peptides. Nanoparticles less than 100 nm in diameter were synthesized using biodegradable diblock poly(lactic-co-glycolic acid)-b-poly(ethylene glycol) using the single-step nanoprecipitation self-assembly method. Encapsulation of Ac2-26 in these nanoparticles resulted in its increasing its half-life in circulation and preferentially targeting it to the site of tissue injury. Administering these particles intravenously resulted in a 30% reduction in neutrophil recruitment to the site of tissue injury in a hind-limb ischemia-reperfusion injury murine model, which could help resolve the inflammatory response more quickly.71
2.2 Targeting the Adaptive Immune System
The adaptive immune system comprises T-cells and B-cells. These cell types express a large diversity of clonal antigen receptors, permitting recognition of a wide repertoire of antigens expressed by foreign pathogens or cancer cells. Furthermore, cells of the adaptive immune system differentiate to become memory cells that ‘remember’ previous antigen exposures and launch a rapid immune response against previously encountered antigens. This property is known as immunologic memory, a hallmark of the adaptive immune system4.
2.2.1 T-cells
T-cells play a central role in the immune system’s ability to eliminate intracellular pathogens and tumors. Current cancer immunotherapies showing significant objective responses in the clinic such as treatment with checkpoint blockade antibodies or adoptive T-cell transfer are largely reliant on the ability of cytotoxic CD8+ T-cells to infiltrate tumors and destroy cancer cells, as are many other cancer immunotherapy strategies2. On the other hand, aberrant T-cell responses contribute to serious autoimmune diseases including Type 1 diabetes and multiple sclerosis.72 Thus, strategies to modulate the function of T-cells, either enhancing or suppressing their function, have been sought in a variety of disease settings. Depending on the therapeutic context, T-cells can be modified to carry cargo that either modify their own function or modulate the function of cells with which they interact.
Engineering T-cell function in cancer immunotherapy
Tumor-antigen specific T-cells that naturally arise in a patient (endogenous T-cells), expanded by a cancer vaccine, or artificially introduced by adoptive cell therapy can potentially attack and destroy cancer cells. However, strategies to expand these T-cells specifically in vivo or to block the immunosuppressive signals they face in the tumor microenvironment are of importance for maximizing the efficacy of immunotherapies. Nanoparticle delivery agents have been explored to address these issues and enhance the function of T-cell-based immunity in several ways.
One of the most promising clinical strategies for treating advanced melanoma and certain leukemias is via adoptive cell therapy (ACT).20,73 ACT involves transferring large numbers of autologous tumor-specific T-cells expanded ex vivo back into patients to mediate tumor regression. A key step in this treatment is the rapid expansion of antigen-specific T-cells in culture. Perica et al. designed artificial nano-APCs using dextran-coated iron oxide particles with surface coupled MHC-Ig dimers and anti-CD28 antibodies designed to allow magnetic field-based aggregation of particles bound to T-cell receptors (TCRs). Ex vivo stimulation of T-cells with these particles in the presence of a magnetic field enhanced TCR clustering, reduced the threshold of activation of T-cells and improved the efficacy of adoptive T-cell therapy (Fig. 5 A, B).74 A startup company, NexImmune, is working to develop this technology for adoptive T-cell therapy and other clinical applications.
Figure 5.
Different nanoparticle based strategies to improve adoptive T-cell transfer (A) Schematic of iron-dextran nanoparticles functionalized with T-cell activating proteins (nano-APCs) stimulating T-cell receptor signaling in the absence or presence of a magnetic field (B) Adoptive transfer of magnet-enhanced nano-APC activated T cells increased survival compared to no magnet and control groups. Mice were censored if dead or tumors were > 150 mm2. (p<0.001 by Mantel Cox log-rank test)74 Reprinted with permission from reference 74. Copyright 2014 American Chemical Society. (C) Schematic of maleimide-based conjugation to cell surface thiols. MBP-PE: 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[4– (p-maleimidophenyl)butyamide] (D) Confocal microscopy images of CD8+ effector T-cells immediately after conjugation with fluorescent 1,1-dioctadecyl-3,3,3,3-tetramethylindodicarbocyanine (DiD)-labeled multilamellar lipid nanoparticles (top) and after 4 days of in vitro expansion (bottom) (D) Scale bar, 2 µm (E) Survival of mice after adoptive T-cell therapy is enhanced with nanoparticle conjugated T-cells illustrated by Kaplan-Meir curves (n=6 per group)79 Reprinted with permission from reference 79. Copyright 2010 Nature America, Inc. (F) T-cell targeted liposome with surface attached anti-Thy1.1 or IL-2Fc (G) Representative histograms of liposomes labeling antigen-specific adoptively transferred or endogenous CD8+ T-cells 48 hours after adoptive transfer and 24 hours after liposome injection.83 Reprinted with permission from reference 83. Copyright 2013 Elsevier B.V.
A second approach to enhance ACT is to functionalize T-cells with nanoparticles that can influence their function in vivo. Following T-cell injection, patients receiving ACT are often treated with systemic adjuvant drugs like interleukin-2 (IL-2).75 In preclinical models a variety of supporting cytokines or immunomodulatory agents have been tested as systemic supporting drugs that can promote continued expansion of the transferred T-cells in vivo, improve T-cell survival, enhance T-cell resistance to immunosuppression, or increase T-cell effector functions.76,77 However, cytokines like IL-2 and other immunomodulators often elicit severe toxicity when administered systemically, due to nonspecific lymphocyte activation in the circulation and pleiotropic effects on other cells.76,78 To better focus the influence of supporting drugs on the donor T-cells themselves, Stephan et al. engineered cytokine encapsulating multi-lamellar lipid nanoparticles and chemically conjugated them to T-cells ex vivo prior to adoptive transfer.79 Unexpectedly, linking nanoparticles to free thiols on T-cell surface proteins (Fig 5C) resulted in minimal particle internalization over several days in culture (Fig 5D). This enabled the nanocarriers to continuously release encapsulated cytokines for engineered autocrine stimulation of cell surface receptors. When T-cells were loaded with nanoparticle “backpacks” carrying stimulatory cytokines, this approach enabled 80-fold increased T-cell expansion in vivo and significant enhancements in the efficacy of ACT, without toxicity (Fig. 5E).79 It was subsequently demonstrated that this cell surface conjugation strategy leads to coupling of particles to receptors known to be trafficked to the immune synapse formed at the contact between T-cells and tumor cells during tumor cell killing.80 Thus, as particle-decorated T-cells engaged tumor cells, the cell-bound nanoparticles were shown to be transported over the cell surface into the T-cell/tumor cell interface, enabling synapse-directed drug delivery.80
A third strategy is to use nanoparticles to deliver stimulatory or protective cues to T-cells directly in the tumor microenvironment. Kwong et al. designed immunostimulatory liposomes comprising PEGylated liposomes with surface-conjugated IL-2 and anti-CD137 (a co-stimulatory receptor up-regulated on activated T-cells).81 These particles were designed to enable high doses of IL-2 and anti-CD137 to be injected into tumors and remain localized at the tumor site without systemic dissemination. This allowed local T-cell stimulation without systemic toxicity. Upon local intra-tumoral injection into established melanomas, this liposomal therapy resulted in improved ratios of tumor infiltrating CD8+ T-cells to regulatory T-cells (T-regs), cured 70% of treated animals, and primed T-cells that traveled away from the injection site to suppress the growth of distal (untreated) tumors. Importantly, intratumoral injection of anti-CD137 and IL-2 anchored on liposomes allowed for non-toxic delivery of doses of anti-CD137 and IL-2 that would be lethal if administered systemically.26
Nanoparticles can also accumulate in tumors following systemic administration, due to the combination of leaky tumor vasculature and dysfunctional lymphatic clearance in many tumors (EPR effect).65 Park et al. designed a biodegradable hybrid core-shell delivery platform comprising nanoscale liposome-encapsulated polymer nanogels (nLgs). By incorporating a small molecule drug chelator (β-cyclodextrin) into the nanogel network, nLgs could encapsulate both a hydrophilic cytokine (IL-2) and a hydrophobic small molecule drug that inhibits the TGF-β receptor–I. TGF-β is one of the major negative regulatory signals produced in tumors,82 and these combination drug nLGs simultaneously provided cues to drive T-cell and NK-cell expansion while blocking a key immunosuppressive pathway. Systemic administration of these nLgs in melanoma tumor bearing mice resulted in curing 40% of the animals.82
A final approach to target T-cells is to exploit the fact that lymphocytes continuously re-circulate through the blood, and directly target these cells in the circulation. Zheng et al. synthesized PEGylated liposomes conjugated either to IL-2 or antibody fragments against an innocuous T-cell surface protein (Thy1) to target adoptively transferred T-cells in vivo.83 When systemically administered, both IL-2 and anti-Thy1 targeted liposomes to adoptively transferred T-cells in vivo (Fig. 5F, G). The advantage of targeting a circulating cell type was evident in these data, as nearly 100% of the target cells were labeled following a single injection of liposomes. Notably, IL-2-liposomes were shown to stimulate greater ACT T-cell proliferation in vivo compared to equivalent soluble doses of the cytokine.83 An alternative to using antibodies or cytokines to target lymphocytes could be to use oligonucleotide aptamers.84 Recently, McNamara et al. reported the generation of aptamers that bind to the T-cell costimulatory receptor CD137 and are capable of binding to T-cells and delivering a stimulatory signal.85
Engineering T-cell function in autoimmunity
During αβ TCR rearrangement, throughout T-cell development, T-cells expressing TCRs that recognize self-proteins can be generated. Such autoreactive T-cells are not always completely eliminated before leaving the thymus, but are usually kept under control through multiple mechanisms of tolerance that act in the peripheral tissues.86 If these normal tolerance mechanisms fail, autoreactive T-cells can attack healthy tissues, giving rise to autoimmune diseases like multiple sclerosis, rheumatoid arthritis and type I diabetes.72 An ideal therapy for autoimmune disease would inhibit autoreactive immune cells without non-specifically diminishing the capacity of the immune system to respond to dangerous microbes. Nanoparticle therapeutics show promise for enabling such selective re-regulation of autoreactive T-cells.
Type I diabetes is caused by polyspecific CD8+ T-cells that recognize multiple epitopes expressed by pancreatic islet cells, and thus an antigen-specific therapeutic strategy would potentially require eliminating or tolerizing CD8+ T-cells with many different specificities. Tsai et al. discovered that stimulation of self-antigen specific CD8+ T-cells via iron oxide nanoparticles conjugated with autoantigen peptide-MHC complexes (pMHC-NPs) led to the expansion of a population of autoregulatory memory-like T-cells. These autoregulatory memory-like T-cells prevented the activation of autoreactive CD8+ T-cells through killing of APCs that present auto-antigens.87 The promotion of a suppressive/regulatory phenotype in these memory-like T-cells may be a result of TCR crosslinking by pMHC-NPs in the absence of the additional co-stimulatory signals T-cells would normally need to receive from dendritic cells for a productive activating immune response. Importantly, once expanded, pMHC-NP-induced autoregulatory T-cells could inhibit the activation of poly-specific autoreactive CD8+ T-cell populations, thereby enabling establishment of normal glycemic levels in non-obese diabetic (NOD) mice.87
The identification of cell surface proteins uniquely expressed by autoreactive T-cells enables nanoparticle-based targeting strategies to directly modulate the function of these cells. For example, the Kv1.3 potassium channel, responsible for ion transport during T-cell activation,88 was identified as being expressed at elevated levels on autoreactive CD45RO+CCR7− T-effector memory cells (TEM) compared to non-autoreactive T-cells. Exploiting this finding, Hadju et al. designed liposomes encapsulating protamine sulfate complexed with siRNA against the Kv1.3 channel, and targeted the liposomes via anti- CD45RO antibodies. These anti-CD45RO Kv1.3 siRNA-nanoparticles specifically bound to TEM cells in in vitro mixed cultures and resulted in reduced activation of TEM cells compared to anti-CD45RO scrambled-siRNA control nanoparticles. By specifically targeting Kv1.3 siRNA to TEM cells the influence of these NPs on other cell types expressing the Kv1.3 channel like neurons, adipose cells and macrophages is minimized. However, the efficacy of these targeted siRNA-carrying liposomes remains to be tested in vivo.89
Delivering cytotoxic drugs like doxorubicin specifically to autoreactive T-cells can result in their elimination. Fahmy et al. demonstrated this concept using PAMAM dendrimers encapsulating doxorubicin that were targeted to T-cells using antibodies or specific peptide-MHC complexes.90 Similarly, delivering specific immunomodulators that influence the differentiation of T-cells can be used to manipulate T-cell development in vivo. CD4+ T-cell-targeted PLGA particles encapsulating leukemia inhibitory factor (LIF) caused increased expression of FoxP3 and promoted CD4+ T-cell differentiation toward a regulatory lineage. These induced T-regs could induce peripheral tolerance and transplantation tolerance in vivo91.
Engineering T-cell function in infectious Disease
In HIV infection, CD4+ T-cells are a primary target of the virus. This has motivated a number of nanoparticle-based strategies to target therapeutic agents like anti-viral siRNA or antiretroviral drugs to CD4+ T-cells to block HIV replication. For example, lipid nanoparticles (LNPs) encapsulating anti-retroviral drug idinavir were targeted to CD4+ T-cells using peptides that recognize the CD4 co-receptor. Pre-treatment of CD4+ T-cells with targeted LNPs resulted in reduced number of infected cells compared to non-targeted LNPs in vitro92. In another approach, highly branched carbosilane dendrimers were functionalized to deliver siRNA into CD4+ T-cells and macrophages (a second target of HIV infection).93 However, these delivery platforms need further testing in in vivo models of HIV infection. Promising in vivo data using dendrimers to deliver siRNA into astrocytes suggest that this platform could be used to treat HIV infections in vivo.94
2.2.2 B-cells
The vast majority of licensed vaccines protect through the induction of neutralizing antibody responses.9 Thus, the design of nanoparticles to enhance the engagement and activation of antigen-specific B-cells is of great relevance for vaccine development. Microbes are intrinsically biological particles (nanoparticles or microparticles), often displaying densely-arrayed repetitive copies of surface antigens involved in target cell binding; B-cells have evolved to recognize and respond to such structural features. Crosslinking of B-cell receptors by repetitively-arrayed antigen promotes signaling through the B-cell receptor: while binding of monovalent protein antigens can trigger partial signaling through BCRs,95 multimerized antigens trigger greater signaling, antigen internalization, and processing of antigen for presentation to CD4+ T-cells– a requirement for B-cells to receive T-cell help for antibody production.96,97 Generation of T-dependent antigen-specific antibody responses has also been shown to require direct stimulation of Toll-like receptors (TLRs) in B cells.98,99 For T-independent antigens such as bacterial polysaccharides (which cannot be presented to T-cells), early studies estimated that ~20 repetitive epitopes with uniform spacing of ~12 nm were required to maximally stimulate antibody responses in vivo.100,101 Antigens displayed in rigid, closely spaced arrays on particles (as naturally present on many viruses) also stimulate extremely potent humoral responses to T-cell-dependent antigens. This is exemplified by the response to virus-like particles (VLPs), where self-assembled protein nanoparticles based on the capsids of viruses (e.g. hepatitis B, papilloma viruses) or bacteriophages (e.g. Qβ, MS2, AP205) trigger long-lived, high-avidity antibody responses following, in some cases, only a single injection.102–104 Indeed, this biology has motivated the use of recombinant VLPs as the basis of the licensed hepatitis B and human papilloma virus vaccines,105–107 and for many other vaccines currently in clinical trials.108 Even for aluminum salts, which are still the most widely used particulate adjuvants for human vaccines in the United States,109 vaccine efficacy has been directly linked to the strength of antigen binding to these inorganic particles.110 For example, for a recombinant smallpox L1-protein subunit vaccine, binding of antigen to alum particles was required for optimal immune responses.111 Phosphate buffer treatment, which changed the surface charge of the aluminum particulates from positive to negative prior to vaccination, disrupted protein binding and reduced serum IgG titers, leading to increased morbidity and weight loss (an indirect measure of disease state) upon vaccinia virus challenge.
The immune response to native microbes and vaccines described above has motivated the study of synthetic nanoparticles that incorporate similar structural features in antigen display to promote humoral immunity. Engineered nanoparticles offer flexibility over the mode of particulate antigen display (e.g. encapsulated, non-covalently surface displayed, surface tethered).112–114 Antigen encapsulation has widely been used to load large amounts of antigen into particle cores and also acts as a means to shield protein payloads from proteases and other denaturants prior to immune cell targeting. While some techniques have been employed to improve antigen loading, this approach generally suffers from poor control over the degree of encapsulation and varies widely based on the particle platform and antigen properties (i.e. hydrophobicity, size, charge). Alternatively, surface display of protein and peptide antigens on nanoparticle surfaces mimicking viral and bacterial pathogens can potentially bind to and activate antigen-specific B cells more readily than encapsulated antigens, which by definition must be released to interact with B-cells (Fig 6). This has been demonstrated unequivocally by Friede et al. by showing that liposomes with surface bound peptide antigens elicited a much stronger B-cell response compared to liposomes with encapsulated peptide antigens at equivalent doses.115 Furthermore, covalently conjugated protein on the surface of calcium phosphate nanoparticles promoted BCR crosslinking in vitro and was 100-fold more efficient in activating antigen-specific B cells compared to soluble protein.116 While surface display requires an additional conjugation step post-particle formulation, this allows for higher control over the degree of coupling based on the conjugation chemistry and antigen of interest, as well as the use of particles made using harsh solvents. For particle surface-displayed antigens, many physicochemical factors are expected to influence the ability of nanoparticles to engage B-cells. For example, Stefanick et al. described how peptide valency, peptide linker length, peptide hydrophilicity, poly(ethylene glycol) (PEG) coating density, and PEG linker length can dramatically alter cellular uptake for peptide-functionalized liposomes binding to target cells, highlighting the importance of each these design elements on effective particle binding and uptake.117,118
Figure 6.
Antigens bound to the surface of liposomes generate a significantly higher antibody tire. Mice were immunized with liposomes containing 2 µg MPLA associated with peptide. (a) no peptide (b) 60 µg peptide internally encapsulated in 5 µmol lipid (c) 65 µg peptide internally encapsulated in 1 µmol lipid (d) 60 µg peptide covalently bound to liposome surface (I µmol lipid) (e) 60 µg peptide co-injected with liposomes (1 µmol lipid) to which no peptide is bound (f) peptide conjugated to ovalbumin in Freund’s complete adjuvant with boosters in Freund’s incomplete adjuvant. IgG titer was measured after the third injection with an interval of three weeks between each injection.115 Reprinted with permission from reference 115 Copyright 1993 Pergamon Press Ltd.
Lipid-coated PLGA nanoparticles with only 10 ng of surface-displayed ovalbumin (OVA), a commonly used model protein antigen, elicited detectable serum IgG titers in vivo after a single immunization and further elicited 1000-fold higher titers compared to soluble OVA combined with adjuvant 1-week post-boost immunization.119 Nanoparticle surface-displayed antigen can also result in increased germinal center formation and, as a consequence, higher affinity antibodies compared to soluble antigen immunization.120 More recently, gold nanoparticle-conjugated glycoprotein antigens have shown efficacy in non-human primate studies against glanders.121 While vaccination did not result in a survival benefit upon bacterial challenge, those animals that were vaccinated and survived had significantly higher LPS-specific IgG titers compared to those who did not survive.
3 Overcoming tissue barriers for vaccines and immunotherapies with nanoparticles
A key aspect to the design of effective vaccines and immunotherapies is the ability to overcome relevant tissue barriers and efficiently deliver the therapeutic payload to a particular tissue destination. The tissue barriers most often encountered in vaccine/immunotherapeutic design are mucosal and epithelial sites, and the frequently-targeted tissue sites for vaccines and immunotherapies are lymph nodes and tumors. In vaccination, it is often desirable to specifically initiate immune responses at the lymph nodes draining mucosal portals of entry of the target pathogen, because T-cells and B-cells activated in these lymph nodes are programmed to home back to the local mucosal tissue.122,123 For example, to promote protection from a respiratory pathogen such as influenza, pulmonary vaccination (intranasal or airway delivery of vaccines) is of interest, because this route of administration primes T-cells that home to the lung where they can become resident memory cells to protect the airway mucosa.124 The unique and effective ways in which nanoparticles have been utilized to overcome tissue barriers and/or to enhance tissue delivery will be reviewed in this section. As will be appreciated from this discussion, some of the effective roles for nanoparticles in these applications are generic properties relevant for delivery of any drug across these barriers, and other aspects relate specifically to the interaction of nanoparticles with the immune system.
3.1 Targeting therapeutics and vaccines to lymph nodes
Lymph nodes are the sites of lymphocyte priming by antigen presenting cells (APCs) and subsequent adaptive immune responses including T-cell differentiation, formation of germinal centers (where B cells undergo affinity maturation), and ultimately, the generation of long-term immunological memory. Therefore, it is cogent to hypothesize that direct delivery of immunotherapeutics/vaccines to lymph nodes may be more effective than distal administration to peripheral tissues. Indeed, extensive work by Johansen and colleagues has demonstrated the value of vaccine delivery to lymph nodes; for example, direct intralymphatic immunization substantially increased the ability of the Baccillus Calmette-Guérin (BCG) tuberculosis vaccine to generate BCG-specific CD8+ and CD4+ T cell responses and sustained protection against tuberculosis challenge, whereas subcutaneous immunization with a 100-fold higher dose did not.125 Synthetic nanoparticles can further modulate the response to intranodal injections, and offer an alternative means to achieve lymph node targeting of vaccines and immunotherapies.
3.1.1 Intranodal administration of nanoparticles
The most straightforward method to deliver vaccines to a lymph node is to directly inject intranodally. Ultrasound-guided intranodal administration of immunotherapies has been used in multiple phase 1 clinical trials.126–129 Although this can be performed with soluble therapeutics, use of nanoparticulate antigen is advantageous because nanoparticles reduce the rate of vaccine/drug clearance from the lymphoid tissue and facilitate antigen uptake by antigen presenting cells.130 For example, Jewell et al. reported that intranodal injection of the TLR-3 agonists poly(inosinic:cytidylic acid) (polyIC) in particulate form substantially prolonged persistence of polyIC in lymph nodes when compared to soluble polyIC.131 In an immunization model, intranodal injection of ovalbumin-loaded particles induced much stronger humoral and cellular immune responses than intranodal injection of soluble ovalbumin.131 In another study, the intranodal injection of nanoparticulate ovalbumin and CpG substantially enhanced the IgG2A antibody response over intranodal injection of soluble vaccine.132 Thus, even on direct administration to lymphoid tissues, nanoparticle formulations can alter and enhance the response to vaccines.
3.1.2 Size-based LN targeting
Although intranodal injection may be useful for immunotherapeutic applications, it is impractical for widespread prophylactic vaccination settings. An alternative is to deliver materials to lymph nodes through lymphatic drainage of interstitial fluid from peripheral tissue injection sites. The fate of soluble materials following parenteral injection is highly dependent on physical size; the blood absorbs ~10-fold more fluid from tissues than lymph, so molecules small enough to enter blood vessels predominately clear to the blood. In general, materials larger than approximately 9 nm in diameter preferentially drain to lymphatics, whereas molecules/particles smaller than ~6 nm drain to the blood (Fig. 7).133 Conversely, very large solid particles (greater than ~50–100 nm) tend to become trapped in the extracellular matrix and cannot freely drain to lymphatics, but can be phagocytosed and transported to lymph nodes by DCs present in the tissue or monocytes that emigrate from the blood.134 Thus, nanoparticles of appropriate size can be efficient synthetic vehicles to deliver vaccines or immuontherapeutics to lymph nodes.
Fig. 7.
Relationship between molecular weight and uptake into lymphatics following subcutaneous injection for a series of globular proteins and small molecules in a sheep model. Data for fluorodeoxyuridine (FuDu), inulincytochrome (CyC), interferon-α (IFN), human growth hormone (hGH), soluble insulin, r metHu-Leptin (Lep), an analogue of Leptin (Lep*), epoietin alfa (EPO), darbepoetin alfa (DA) and a high molecular weight protein (HMwP) Reproduced from Kaminska and Porter133 with permission. Reprinted with permission from reference 133. Copyright 2011 Elsevier B.V.
The optimum size of particles for lymph node targeting have been examined in a number of studies. Hubbell, Swartz, and colleagues compared the ability of 20–100 nm PEGylated poly(propylene sulfide) nanoparticles to drain to lymph nodes and to stimulate immune responses. Larger particles with mean diameters of 100 nm were only 10% as efficient as the smallest particles at draining to lymph nodes and 20–45 nm diam. particles subsequently induced the strongest immune responses.135,136 While smaller particles achieve more efficient diffusion through peripheral tissue to reach lymphatics, Kourtis et al. also demonstrated in the same system that a portion of very small particles (30 nm diam.) are not necessarily trapped in the draining lymph nodes, and can be detected at substantial concentrations in the peripheral blood within ~12 hr following intradermal injection.137 This finding suggests that at least for these phagocytosis-resistant PEGylated materials, very small particles can pass through the lymph nodes without capture, reaching the thoracic duct (and thereby enter the blood). Using model monodisperse polystyrene particles of different sizes, Fifis et al.138,139 and Manolova et al.134, reached similar conclusions, finding that particles less than 50 nm in diameter were most effective in lymph node trafficking and targeting lymph node-resident dendritic cells. While these studies seem to suggest that particles of ~200 nm diam. or larger can only reach lymph nodes through cell-mediated transport or through hydrodynamic/swelling effects caused by the injections themselves, a recent study by Gerner et al. showed that 200 nm nanoparticles applied passively to disrupted ear skin of mice were rapidly trafficked to lymph nodes through lymph, independent of cellular uptake.140 Thus, the precise size limits for lymphatic targeting of solid particles likely depend on both the tissue site and route of administration. Importantly, nanoparticle aspect ratio has also been identified as a key parameter in influencing lymph node draining of solid nanoparticles. Utilizing a unique mold-based particle fabrication process termed PRINT (i.e. particle replication in nonwetting templates), Mueller et al. identified anionic 80×180 nm cylindrical particles as having higher levels of lymph node drainage and subsequent APC uptake compared to other rod-like and spherical particles.141
Size limits for efficient lymphatic uptake are also altered when flexible particles are studied, rather than rigid solid particles. For example, liposomes with surface-displayed peptide antigens as large as 150 – 200 nm show substantial lymph node uptake following s.c. injection in mice, and induced 15–20-fold stronger antibody responses than smaller 65 nm vesicles.142 Increasing liposome size has been associated with decreasing drainage from peripheral injection sites but increased trapping in lymph nodes.143 This competing interplay between injection site drainage and lymph node retention suggests there exists an optimum nanoparticle size, which may vary with material composition and should be determined for each nanoparticle delivery system– these size ranges are a useful rule of thumb, but lymphatic uptake will be further influenced by the surface chemistry of the particle, antigen dose and type, and immunization route.144,145
For vaccines, lymph node targeting of adjuvant compounds is equally if not more important than lymphatic uptake of antigen, because potent adjuvants that distribute into the blood or pass through lymph nodes to reach the systemic circulation can induce unacceptable systemic inflammatory toxicity. Nanoparticle targeting of adjuvants to lymph nodes can thus increase both efficacy and safety. For example, a study of 3M-052, an adjuvant molecule that binds to Toll-like receptor 7/8, demonstrated that loading of this compound in liposomes promoted dose-sparing of the adjuvant and avoidance of systemic inflammation.146 Similarly, Ilyinskii et al. demonstrated that s.c. administration of the TLR7/8 agonist resiquimod in soluble form elicited pronounced systemic inflammatory cytokines and no local cytokine induction in draining lymph nodes, while the same compound delivered in PEGylated PLGA nanoparticles elicited strong localized cytokine induction in draining lymph nodes but no systemic inflammatory toxicity.147 Similarly, nanoparticle formulation is being explored as a method to enhance the lymph node drainage and retention of antiretroviral drugs, and lipid complexes were reported to efficiently deliver the antiretroviral drug indinavir to lymph nodes after subcutaneous injection.148,149
3.1.3 Promoting vaccine/therapeutic capture in lymph nodes
On reaching lymph nodes through the lymphatics, nanoparticles are often either captured by macrophages or dendritic cells lining the subcapsular sinus or medullary sinuses of the lymph node.150–152 In some cases, nanoparticles have been shown to accumulate and persist in lymph nodes for extended periods, which may contribute to enhanced immune responses to particulate vaccines.153 Recently, several groups have expanded upon the size-based targeting strategy of nanoparticles to also incorporate specific ligands to promote vaccine particle capture by specific cell types in lymph nodes. Inclusion of mannose on the surface of PEGylated liposomes containing encapsulated antigen enhanced the binding to and uptake into APCs that express the mannose receptor. Subsequently, mannose nanoparticles generated stronger humoral immune responses than unlabeled nanoparticles.154 The cell surface receptors CD40, DEC-205 and CD11c expressed by dendritic cells have also been targeted for enhanced lymph node delivery. PLGA nanoparticles conjugated with antibodies against each of these molecules all exhibited enhanced dendritic cell uptake and T-cell stimulatory capacity in vitro in comparison to untargeted nanoparticles.155 In a subsequent study, the presence of anti-CD40 on nanoparticles (NP-CD40) improved dendritic cell uptake of antigen in draining lymph nodes after subcutaneous administration in mice and modestly improved both therapeutic and prophylactic treatment efficacies in a tumor model (Fig. 8).156 Larger doses of co-delivered TLR agonists – which were shown to be essential for DC maturation after nanoparticulate antigen uptake – will likely further enhance these immune responses. The composition of the nanoparticle itself can also influence particle uptake in lymphoid tissues. For example, cholesteryl pullulan nanogels were shown to be selectively engulfed by medullary macrophages in draining lymph nodes after subcutaneous injection.157 In comparison to soluble antigen formulated in Incomplete Freund’s Adjuvant, antigen encapsulated in these hydrogel nanoparticles substantially delayed tumor growth in a cancer therapeutic model.157
Figure 8.
Vaccination with PLGA-αCD40 nanoparticles prolong survival after tumor inoculation. Vaccines consisted of immunization in the right flank with 10 µg OVA encapsulated in nanoparticles displaying αCD40 mAb (NP-CD40), or isotype control antibody (NP-iso) at 7 days prior to tumor inoculation in the prophylactic model (A), or 7 and 17 days post-tumor inoculation in the therapeutic model (B). Tumor inoculations consisted of 2×105 B16-OVA tumor cells injected s.c. in the left flank. Adapted from Rosalia et al.156 Reprinted with permission from reference 156. Copyright 2014 Elsevier Ltd.
3.2 Nanoparticles to penetrate mucosal and epithelial barriers for vaccines and immunomodulation
Most current human vaccines are administered parenterally, resulting in strong systemic immune responses and weak to non-existent mucosal immune responses. Yet most pathogens infect through mucosal tissues,158,159 and the establishment of mucosa-homing T-cells and B-cells through mucosal immunization can be a key component of vaccine efficacy.160–162 The inductive sites of the mucosal immune system consist of both mucosa-associated lymphoid tissues (MALT) and mucosa-draining lymph nodes, and include the tonsils in the upper respiratory airway and Peyer’s patches in the intestines. Although the composition and structure of mucosal tissue varies depending on location (oral/intestinal tract, nasal/respiratory tract, genital tract), it can generally be classified as either type I or type II mucosal tissue. Gut-associated lymphoid tissue (GALT), nasal-associated lymphoid tissue, and uterine mucosal tissue of the female genital tract (FGT) are all type I mucosal tissue, while vaginal mucosal tissue is type II.163 Type I mucosae consists of simple columnar epithelium linked by tight gap junctions while type II mucosae is lined with multilayer stratified squamous epithelium.164 IgA in the main predominant immunoglobulin produced in type I mucosal tissues, but IgG is the primary immunoglobulin of type II mucosal tissues.
For vaccines, local administration initiates immune responses concentrated at the mucosal site, but mucosal vaccine development is complicated by the same safety vs. efficacy considerations of parenteral vaccines. Furthermore, delivery of a vaccine to the inductive sites of the mucosal immune system (draining lymph nodes and MALT), requires mucus penetration, crossing the epithelial barrier, and eventual drainage to local lymph nodes. Nanoparticles are attractive mucosal vaccine/immunotherapy delivery vehicles due to the enhanced uptake by APCs of particulate antigen, the preferential draining of nanoparticles to lymphatics rather than to the blood stream (as discussed in the previous section), and lastly, depending on size and composition, the ability of nanoparticles to diffuse through mucus and cross mucosal barriers.
3.2.1 Airways and nasal mucosa
Intranasal administration is needle free and noninvasive, and allows access to the rich immunological environment of the respiratory mucosa. The epithelium of the airways and nasal mucosa contain Microfold cells (M-cells), which transcytose particulate matter across the epithelial layer to underlying lymphoid follicles of the MALT.165 The first studies to suggest a potent role for nanoparticles in pulmonary vaccines were published in the late 1990s. An early HIV vaccine study demonstrated that a gp160 DNA-based intranasal vaccine induced stronger serum and mucosal antibody titers than the equivalent intramuscular vaccine. Furthermore, co-administration with cationic liposomes—to which the negatively charged DNA antigen will bind—adjuvanted both humoral and cell-mediated vaccine-specific immune activity.166 Two years later, Klavinskis et al. reported that after intranasal administration of plasmid DNA-lipid nanocomplexes, DNA was distributed in the respiratory tract, draining lymph nodes and spleen. This nanoparticulate DNA vaccine induced serum and mucosal antibodies significantly superior to those produced by unformulated DNA.167
Several strategies have been pursued to further develop strategies for effective pulmonary vaccine/immunotherapy treatment using nanoparticles. An anatomic approach is to deliver nanoparticles to the deep lung, targeting the large population of dendritic cells lining the alveoli (air sacs of the lung), which actively extend processes into the alveolar lumen to survey for microbes.168 Using crosslink-stabilized lipid nanocapsules loaded with antigen and TLR agonist adjuvants, Li et al. demonstrated that nanocapsules (ICMVs) were captured much more efficiently than soluble vaccines by APCs in the lungs following pulmonary instillation (mimicking aerosol administration).169 This led to greatly increased and prolonged antigen presentation in lung-draining lymph nodes, and transformed an antigen/adjuvant combination that was completely non-protective against a viral challenge as a soluble formulation to a 100% protective vaccine (Fig. 9). In another example, Vicente et al. developed lipid-chitosan hybrid nanoparticles consisting of an oily core loaded with imiquimod and surrounded by a phospholipid layer and a chitosan coating onto which a hepatitis B protein antigen was adsorbed. These nanoparticles were taken up by macrophages and generated long-lived antigen-specific antibody titers.170 Consistent with the idea that nanoparticle capture by APCs is particularly efficient in the deep respiratory tract, Sanders et al. demonstrated that antigen formulated with ISCOMATRIX nanoparticles (lipid vesicles consisting of phospholipid, cholesterol and saponin) induced much stronger immune responses when delivered to the total respiratory tract rather than only the upper respiratory airways.171
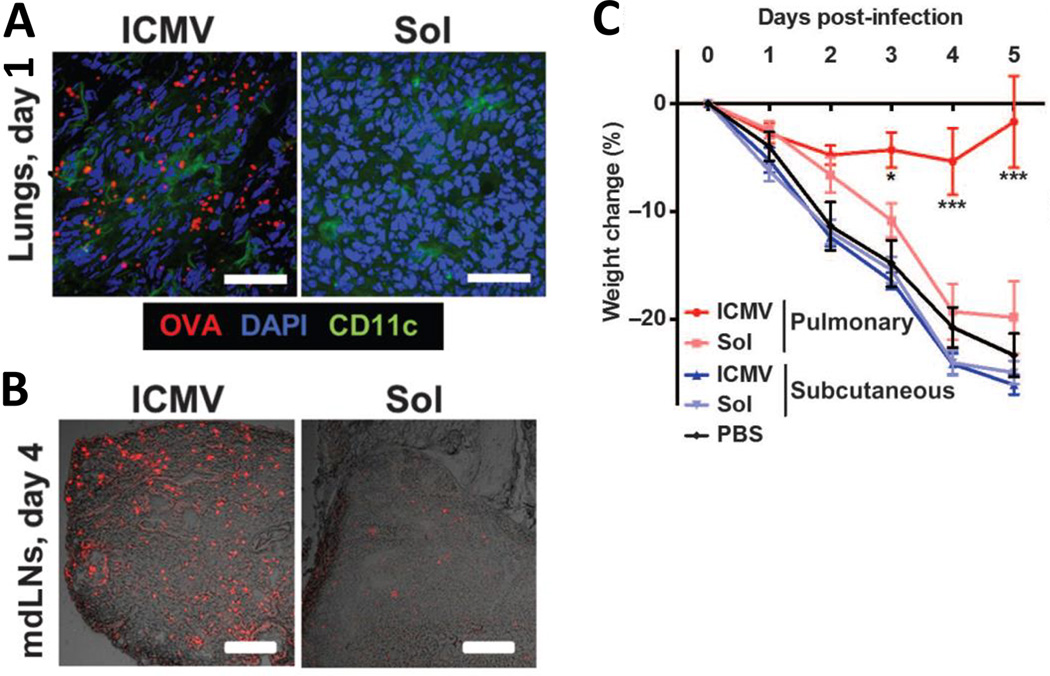
Figure 9.
Nanoparticles targeted to lung DCs enhance vaccine uptake and protect against infection challenge. (A & B) C57BL/6 mice were immunized intratracheally with OVA in lipid nanoparticle (ICMV) or soluble formulations. Representative cryosections after intratracheal immunization with fluorescent OVA (red) from lungs on day 1 (A; scale bars, 50 µm) and mediastinal draining lymph nodes (mdLNs) on day 4 (B; scale bars, 200 µm). (C) C57BL/6 mice were immunized intratracheally or subcutaneously with a peptide vaccine in nanoparticle (ICMV) or soluble forms on days 0 and 28, then challenged by intratracheal administration of vaccinia virus (1×106 PFU) on day 42; body weight changes were tracked over time. Adapted from Li et al.169 Reprinted with permission from reference 169. Copyright 2013 American Association for the Advancement of Science.
A second strategy that has shown promise for enhancing vaccine uptake by immune cells at pulmonary mucosal surfaces has been to employ nanoparticles that are mucoadhesive, to increase the particles’ residence time at the epithelial surface. Using cationic cholesteryl pullulan nanogels (cCHPs) as vaccine carriers, Nochi et al. demonstrated increased retention in the nasal mucosa and uptake of antigen by mucosal dendritic cells (Fig. 10). This enhanced antigen delivery translated to significantly increased mucosal antibody responses and protection from Clostridium botulinum challenge.172
Figure 10.
Cationic nanogels facilitate effective delivery of vaccine antigen into the nasal mucosa. Antigen (Clostridium botulinum type-A neurotoxin BoHc/A) was administered intranasally in cationic nanogels (cCHP-BoHc/A) or in soluble form (BoHc/A) and nasal epithelium tissue sections were collected over time. Green fluorescence refers to the antigen, BoHc/A, red fluorescence refers to the nanogels, and nuclei are represented in blue. Adapted from Nochi et al.172 Reprinted with permission from reference 172. Copyright 2010 Macmillan Publishers Ltd.
An orthogonal approach is to design nanoparticle carriers to efficiently diffuse through mucus and reach the underlying epithelium, where capture by APCs or transcytosis by epithelial cells could provide effective uptake of vaccines/immunomodulators. A dense surface coating of poly(ethylene glycol) on nanoparticles has been reported by the Hanes and Saltzman groups to enhance the mucus-penetrating abilities of nanoparticles.173,174 Though not explicitly tested for mucus-penetration efficiency, vaccines prepared by the Hubbell and Swartz labs using PEGylated poly(propylene sulfide) nanoparticles (PPS-NPs) surface-conjugated with protein antigen/molecular adjuvants may gain at least some of their efficacy from fulfilling these design criteria for effective mucus penetration. Intranasal administration of 50 nm PPS-NPs resulted in the deposition of particles in the nasal tissue, enhanced CD8+ T-cell responses in the lung and spleen, and enhanced mucosal antibody production in comparison to soluble antigen.175 The co-conjugation of the danger signal flagellin to PPS-NPs also increased antibody titers to the vaccine antigen. Furthermore, 30 nm PPS-NPs loaded with ovalbumin, mixed with CpG, and administered via pulmonary delivery induced significantly enhanced cytotoxic CD8+ T-cell responses, providing enhanced protection against an influenza virus challenge compared to soluble pulmonary vaccine.176 Interestingly, in contrast to results obtained for peripheral lymph node targeting following parenteral injection, a comparison of intranasal administration of large (200 nm) vs. small (30 nm) PPS-NPs conjugated to the model antigen ovalbumin revealed that 200 nm PPS-NPs more effectively delivered ovalbumin to MHC class I and MHC class II antigen presentation pathways of APCs, enhanced lung CD4+ T-cell responses, and increased systemic and mucosal antibody titers compared to 30 nm PPS-NPs.177 These results suggest that cell-mediated transport of particulate antigens to lymph nodes may be particularly important or more efficient for mucosal immune responses compared to direct lymph node targeting at these barrier sites.
In addition to mucus penetration, nanoparticles have also been designed to promote binding to epithelial cells and transport across the epithelial barrier to enhance pulmonary vaccine responses. Heparin-binding hemagglutinin adhesion (HBHA) protein is known to bind to heparin-sulfate-containing receptors on lung epithelial cells. Antigen-HBHA constructs adsorbed onto the surface of wax nanoparticles induced stronger humoral and cellular immune responses after intranasal administration than antigen-only nanoparticles or soluble antigen-HBHA protein fusions.178
Nanoparticle vehicles have also been designed to more actively promote transit across the epithelial barrier at mucosal surfaces. Chitosan is a biocompatible cationic polysaccharide that is mucoadhesive and capable of reversibly opening epithelial tight gap junctions.179 Following the demonstration of enhanced systemic uptake of insulin when intranasally administered in chitosan nanoparticles180, there has been considerable interest in the development of chitosan nanoparticles as pulmonary delivery vehicles for a wide range of applications. Intranasal administration of chitosan-DNA nanoparticles expressing the streptococcus pneumonia surface antigen A generated stronger mucosal and systemic antibody titers than naked DNA.181 Encapsulated influenza protein antigen in chitosan nanoparticles was shown to induce stronger mucosal and systemic anti-vaccine antibody titers than soluble antigen alone. Furthermore, chitosan nanoparticles conferred 100% protection against lethal intranasal influenza challenge.182 Bal et al. undertook a comprehensive study of the humoral effects of immunomodulators when co-encapsulated with antigen in chitosan nanoparticles in intranasal or intradermal vaccination models. As expected, nanoparticulate antigen induced stronger antibody responses regardless of immunization route, but the potency of immunomodulators—CpG, lipopolysaccharide, PAM3CSK4, muramyldipeptide, and cholera toxin subunit B—was dependent on the immunization route.183 These results likely reflect different APC populations residing in different tissues, and highlight the necessity of matching optimal immunomodulators to the immunization route in vaccine and immunotherapy development.
In addition to chitosan, PPS, and lipid nanoparticles, several other nanoparticle formulations are currently in development as pulmonary vaccine delivery systems. Silica particles taken up by APCs can activate an intracellular inflammatory signaling pathway known as the inflammasome.184,185 Exploiting this intrinsic adjuvant property of silica particles, intratracheal administration of soluble influenza protein antigen co-formulated with silica nanoparticles generated stronger mucosal antibody responses but weaker systemic antibody responses than intraperitoneal administration of influenza protein formulated in alum.186 In an interesting example of long-term antigen release, polyanhydride nanoparticles with encapsulated antigen were designed to release antigen over a 28 day period.187 When immunized via intranasal administration, mice receiving antigen-loaded nanoparticles produced sustained titers of high avidity antibodies and were protected against Yersinia pestis challenge.
A critical consideration for pulmonary vaccine administration is the potential for the induction of dangerous airway inflammation or fibrotic responses. By targeting phagocytic dendritic cells, nanoparticles may help to ensure that only danger sensor receptors on innate cells critical to induction of the desired immune response are stimulated and avoid triggering lung epithelial cells themselves. Importantly, a recent study comparing the surface hydrophobicity of nanoparticles and the resulting histopathology after intratracheal administration demonstrated that hydrophobic nanoparticles were not biocompatible while hydrophilic nanoparticles (coated with PEG or lecithin) were well tolerated in lung tissue.188 Hydrophilic nanoparticles have also been shown to have increased plasma bioavailability after oral or intranasal administration, suggesting hydrophilicity is a key component for antigen transport across mucosal barriers.189,190
3.2.2 Gastrointestinal tract
The oral delivery route offers a multitude of advantages for vaccines and immunotherapy treatment: it dispenses with risks from needle use (or re-use); is amenable to self-administration with high patience compliance; and for vaccines, leads to the induction of both systemic and mucosal immunity. However, oral immunomodulators must first survive exposure to the stomach pH, proteolytic enzymes and bile salts in the gastrointestinal (GI) tract, and then transit through mucus and the gut epithelium to reach the gut-associated lymphoid tissue (GALT). Nanoparticles offer opportunities to both protect vaccines/immunotherapeutics from degradation, and effectively transport particles across the intestinal lumen. With respect to degradation protection, encapsulation of antigen inside polymeric nanoparticles has been the predominant approach. Understanding the stability of any given antigen/nanoparticle system when exposed to gastric and intestinal fluid should be a key component in the design of a novel oral vaccine.191 As this chapter is centered upon the use of nanoparticles to overcome tissue barriers, the rest of this section will focus on methods currently in development to use nanoparticles as intestinal lumen transporters.
In 1997, Desai et al. first observed that 100 nm PLGA nanoparticles were taken up by intestinal tissues much more efficiently than soluble coumarin-6 dye or 500 nm – 5 µm particles.192 This size-dependent uptake suggested that nanoparticles may be efficacious delivery vehicles for materials that are not readily absorbed in the intestinal tract, and subsequent particulate delivery systems have primarily utilized nanoparticles in the 100 – 500 nm diam. range. For example, oral administration of 470 nm mesoporous carbon nanoparticles (C1) loaded with antigen induced serum antibody responses nearly equivalent to intramuscular administration of antigen emulsified in Complete Freund’s Adjuvant, a gold-standard experimental adjuvant that is too toxic for human use (Fig. 11).193 On the other end of the size spectrum, chitosan-functionalized gold nanoparticles have also been investigated as potential oral vaccine delivery systems. Chitosan nanoparticles 20–40 nm in diam. with surface-adsorbed antigen generated stronger titers than soluble antigen following oral immunization, and the co-adsorption of saponin-containing adjuvants further enhanced humoral responses elicited by these particles.194,195
Figure 11.
Carbon nanoparticles for oral vaccination. (A) Schematic of carbonization mechanism for formation of mesoporous carbon nanoparticles (C1). (B) Mean serum anti-BSA IgG profile of mice after: i.m. administration of BSA emulsified in Complete Freund’s Adjuvant (FCA), oral administration of soluble BSA, or oral administration of BSA loaded in carbon nanoparticles (C1). Prime and boost immunizations were spaced three weeks apart. Adapted from Wang et al.193 Reprinted with permission from reference 193. Copyright 2011 Elsevier B.V.
An important mechanism for particle uptake from the gut lumen into the GALT is proposed to be via microfold cells (M-cells), a specialized epithelial cell that overlies lymphoid tissue structures of the small intestine known as Peyer’s patches. M-cells efficiently internalize and transcytose particulate antigens across the gut epithelium into basolateral pockets where antigen presenting cells reside, thereby playing a key role in initiating adaptive immune responses in the gut.196 Targeting M-cells has been a major focus of oral vaccine development. Various lectins which bind specifically to M-cells have been investigated for use as M-cell targeted vaccines, and both lectin-coated PLGA particles and liposomes have been shown to enhance uptake of encapsulated antigen into M-cells and induce stronger humoral immune responses following oral delivery than unmodified particles.197,198 In an alternative approach, PLGA nanoparticles have been functionalized with RGD analogs in order to target β1 integrins on the apical surface of M-cells; this functionalization enhanced nanoparticle uptake and humoral immune responses after intraduodenal administration.199 Slütter et al. demonstrated that N-trimethylated chitosan nanoparticles enhanced antigen transport in an M-cell dependent manner and promoted dendritic cell activation in vitro, although this did not impact the humoral response in vivo after intraduodenal administration.200 One significant disadvantage of chitosan nanoparticles is that chitosan rapidly dissolves at pH 1.2 – 2.0 and thus chitosan NPs would be expected to dissolve in the stomach.201 One solution to this challenge reported by Jain et al. was to encapsulate antigen-loaded chitosan nanoparticles within liposome- or niosome-microparticles to protect the NPs during transit through the stomach.202 In comparison to “naked” chitosan NPs, encapsulated particles efficiently retained antigen when exposed to simulated gastric fluid (pH 1.2) and induced significantly higher serum IgG titers after oral immunization. An alternative method to stabilize chitosan nanoparticles is to crosslink the chitosan polymers. Harde et al. developed a method to tandem crosslink chitosan nanoparticles using tripolyphosphate followed by glutaraldehyde.203 These crosslinked chitosan nanoparticles exhibited greater stability in acidic environments, enhanced uptake by antigen-presenting cells, greater intestinal permeation, and significantly stronger systemic and mucosal antibody responses than unmodified chitosan nanoparticles after oral administration.203
M-cells are particularly attractive targets for nanoparticle delivery because of their specific localization over gut lymphoid tissues, but this also means that these cells make up a relatively small portion of the total gut epithelium. Thus, other routes for receptor-specific uptake of have also been explored, targeting receptors expressed on normal gut epithelial cells: Thiamine – a member of the vitamin B family – is absorbed from the intestinal lumen in a specialized, carrier-dependent mechanism.204 Thiamine-coated poly(methyl vinyl ether-co-maleic anhydride) nanoparticles have been shown to adhere to gut mucosa in a thiamine dependent manner and when administered orally, thiamine-coated nanoparticles induced substantially stronger humoral responses than plain nanoparticles.205
The co-delivery of TLR-4 or TLR-2 agonists may also be an effective method to enhance transport across the intestinal lumen. TLR-4 agonism has been reported to increase intestinal epithelial tight junction permeability206 and administration of lipopolysaccharide, a TLR-4 ligand, enhanced intestinal adsorption of microparticles.207 In addition, TLR-2 agonism has shown to enhance the transcytosis of microparticles by M-cells in a dose-dependent manner207 as well as induce the migration of dendritic cells into the follicle-associated epithelium of the GALT.208 These observations suggest TLR-4 and TLR-2-based adjuvants may play a role in the intestinal fate of nanoparticulate vaccines and should be taken into account when designing future oral vaccines. Conventional wisdom has held that mucoadhesive nanoparticles will enhance the intestinal adsorption of nanoparticles, however recent studies are challenging this assumption. Maisel et al. demonstrated that PEGylation of polystyrene nanoparticles eliminated the mucoadhesiveness of polystyrene nanoparticles and enabled widespread nanoparticle penetration of the intestinal tract in both healthy and ulcerative colitis mouse models.173 PEGylation has also been demonstrated to enhance the intestinal tract permeation of solid lipid nanoparticles (SLNs), as shown in Fig. 12.209 Whether or not this enhanced intestinal adsorption of antigen via hydrophilic, mucus penetrating particles enhances immune responses remains to be seen.
Figure 12.
Distribution of solid lipid nanoparticles (SLN) and PEGylated solid lipid nanoparticles (pSLN) in small intestinal villi viewed at different magnifications. Blue fluorescence indicates the nuclei of small intestinal cells and green fluorescence indicates nanoparticles. Adapted from Yuan et al.209 Reprinted with permission from reference 209. Copyright 2013 American Chemical Society.
Efficient delivery to the lower gastrointestinal tract without pH-dependent or enzymatic degradation of antigen in the proximal gut still remains a critical hurdle in the development of an oral vaccine. To target the large intestine, Zhu et al. coated PLGA nanoparticles with an anionic copolymer (Eudragit FS30D) that is soluble only at pH > 7.0, a pH only present in the terminal ileum.210 Particles were taken up in the large intestine and not the small intestine, and oral administration of antigen-loaded particles induced stronger mucosal immune responses than intra-colorectal administration of soluble antigen.210 This two-stage delivery approach demonstrates that pH-dependent release of nanoparticles within targeted regions of the GI tract may be an effective method to control the location of antigen delivery within the digestive system for future oral nanoparticulate vaccines.
3.2.3 Reproductive tract
The female genital tract (FGT) possesses several distinctive characteristics that require thoughtful design of FGT-targeted vaccines/immunotherapy. Importantly, the mucosal surface of the lower FGT tract (vagina) consists of a loosely-connected multilayer squamous epithelium, while the mucosal surface of the upper FGT tract (uterus, cervix, and fallopian tubes) is a single layer of pseudo-squamous and simple columnar epithelia.211 These columnar epithelial cells of the upper FGT form tight junctions to maintain the integrity of the mucosal monolayer.212 The FGT lacks an equivalent of the M-cells present in the intestinal and nasal tracts. FGT epithelial cells act as both a physical barrier from the external environment and as innate immune sentinels; both uterine and vaginal epithelial cells express Toll-like receptors 1–9 and beta-defensins 1, 2, and 4.213 Activation of TLRs on FGT epithelial cells induces the secretion of the chemoattractant CCL-2, which in turn recruits immune cells into the FGT and initiates an inflammatory response. Furthermore, FGT epithelial cells are non-professional APCs, capable of presenting antigen to CD4+ T-cells.214 Beneath the mucosal surface, the lamina propria of the FGT contains a cadre of immune cells including CD4+ and CD8+ T cells, B cells, plasma cells, DCs, macrophages, Langerhans cells (vagina), NK cells (uterus) and regulatory T-cells (uterus).215 Upon infection, Langerhans cells and DCs recognize pathogens, mature, and migrate to draining lymph nodes to prime naïve B and T cells.122 There are three significant barriers to effective vaginal delivery of immunotherapies: penetrating mucus, crossing the epithelial layer, and subsequently trafficking to the draining lymph node. As the suitability of nanoparticles to enable efficient lymph node delivery has already been discussed, this section will briefly discuss the development of mucus-penetrating particles and epithelial crossing particles for FGT-targeted therapies.
Mucus-penetrating particles possess hydrophilic surface-coatings that minimize interactions with mucus. The polymer PEG has alternatively been reported to be mucoadhesive or to be mucus-penetrating.216,217 To reconcile these observations, a study by Wang et. al. demonstrated that low molecular weight PEG at high surface-coating densities penetrates cervical-vaginal mucus (CVM) but high molecular weight PEG adheres to CVM in vitro.218 Further in vivo studies compared conventional polystyrene nanoparticles (CPs) to CPs with a high density coating of low molecular weight PEG to be mucus-penentrating particles (MPPS) and revealed that MPPs were uniformly distributed across the murine vaginal epithelium while CPs were aggregated in vaginal mucus.219 Furthermore, MPPs were retained in the cervicovaginal tract whereas CPs were almost entirely flushed out in 6 hours, as shown in Fig. 13.219 Additional studies demonstrated that delivery of MPPs in hypotonic solution rather than isotonic solution further enhanced vaginal distribution and retention of the particles.220 MPPs loaded with the chemotherapeutic drug paclitaxel was demonstrated to control orthotopic TC-1 cervical tumor growth better and prolong mouse survival compared to paclitaxel-loaded CPs or Taxol (the clinical formulation of paclitaxel). PLGA nanoparticles have also been shown to penetrate CVM when coated with PEG174 and to induce long-lived systemic and local humoral immune responses after intravaginal administration221; these results suggest that MPPs could be effective delivery vehicles for vaginal vaccines.
Figure 13.
MPPs are retained in the cervicovaginal tract. (A) Overlay of particle fluorescence intensity and photographic images for conventional nanoparticles (CPs) and mucus-penetrating particles (MPPs) in the entire cervicovaginal tract tissue. (B) Percent of particles remaining over time on the basis of quantification of particle fluorescence in (A). Adapted from Ensign et al.219 Reprinted with permission from reference 219. Copyright 2012 American Association for the Advancement of Science.
3.3 Nanoparticle delivery in the skin
Transcutaneous immunization – vaccination through the skin—is an attractive alternative to needle-based immunizations due to its simplicity and potential for pain-free administration. The epidermis is a rich immunological site containing large numbers of epidermal Langerhans cells (skin-resident dendritic cells) that capture antigen and then migrate to draining lymph nodes to initiate adaptive immune responses222. A study of amorphous silica nanoparticles demonstrated that uptake by Langerhans cells in the skin was positively correlated with nanoparticle size, with 200 nm diameter nanoparticles exhibiting the most efficient uptake.223 In keeping with this observation, elastic liposomes designed to be capable of permeating through skin induced stronger systemic and salivary antibody responses than soluble antigen224 or antigen-loaded chitosan nanoparticles when applied topically to the skin, and modestly enhanced anti-tumor immunity.225 Mittal et al. induced strong cellular and humoral immune responses when a cyclic dinucleotide adjuvant – cyclic-di-AMP – was included with antigen-loaded chitosan-PLGA nanoparticles and administered transcutaneously.226
Permeation of the hydrophobic stratum corneum to reach the epidermis is a considerable barrier to effective transcutaneous immunization. Protein transduction domains (PTDs) are commonly used as a skin permeation enhancer for macromolecules. Kitaoka et al. demonstrated that inclusion of the PTD polyarginine (R6) in a solid-in-oil nanodispersion (S/O) antigen delivery system generated higher systemic antibody responses than polyarginine-free nanodispersions or soluble antigen and polyarginine (Fig. 14).227
Figure 14.
Anti-OVA mouse serum IgG response to 100 µg OVA in ddy mice (n = 5 mice) after transcutaneous application of OVA in S/O nanodispersions or in PBS with (+) or without (-) polyarginine (R6), or OVA and R6 in separate nanodispersions (OVA S/O and R6 S/O), compared to subcutaneous injection of 100 µg soluble OVA in PBS. Adapted from Kitaoka et al.227 Reprinted with permission from reference 227. Copyright 2013 Elsevier B.V.
4 Modulating antigen presenting cells and innate immunity
Antigen presenting cells play a crucial role in the response to vaccines and immunotherapies, whether the goal is to promote immunity or tolerance. Among APCs, dendritic cells are particularly important for the primary immune response because they govern the activation of CD4+ and CD8+ T-cells, which provide help for antibody responses and exert direct cytotoxic activity on infected/transformed cells, respectively (Fig. 15).6,228 Adjuvants incorporated into vaccines are often designed to act specifically on DCs, as regulation of DC activation determines the outcome of vaccination. Thus, a final key application of synthetic nanoparticles in immune engineering is modulation of APC function. Nanoparticles can act on APCs in several distinct ways, by encapsulating or displaying danger signals that promote DC activation, by triggering particle-specific immune recognition and antigen processing, delivering danger signals, or via intrinsic immunomodulatory effects on APCs. By “programming” the activation state of DCs or other APCs, nanoparticle formulations directly impact the induction of cellular and humoral immunity to vaccines and immunotherapies.
Figure 15.
A schematic view of antigen processing and presentation in dendritic cells (DCs). DC activity is initiated by pathogen-associated molecular patterns (PAMPs) and/or danger-associated molecular patterns (DAMPs) that are recognized by pattern recognition receptors (PRRs) on the DC surface. As depicted on the right, antigen and adjuvant containing nanoparticles are internalized by DCs through cell internalization pathways such as phagocytosis. Exogenous antigen is processed by proteases in endocytic vesicles (endosomes) and eventually loaded onto MHC Class II molecules found in vesicles targeted to endosomes that carry potential ligands. Once peptide is loaded onto MHC II molecules, the peptide-MHC II complex is trafficked to the cell membrane where it presents the antigen to CD4+ T-cells bearing a cognate T-cell receptor (TCR). Activated CD4+ T-cells can further initiate B-cell activation and other immune effector functions. Depicted on the left are the two alternate pathways through which antigens can be loaded onto MHC Class I molecules. In the classical MHC I loading pathway, cytosolic or endogenous antigens are processed through the proteasome. Resulting peptide fragments are transported to the endoplasmic reticulum (ER) where they are loaded onto MHC Class I molecules. In certain DCs, exogenous antigens can be loaded onto MHC Class I molecules (‘cross-presented’) when antigens from endosomes are released into the cytoplasm or when trafficked to special vacuoles (not depicted). Resulting peptide-MHC l complexes are similarly trafficked to the membrane where they can interact with CD8+ T-cells bearing cognate TCRs. Activated CD8+ T-cells have cytotoxic activity and can kill infected cells that present cognate peptide-MHC I complexed antigens. DCs also express MHC Class I-like molecules called CD1d which present lipid antigens like a-galactosylceramide (a-Gal-Cer) to invariant TCRs on NKT cells. On the lower right side, nanoparticles carrying α-Gal-Cer are internalized, processed and lipid antigen is presented on CD1d to TCRs on NKT cells. NKT activation further leads to NK cell transactivation.
4.1 Nanoparticulate antigen delivery to DCs to promote T-cell immunity
4.1.1 Enhancing antigen presentation to CD4+ T-cells
CD4+ T-cells are also known as helper T-cells since they provide required signals to B-cells to promote class switching and affinity maturation of the antibody response, instruct dendritic cells to maximize the induction of memory cells during CD8+ T-cell activation, and provide cytokines to instruct macrophages and other innate immune cells to clear infections. Dendritic cells serve as a gatekeeper on all of these processes, as they present peptide antigens bound to class II MHC molecules on their surfaces that activate CD4+ T-cells to carry out all of these functions. A role for nanoparticle carriers in promoting antigen delivery to APCs for CD4+ T-cell priming was first shown in the early 1990s, when it was demonstrated that nanoparticles (liposomes) carrying antigen designed to be stable on internalization by APCs until they reached acidic intracellular lysosomal compartments could promote antigen processing and presentation to CD4+ T-cells.229 Since then it has been shown that particle size and composition influence the capacity of nanoparticles to promote antigen delivery to class II MHC loading pathways.230 For example, OVA conjugated to 200 nm particles resulted in increased class I and II MHC presentation, CD4+ T cell responses in the lungs, systemic and musical immunity and, importantly, a higher percentage of antigen-specific polyfunctional CD4+ T cells compared to OVA conjugated to 30 nm particles.177 For liposomes, lipid bilayer composition has been shown to dictate dendritic cell interactions- cationic liposomes containing antigen are readily internalized, while anionic liposome internalization can be improved upon addition of DC targeting moieties such as mannosylated phosphotidylserine.231 Taking advantage of their propensity for internalization into various cell types and large surface area available for chemical modification, single-walled carbon nanotubes have recently been utilized as novel vaccine carriers.232 Mice immunized with 50–400 nm peptide-conjugated carbon nanotubes along with a water-in-oil emulsion adjuvant induced peptide-specific IgG responses that were not present following immunization with the peptide and adjuvant alone.232
Nanoparticles also enable compartmentalization of the epitopes targeted by B-cell and helper T-cell responses. Minimal B-cell epitopes (e.g., short peptides) are of interest for eliciting highly focused antibody responses against a chosen target antigen. However, these short peptides may not be recognized by T-cells, and thus separate peptide antigens (helper epitopes) may need to be co-delivered to APCs to prime helper T-cell responses to such vaccines. Traditionally such B cell antigens are conjugated to carrier proteins capable of eliciting strong T-cell responses, such as diphtheria toxoid233 or keyhole limpet hemocyanin (KLH),234 or more recently linked directly to small T-cell help peptides.235 However, several disadvantages exist with these methods, including competing antibody responses to the carrier protein itself, antibodies developed to the conjugation site or irrelevant antibodies towards improperly oriented antigens, and limited conjugation sites for antigen loading and multivalency.236 This problem can be solved by a strategy know as “instructural help”: antigens encapsulated within nanoparticles are inaccessible to B-cells until released, but upon release from particles following internalization by B-cells or dendritic cells are readily processed for loading onto MHC molecules for presentation to T-cells. Thus, particles encapsulating helper T-cell antigens but surface-displaying B-cell epitopes can be used to foster a B-cell response that is focused on the desired B-cell antigen without competition from B-cells recognizing the helper epitope. This has been demonstrated in the case of lipid nanoparticle vaccines displaying surface-bound peptides derived from the gp41 envelope protein of HIV (as a B-cell target), but containing encapsulated T-helper epitope peptides (Fig. 16).142 Encapsulation of the helper epitopes permitted efficient T-cell help to be provided in the vaccine while limiting the antibody response raised against the encapsulated T-cell peptides; by contrast strong humoral responses were elicited against the surface-displayed gp41 peptide.142
Figure 16.
Liposomes with surface-displayed B cell epitope (membrane-proximal external region, MPER) and encapsulated T-cell epitope (HIV30) promote strong B-cell responses against MPER while minimizing off-target responses against the helper epitope. (A) Schematic of 3 forms of T-helper epitope incorporation in MPER liposomes. Only in the case of TCEP cleaved external HIV30 is the T-helper epitope displayed solely intrastructurally. (B) Mice were immunized with 150 nm MPER liposomes with soluble or incorporated HIV30 with co-delivered adjuvant-containing liposomes. Shown are serum anti-MPER and anti-HIV30 IgG titers 7 days post-boost. Liposomes with “hidden” T helper HIV30 epitopes (cleaved HIV30) elicited greatly reduced antibody responses against the helper sequence. Adapted from Hanson et al.142 Reprinted with permission from reference 142. Copyright 2014 Elsevier Ltd.
4.1.2 Particulate delivery for cross presentation of protein antigens
For most prophylactic vaccines success is measured by the ability to produce long-lasting antibody responses to block infection. However, for some viruses like HIV, intracellular pathogens, and cancer, CD8+ T-cell responses are required to act synergistically with humoral immunity to eliminate infected cells or destroy tumors. This presents a challenge as typically only live infections elicit strong CD8+ T-cell priming. Soluble antigens acquired by DCs from the extracellular environment are internalized into endolysosomal compartments, broken down into peptides, and loaded almost exclusively onto class II MHC molecules for presentation to CD4+ helper T-cells. By contrast, class I MHC molecules that present peptides to CD8+ killer T-cells are usually only loaded with antigens located in the cytosol of DCs.237 Because cytosolic localization of antigens only occurs in special situations (e.g., infection of the DC itself with a virus or intracellular pathogen), this requirement would appear to strongly limit the occurrence of CD8+ T-cell responses in most settings. However, antigen loading onto class I MHC can also happen in a second circumstance: when DCs or macrophages phagocytose particulate antigen.237,238 Phagocytosis of antigen associated with dying cells, microparticles, or nanoparticles triggers professional APCs to shuttle a fraction of the antigen to the cytosol or to deliver it to special vacuoles where class I MHC loading can occur.239 This process, called cross presentation, was first demonstrated for antigens conjugated to synthetic iron oxide or polymer beads,240–243 but has since been demonstrated to occur for diverse synthetic particles composed of materials ranging from gold to calcium phosphate to solid polymers to hydrogels to lipids, and occurs irrespective of whether antigen is encapsulated in the particle or surface-conjugated.244–248 Although the earliest studies suggested an optimal particle size of several hundred nanometers for triggering cross presentation, more recent work has provided evidence for enhanced cross presentation of protein antigens conjugated to nanoparticles as small as 7–11 nm mean diameter.249,250 Efficient cross presentation leading to in vivo CD8+ T-cell expansion comparable to live viral infections has been reported with stabilized lipid nanocapsules termed ICMVs (interbilayer-crosslinked multilamellar vesicles)169,251 and also with multilamellar lipid vesicles loaded with a strong T-cell-inducing adjuvant (poly(I:C)) and surface-absorbed antigen.252 The ICMV technology has been licensed to Vedantra Pharmaceuticals, which aims to translate this nanocapsule approach to human clinical trials.253
Although early studies covalently conjugated proteins to solid particles, cross presentation can be optimized by engineering particle-antigen linkages to be labile in the endosomal pathway. For example, antigen coupled to pluronic-stabilized poly(propylene sulfide) nanoparticles via a reduction-sensitive disulfide linkage specifically allowed for the release of antigen within the reductive environment of the endosome in APCs after uptake leading to enhanced in vitro and in vivo CD8+ T cell stimulation compared to non-degradable linkers.254,255 In pulmonary vaccination, these nanoparticles efficiently promoted cross presentation resulting in a 10-fold enhanced effector CD8+ T-cell frequency in the lungs.176 Stano et al. carried out a comparative study of vaccination with antigen conjugated to 30 nm solid poly(propylene sulfide) (PPS) nanoparticles vs. the same antigen encapsulated in 125 nm PPS-based polymersomes (Fig. 17).256 While PPS NPs promoted robust CD8+ T-cell responses, polymersomes with encapsulated antigen induced enhanced frequencies of antigen-specific CD4+ T cells in the spleen, lymph nodes, and lungs, but were inefficient at promoting CD8+ T-cells in comparison to solid nanoparticles.256 pH-sensitive micelles carrying antigen and adjuvant have also been utilized to enhance cross presentation to CD8+ T-cells, improving both cellular and humoral responses.257,258
Figure 17.
(A) Schematic describing chemical and physical differences between solid-core NPs and water-core polymersomes (PS). Mice were immunized s.c. 3 times at 2-week intervals with various OVA formulations. One week after the final immunization, splenocytes, lymph nodes cells and lung leukocytes were isolated and restimulated ex vivo with OVA for 6 hours. PSs were more effective than NPs in inducing cytokine-secreting CD4+ T-cells (B) while NPs were more effective in inducing cytokine-secreting CD8+ T-cells (C). Co-administration of both particle types elicited T-cell immunity characteristic of the two particles at the same time. Adapted from Stano et al.256 Reprinted with permission from reference 256. Copyright 2013 Elsevier Ltd.
Nanoparticles have also been demonstrated to have potential as platforms for cross presentation of complex antigen mixtures in DCs, as exemplified by the entrapment of tumor lysate proteins, leading to enhanced tumor-specific CD8+ T-cell priming relative to soluble lysate mixtures.259 Novel strategies for displaying antigens on nanoparticles also have great potential for enabling more effective cross presentation to T-cells. For example, Fang et al. developed a method to coat biodegradable polymer nanoparticles with membranes derived from tumor cells.260 This approach promoted antigen uptake and cross presentation by dendritic cells to tumor antigen-specific T-cells, and is especially attractive because it in principle allows the entire repertoire of tumor cell surface antigens to be delivered to DCs. This approach can also be used to directly target tumor cells and vasculature for immunotherapies.261 Alternatively, the delivery of minimal peptides has been shown to help avoid tolerance and T-cell mediated anergy by minimizing peptide loading on unintended class I MHC expressing cells.139,262–264
The strategies discussed above all rely on an intrinsic pathway for delivery of antigen to class I MHC following uptake of particulate antigen, which seems to be largely independent of particle chemistry and only weakly sensitive to particle size. However, nanoparticles can also promote cross presentation more directly following internalization, via endosome/phagosome disruption and direct delivery of antigen to the cytosol, where they can be processed by the normal MHC I loading pathways. For this approach, most if not all of the common strategies employed for cytosolic drug delivery have been applied to promote antigen cross presentation. For example, Hu et al. developed core-shell gel nanoparticles with a pH-responsive core and nontoxic hydrophilic shell capable of efficient cytosolic delivery of membrane impermeable molecules via endosomal disruption through the proton sponge effect (studied for many years in nucleic acid delivery materials).265 When loaded with OVA protein these particles were shown to promote priming of CD8+ T-cells at 100-fold lower doses compared to soluble protein.266 Going a step further to promote efficient antigen release from particles in the cytosol, bioreducible alginate-poly(ethylene imine) nanogels carrying protein antigen have been synthesized. These particles were designed to disrupt endosomes through the proton sponge effect of PEI, and subsequently dissolve in the reducing conditions of the cytosol.267 These nanogels more strongly promoted intracellular antigen degradation and cytosolic release, robustly enhancing antibody responses and increased tumor cell lysis in an in vitro CD8+ T-cell cytotoxicity assay compared to non-reducible nanogels.267 Endosomolytic and pH-responsive micelles additionally have the capability to uniformly surface display antigen and incorporate adjuvant.257,258 These micelles demonstrated enhanced cross-presentation in vitro and in vivo as a function of micelle-mediated enhancements in intracellular antigen retention and cytosolic antigen accumulation. Surface-engineered gold nanorods268 and acetylated dextran nanoparticles269 have similarly been shown to osmotically rupture endosomes to deliver antigen to the cytosol. Intracellular targeting of the endoplasmic reticulum (ER) is another approach that has been shown to promote cross-presentation- following ER-endosome fusion nanoparticles are shuttled to the cytosol.270,271 Polymer-modified liposomes that destabilize at low pH have also been reported to mediate efficient cytosolic delivery of its cargo via endosome fusion and disruption.272,273 Additionally, one could imagine direct endosome destabilizing techniques to be applied to nanoparticle-mediated antigen delivery to the cytosol. For example, photochemical destabilization of endosomes for cytosolic delivery has been shown for antigens co-delivered with a photosensitizer that will cause a series of reactions to rupture endosomes upon light exposure to significantly enhance cross-presentation compared to soluble antigen alone.274
4.1.3 Modulating antigen presentation to invariant natural killer T-cells
While most cells of the adaptive immune system recognize foreign protein antigens, a subset of T-cells known as invariant natural killer T (iNKT) cells have evolved to recognize lipid antigens presented by CD1d receptors expressed on APCs. A potent lipid antigen that stimulates iNKT cells is the glycolipid α-galactosylceramide (α-GalCer), and this molecule has been proposed as an adjuvant compound to trigger anti-viral and anti-tumor responses from iNKT cells. Analogous to “normal” T-cell activation, α-GalCer and other lipid ligands for iNKT cell receptors must be captured and presented to the iNKT cells on the non-classical MHC molecule CD1d at the surface of APCs. Intravenous injection of α-GalCer leads to iNKT hypo-responsiveness, which is believed to be due in part to the non-selective nature of APCs that capture and present α-GalCer to iNKT cells following systemic administration. This can be prevented by targeting α-GalCer to specific subsets of APCs to activate iNKT cells in the right context. Fernandez et al. co-targeted α-GalCer and the model antigen ovalbumin to DCs by packaging them in PLGA nanoparticles with surface-conjugated anti-DEC205. This strategy allowed for maximal stimulation of iNKT cells as shown by increased cytokine release, expansion of antigen-specific CD8+ T-cells, and increased anti-Ova IgG titers. Furthermore, targeting of α-GalCer to CD8+ DCs abrogated the hypo-responsiveness of iNKT cells upon re-stimulation.275 Another method to target α-GalCer to DCs is to target the C-type lectin receptors (CLRs) expressed on the surface of DCs. CLRs recognize mannose receptors at the end of carbohydrate ligands and trigger phagocytosis. When α-GalCer is encapsulated in oligomannose-coated liposomes, these liposomes are preferentially taken up by dendritic cells resulting in enhanced iNKT activation and proliferation.276 Octaarginine or R8 is a cell-penetrating peptide, which when attached to the surface of liposomes promotes uptake in dendritic cells by macropinocytocis.277 PEGylated R8-liposomes encapsulating α-GalCer were shown to elicit potent iNKT cell activation in vivo, resulting in prophylactic control of tumor cell growth.278
4.1.4 Targeting antigen to APCs and regulating kinetics of antigen release
A final function of nanoparticles in regulating T-cell responses is to regulate the availability of antigen in or around antigen presenting cells. Sustained exposure to antigen and, consequently, presentation on class I and II MHC complexes has been shown to promote prolonged CD4+ and CD8+ T cell activation and cytokine expression. Nanoparticles that encapsulate antigen for sustained release either in the extracellular environment or within DCs can promote T-cell responses. Shen et al. demonstrated enhanced and prolonged cross presentation of antigen loaded on PLGA nanoparticles in vitro relative to soluble antigen or antigen bound to latex beads.279 PLGA particles were able to facilitate endosomal escape of antigen into the cytosol and also served as an intracellular reservoir. In a more recent comparative study of PLGA nanoparticles and liposomes, Demento et al. demonstrated sustained release of antigen from PLGA nanoparticles resulted in enhanced recall of CD8+ T-cell responses following bacterial challenge, compared to fast-releasing liposomes (Fig. 18).280 Furthermore, targeting moieties can be added to the surface of nanoparticles to directly engage cell-surface receptors on APCs and enhance uptake, effectively maximizing exposure to antigen.281 In addition, while sustained exposure to antigens in chronic inflammatory states, such as cancer, autoimmunity, and chronic inflammation, has been shown to induce tolerance and/or T cell exhaustion282,283, thus far there are no examples in the literature describing such undesirable effects from controlled antigen release by synthetic nanoparticles. This is likely due to several factors including (1) transient exposure to antigen, (2) antigen dose, and (3) the additional presence of a danger signal. In addition to the above examples, Hanlon et al. described how controlled released of tumor antigens from nanoparticles loaded onto dendritic cells can drive anti-tumor responses, while delivery of naked tumor lysate induced T cell profiles characteristic of tolerization and exhaustion.284
Figure 18.
Sustained-release PLGA nanoparticles induce stronger immune responses than fast-release liposomes. (A) OVA release profiles from liposomes and PLGA nanoparticles incubated in PBS at 37°C. (B) Splenocytes from mice (n=3)—immunized subcutaneously on day 0 and challenged on day 90 with i.v. 5 ×104 CFU of OVA-expressing L. monocytogenes —were collected on day 7 post-challenge and pulsed with SIINFEKL. Activated antigen-specific CD8+CD44+ T-cells were determined using a SIINFEKL tetramer. Adapted from Demento et al.280 Reprinted with permission from reference 280. Copyright 2012 Elsevier Ltd.
4.2 Nanoparticles as adjuvants
Complementary to their potential roles in presenting antigen to B-cells or delivering antigen to appropriate antigen processing pathways in antigen presenting cells, nanoparticles can also be designed to provide immunity- or tolerance-promoting secondary signals that are required to instruct the immune system as to the type of immune response to be mounted against the encountered antigen. In the former case, these immunity-promoting cues are often referred to as “danger signals”, as they are requisite cues that inform responding immune cells that a foreign antigen is dangerous (as opposed to being an innocuous environmental or food-derived antigen) and a protective immune response should be mounted.285 These are the cues needed for prophylactic or therapeutic vaccines. By contrast, counter-regulatory signals can be provided by nanoparticles that instruct the immune system to become tolerant to the encountered antigen. Such tolerizing signals could be used to block allergies, to counter xenoresponses to therapeutic proteins, or to treat autoimmune disease. Provision of these signals can be achieved in at least two ways: (i) nanoparticles can encapsulate compounds that stimulate the appropriate pro- or anti-immunity pathway, or (ii) the nanoparticle itself, by virtue of its structure or composition, can itself stimulate these pathways. This section will discuss these two broad strategies for nanoparticle adjuvants.
4.2.1 Nanoparticle delivery of molecular adjuvants
Danger signals in the induction of the immune response
The danger signals that drive protective immunity in prophylactic or therapeutic vaccines are characterized as either (1) pathogen-associated molecular patterns (PAMPs; e.g. lipopolysaccharides or flagellin from bacteria, double-stranded RNA from viruses, or cell wall components from yeast and fungi) which are evolutionarily conserved molecular motifs unique to pathogens and absent from human tissue, or (2) so-called damage-associated molecular patterns (DAMPs; i.e. molecular signatures of tissue damage such as degraded heparan sulfate or uric acid released from dying cells).286,287 These compounds are recognized by pattern recognition receptors (PRRs) expressed by immune cells, especially antigen presenting cells, and instruct APCs to produce inflammatory cytokines and to up-regulate co-stimulatory receptors and antigen presentation machinery to produce productive T- and B-cell priming. The most well studied class of these receptors is the Toll-like receptors (TLRs). These natural components or synthetic compounds that act on their receptors are molecular adjuvants that can drive tailored immune responses through defined signaling pathways. Current evidence suggests that danger signaling is critical for an optimal immune response– adaptive immune responses to vaccines are dependent on danger signals.98,288–290 Even virus-like particles, despite their potent ability to engage B-cells as discussed above, are thought to rely on contaminating Toll-like receptor ligands for their immunogenicity.291
Enhancing molecular adjuvants through nanoparticle delivery
Nanoparticle delivery of molecular adjuvants can play several roles in vaccine design. The first is to enhance the potency and efficacy of adjuvants, through a number of distinct mechanisms. Nanoparticle delivery can concentrate adjuvants at a tissue level within lymphoid organs, forming intranodal depots and prolonging exposure in this important site of action (without necessarily eliciting toxic systemic exposure).131,146,153 This is especially relevant for small-molecule adjuvant compounds (e.g., imidazoquinolines that trigger TLRs 7 and 8), which are usually cleared rapidly to the bloodstream on injection. In the case of cytokines, which can also act as molecular adjuvants, it is essential to avoid systemic exposure. St. John et al. circumvented this issue by encapsulating various cytokines in synthetic mast-cell granule-like nanoparticles composed of chitosan and heparin that efficiently drained to lymph nodes and were able to effectively adjuvant vaccination with hemagglutinin.292 More recently, Hanson et al. showed a significant dose-sparing effect of cyclic dinucleotide small molecule adjuvants (agonists of cytosolic stimulator of IFN genes, STING) when loaded inside liposomal nanoparticles.293 Without compromising the humoral response towards the co-delivered antigen, systemic cytokine induction and toxicity was avoided.293 Such nanoparticle-mediated targeting of adjuvants to lymph nodes in vaccination increases both efficacy and safety, both by blocking systemic distribution of the adjuvant and by permitting significant dose-sparing (up to 250-fold).146,147,153,293,294 Targeted delivery of TLR ligands in nanoparticles to DCs has also been shown to strongly enhance adjuvanticity.295
Particle encapsulation can also act to concentrate danger signals at the single cell level. As with antigens, nanoparticles’ natural tropism for phagocytic cells can concentrate adjuvants within receptor-bearing endosomal compartments or near the cell surface. Though the importance of local adjuvant concentration on danger signal receptor engagement and signaling has not yet been studied this is likely to play a role in the response to nanoparticle adjuvants. For example, co-encapsulation of TLR agonists and antigens in PLGA or calcium phosphate nanoparticles has been shown to enhance antigen uptake, APC activation, T-cell priming, and protection against infectious challenge in vivo.245,262,296,297 Co-encapsulation of antigen and adjuvant on nanoparticles also allows for co-localization of these compounds within the same endosomal/phagosomal compartments which has been shown to be a requirement for physically instructing dendritic cells to present the associated foreign antigen in vitro,298–300 although evidence exists for phagosome maturation in the absence of TLRs albeit at a slower constitutive rate.301,302 This was exemplified in the early 1990s by Friede et al. who compared TLR4 agonist, MPLA, delivery in the same or separate liposomes with peptide-antigen and found that MPLA delivered in the same nanoparticle resulted in significantly higher antigen-specific titers compared to delivery of MPLA in separate particles.115 Notably, several examples exist for which small nanoparticles at sufficient dosing can co-localize together in the same APC even if antigen and adjuvant are carried by separate particles, as illustrated by de Titta et al. for 30 nm poly(propylene sulfide) particles with surface-conjugated antigen mixed with particles functionalized with the TLR9 agonist CpG DNA.303
Nanoparticle delivery may also qualitatively change the biology of molecular adjuvants. For example, studies of adjuvants triggering the danger sensor TLR4 have suggested that TLR4 signaling cannot provide a suitable danger signal for CD8+ T-cell responses.304 Early studies of TLR4 ligand (LPS) incorporation into empty liposomes also reported decreased LPS potency compared to soluble LPS in vitro.305 By contrast, TLR4 ligands co-encapsulated with antigen in lipid nanoparticles have been shown to trigger robust cross presentation and CD8+ T-cell priming in vivo in mice.169 It is also known that combinations of danger signals can exhibit powerful synergy in activating APCs.306–308 Particle co-encapsulation of combinations of danger signals provides the opportunity for maximizing this synergy. This has been dramatically illustrated with PLGA nanoparticles co-encapsulating TLR4 and TLR7 small molecule adjuvants- co-delivery of these adjuvant compounds in PLGA resulted in a synergistic enhancement in comparison to either one of the TLR ligands delivered alone in separate particles (Fig. 19).309 Extremely long-lived germinal centers following vaccination were observed, resulting in high-titer and durable antibody responses. Importantly, these results have been recapitulated in non-human primates, setting the stage for clinical testing of this strategy for enhancing vaccines.309
Figure 19.
Immunization with two TLR agonists induces persistent germinal centers and long-lived antibody-forming cells in draining lymph nodes. C57BL/6 mice were immunized with OVA encapsulated in nanoparticles with TLR-4 agonist (MPL) and TLR-7 agonist (R837) plus antigen. (A) Antibody titers 4 weeks post-prime. (B) Germinal centers (GCs) were counted in draining lymph node (LN) sections over time. (C) ELISPOT assay quantification of antibody-secreting cells in lymph nodes over time. Adapted from Kasturi et al.309 Reprinted with permission from reference 309. Copyright 2011 Macmillan Publishers Ltd.
Using nanoparticles to promote tolerance
In the absence of danger signals, the presentation of self-antigens on APCs can induce tolerance. One strategy to induce peripheral tolerance of auto-reactive T-cells is to intravenously inject fixed, self-antigen-pulsed apoptotic splenocytes.310 This form of antigen delivery mimics the processing of self-antigens from dying host cells and results in the presentation of self-antigens on APCs in the absence of co-stimulatory signals. This therapy induces antigen specific tolerance of T-cell clones and the generation of new regulatory T-cells.311 However, the requirement of manipulating live patient cells for this treatment makes this approach complex for clinical translation. Nanoparticle delivery of self-antigens to APCs has recently been investigated as an alternative strategy to promote tolerance.312 Hunter et al. employed biodegradable PLGA nanoparticles to systemically deliver antigens to APCs in the spleen. When PLGA particles conjugated with auto-antigenic peptides were administered intravenously, they prevented the onset of experimental autoimmune encephalitis (EAE), a model of human multiple sclerosis (MS) and further ameliorated the symptoms of established EAE.313 Another approach is to co-deliver auto-antigens together with immunoregulatory drugs that promote a tolerogenic response. PLGA nanoparticles delivering either peptide or protein antigens together with the Treg-promoting drug rapamycin inhibited CD4+ and CD8+ T-cell activation while increasing the number of regulatory T-cells.314 Selecta Biosciences, which is developing this platform, has successfully completed a phase I clinical trial of PLGA nanoparticle vaccines in a different indication315, demonstrating that these materials can be GMP manufactured for clinical translation of this strategy. Recently, nanogels carrying self-antigens have shown superior tolerance induction compared to the delivery of soluble antigens or PLGA-loaded antigens, as a result of their increased internalization by DCs.316,317 Yeste et al. described a gold nanoparticle-based system that allowed for the co-delivery of multiple factors to DCs that synergized to induce antigen-specific regulatory T-cell activation leading to suppression of experimental autoimmune encephalomyelitis.318
The strategies just described all rely on targeting of antigens to APCs under conditions providing tolerogenic cues during antigen presentation to T-cells. Another approach is to directly deliver peptide-MHC ligands to T-cells via nanoparticles, in the absence of any other costimulatory signals. Tsai et al. demonstrated that peptide-MHC displaying nanoparticles could expand regulatory CD8+ T cells, a novel regulatory cell phenotype, and eventually restored normoglycemia in a humanized model of diabetes.319
Enhancing vaccine safety
Finally, the most critical issue with molecular adjuvants is safety. As noted above, a number of small-molecule danger signal compounds are problematic adjuvants for human use, due to their rapid dissemination into the systemic circulation on injection, leading to toxic systemic inflammation.146 Nanoparticle conjugation or encapsulation completely alters the fate of these compounds, promoting their targeting to lymphoid organs as discussed above, and blocking their trafficking into the systemic circulation. In addition, particle delivery may focus their action primarily on phagocytic cells (macrophages and dendritic cells) which may be beneficial for avoiding nonspecific polyclonal stimulation (e.g., of B-cells).
4.2.2 Intrinsic adjuvant properties of nanoparticles
Activating complement
The complement system – an ancient component of innate immunity – comprised of three distinct pathways (classical, lectin, and alternative), initiates a wealth of host responses including inflammatory responses which activate adaptive immunity and, thus, activation of complement may serve as an adjuvant strategy for vaccines and immunotherapeutics.320 While nanoparticles have been shown to be capable of activating complement, this is dependent on the surface chemistry of the particles.135,321–323 Reddy et al. reported that pluronic-stabilized poly(propylene sulfide) nanoparticles activated complement in a hydroxyl group-dependent manner and this activation induced the maturation of dendritic cells and enhanced cellular immunity when antigen was conjugated to the nanoparticles.135 Surface charge and carboxylation was later shown to further modulate complement activation.322 Alteration of the architecture of poly(ethylene oxide) chains on the surface of nanoparticles has also been shown to switch complement activation between classical and lectin pathways.323 The effects of this complement pathway switch on the adjuvant ability of nanoparticles remains to be elucidated.
Induction of autophagy
Recently it has begun to be recognized that nanoparticles may be capable of intrinsic adjuvant activity, deriving from their structure and/or composition, providing an entirely novel way for materials chemistry to be used to create new vaccines. One pathway for nanoparticle-mediated adjuvant action is induction of autophagy in antigen presenting cells. Autophagy is the process of “self-eating”– a process whereby cells engulf cytosolic contents into special membrane-bounded compartments known as autophagosomes, to degrade and recycle proteins during starvation or cellular stress. Autophagy is becoming recognized as an important player in diverse biological processes, including induction of the immune response.324 For example, Ravindran et al. recently demonstrated that virus-mediated signaling induced autophagy in dendritic cells, leading to enhanced CD4+ and CD8+ T-cell responses.325 This was the first study to demonstrate the link between the virus-induced integrated stress responses in DCs to the adaptive immune response. In parallel, studies of nanoparticle interactions with both tumor and normal cells have revealed that nanoparticle uptake can induce autophagy.326 Bringing these two observations together, Li et al. showed that alumina nanoparticles conjugated with antigen, which were shown to induce and, more importantly, require autophagy in dendritic cells to effectively cross present antigen to T cells, strongly promoted cellular and humoral immune responses in vivo.327
Activation of the inflammasome
A second pathway by which nanoparticles can directly provide danger signal effects is through activation of the inflammasome in APCs. Inflammasomes are cytosolic protein complexes that form in response to infection or stress, likely as a result of phagosomal/lysosomal rupture or destabilization, and further mediate inflammatory responses.328,329 Alum mediated adjuvancy has been correlated to activation of the NALP3 inflammasome in DCs,330,331 although some follow-up studies reported that the NALP3 inflammasome was not directly required for its adjuvant action.332,333 Similarly, other particulate vaccines have been demonstrated to activate the NALP3 inflammasome,334 including PLGA335,336 and gold.337 Several groups have shown that the induction of immunity through activation of the inflammasome by solid particles is dependent on several particle characteristics including particle chemistry, size, and shape.337–339 Using a comprehensive library of aluminum-based nanoparticles, Sun et al. demonstrated that increased aspect ratio and surface hydroxyl content correlated with the extent of lysosome damage, inflammasome activation and IL-1β cytokine production.339 A study comparing various size and shape gold nanoparticles similarly revealed rod-like particles were the most effective inflammasome activators, although spherical and cubic particles significantly induced inflammatory cytokine production.337 Based on the collated evidence of diverse particle types and chemistries triggering the inflammasome, it seems likely that the inflammasome may play a role in all nanoparticle-based vaccines.
Hydrophobicity as a danger signal
A third means for intrinsic adjuvant activity in nanomaterials is via hydrophobicity. Matzinger first proposed that the exposure of hydrophobic moieties, characteristic of unfolded proteins, microbes, and dying cells, could represent an important evolutionarily-conserved danger signal.340 Recently, evidence in support of this concept from synthetic nanoparticles has begun to emerge. Nanoparticles composed of amphiphilic poly(γ-glutamic acid) showed increased uptake and activation of DCs in vitro and cellular responses in vivo as a function of increased side-chain hydrophobicity.341 In agreement, increased surface hydrophobicity on gold nanoparticles has been shown to directly correlate to increased inflammatory cytokine expression levels both in vitro and in vivo.342 Petersen et al. demonstrated using amphiphilic polyanhydride nanoparticles that polymer hydrophobicity, in addition to several other factors including presentation of oxygen-rich molecular patterns and structural features, influenced innate immune responses.343
5 CONCLUSIONS
As shown by the many examples covered in this review, nanoparticle technologies offer a great breadth of new ways to tune specific immune responses for prevention or treatment of disease. Many of these new strategies for vaccines and immunotherapy were inspired by the availability of novel nanomaterials with new functionalities and capabilities. This trend should continue for the foreseeable future as many classes of new materials have yet to be studied in depth and may have great potential in this field. Examples include DNA nanostructures that can present complex, three-dimensional multivalent motifs to engage and organize immune cell receptors344–346, nanoparticles with the capacity to embed within or penetrate cell membranes347,348, and novel self-assembling materials that can form complex, hierarchical nanoparticles.349–351 While still in its infancy, the field of immune engineering, with nanoparticle technologies as key tools, is moving rapidly with a number of technologies moving beyond small animal models to non-human primate testing or other large animal models and small early-stage clinical trials.121,309,352–356 Important lessons for this effort can be gleaned from the field of cancer nanotechnology, chief among these being the importance of manufacturing– elegant materials that cannot be produced reliably or at large scale by commercial processes cannot survive the translation to clinical implementation. The availability of robust synthesis strategies must be a driving consideration right from the earliest stage of devising new concepts. These considerations will ensure that the field of synthetic nanoparticles for vaccines and immunotherapy reaches its full potential for clinical impact in public health and medicine.
Acknowledgments
This work was supported in part by the NIH (AI111860, CA174795, CA172164, AI091693, AI095109), the Dept. of Defense (award W911NF-13-D-0001 and W911NF-07-D-0004), the Bill and Melinda Gates Foundation, and the Ragon Institute of MGH, MIT, and Harvard. DJI is an investigator of the Howard Hughes Medical Institute.
References
- 1.Smith DM, Simon JK, Baker JR., Jr Applications of Nanotechnology for Immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer Immunotherapy Comes of Age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheridan BS, Lefrançois L. Regional and Mucosal Memory T Cells. Nat. Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles A Janeway J, Travers P, Walport M, Shlomchik MJ. Principles of Innate and Adaptive Immunity. 2001 [Google Scholar]
- 5.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, Fong L. Improved Survival with T Cell Clonotype Stability after Anti-CTLA-4 Treatment in Cancer Patients. Sci Transl Med. 2014;6:238ra70. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Banchereau J. Taking Dendritic Cells into Medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 7.Mellman I, Steinman RM. Dendritic Cells: Specialized and Regulated Antigen Processing Machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Amanna IJ, Slifka MK. Contributions of Humoral and Cellular Immunity to Vaccine-Induced Protection in Humans. Virology. 2011;411:206–215. doi: 10.1016/j.virol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RP, Jabri B. Vaccine against Autoimmune Disease: Antigen-Specific Immunotherapy. Curr Opin Immunol. 2013;25:410–417. doi: 10.1016/j.coi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamborsky J, Kroger A, Wolfe C, editors. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Washington D.C.: Public Health Foundation; 2015. [Google Scholar]
- 13.Centers for Disease Control and Prevention: Vaccines and Immunizations Publications. Parents guide to childhood immunization. [accessed Feb 1, 2015]; http://www.cdc.gov/vaccines/pubs/parents-guide/downloads/parents-guide-part3.pdf#page=10.
- 14.Wu TY, Singh M, Miller AT, Gregorio E, De Doro F, Oro UD, Skibinski DAG, Mbow ML, Bufali S, Herman AE, et al. Rational Design of Small Molecules as Vaccine Adjuvants. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009980. [DOI] [PubMed] [Google Scholar]
- 15.Rappuoli R, Aderem A. A 2020 Vision for Vaccines against HIV, Tuberculosis and Malaria. Nature. 2011;473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 16.Burchill MA, Tamburini BA, Pennock ND, White JT, Kurche JS, Kedl RM. T Cell Vaccinology: Exploring the Known Unknowns. Vaccine. 2013;31:297–305. doi: 10.1016/j.vaccine.2012.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich P-Y, Mendrzyk R, et al. Multipeptide Immune Response to Cancer Vaccine IMA901 after Single-Dose Cyclophosphamide Associates with Longer Patient Survival. Nat. Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 18.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic Cancer Vaccines: Are We There Yet? Immunol. Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couzin-Frankel J. Breakthrough of the Year 2013. Cancer Immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 20.Restifo NP, Dudley ME, Rosenberg SA. Adoptive Immunotherapy for Cancer: Harnessing the T Cell Response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2011;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis ME, Chen ZG, Shin DM. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 25.Irvine DJ, Swartz MA, Szeto GL. Engineering Synthetic Vaccines Using Cues from Natural Immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ. Localized Immunotherapy via Liposome-Anchored Anti-CD137 + IL-2 Prevents Lethal Toxicity and Elicits Local and Systemic Antitumor Immunity. Cancer Res. 2013;73:1547–1558. doi: 10.1158/0008-5472.CAN-12-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong B, Liu H, Irvine DJ. Induction of Potent Anti-Tumor Responses While Eliminating Systemic Side Effects via Liposome-Anchored Combinatorial Immunotherapy. Biomaterials. 2011;32:5134–5147. doi: 10.1016/j.biomaterials.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-Based Programming of Lymph-Node Targeting in Molecular Vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay D, Kwon B, Chen L, et al. Cytokine-Mediated Disruption of Lymphocyte Trafficking, Hemopoiesis, and Induction of Lymphopenia, Anemia, and Thrombocytopenia in Anti-CD137-Treated Mice. J. Immunol. 2007;178:4194–4213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of Single-Dose Interleukin-12 Exposure on Interleukin-12-Associated Toxicity and Interferon-Gamma Production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 31.Di Giacomo AM, Biagioli M, Maio M. The Emerging Toxicity Profiles of Anti-CTLA-4 Antibodies across Clinical Indications. Semin Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Salata O. Applications of Nanoparticles in Biology and Medicine. J. Nanobiotechnology. 2004;2:3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi C, Pamer EG. Monocyte Recruitment during Infection and Inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray PJ, Wynn TA. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez FO, Gordon S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinheiro M, Lucio M, Lima JLFC, Reis S. Liposomes As Drug Delivery Systems for the Treatment of TB: Passive & Active Targeting of Liposomes as Drug Delivery Systems for the Treatment of TB. Nanomedicine. 2011;6:1413–1428. doi: 10.2217/nnm.11.122. [DOI] [PubMed] [Google Scholar]
- 37.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson Ja, Chaubal M, et al. Development of a Macrophage-Based Nanoparticle Platform for Antiretroviral Drug Delivery. Blood. 2006;108:2827–2835. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman HE. Macrophage Delivery of Nanoformulated Antiretroviral Drug to the Brain in a Murine Model of neuroAIDS. J. Immunol. 2009;183:661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van ’t Klooster G, Hoeben E, Borghys H, Looszova A, Bouche M-P, van Velsen F, Baert L. Pharmacokinetics and Disposition of Rilpivirine (TMC278) Nanosuspension as a Long-Acting Injectable Antiretroviral Formulation. Antimicrob. Agents Chemother. 2010;54:2042–2050. doi: 10.1128/AAC.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JM, Wilson WR. Exploiting Tumour Hypoxia in Cancer Treatment. Nat. Rev. Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 41.Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, Lee JS, Lee JS, Park HJ, Song SY, et al. Use of Macrophages to Deliver Therapeutic and Imaging Contrast Agents to Tumors. Biomaterials. 2012;33:4195–4203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Alizadeh D, Zhang L, Hwang J, Schluep T, Badie B. Tumor-Associated Macrophages Are Predominant Carriers of Cyclodextrin-Based Nanoparticles into Gliomas. Nanomedicine. 2010;6:382–390. doi: 10.1016/j.nano.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Champion JA, Mitragotri S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu SS, Lau CM, Thomas SN, Jerome WG, Maron DJ, Dickerson JH, Hubbell JA, Giorgio TD. Size- and Charge-Dependent Non-Specific Uptake of PEGylated Nanoparticles by Macrophages. Int. J. Nanomedicine. 2012;7:799–813. doi: 10.2147/IJN.S28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fröhlich E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasegawa T, Hirota K, Tomoda K, Ito F, Inagawa H, Kochi C, Soma G-I, Makino K, Terada H. Phagocytic Activity of Alveolar Macrophages toward Polystyrene Latex Microspheres and PLGA Microspheres Loaded with Anti-Tuberculosis Agent. Colloids Surf. B. Biointerfaces. 2007;60:221–228. doi: 10.1016/j.colsurfb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Takano S, Aramaki Y, Tsuchiya S. Physicochemical Properties of Liposomes Affecting Apoptosis Induced by Cationic Liposomes in Macrophages. Pharm. Res. 2003;20:962–968. doi: 10.1023/a:1024441702398. [DOI] [PubMed] [Google Scholar]
- 48.Champion JA, Walker A, Mitragotri S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm. Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Control. Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Tomita Y, Rikimaru-Kaneko A, Hashiguchi K, Shirotake S. Effect of Anionic and Cationic N-Butylcyanoacrylate Nanoparticles on NO and Cytokine Production in Raw264.7 Cells. Immunopharmacol. Immunotoxicol. 2011;33:730–737. doi: 10.3109/08923973.2011.565345. [DOI] [PubMed] [Google Scholar]
- 51.Moghimi SM, Hunter AC, Murray JC. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 52.Dolina JS, Sung S-SJ, Novobrantseva TI, Nguyen TM, Hahn YS. Lipidoid Nanoparticles Containing PD-L1 siRNA Delivered In Vivo Enter Kupffer Cells and Enhance NK and CD8(+) T Cell-Mediated Hepatic Antiviral Immunity. Mol. Ther. Nucleic Acids. 2013;2:e72. doi: 10.1038/mtna.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 Promote Tolerance by Blocking the TCR-Induced Stop Signal. Nat. Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, de la Barrera SS, García VE. Role Played by the Programmed Death-1-Programmed Death Ligand Pathway during Innate Immunity against Mycobacterium Tuberculosis. J. Infect. Dis. 2010;202:524–532. doi: 10.1086/654932. [DOI] [PubMed] [Google Scholar]
- 55.Benson DM, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, et al. The PD-1/PD-L1 Axis Modulates the Natural Killer Cell versus Multiple Myeloma Effect: A Therapeutic Target for CT-011, a Novel Monoclonal Anti-PD-1 Antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okazaki T, Honjo T. The PD-1-PD-L Pathway in Immunological Tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, et al. Therapeutic siRNA Silencing in Inflammatory Monocytes in Mice. Nat. Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, et al. A Statin-Loaded Reconstituted High-Density Lipoprotein Nanoparticle Inhibits Atherosclerotic Plaque Inflammation. Nat. Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beduneau A, Ma Z, Grotepas CB, Kabanov A, Rabinow BE, Gong N, Mosley RL, Dou H, Boska MD, Gendelman HE. Facilitated Monocyte-Macrophage Uptake and Tissue Distribution of Superparmagnetic Iron-Oxide Nanoparticles. PLoS One. 2009;4:1–12. doi: 10.1371/journal.pone.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laroui H, Viennois E, Xiao B, Canup BSB, Geem D, Denning TL, Merlin D. Fab’-Bearing siRNA TNFα-Loaded Nanoparticles Targeted to Colonic Macrophages Offer an Effective Therapy for Experimental Colitis. J. Control. Release. 2014;186:41–53. doi: 10.1016/j.jconrel.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wijagkanalan W, Kawakami S, Higuchi Y, Yamashita F, Hashida M. Intratracheally Instilled Mannosylated Cationic liposome/NFκB Decoy Complexes for Effective Prevention of LPS-Induced Lung Inflammation. J. Control. Release. 2011;149:42–50. doi: 10.1016/j.jconrel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived from Ly6C(high) Monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 63.Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET Imaging of Tumor Associated Macrophages Using Mannose Coated 64Cu Liposomes. Biomaterials. 2012;33:7785–7793. doi: 10.1016/j.biomaterials.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In Vivo Biodistribution and Highly Efficient Tumour Targeting of Carbon Nanotubes in Mice. Nat. Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 65.Maeda H. The Enhanced Permeability and Retention (EPR) Effect in Tumor Vasculature: The Key Role of Tumor-Selective Macromolecular Drug Targeting. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 66.Smith BR, Zavaleta C, Rosenberg J, Tong R, Ramunas J, Liu Z, Dai H, Gambhir SS. High-Resolution, Serial Intravital Microscopic Imaging of Nanoparticle Delivery and Targeting in a Small Animal Tumor Model. Nano Today. 2013;8 doi: 10.1016/j.nantod.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith BR, Ghosn EEB, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS. Selective Uptake of Single-Walled Carbon Nanotubes by Circulating Monocytes for Enhanced Tumour Delivery. Nat. Nanotechnol. 2014;9:481–487. doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kolaczkowska E, Kubes P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 69.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil Function in Inflammation and Inflammatory Diseases. Rheumatology (Oxford) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Li J, Cho J, Malik AB. Prevention of Vascular Inflammation by Nanoparticle Targeting of Adherent Neutrophils. Nat. Nanotechnol. 2014;9:204–210. doi: 10.1038/nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Development and in Vivo Efficacy of Targeted Polymeric Inflammation-Resolving Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai S, Santamaria P. MHC Class II Polymorphisms, Autoreactive T-Cells, and Autoimmunity. Front. Immunol. 2013;4:321. doi: 10.3389/fimmu.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive Immunotherapy for Cancer or Viruses. Annu. Rev. Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic Field-Induced T Cell Receptor Clustering by Nanoparticles Enhances T Cell Activation and Stimulates Antitumor Activity. ACS Nano. 2015;8:2252–2260. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T Cell Therapy Using Antigen-Specific CD8+ T Cell Clones for the Treatment of Patients with Metastatic Melanoma: In Vivo Persistence, Migration, and Antitumor Effect of Transferred T Cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosalia RA, Arenas-Ramirez N, Bouchaud G, Raeber ME, Boyman O. Use of Enhanced Interleukin-2 Formulations for Improved Immunotherapy against Cancer. Curr. Opin. Chem. Biol. 2014;23:39–46. doi: 10.1016/j.cbpa.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in Regulating CD8+ T Cell Expansion and Function. J. Exp. Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Panelli MC, White R, Foster M, Martin B, Wang E, Smith K, Marincola FM. Forecasting the Cytokine Storm Following Systemic Interleukin (IL)-2 Administration. J. Transl. Med. 2004;2:17. doi: 10.1186/1479-5876-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic Cell Engineering with Surface-Conjugated Synthetic Nanoparticles. Nat. Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-Directed Delivery of Immunomodulators Using T-Cell-Conjugated Nanoparticles. Biomaterials. 2012;33:5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vinay DS, Kwon BS. Role of 4-1BB in Immune Responses. Semin. Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 82.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, et al. Combination Delivery of TGF-Β Inhibitor and IL-2 by Nanoscale Liposomal Polymeric Gels Enhances Tumour Immunotherapy. Nat. Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ. In Vivo Targeting of Adoptively Transferred T-Cells with Antibody- and Cytokine-Conjugated Liposomes. J. Control. Release. 2013;172:426–435. doi: 10.1016/j.jconrel.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ni X, Castanares M, Mukherjee A, Lupold SE. Nucleic Acid Aptamers: Clinical Applications and Promising New Horizons. Curr. Med. Chem. 2011;18:4206–4214. doi: 10.2174/092986711797189600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, Sullenger B, Gilboa E. Multivalent 4-1BB Binding Aptamers Costimulate CD8+ T Cells and Inhibit Tumor Growth in Mice. J. Clin. Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xing Y, Hogquist KA. T-Cell Tolerance: Central and Peripheral. Cold Spring Harb. Perspect. Biol. 2012;4:a006957. doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P. Reversal of Autoimmunity by Boosting Memory-like Autoregulatory T Cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 88.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, et al. Kv1.3 Channels Are a Therapeutic Target for T Cell-Mediated Autoimmune Diseases. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hajdu P, Chimote AA, Thompson TH, Koo Y, Yun Y, Conforti L. Functionalized Liposomes Loaded with siRNAs Targeting Ion Channels in Effector Memory T Cells as a Potential Therapy for Autoimmunity. Biomaterials. 2013;34:10249–10257. doi: 10.1016/j.biomaterials.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fahmy TM, Schneck JP, Saltzman WM. A Nanoscopic Multivalent Antigen-Presenting Carrier for Sensitive Detection and Drug Delivery to T Cells. Nanomedicine. 2007;3:75–85. doi: 10.1016/j.nano.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 91.Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM. Modulation of CD4+ T Lymphocyte Lineage Outcomes with Targeted, Nanoparticle-Mediated Cytokine Delivery. Mol. Pharm. 2011;8:143–152. doi: 10.1021/mp100203a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Endsley AN, Ho RJY. Enhanced Anti-HIV Efficacy of Indinavir after Inclusion in CD4-Targeted Lipid Nanoparticles. J. Acquir. Immune Defic. Syndr. 2012;61:417–424. doi: 10.1097/QAI.0b013e3182653c1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perisé-Barrios AJ, Jiménez JL, Domínguez-Soto A, de la Mata FJ, Corbí AL, Gomez R, Muñoz-Fernandez MÁ. Carbosilane Dendrimers as Gene Delivery Agents for the Treatment of HIV Infection. J. Control. Release. 2014;184:51–57. doi: 10.1016/j.jconrel.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 94.Serramía MJ, Álvarez S, Fuentes-Paniagua E, Clemente MI, Sánchez-Nieves J, Gómez R, de la Mata J, Muñoz-Fernández MÁ. In Vivo Delivery of siRNA to the Brain by Carbosilane Dendrimer. J. Control. Release. 2015;200C:60–70. doi: 10.1016/j.jconrel.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 95.Kim Y-M, Pan JY-J, Korbel Ga, Peperzak V, Boes M, Ploegh HL. Monovalent Ligation of the B Cell Receptor Induces Receptor Activation but Fails to Promote Antigen Presentation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3327–3332. doi: 10.1073/pnas.0511315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minguet S, Dopfer EP, Schamel WWA. Low-Valency, but Not Monovalent, Antigens Trigger the B-Cell Antigen Receptor (BCR) Int. Immunol. 2010;22:205–212. doi: 10.1093/intimm/dxp129. [DOI] [PubMed] [Google Scholar]
- 97.Bachmann MF, Hoffman Rohrer U, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The Influence of Antigen Organization on B Cell Responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 98.Pasare C, Medzhitov R. Control of B-Cell Responses by Toll-like Receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 99.Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, Huber M, Schamel WW. Enhanced B-Cell Activation Mediated by TLR4 and BCR Crosstalk. Eur. J. Immunol. 2008;38:2475–2487. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- 100.Dintzis RZ, Middleton MH, Dintzis HM. Studies on the Immunogenicity and Tolerogenicity of T-Independent Antigens. J. Immunol. 1983;131:2196–2203. [PubMed] [Google Scholar]
- 101.Dintzis HM, Dintzis RZ, Vogelstein B. Molecular Determinants of Immunogenicity: The Immunon Model of Immune Response. Proc. Natl. Acad. Sci. 1976;73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chackerian B, Durfee MR, Schiller JT. Anergy in a B Cell Receptor Transgenic Mouse Model 1. J. Immunol. Immunol. 2011;2008:5816–5825. doi: 10.4049/jimmunol.180.9.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chackerian B, Lowy DR, Schiller JT. Conjugation of a Self-Antigen to Papillomavirus-like Particles Allows for Efficient Induction of Protective Autoantibodies. J. Clin. Invest. 2001;108:415–423. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gatto D, Martin SW, Bessa J, Pellicioli E, Saudan P, Hinton HJ, Bachmann MF. Regulation of Memory Antibody Levels: The Role of Persisting Antigen versus Plasma Cell Life Span. J. Immunol. 2007;178:67–76. doi: 10.4049/jimmunol.178.1.67. [DOI] [PubMed] [Google Scholar]
- 105.Zeltins A. Construction and Characterization of Virus-like Particles: A Review. Mol. Biotechnol. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lico C, Marusic C, Capuano F, Buriani G, Benvenuto E, Baschieri S. Innovation in Vaccinology. 2012 [Google Scholar]
- 107.Rodríguez-Limas Wa, Sekar K, Tyo KEJ. Virus-like Particles: The Future of Microbial Factories and Cell-Free Systems as Platforms for Vaccine Development. Curr. Opin. Biotechnol. 2013;24:1089–1093. doi: 10.1016/j.copbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang L-J, Dowd KA, Mendoza FH, Saunders JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME, et al. Safety and Tolerability of Chikungunya Virus-like Particle Vaccine in Healthy Adults: A Phase 1 Dose-Escalation Trial. Lancet. 2014;384:2046–2052. doi: 10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- 109.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New Adjuvants for Human Vaccines. Curr Opin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Morefield GL, Sokolovska A, Jiang D, Hogenesch H, Robinson JP, Hem SL. Role of Aluminum-Containing Adjuvants in Antigen Internalization by Dendritic Cells in Vitro. Vaccine. 2005;23:1588–1595. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 111.Xiao Y, Zeng Y, Alexander E, Mehta S, Joshi SB, Buchman GW, Volkin DB, Middaugh CR, Isaacs SN. Adsorption of Recombinant Poxvirus L1-Protein to Aluminum hydroxide/CpG Vaccine Adjuvants Enhances Immune Responses and Protection of Mice from Vaccinia Virus Challenge. Vaccine. 2013;31:319–326. doi: 10.1016/j.vaccine.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watson DS, Endsley AN, Huang L. Design Considerations for Liposomal Vaccines: Influence of Formulation Parameters on Antibody and Cell-Mediated Immune Responses to Liposome Associated Antigens. Vaccine. 2012;30:2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sah H, Thoma LA, Desu HR, Sah E, Wood GC. Concepts and Practices Used to Develop Functional PLGA-Based Nanoparticulate Systems. Int. J. Nanomedicine. 2013;8:747–765. doi: 10.2147/IJN.S40579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moyle PM, Toth I. Modern Subunit Vaccines: Development, Components, and Research Opportunities. ChemMedChem. 2013;8:360–376. doi: 10.1002/cmdc.201200487. [DOI] [PubMed] [Google Scholar]
- 115.Friede M, Muller S, Briand JP, Van Regenmortel MH, Schuber F. Induction of Immune Response against a Short Synthetic Peptide Antigen Coupled to Small Neutral Liposomes Containing Monophosphoryl Lipid A. Mol. Immunol. 1993;30:539–547. doi: 10.1016/0161-5890(93)90028-a. [DOI] [PubMed] [Google Scholar]
- 116.Temchura VV, Kozlova D, Sokolova V, Überla K, Epple M. Targeting and Activation of Antigen-Specific B-Cells by Calcium Phosphate Nanoparticles Loaded with Protein Antigen. Biomaterials. 2014;35:6098–6105. doi: 10.1016/j.biomaterials.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 117.Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. A Systematic Analysis of Peptide Linker Length and Liposomal Polyethylene Glycol Coating on Cellular Uptake of Peptide-Targeted Liposomes. ACS Nano. 2013;7:2935–2947. doi: 10.1021/nn305663e. [DOI] [PubMed] [Google Scholar]
- 118.Stefanick JF, Ashley JD, Bilgicer B. Enhanced Cellular Uptake of Peptide-Targeted Nanoparticles through Increased Peptide Hydrophilicity and Optimized Ethylene Glycol Peptide-Linker Length. ACS Nano. 2013;7:8115–8127. doi: 10.1021/nn4033954. [DOI] [PubMed] [Google Scholar]
- 119.Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H, Irvine DJ. Robust IgG Responses to Nanograms of Antigen Using a Biomimetic Lipid-Coated Particle Vaccine. J. Control. Release. 2012;157:354–365. doi: 10.1016/j.jconrel.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-Displaying Lipid-Enveloped PLGA Nanoparticles as Delivery Agents for a Plasmodium Vivax Malaria Vaccine. PLoS One. 2012;7:e31472. doi: 10.1371/journal.pone.0031472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Torres AG, Gregory AE, Hatcher CL, Vinet-Oliphant H, Morici LA, Titball RW, Roy CJ. Protection of Non-Human Primates against Glanders with a Gold Nanoparticle Glycoconjugate Vaccine. Vaccine. 2015;33:686–692. doi: 10.1016/j.vaccine.2014.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iwasaki A. Antiviral Immune Responses in the Genital Tract: Clues for Vaccines. Nat. Rev. Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iwasaki A. Mucosal Dendritic Cells. Annu. Rev. Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 124.Clark RA. Resident Memory T Cells in Human Health and Disease. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.3010641. 269rv1–rv269rv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waeckerle-Men Y, Bruffaerts N, Liang Y, Jurion F, Sander P, Kündig TM, Huygen K, Johansen P. Lymph Node Targeting of BCG Vaccines Amplifies CD4 and CD8 T-Cell Responses and Protection against Mycobacterium Tuberculosis. Vaccine. 2013;31:1057–1064. doi: 10.1016/j.vaccine.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 126.Weber J, Boswell W, Smith J, Hersh E, Snively J, Diaz M, Miles S, Liu X, Obrocea M, Qiu Z, et al. Phase 1 Trial of Intranodal Injection of a Melan-A/MART-1 DNA Plasmid Vaccine in Patients with Stage IV Melanoma. J. Immunother. 31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 127.Maier T, Tun-Kyi A, Tassis A, Jungius K-P, Burg G, Dummer R, Nestle FO. Vaccination of Patients with Cutaneous T-Cell Lymphoma Using Intranodal Injection of Autologous Tumor-Lysate-Pulsed Dendritic Cells. Blood. 2003;102:2338–2344. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 128.Gilliet M, Kleinhans M, Lantelme E, Schadendorf D, Burg G, Nestle FO. Intranodal Injection of Semimature Monocyte-Derived Dendritic Cells Induces T Helper Type 1 Responses to Protein Neoantigen. Blood. 2003;102:36–42. doi: 10.1182/blood-2002-07-2274. [DOI] [PubMed] [Google Scholar]
- 129.Adamina M, Rosenthal R, Weber WP, Frey DM, Viehl CT, Bolli M, Huegli RW, Jacob AL, Heberer M, Oertli D, et al. Intranodal Immunization with a Vaccinia Virus Encoding Multiple Antigenic Epitopes and Costimulatory Molecules in Metastatic Melanoma. Mol. Ther. 2010;18:651–659. doi: 10.1038/mt.2009.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silva AL, Rosalia RA, Varypataki E, Sibuea S, Ossendorp F, Jiskoot W. Poly-(lactic-Co-Glycolic-Acid)-Based Particulate Vaccines: Particle Uptake by Dendritic Cells Is a Key Parameter for Immune Activation. Vaccine. 2015;33:847–854. doi: 10.1016/j.vaccine.2014.12.059. [DOI] [PubMed] [Google Scholar]
- 131.Jewell CM, Bustamante Lopez SC, Irvine DJ. In Situ Engineering of the Lymph Node Microenvironment via Intranodal Injection of Adjuvant-Releasing Polymer Particles. Proc. Natl. Acad. Sci. 2011;108:15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Slütter B, Bal SM, Ding Z, Jiskoot W, Bouwstra JA. Adjuvant Effect of Cationic Liposomes and CpG Depends on Administration Route. J. Control. Release. 2011;154:123–130. doi: 10.1016/j.jconrel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 133.Kaminskas LM, Porter CJH. Targeting the Lymphatics Using Dendritic Polymers (dendrimers) Adv. Drug Deliv. Rev. 2011;63:890–900. doi: 10.1016/j.addr.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 134.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles Target Distinct Dendritic Cell Populations according to Their Size. Eur. J. Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 135.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting Lymphatic Transport and Complement Activation in Nanoparticle Vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 136.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In Vivo Targeting of Dendritic Cells in Lymph Nodes with Poly(propylene Sulfide) Nanoparticles. J. Control. Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 137.Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, Swartz MA. Peripherally Administered Nanoparticles Target Monocytic Myeloid Cells, Secondary Lymphoid Organs and Tumors in Mice. PLoS One. 2013;8:e61646. doi: 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IFC, Plebanski M. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. J. Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 139.Fifis T, Mottram P, Bogdanoska V, Hanley J, Plebanski M. Short Peptide Sequences Containing MHC Class I And/or Class II Epitopes Linked to Nano-Beads Induce Strong Immunity and Inhibition of Growth of Antigen-Specific Tumour Challenge in Mice. Vaccine. 2004;23:258–266. doi: 10.1016/j.vaccine.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 140.Gerner MY, Torabi-Parizi P, Germain RN. Strategically Localized Dendritic Cells Promote Rapid T Cell Responses to Lymph-Borne Particulate Antigens. Immunity. 2014;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 141.Mueller SN, Tian S, DeSimone JM. Rapid and Persistent Delivery of Antigen by Lymph Node Targeting PRINT Nanoparticle Vaccine Carrier To Promote Humoral Immunity. Mol. Pharm. 2015;12:1356–1365. doi: 10.1021/mp500589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hanson MC, Abraham W, Crespo MP, Chen SH, Liu H, Szeto GL, Kim M, Reinherz EL, Irvine DJ. Liposomal Vaccines Incorporating Molecular Adjuvants and Intrastructural T-Cell Help Promote the Immunogenicity of HIV Membrane-Proximal External Region Peptides. Vaccine. 2015;33:861–868. doi: 10.1016/j.vaccine.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oussoren C, Zuidema J, Crommelin DJA, Storm G. Lymphatic Uptake and Biodistribution of Liposomes after Subcutaneous Injection. II. Influence of Liposomal Size, Lipid Composition and Lipid Dose. Biochim. Biophys. Acta - Biomembr. 1997;1328:261–272. doi: 10.1016/s0005-2736(97)00122-3. [DOI] [PubMed] [Google Scholar]
- 144.Oyewumi MO, Kumar A, Cui Z. Nano-Microparticles as Immune Adjuvants: Correlating Particle Sizes and the Resultant Immune Responses. Expert Rev. Vaccines. 2010;9:1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yan S, Gu W, Xu ZP. Re-Considering How Particle Size and Other Properties of Antigen-Adjuvant Complexes Impact on the Immune Responses. J. Colloid Interface Sci. 2013;395:1–10. doi: 10.1016/j.jcis.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 146.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine Adjuvant Activity of 3m-052: An Imidazoquinoline Designed for Local Activity without Systemic Cytokine Induction. Vaccine. 2011;29:5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 147.Ilyinskii PO, Roy CJ, O’Neil CP, Browning Ea, Pittet La, Altreuter DH, Alexis F, Tonti E, Shi J, Basto Pa, et al. Adjuvant-Carrying Synthetic Vaccine Particles Augment the Immune Response to Encapsulated Antigen and Exhibit Strong Local Immune Activation without Inducing Systemic Cytokine Release. Vaccine. 2014;32:2882–2895. doi: 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kinman L, Brodie SJ, Tsai CC, Bui T, Larsen K, Schmidt A, Anderson D, Morton WR, Hu S-L, Ho RJY. Lipid-Drug Association Enhanced HIV-1 Protease Inhibitor Indinavir Localization in Lymphoid Tissues and Viral Load Reduction: A Proof of Concept Study in HIV-2287-Infected Macaques. J. Acquir. Immune Defic. Syndr. 2003;34:387–397. doi: 10.1097/00126334-200312010-00005. [DOI] [PubMed] [Google Scholar]
- 149.Freeling JP, Ho RJY. Anti-HIV Drug Particles May Overcome Lymphatic Drug Insufficiency and Associated HIV Persistence. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2512–E2513. doi: 10.1073/pnas.1406554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Junt T, Moseman EA, Iannacone M, Massberg S, Lang Pa, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular Sinus Macrophages in Lymph Nodes Clear Lymph-Borne Viruses and Present Them to Antiviral B Cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 151.Carrasco YR, Batista FD. B Cells Acquire Particulate Antigen in a Macrophage-Rich Area at the Boundary between the Follicle and the Subcapsular Sinus of the Lymph Node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 152.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular Encounter and Complement-Dependent Transport of Immune Complexes by Lymph Node B Cells. Nat. Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 153.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing Humoral Responses to a Malaria Antigen with Nanoparticle Vaccines That Expand Tfh Cells and Promote Germinal Center Induction. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang C, Liu P, Zhuang Y, Li P, Jiang B, Pan H, Liu L, Cai L, Ma Y. Lymphatic-Targeted Cationic Liposomes: A Robust Vaccine Adjuvant for Promoting Long-Term Immunological Memory. Vaccine. 2014;32:5475–5483. doi: 10.1016/j.vaccine.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 155.Cruz LJ, Rosalia RA, Kleinovink JW, Rueda F, Löwik CWGM, Ossendorp F. Targeting Nanoparticles to CD40, DEC-205 or CD11c Molecules on Dendritic Cells for Efficient CD8(+) T Cell Response: A Comparative Study. J. Control. Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 156.Rosalia RA, Cruz LJ, Duikeren S Van, Tromp AT, Oostendorp J, Silva AL, Jiskoot W, Gruijl T De, Clemens L, Burg SH Van Der, et al. CD40-Targeted Dendritic Cell Delivery of PLGA-Nanoparticle Vaccines Induce Potent Anti-Tumor Responses. Biomaterials. 2015;40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 157.Muraoka D, Harada N, Hayashi T, Tahara Y, Momose F, Sawada S, Mukai S, Akiyoshi K, Shiku H. Nanogel-Based Immunologically Stealth Vaccine Targets Macrophages in the Medulla of Lymph Node and Induces Potent Antitumor Immunity. ACS Nano. 2014;8:9209–9218. doi: 10.1021/nn502975r. [DOI] [PubMed] [Google Scholar]
- 158.Cerutti A, Chen K, Chorny A. Immunoglobulin Responses at the Mucosal Interface. Annu. Rev. Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brandtzaeg P. Induction of Secretory Immunity and Memory at Mucosal Surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 160.Hanniffy SB, Carter AT, Hitchin E, Wells JM. Mucosal Delivery of a Pneumococcal Vaccine Using Lactococcus Lactis Affords Protection against Respiratory Infection. J. Infect. Dis. 2007;195:185–193. doi: 10.1086/509807. [DOI] [PubMed] [Google Scholar]
- 161.Ralli-Jain P, Tifrea D, Cheng C, Pal S, de la Maza LM. Enhancement of the Protective Efficacy of a Chlamydia Trachomatis Recombinant Vaccine by Combining Systemic and Mucosal Routes for Immunization. Vaccine. 2010;28:7659–7666. doi: 10.1016/j.vaccine.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kuznetsov VA, Stepanov VS, Berzofsky JA, Belyakov IM. Assessment of the Relative Therapeutic Effects of Vaccines on Virus Load and Immune Responses in Small Groups at Several Time Points: Efficacy of Mucosal and Subcutaneous Polypeptide Vaccines in Rhesus Macaques Exposed to SHIV. J. Clin. Virol. 2004;31(Suppl 1):S69–S82. doi: 10.1016/j.jcv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 163.Gebril A, Alsaadi M, Acevedo R, Mullen AB, Ferro VA. Optimizing Efficacy of Mucosal Vaccines. Expert Rev. Vaccines. 2012;11:1139–1155. doi: 10.1586/erv.12.81. [DOI] [PubMed] [Google Scholar]
- 164.Woodrow Ka, Bennett KM, Lo DD. Mucosal Vaccine Design and Delivery. Annu. Rev. Biomed. Eng. 2012;14:17–46. doi: 10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
- 165.Riese P, Sakthivel P, Trittel S, Guzmán CA. Intranasal Formulations: Promising Strategy to Deliver Vaccines. Expert Opin. Drug Deliv. 2014;11:1619–1634. doi: 10.1517/17425247.2014.931936. [DOI] [PubMed] [Google Scholar]
- 166.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J, Wahren B, Okuda K. Intranasal Immunization of a DNA Vaccine with IL-12- and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)-Expressing Plasmids in Liposomes Induces Strong Mucosal and Cell-Mediated Immune Responses against HIV-1 Antigens. J. Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 167.Klavinskis LS, Barnfield C, Gao L, Parker S. Intranasal Immunization with Plasmid DNA-Lipid Complexes Elicits Mucosal Immunity in the Female Genital and Rectal Tracts. J. Immunol. 1999;162:254–262. [PubMed] [Google Scholar]
- 168.Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, Krummel MF. Spatiotemporally Separated Antigen Uptake by Alveolar Dendritic Cells and Airway Presentation to T Cells in the Lung. J. Exp. Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, Seidman Ma, Yen M, Im E-J, Foley MH, Barouch DH, et al. Generation of Effector Memory T Cell-Based Mucosal and Systemic Immunity with Pulmonary Nanoparticle Vaccination. Sci. Transl. Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vicente S, Peleteiro M, Díaz-Freitas B, Sanchez A, González-Fernández Á, Alonso MJ. Co-Delivery of Viral Proteins and a TLR7 Agonist from Polysaccharide Nanocapsules: A Needle-Free Vaccination Strategy. J. Control. Release. 2013;172:773–781. doi: 10.1016/j.jconrel.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 171.Sanders MT, Deliyannis G, Pearse MJ, McNamara MK, Brown LE. Single Dose Intranasal Immunization with ISCOMATRIX Vaccines to Elicit Antibody-Mediated Clearance of Influenza Virus Requires Delivery to the Lower Respiratory Tract. Vaccine. 2009;27:2475–2482. doi: 10.1016/j.vaccine.2009.02.054. [DOI] [PubMed] [Google Scholar]
- 172.Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, et al. Nanogel Antigenic Protein-Delivery System for Adjuvant-Free Intranasal Vaccines. Nat. Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 173.Maisel K, Ensign L, Reddy M, Cone R, Hanes J. Effect of Surface Chemistry on Nanoparticle Interaction with Gastrointestinal Mucus and Distribution in the Gastrointestinal Tract Following Oral and Rectal Administration in the Mouse. J. Control. Release. 2015;197:48–57. doi: 10.1016/j.jconrel.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cu Y, Booth CJ, Saltzman WM. In Vivo Distribution of Surface-Modified PLGA Nanoparticles Following Intravaginal Delivery. J. Control. Release. 2011;156:258–264. doi: 10.1016/j.jconrel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stano A, van der Vlies AJ, Martino MM, Swartz MA, Hubbell JA, Simeoni E. PPS Nanoparticles as Versatile Delivery System to Induce Systemic and Broad Mucosal Immunity after Intranasal Administration. Vaccine. 2011;29:804–812. doi: 10.1016/j.vaccine.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 176.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA. Nanoparticle Conjugation of Antigen Enhances Cytotoxic T-Cell Responses in Pulmonary Vaccination. Proc. Natl. Acad. Sci. 2011;108:E989–E997. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stano A, Nembrini C, Swartz MA, Hubbell JA, Simeoni E. Nanoparticle Size Influences the Magnitude and Quality of Mucosal Immune Responses after Intranasal Immunization. Vaccine. 2012;30:7541–7546. doi: 10.1016/j.vaccine.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 178.Stylianou E, Diogo GR, Pepponi I, van Dolleweerd C, Arias MA, Locht C, Rider CC, Sibley L, Cutting SM, Loxley A, et al. Mucosal Delivery of Antigen-Coated Nanoparticles to Lungs Confers Protective Immunity against Tuberculosis Infection in Mice. Eur. J. Immunol. 2014;44:440–449. doi: 10.1002/eji.201343887. [DOI] [PubMed] [Google Scholar]
- 179.Yeh T-H, Hsu L-W, Tseng MT, Lee P-L, Sonjae K, Ho Y-C, Sung H-W. Mechanism and Consequence of Chitosan-Mediated Reversible Epithelial Tight Junction Opening. Biomaterials. 2011;32:6164–6173. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 180.Fernandez-Urrusuno R, Calvo P, Remunan-Lopez C, Vila-Jato J, Alonso M. Enhancement of Nasal Absorption of Insulin Using Chitosan Nanoparticles. Pharm. Res. 1999;16:1576–1581. doi: 10.1023/a:1018908705446. [DOI] [PubMed] [Google Scholar]
- 181.Xu J, Dai W, Wang Z, Chen B, Li Z, Fan X. Intranasal Vaccination with Chitosan-DNA Nanoparticles Expressing Pneumococcal Surface Antigen a Protects Mice against Nasopharyngeal Colonization by Streptococcus Pneumoniae. Clin. Vaccine Immunol. 2011;18:75–81. doi: 10.1128/CVI.00263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sawaengsak C, Mori Y, Yamanishi K, Mitrevej A, Sinchaipanid N. Chitosan Nanoparticle Encapsulated Hemagglutinin-Split Influenza Virus Mucosal Vaccine. AAPS PharmSciTech. 2014;15:317–325. doi: 10.1208/s12249-013-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bal SM, Slütter B, Verheul R, Bouwstra Ja, Jiskoot W. Adjuvanted, Antigen Loaded N-Trimethyl Chitosan Nanoparticles for Nasal and Intradermal Vaccination: Adjuvant- and Site-Dependent Immunogenicity in Mice. Eur. J. Pharm. Sci. 2012;45:475–481. doi: 10.1016/j.ejps.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 184.Davis MJ, Swanson JA. Technical Advance: Caspase-1 Activation and IL-1β Release Correlate with the Degree of Lysosome Damage, as Illustrated by a Novel Imaging Method to Quantify Phagolysosome Damage. J. Leukoc. Biol. 2010;88:813–822. doi: 10.1189/jlb.0310159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate Immune Activation through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nehaus V, Chichester JA, Ebensen T, Schwarz K, Hartman CE, Shoji Y, Guzmán CA, Yusibov V, Sewald K, Braun A. A New Adjuvanted Nanoparticle-Based H1N1 Influenza Vaccine Induced Antigen-Specific Local Mucosal and Systemic Immune Responses after Administration into the Lung. Vaccine. 2014;32:3216–3222. doi: 10.1016/j.vaccine.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 187.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a Protective Single-Dose Intranasal Nanoparticle-Based Vaccine Platform for Respiratory Infectious Diseases. PLoS One. 2011;6:1–8. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jones M-C, Jones Sa, Riffo-Vasquez Y, Spina D, Hoffman E, Morgan A, Patel A, Page C, Forbes B, Dailey LA. Quantitative Assessment of Nanoparticle Surface Hydrophobicity and Its Influence on Pulmonary Biocompatibility. J. Control. Release. 2014;183:94–104. doi: 10.1016/j.jconrel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 189.Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid Nanocapsules: A New Platform for Nanomedicine. Int. J. Pharm. 2009;379:201–209. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 190.Tobio M, Gref R, Sanchez A, Langer R, Alonso M. Stealth PLA-PEG Nanoparticles as Protein Carriers for Nasal Administration. Pharm. Res. 1998;15:270–275. doi: 10.1023/a:1011922819926. [DOI] [PubMed] [Google Scholar]
- 191.Plapied L, Duhem N, des Rieux A, Préat V. Fate of Polymeric Nanocarriers for Oral Drug Delivery. Curr. Opin. Colloid Interface Sci. 2011;16:228–237. [Google Scholar]
- 192.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal Uptake of Biodegradable Microparticles: Effect of Particle Size. Pharm. Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 193.Wang T, Zou M, Jiang H, Ji Z, Gao P, Cheng G. Synthesis of a Novel Kind of Carbon Nanoparticle with Large Mesopores and Macropores and Its Application as an Oral Vaccine Adjuvant. Eur. J. Pharm. Sci. 2011;44:653–659. doi: 10.1016/j.ejps.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 194.Barhate G, Gautam M, Gairola S, Jadhav S, Pokharkar V. Quillaja Saponaria Extract as Mucosal Adjuvant with Chitosan Functionalized Gold Nanoparticles for Mucosal Vaccine Delivery: Stability and Immunoefficiency Studies. Int. J. Pharm. 2013;441:636–642. doi: 10.1016/j.ijpharm.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 195.Barhate G, Gautam M, Gairola S, Jadhav S, Pokharkar V. Enhanced Mucosal Immune Responses against Tetanus Toxoid Using Novel Delivery System Comprised of Chitosan-Functionalized Gold Nanoparticles and Botanical Adjuvant: Characterization, Immunogenicity, and Stability Assessment. J. Pharm. Sci. 2014;103:3448–3456. doi: 10.1002/jps.24161. [DOI] [PubMed] [Google Scholar]
- 196.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) Cells: Important Immunosurveillance Posts in the Intestinal Epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gupta PN, Vyas SP. Investigation of Lectinized Liposomes as M-Cell Targeted Carrier-Adjuvant for Mucosal Immunization. Colloids Surf. B. Biointerfaces. 2011;82:118–125. doi: 10.1016/j.colsurfb.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 198.Gupta PN, Khatri K, Goyal AK, Mishra N, Vyas SP. M-Cell Targeted Biodegradable PLGA Nanoparticles for Oral Immunization against Hepatitis B. J. Drug Target. 2007;15:701–713. doi: 10.1080/10611860701637982. [DOI] [PubMed] [Google Scholar]
- 199.Fievez V, Plapied L, des Rieux A, Pourcelle V, Freichels H, Wascotte V, Vanderhaeghen M-L, Jerôme C, Vanderplasschen A, Marchand-Brynaert J, et al. Targeting Nanoparticles to M Cells with Non-Peptidic Ligands for Oral Vaccination. Eur. J. Pharm. Biopharm. 2009;73:16–24. doi: 10.1016/j.ejpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 200.Slütter B, Plapied L, Fievez V, Sande MA, des Rieux A, Schneider Y-J, Van Riet E, Jiskoot W, Préat V. Mechanistic Study of the Adjuvant Effect of Biodegradable Nanoparticles in Mucosal Vaccination. J. Control. Release. 2009;138:113–121. doi: 10.1016/j.jconrel.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 201.Hejazi R, Amiji M. Chitosan-Based Gastrointestinal Delivery Systems. J. Control. Release. 2003;89:151–165. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 202.Jain S, Sharma RK, Vyas SP. Chitosan Nanoparticles Encapsulated Vesicular Systems for Oral Immunization: Preparation, in-Vitro and in-Vivo Characterization. J. Pharm. Pharmacol. 2006;58:303–310. doi: 10.1211/jpp.58.3.0003. [DOI] [PubMed] [Google Scholar]
- 203.Harde H, Agrawal AK, Jain S. Development of Stabilized Glucomannosylated Chitosan Nanoparticles Using Tandem Crosslinking Method for Oral Vaccine Delivery. Nanomedicine (Lond) 2014;9:2511–2529. doi: 10.2217/nnm.13.225. [DOI] [PubMed] [Google Scholar]
- 204.Dudeja PK, Tyagi S, Gill R, Said HM. Evidence for a Carrier-Mediated Mechanism for Thiamine Transport to Human Jejunal Basolateral Membrane Vesicles. Dig. Dis. Sci. 2003;48:109–115. doi: 10.1023/a:1021794600864. [DOI] [PubMed] [Google Scholar]
- 205.Salman HH, Gamazo C, Agüeros M, Irache JM. Bioadhesive Capacity and Immunoadjuvant Properties of Thiamine-Coated Nanoparticles. Vaccine. 2007;25:8123–8132. doi: 10.1016/j.vaccine.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 206.Cario E, Gerken G, Podolsky DK. Toll-like Receptor 2 Enhances ZO-1-Associated Intestinal Epithelial Barrier Integrity via Protein Kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 207.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs Regulate the Gatekeeping Functions of the Intestinal Follicle-Associated Epithelium. J. Immunol. 2006;176:4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 208.Chabot SM, Chernin TS, Shawi M, Wagner J, Farrant S, Burt DS, Cyr S, Neutra MR. TLR2 Activation by Proteosomes Promotes Uptake of Particulate Vaccines at Mucosal Surfaces. Vaccine. 2007;25:5348–5358. doi: 10.1016/j.vaccine.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 209.Yuan H, Chen C-Y, Chai G-H, Du Y-Z, Hu F-Q. Improved Transport and Absorption through Gastrointestinal Tract by PEGylated Solid Lipid Nanoparticles. Mol. Pharm. 2013;10:1865–1873. doi: 10.1021/mp300649z. [DOI] [PubMed] [Google Scholar]
- 210.Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, Kirk J, Eppler B, Klinman DM, Sui Y, et al. Large Intestine-Targeted, Nanoparticle-Releasing Oral Vaccine to Control Genitorectal Viral Infection. Nat. Med. 2012;18:1291–1296. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vajdy M, editor. Immunity Against Mucosal Pathogens. Springer Netherlands: Dordrecht; 2008. [Google Scholar]
- 212.Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR. Innate Immunity in the Female Reproductive Tract: Role of Sex Hormones in Regulating Uterine Epithelial Cell Protection Against Pathogens. Curr. Womens. Health Rev. 2008;4:102–117. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Soboll G, Schaefer TM, Wira CR. Effect of Toll-like Receptor (TLR) Agonists on TLR and Microbicide Expression in Uterine and Vaginal Tissues of the Mouse. Am. J. Reprod. Immunol. 2006;55:434–446. doi: 10.1111/j.1600-0897.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 214.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial Cells in the Female Reproductive Tract: A Central Role as Sentinels of Immune Protection. Am. J. Reprod. Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 215.Yeaman GR, White HD, Howell A, Prabhala R, Wira CR. The Mucosal Immune System in the Human Female Reproductive Tract: Potential Insights into the Heterosexual Transmission of HIV. AIDS Res. Hum. Retroviruses. 1998;14(Suppl 1):S57–S62. [PubMed] [Google Scholar]
- 216.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang Y-Y, Cone R, Hanes J. Rapid Transport of Large Polymeric Nanoparticles in Fresh Undiluted Human Mucus. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bures P, Huang Y, Oral E, Peppas NA. Surface Modifications and Molecular Imprinting of Polymers in Medical and Pharmaceutical Applications. J. Control. Release. 2001;72:25–33. doi: 10.1016/s0168-3659(01)00259-0. [DOI] [PubMed] [Google Scholar]
- 218.Wang Y-Y, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles That “Slip” through the Human Mucus Barrier. Angew. Chem. Int. Ed. Engl. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ensign LM, Tang BC, Wang Y-Y, Tse TA, Hoen T, Cone R, Hanes J. Mucus-Penetrating Nanoparticles for Vaginal Drug Delivery Protect Against Herpes Simplex Virus. Sci. Transl. Med. 2012;4:138ra79. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ensign LM, Hoen TE, Maisel K, Cone RA, Hanes JS. Enhanced Vaginal Drug Delivery through the Use of Hypotonic Formulations That Induce Fluid Uptake. Biomaterials. 2013;34:6922–6929. doi: 10.1016/j.biomaterials.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kuo-Haller P, Cu Y, Blum J, Appleton JA, Saltzman WM. Vaccine Delivery by Polymeric Vehicles in the Mouse Reproductive Tract Induces Sustained Local and Systemic Immunity. Mol. Pharm. 2010;7:1585–1595. doi: 10.1021/mp100009e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Glenn GM, Kenney RT, Ellingsworth LR, Frech SA, Hammond SA, Zoeteweij JP. Transcutaneous Immunization and Immunostimulant Strategies: Capitalizing on the Immunocompetence of the Skin. Expert Rev. Vaccines. 2003;2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 223.Rancan F, Gao Q, Graf C, Troppens S, Hadam S, Hackbarth S, Kembuan C, Blume-Peytavi U, Rühl E, Lademann J, et al. Skin Penetration and Cellular Uptake of Amorphous Silica Nanoparticles with Variable Size, Surface Functionalization, and Colloidal Stability. ACS Nano. 2012;6:6829–6842. doi: 10.1021/nn301622h. [DOI] [PubMed] [Google Scholar]
- 224.Mishra D, Dubey V, Asthana A, Saraf DK, Jain NK. Elastic Liposomes Mediated Transcutaneous Immunization against Hepatitis B. Vaccine. 2006;24:4847–4855. doi: 10.1016/j.vaccine.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 225.Li N, Peng L-H, Chen X, Zhang T-Y, Shao G-F, Liang W-Q, Gao J-Q. Antigen-Loaded Nanocarriers Enhance the Migration of Stimulated Langerhans Cells to Draining Lymph Nodes and Induce Effective Transcutaneous Immunization. Nanomedicine. 2014;10:215–223. doi: 10.1016/j.nano.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 226.Mittal A, Schulze K, Ebensen T, Weißmann S, Hansen S, Lehr CM, Guzman CA. Efficient Nanoparticle-Mediated Needle-Free Transcutaneous Vaccination via Hair Follicles Requires Adjuvantation. Nanomedicine. 2014;11:147–154. doi: 10.1016/j.nano.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 227.Kitaoka M, Imamura K, Hirakawa Y, Tahara Y, Kamiya N, Goto M. Needle-Free Immunization Using a Solid-in-Oil Nanodispersion Enhanced by a Skin-Permeable Oligoarginine Peptide. Int. J. Pharm. 2013;458:334–339. [PubMed] [Google Scholar]
- 228.Banchereau J, Steinman RM. Dendritic Cells and the Control of Immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 229.Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-Encapsulated Antigens Are Processed in Lysosomes, Recycled, and Presented to T Cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- 230.Bachmann MF, Jennings GT. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 231.Foged C, Arigita C, Sundblad A, Jiskoot W, Storm G, Frokjaer S. Interaction of Dendritic Cells with Antigen-Containing Liposomes: Effect of Bilayer Composition. Vaccine. 2004;22:1903–1913. doi: 10.1016/j.vaccine.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 232.Villa CH, Dao T, Ahearn I, Fehrenbacher N, Casey E, Rey DA, Korontsvit T, Zakhaleva V, Batt CA, Philips MR, et al. Single-Walled Carbon Nanotubes Deliver Peptide Antigen into Dendritic Cells and Enhance IgG Responses to Tumor-Associated Antigens. ACS Nano. 2011;5:5300–5311. doi: 10.1021/nn200182x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xu Q-H, Zhao X-N, Cheng J-P, Wei C-H, Zhang Q-H, Rong K-T. Influence of Carrier Proteins on the Immunologic Response to Haptenic Antitetrodotoxin Vaccine. Bioconjug. Chem. 2006;17:1508–1513. doi: 10.1021/bc060083u. [DOI] [PubMed] [Google Scholar]
- 234.Helling F, Shang A, Calves M, Zhang S, Ren S, Yu RK, Oettgen HF, Livingston PO. GD3 Vaccines for Melanoma: Superior Immunogenicity of Keyhole Limpet Hemocyanin Conjugate Vaccines. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 235.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons G-J. Robust Immune Responses Elicited by a Fully Synthetic Three-Component Vaccine. Nat. Chem. Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Black M, Trent A, Tirrell M, Olive C. Advances in the Design and Delivery of Peptide Subunit Vaccines with a Focus on Toll-like Receptor Agonists. Expert Rev. Vaccines. 2010;9:157–173. doi: 10.1586/erv.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Joffre OP, Segura E, Savina A, Amigorena S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 238.Brode S, MacAry PA. Cross-Presentation: Dendritic Cells and Macrophages Bite off More than They Can Chew! Immunology. 2004;112:345–351. doi: 10.1111/j.1365-2567.2004.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Amigorena S, Savina A. Intracellular Mechanisms of Antigen Cross Presentation in Dendritic Cells. Curr. Opin. Immunol. 2010;22:109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 240.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient Major Histocompatibility Complex Class I Presentation of Exogenous Antigen upon Phagocytosis by Macrophages. Proc. Natl. Acad. Sci. U. S. A. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kovacsovics-Bankowski M, Rock KL. A Phagosome-to-Cytosol Pathway for Exogenous Antigens Presented on MHC Class I Molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 242.Reis e Sousa C, Germain RN. Major Histocompatibility Complex Class I Presentation of Peptides Derived from Soluble Exogenous Antigen by a Subset of Cells Engaged in Phagocytosis. J. Exp. Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Harding CV, Song R. Phagocytic Processing of Exogenous Particulate Antigens by Macrophages for Presentation by Class I MHC Molecules. J. Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 244.Jain S, Yap WT, Irvine DJ. Synthesis of Protein-Loaded Hydrogel Particles in an Aqueous Two-Phase System for Coincident Antigen and CpG Oligonucleotide Delivery to Antigen-Presenting Cells. Biomacromolecules. 2005;6:2590–2600. doi: 10.1021/bm0503221. [DOI] [PubMed] [Google Scholar]
- 245.Hamdy S, Elamanchili P, Alshamsan A, Molavi O, Satou T, Samuel J. Enhanced Antigen-Specific Primary CD4+ and CD8+ Responses by Codelivery of Ovalbumin and Toll-like Receptor Ligand Monophosphoryl Lipid A in poly(D,L-Lactic-Co-Glycolic Acid) Nanoparticles. J. Biomed. Mater. Res. Part A. 2007;81A:652–662. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- 246.Taneichi M, Ishida H, Kajino K, Tanaka Y, Kasai M, Nishida M, Yamamura H, Uchida T, Alerts E. Antigen Chemically Coupled to the Surface of Liposomes Are Cross-Presented to CD8 + T Cells and Induce Potent Antitumor Immunity. J. Immunol. 2006;177:2324–2330. doi: 10.4049/jimmunol.177.4.2324. [DOI] [PubMed] [Google Scholar]
- 247.Tanaka Y, Taneichi M, Kasai M, Kakiuchi T, Uchida T. Liposome-Coupled Antigens Are Internalized by Antigen-Presenting Cells via Pinocytosis and Cross-Presented to CD8 T Cells. PLoS One. 2010;5:e15225. doi: 10.1371/journal.pone.0015225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Arias Ma, Loxley A, Eatmon C, Van Roey G, Fairhurst D, Mitchnick M, Dash P, Cole T, Wegmann F, Sattentau Q, et al. Carnauba Wax Nanoparticles Enhance Strong Systemic and Mucosal Cellular and Humoral Immune Responses to HIV-gp140 Antigen. Vaccine. 2011;29:1258–1269. doi: 10.1016/j.vaccine.2010.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee IH, Kwon HK, An S, Kim D, Kim S, Yu MK, Lee JH, Lee TS, Im SH, Jon S. Imageable Antigen-Presenting Gold Nanoparticle Vaccines for Effective Cancer Immunotherapy in Vivo. Angew. Chemie - Int. Ed. 2012;51:8800–8805. doi: 10.1002/anie.201203193. [DOI] [PubMed] [Google Scholar]
- 250.Ahn S, Lee IH, Kang S, Kim D, Choi M, Saw PE, Shin EC, Jon S. Gold Nanoparticles Displaying Tumor-Associated Self-Antigens as a Potential Vaccine for Cancer Immunotherapy. Adv. Healthc. Mater. 2014;3:1194–1199. doi: 10.1002/adhm.201300597. [DOI] [PubMed] [Google Scholar]
- 251.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Ho Um S, Khant H, et al. Interbilayer-Crosslinked Multilamellar Vesicles as Synthetic Vaccines for Potent Humoral and Cellular Immune Responses. Nat. Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nordly P, Rose F, Christensen D, Nielsen HM, Andersen P, Agger EM, Foged C. Immunity by Formulation Design: Induction of High CD8+ T-Cell Responses by poly(I:C) Incorporated into the CAF01 Adjuvant via a Double Emulsion Method. J. Control. Release. 2011;150:307–317. doi: 10.1016/j.jconrel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 253.Li AV, Eby JK, DeMuth PC, Irvine DJ. Novel Synthetic Vesicle for Rapid in Vivo Expansion of CD8 T Cells Can Significantly Improve Checkpoint Inhibitor Therapy. In; American Association for Cancer Research Annual Meeting; 2015. [Google Scholar]
- 254.Van der Vlies AJ, O’Neil CP, Hasegawa U, Hammond N, Hubbell JA. Synthesis of Pyridyl Disulfide-Functionalized Nanoparticles for Conjugating Thiol -Containing Small Molecules, Peptides and Proteins.pdf. Bioconjug. Chem. 2010;21:653–662. doi: 10.1021/bc9004443. [DOI] [PubMed] [Google Scholar]
- 255.Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen Delivery to Dendritic Cells by Poly(propylene Sulfide) Nanoparticles with Disulfide Conjugated Peptides: Cross-Presentation and T Cell Activation. Vaccine. 2010;28:7897–7906. doi: 10.1016/j.vaccine.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 256.Stano A, Scott EA, Dane KY, Swartz MA, Hubbell JA. Tunable T Cell Immunity towards a Protein Antigen Using Polymersomes vs. Solid-Core Nanoparticles. Biomaterials. 2013;34:4339–4346. doi: 10.1016/j.biomaterials.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 257.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee C, Opara C, Convertine A, Stayton PS. pH-Responsive Nanoparticle Vaccines for Dual-Delivery of Antigens and Immunostimulatory Oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Keller S, Wilson JT, Patilea GI, Kern HB, Convertine AJ, Stayton PS. Neutral Polymer Micelle Carriers with pH-Responsive, Endosome-Releasing Activity Modulate Antigen Trafficking to Enhance CD8+ T Cell Responses. J. Control. Release. 2014;191:24–33. doi: 10.1016/j.jconrel.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Polymer Nanoparticles for Immunotherapy from Encapsulated Tumor-Associated Antigens and Whole Tumor Cells. Mol. Pharm. 2007;4:47–57. doi: 10.1021/mp060107e. [DOI] [PubMed] [Google Scholar]
- 260.Fang RH, Hu CJ, Luk BT, Gao W, Copp JA, Tai Y, Connor DEO, Zhang L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Modery-Pawlowski CL, Gupta A. Sen. Heteromultivalent Ligand-Decoration for Actively Targeted Nanomedicine. Biomaterials. 2014;35:2568–2579. doi: 10.1016/j.biomaterials.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 262.Elamanchili P, Lutsiak CME, Hamdy S, Diwan M, Samuel J. “Pathogen-Mimicking” Nanoparticles for Vaccine Delivery to Dendritic Cells. J. Immunother. 2007;30:378–395. doi: 10.1097/CJI.0b013e31802cf3e3. [DOI] [PubMed] [Google Scholar]
- 263.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-Delivery of Cancer-Associated Antigen and Toll-like Receptor 4 Ligand in PLGA Nanoparticles Induces Potent CD8+ T Cell-Mediated Anti-Tumor Immunity. Vaccine. 2008;26:5046–5057. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 264.Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, Reinhold B, Keskin DB, Reinherz EL, Sasada T. Induction of Anti-Tumor Cytotoxic T Cell Responses through PLGA-Nanoparticle Mediated Antigen Delivery. Biomaterials. 2011;32:3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 265.Hu Y, Litwin T, Nagaraja, a R, Kwong B, Katz J, Watson N, Irvine DJ. Cytosolic Delivery of Membrane Impermeable Molecules in Dendritic Cells Using pH Responsive Core-Shell Nanoparticles. Nano Lett. 2007 doi: 10.1021/nl071542i. [DOI] [PubMed] [Google Scholar]
- 266.Hu Y, Atukorale PU, Lu JJ, Moon JJ, Um SH, Cho EL, Wang Y, Chen J, Irvine DJ. Cytosolic Delivery Mediated via Electrostatic Surface Binding of Protein, Virus, or siRNA Cargos to Ph-Responsive Core-Shell Gel Particles. Biomacromolecules. 2009;10:756–765. doi: 10.1021/bm801199z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, Liu L, Wang C, Cai L, Ma Y. Bioreducible Alginate-Poly(ethylenimine) Nanogels as an Antigen-Delivery System Robustly Enhance Vaccine-Elicited Humoral and Cellular Immune Responses. J. Control. Release. 2013;168:271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 268.Xu L, Liu Y, Chen Z, Li W, Liu Y, Wang L, Liu Y, Wu X, Ji Y, Zhao Y, et al. Surface-Engineered Gold Nanorods: Promising DNA Vaccine Adjuvant for HIV-1 Treatment. Nano Lett. 2012;12:2003–2012. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 269.Broaders KE, Cohen Ja, Beaudette TT, Bachelder EM, Fréchet JMJ. Acetalated Dextran Is a Chemically and Biologically Tunable Material for Particulate Immunotherapy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5497–5502. doi: 10.1073/pnas.0901592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mukai Y, Yoshinaga T, Yoshikawa M, Matsuo K, Yoshikawa T, Matsuo K, Niki K, Yoshioka Y, Okada N, Nakagawa S. Induction of Endoplasmic Reticulum-Endosome Fusion for Antigen Cross-Presentation Induced by Poly (-Glutamic Acid) Nanoparticles. J. Immunol. 2011;187:6249–6255. doi: 10.4049/jimmunol.1001093. [DOI] [PubMed] [Google Scholar]
- 271.Sneh-Edri H. Intracellular Targeting of PLGA Nanoparticles Encapsulating Antigenic Peptide to the Endoplasmic Reticulum of Dendritic Cells and Its Effect on Antigen Cross- Mol. …. 2011;8:1266–1275. doi: 10.1021/mp200198c. [DOI] [PubMed] [Google Scholar]
- 272.Yuba E, Kojima C, Harada A, Tana, Watarai S, Kono K. pH-Sensitive Fusogenic Polymer-Modified Liposomes as a Carrier of Antigenic Proteins for Activation of Cellular Immunity. Biomaterials. 2010;31:943–951. doi: 10.1016/j.biomaterials.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 273.Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A Liposome-Based Antigen Delivery System Using pH-Sensitive Fusogenic Polymers for Cancer Immunotherapy. Biomaterials. 2013;34:3042–3052. doi: 10.1016/j.biomaterials.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 274.Waeckerle-Men Y, Mauracher A, Håkerud M, Mohanan D, Kündig TM, Høgset A, Johansen P. Photochemical Targeting of Antigens to the Cytosol for Stimulation of MHC Class-I-Restricted T-Cell Responses. Eur. J. Pharm. Biopharm. 2013;85:34–41. doi: 10.1016/j.ejpb.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 275.Macho Fernandez E, Chang J, Fontaine J, Bialecki E, Rodriguez F, Werkmeister E, Krieger V, Ehret C, Heurtault B, Fournel S, et al. Activation of Invariant Natural Killer T Lymphocytes in Response to the Α-Galactosylceramide Analogue KRN7000 Encapsulated in PLGA-Based Nanoparticles and Microparticles. Int. J. Pharm. 2012;423:45–54. doi: 10.1016/j.ijpharm.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 276.Ishii M, Kojima N. Effective Stimulation of Invariant Natural Killer T Cells by Oligomannose-Coated Liposomes. Int. Immunopharmacol. 2013;15:685–692. doi: 10.1016/j.intimp.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 277.Nakamura T, Moriguchi R, Kogure K, Shastri N, Harashima H. Efficient MHC Class I Presentation by Controlled Intracellular Trafficking of Antigens in Octaarginine-Modified Liposomes. Mol. Ther. 2008;16:1507–1514. doi: 10.1038/mt.2008.122. [DOI] [PubMed] [Google Scholar]
- 278.Nakamura T, Yamazaki D, Yamauchi J, Harashima H. The Nanoparticulation by Octaarginine-Modified Liposome Improves Α-Galactosylceramide-Mediated Antitumor Therapy via Systemic Administration. J. Control. Release. 2013;171:216–224. doi: 10.1016/j.jconrel.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 279.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Enhanced and Prolonged Cross-Presentation Following Endosomal Escape of Exogenous Antigens Encapsulated in Biodegradable Nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, Fahmy TM. Role of Sustained Antigen Release from Nanoparticle Vaccines in Shaping the T Cell Memory Phenotype. Biomaterials. 2012;33:4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Saluja SS, Hanlon DJ, Sharp FA, Hong E, Khalil D, Robinson E, Tigelaar R, Fahmy TM, Edelson RL. Targeting Human Dendritic Cells via DEC-205 Using PLGA Nanoparticles Leads to Enhanced Cross-Presentation of a Melanoma-Associated Antigen. Int. J. Nanomedicine. 2014;9:5231–5246. doi: 10.2147/IJN.S66639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Schietinger A, Greenberg PD. Tolerance and Exhaustion: Defining Mechanisms of T Cell Dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T Cell Anergy, Exhaustion, Senescence, and Stemness in the Tumor Microenvironment. Curr. Opin. Immunol. 2013;25:214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hanlon DJ, Aldo PB, Devine L, Alvero AB, Engberg AK, Edelson R, Mor G. Enhanced Stimulation of Anti-Ovarian Cancer CD8(+) T Cells by Dendritic Cells Loaded with Nanoparticle Encapsulated Tumor Antigen. Am. J. Reprod. Immunol. 2011;65:597–609. doi: 10.1111/j.1600-0897.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Matzinger P. The Danger Model: A Renewed Sense of Self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 286.Matzinger P. Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 287.Gallucci S, Matzinger P. Danger Signals: SOS to the Immune System. Curr. Opin. Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 288.Iwasaki A, Medzhitov R. Toll-like Receptor Control of the Adaptive Immune Responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 289.Jin B, Sun T, Yu XH, Yang YX, Yeo AET. The Effects of TLR Activation on T-Cell Development and Differentiation. Clin. Dev. Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lanzavecchia A, Sallusto F. Toll-like Receptors and Innate Immunity in B-Cell Activation and Antibody Responses. Curr. Opin. Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 291.Vanlandschoot P, Van Houtte F, Ulrichts P, Tavernier J, Leroux-Roels G. Immunostimulatory Potential of Hepatitis B Nucleocapsid Preparations: Lipopolysaccharide Contamination Should Not Be Overlooked. J. Gen. Virol. 2005;86:323–331. doi: 10.1099/vir.0.80605-0. [DOI] [PubMed] [Google Scholar]
- 292.St John AL, Chan CY, Staats HF, Leong KW, Abraham SN. Synthetic Mast-Cell Granules as Adjuvants to Promote and Polarize Immunity in Lymph Nodes. Nat. Mater. 2012;11:250–257. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Nanoparticulate STING Agonists Are Potent Lymph Node-Targeted Vaccine Adjuvants. J. Clin. Invest. 2015 doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Diwan M, Elamanchili P, Cao M, Samuel J. Dose Sparing of CpG Oligodeoxynucleotide Vaccine Adjuvants by Nanoparticle Delivery. Curr. Drug Deliv. 2004;1:405–412. doi: 10.2174/1567201043334597. [DOI] [PubMed] [Google Scholar]
- 295.Tacken PJ, Zeelenberg IS, Cruz LJ, Van Hout-Kuijer Ma, Van De Glind G, Fokkink RG, Lambeck AJa, Figdor CG. Targeted Delivery of Toll-like Receptor Ligands to Human and Mouse Dendritic Cells Strongly Enhances Adjuvanticity. Blood. 2011;118:6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 296.Demento SL, Bonafé N, Cui W, Kaech SM, Caplan MJ, Fikrig E, Ledizet M, Fahmy TM. TLR9-Targeted Biodegradable Nanoparticles as Immunization Vectors Protect against West Nile Encephalitis. J. Immunol. 2010;185:2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sokolova V, Knuschke T, Kovtun A, Buer J, Epple M, Westendorf AM. The Use of Calcium Phosphate Nanoparticles Encapsulating Toll-like Receptor Ligands and the Antigen Hemagglutinin to Induce Dendritic Cell Maturation and T Cell Activation. Biomaterials. 2010;31:5627–5633. doi: 10.1016/j.biomaterials.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 298.Blander JM, Medzhitov R. Regulation of Phagosome Maturation by Signals from. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 299.Blander JM, Medzhitov R. Toll-Dependent Selection of Microbial Antigens for Presentation by Dendritic Cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 300.Hoffmann E, Kotsias F, Visentin G, Bruhns P, Savina A, Amigorena S. Autonomous Phagosomal Degradation and Antigen Presentation in Dendritic Cells. Proc. Natl. Acad. Sci. 2012;109:14556–14561. doi: 10.1073/pnas.1203912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yates RM, Russell DG. Phagosome Maturation Proceeds Independently of Stimulation of Toll-like Receptors 2 and 4. Immunity. 2005;23:409–717. doi: 10.1016/j.immuni.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 302.Russell DG, Yates RM. TLR Signalling and Phagosome Maturation: An Alternative Viewpoint. Cell. Microbiol. 2007;9:849–850. doi: 10.1111/j.1462-5822.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 303.Titta A De, Ballester M, Julier Z, Nembrini C, Jeanbart L, Vlies AJ Van Der. Nanoparticle Conjugation of CpG Enhances Adjuvancy for Cellular Immunity and Memory Recall at Low Dose. Proc. Natl. Acad. Sci. 2013;110:19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mandraju R, Murray S, Forman J, Pasare C. Differential Ability of Surface and Endosomal TLRs to Induce CD8 T Cell Responses in Vivo. J. Immunol. 2014;192:4303–4315. doi: 10.4049/jimmunol.1302244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dijkstra JAN, Mellors JW, Ryan JL, Szoka FC. Modulation of the Biological Activity of Bacterial Endotoxin by Incorporation into Liposomes. J. Immunol. 1987;138:2663–2670. [PubMed] [Google Scholar]
- 306.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like Receptor Agonist Combinations Synergistically Trigger a T Helper Type 1-Polarizing Program in Dendritic Cells. Nat. Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bagchi A, Herrup Ea, Warren HS, Trigilio J, Shin H-S, Valentine C, Hellman J. MyD88-Dependent and MyD88-Independent Pathways in Synergy, Priming, and Tolerance between TLR Agonists. J. Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 308.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky Ja. Toll-like Receptor Ligands Synergize through Distinct Dendritic Cell Pathways to Induce T Cell Responses: Implications for Vaccines. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the Magnitude and Persistence of Antibody Responses with Innate Immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Turley DM, Miller SD. Peripheral Tolerance Induction Using Ethylenecarbodiimide-Fixed APCs Uses Both Direct and Indirect Mechanisms of Antigen Presentation for Prevention of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 311.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, et al. Tolerance Induced by Apoptotic Antigen-Coupled Leukocytes Is Induced by PD-L1+ and IL-10–Producing Splenic Macrophages and Maintained by T Regulatory Cells. J. Immunol. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Gharagozloo M, Majewski S, Foldvari M. Therapeutic Applications of Nanomedicine in Autoimmune Diseases: From Immunosuppression to Tolerance Induction. Nanomedicine Nanotechnology, Biol. Med. 2015 doi: 10.1016/j.nano.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 313.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD. A Biodegradable Nanoparticle Platform for the Induction of Antigen-Specific Immune Tolerance for Treatment of Autoimmune Disease. ACS Nano. 2014;8:2148–2160. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Maldonado Ra, LaMothe Ra, Ferrari JD, Zhang A-H, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, et al. Polymeric Synthetic Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance. Proc. Natl. Acad. Sci. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Selecta Biosciences. Safety and Pharmacodynamics of SEL-068 Vaccine in Smokers and Non-Smokers. [accessed May 13, 2015]; https://clinicaltrials.gov/ct2/show/NCT01478893.
- 316.Look M, Stern E, Wang Qa, DiPlacido LD, Kashgarian M, Craft J, Fahmy TM. Nanogel-Based Delivery of Mycophenolic Acid Ameliorates Systemic Lupus Erythematosus in Mice. J. Clin. Invest. 2013;123:1741–1749. doi: 10.1172/JCI65907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Look M, Saltzman WM, Craft J, Fahmy TM. The Nanomaterial-Dependent Modulation of Dendritic Cells and Its Potential Influence on Therapeutic Immunosuppression in Lupus. Biomaterials. 2014;35:1089–1095. doi: 10.1016/j.biomaterials.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yeste a, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-Mediated Codelivery of Myelin Antigen and a Tolerogenic Small Molecule Suppresses Experimental Autoimmune Encephalomyelitis. Proc. Natl. Acad. Sci. 2012;109:11270–11275. doi: 10.1073/pnas.1120611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P. Reversal of Autoimmunity by Boosting Memory-like Autoregulatory T Cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 320.Sarma JV, Ward PA. The Complement System. Cell Tissue Res. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bertholon I, Vauthier C, Labarre D. Complement Activation by Core-Shell Poly(isobutylcyanoacrylate)-Polysaccharide Nanoparticles: Influences of Surface Morphology, Length, and Type of Polysaccharide. Pharm. Res. 2006;23:1313–1323. doi: 10.1007/s11095-006-0069-0. [DOI] [PubMed] [Google Scholar]
- 322.Thomas SN, van der Vlies AJ, O’Neil CP, Reddy ST, Yu SS, Giorgio TD, Swartz MA, Hubbell JA. Engineering Complement Activation on Polypropylene Sulfide Vaccine Nanoparticles. Biomaterials. 2011;32:2194–2203. doi: 10.1016/j.biomaterials.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 323.Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM. Distinct Polymer Architecture Mediates Switching of Complement Activation Pathways at the Nanosphere-Serum Interface: Implications for Stealth Nanoparticle Engineering. ACS Nano. 2010;4:6629–6638. doi: 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- 324.Deretic V, Saitoh T, Akira S. Autophagy in Infection, Inflammation and Immunity. Nat. Rev. Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, Park Y, Jones DP, Chappert P, Davoust J, et al. Vaccine Activation of the Nutrient Sensor GCN2 in Dendritic Cells Enhances Antigen Presentation. Science. 2014;343:313–318. doi: 10.1126/science.1246829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Panzarini E, Dini L. Nanomaterial-Induced Autophagy: A New Reversal MDR Tool in Cancer Therapy? Mol. Pharm. 2014;11:2527–2538. doi: 10.1021/mp500066v. [DOI] [PubMed] [Google Scholar]
- 327.Li H, Li Y, Jiao J, Hu H-M. Alpha-Alumina Nanoparticles Induce Efficient Autophagy-Dependent Cross-Presentation and Potent Antitumour Response. Nat. Nanotechnol. 2011;6:645–650. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zoete MR De, Palm NW, Zhu S, Flavell RA. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014;6:a016287. doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica Crystals and Aluminum Salts Activate the NALP3 Inflammasome through Phagosomal Destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell Ra. Crucial Role for the Nalp3 Inflammasome in the Immunostimulatory Properties of Aluminium Adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. J. Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 332.Franchi L, Núñez G. The Nlrp3 Inflammasome Is Critical for Aluminium Hydroxide-Mediated IL-1beta Secretion but Dispensable for Adjuvant Activity. Eur. J. Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.McKee AS, Munks MW, MacLeod MKL, Fleenor CJ, Van Rooijen N, Kappler JW, Marrack P. Alum Induces Innate Immune Responses through Macrophage and Mast Cell Sensors, but These Sensors Are Not Required for Alum to Act as an Adjuvant for Specific Immunity. J. Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Harris J, Sharp FA, Lavelle EC. The Role of Inflammasomes in the Immunostimulatory Effects of Particulate Vaccine Adjuvants. Eur. J. Immunol. 2010;40:634–638. doi: 10.1002/eji.200940172. [DOI] [PubMed] [Google Scholar]
- 335.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell Ra, et al. Inflammasome-Activating Nanoparticles as Modular Systems for Optimizing Vaccine Efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Sharp Fa, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Pétrilli V, Tschopp J, et al. Uptake of Particulate Vaccine Adjuvants by Dendritic Cells Activates the NALP3 Inflammasome. Proc. Natl. Acad. Sci. U. S. A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 338.Williams GR, Fierens K, Preston SG, Lunn D, Rysnik O, De Prijck S, Kool M, Buckley HC, Lambrecht BN, O’Hare D, et al. Immunity Induced by a Broad Class of Inorganic Crystalline Materials Is Directly Controlled by Their Chemistry. J. Exp. Med. 2014;211:1019–1025. doi: 10.1084/jem.20131768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Sun B, Ji Z, Liao YP, Wang M, Wang X, Dong J, Chang CH, Li R, Zhang H, Nel AE, et al. Engineering an Effective Immune Adjuvant by Designed Control of Shape and Crystallinity of Aluminum Oxyhydroxide Nanoparticles. ACS Nano. 2013;7:10834–10849. doi: 10.1021/nn404211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Seong S-Y, Matzinger P. Hydrophobicity: An Ancient Damage-Associated Molecular Pattern That Initiates Innate Immune Responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 341.Shima F, Akagi T, Uto T, Akashi M. Manipulating the Antigen-Specific Immune Response by the Hydrophobicity of Amphiphilic Poly(γ-Glutamic Acid) Nanoparticles. Biomaterials. 2013;34:9709–9716. doi: 10.1016/j.biomaterials.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 342.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. Nanoparticle Hydrophobicity Dictates Immune Response. J. Am. Chem. Soc. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, Wannemuehler MJ, Narasimhan B. Activation of Innate Immune Responses in a Pathogen-Mimicking Manner by Amphiphilic Polyanhydride Nanoparticle Adjuvants. Biomaterials. 2011;32:6815–6822. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 344.Chou LYT, Zagorovsky K, Chan WCW. DNA Assembly of Nanoparticle Superstructures for Controlled Biological Delivery and Elimination. Nat. Nanotechnol. 2014;9:148–155. doi: 10.1038/nnano.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Gungor B, Yagci FC, Tincer G, Bayyurt B, Alpdundar E, Yildiz S, Ozcan M, Gursel I, Gursel M. CpG ODN Nanorings Induce IFNα from Plasmacytoid Dendritic Cells and Demonstrate Potent Vaccine Adjuvant Activity. Sci. Transl. Med. 2014;6:235ra61. doi: 10.1126/scitranslmed.3007909. [DOI] [PubMed] [Google Scholar]
- 346.Mohri K, Takahashi N, Nishikawa M, Kusuki E, Shiomi T, Takahashi Y, Takakura Y. Increased Immunostimulatory Activity of Polypod-like Structured DNA by Ligation of the Terminal Loop Structures. J. Control. Release. 2012;163:285–292. doi: 10.1016/j.jconrel.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 347.Van Lehn RC, Ricci M, Silva PHJ, Andreozzi P, Reguera J, Voïtchovsky K, Stellacci F, Alexander-Katz A. Lipid Tail Protrusions Mediate the Insertion of Nanoparticles into Model Cell Membranes. Nat. Commun. 2014;5:4482. doi: 10.1038/ncomms5482. [DOI] [PubMed] [Google Scholar]
- 348.Bonnaud C, Monnier CA, Demurtas D, Jud C, Vanhecke D, Montet X, Hovius R, Lattuada M, Rothen-Rutishauser B, Petri-Fink A. Insertion of Nanoparticle Clusters into Vesicle Bilayers. ACS Nano. 2014;8:3451–3460. doi: 10.1021/nn406349z. [DOI] [PubMed] [Google Scholar]
- 349.Zhang S, Sun H-J, Hughes AD, Moussodia R-O, Bertin A, Chen Y, Pochan DJ, Heiney PA, Klein ML, Percec V. Self-Assembly of Amphiphilic Janus Dendrimers into Uniform Onion-like Dendrimersomes with Predictable Size and Number of Bilayers. Proc. Natl. Acad. Sci. U. S. A. 2014;111:9058–9063. doi: 10.1073/pnas.1402858111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Tang R, Kim CS, Solfiell DJ, Rana S, Mout R, Velázquez-Delgado EM, Chompoosor A, Jeong Y, Yan B, Zhu Z-J, et al. Direct Delivery of Functional Proteins and Enzymes to the Cytosol Using Nanoparticle-Stabilized Nanocapsules. ACS Nano. 2013;7:6667–6673. doi: 10.1021/nn402753y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Xia Y, Nguyen TD, Yang M, Lee B, Santos A, Podsiadlo P, Tang Z, Glotzer SC, Kotov NA. Self-Assembly of Self-Limiting Monodisperse Supraparticles from Polydisperse Nanoparticles. Nat. Nanotechnol. 2011;6:580–587. doi: 10.1038/nnano.2011.121. [DOI] [PubMed] [Google Scholar]
- 352.Ataman-Onal Y, Munier S, Ganée A, Terrat C, Durand P-Y, Battail N, Martinon F, Le Grand R, Charles M-H, Delair T, et al. Surfactant-Free Anionic PLA Nanoparticles Coated with HIV-1 p24 Protein Induced Enhanced Cellular and Humoral Immune Responses in Various Animal Models. J. Control. Release. 2006;112:175–185. doi: 10.1016/j.jconrel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 353.Himeno A, Akagi T, Uto T, Wang X, Baba M, Ibuki K, Matsuyama M, Horiike M, Igarashi T, Miura T, et al. Evaluation of the Immune Response and Protective Effects of Rhesus Macaques Vaccinated with Biodegradable Nanoparticles Carrying gp120 of Human Immunodeficiency Virus. Vaccine. 2010;28:5377–5385. doi: 10.1016/j.vaccine.2010.04.110. [DOI] [PubMed] [Google Scholar]
- 354.Scheerlinck J-PY, Gloster S, Gamvrellis A, Mottram PL, Plebanski M. Systemic Immune Responses in Sheep, Induced by a Novel Nano-Bead Adjuvant. Vaccine. 2006;24:1124–1131. doi: 10.1016/j.vaccine.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 355.Tsuji K, Hamada T, Uenaka A, Wada H, Sato E, Isobe M, Asagoe K, Yamasaki O, Shiku H, Ritter G, et al. Induction of Immune Response against NY-ESO-1 by CHP-NY-ESO-1 Vaccination and Immune Regulation in a Melanoma Patient. Cancer Immunol. Immunother. 2008;57:1429–1437. doi: 10.1007/s00262-008-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Fraser CC, Altreuter DH, Ilyinskii P, Pittet L, LaMothe RA, Keegan M, Johnston L, Kishimoto TK. Generation of a Universal CD4 Memory T Cell Recall Peptide Effective in Humans, Mice and Non-Human Primates. Vaccine. 2014;32:2896–2903. doi: 10.1016/j.vaccine.2014.02.024. [DOI] [PubMed] [Google Scholar]