Abstract
Tumors exhibit genomic and phenotypic heterogeneity which has prognostic significance and may influence response to therapy. Imaging can quantify the spatial variation in architecture and function of individual tumors through quantifying basic biophysical parameters such as density or MRI signal relaxation rate; through measurements of blood flow, hypoxia, metabolism, cell death and other phenotypic features; and through mapping the spatial distribution of biochemical pathways and cell signaling networks. These methods can establish whether one tumor is more or less heterogeneous than another and can identify sub-regions with differing biology. In this article we review the image analysis methods currently used to quantify spatial heterogeneity within tumors. We discuss how analysis of intratumor heterogeneity can provide benefit over more simple biomarkers such as tumor size and average function. We consider how imaging methods can be integrated with genomic and pathology data, rather than be developed in isolation. Finally, we identify the challenges that must be overcome before measurements of intratumoral heterogeneity can be used routinely to guide patient care.
Introduction
Malignant tumors are biologically complex and exhibit substantial spatial variation in gene expression, biochemistry, histopathology and macroscopic structure. Cancerous cells not only undergo clonal evolution from a single progenitor cell into more aggressive and therapy resistant cells, but also exhibit branched evolution, whereby each tumor develops and preserves multiple distinct sub-clonal populations (1). This genetic heterogeneity (1, 2), combined with spatial variation in environmental stressors, leads to regional differences in stromal architecture (3) oxygen consumption (4, 5), glucose metabolism (4) and growth factor expression (6). Consequently, tumor subregions develop, each with spatially distinct patterns of blood flow (7, 8), vessel permeability (9), cell proliferation (10), cell death (11) and other features.
Spatial heterogeneity is found between different tumors in individual patients (intertumor heterogeneity) and within each lesion (intratumor heterogeneity). Intratumor heterogeneity is near ubiquitous in malignant tumors, but the extent varies between pre-clinical cancer models and between patients (12). Allowing for these differences, some common themes emerge. Firstly, intratumor heterogeneity is dynamic. For example, variations in tumor pO2 fluctuate over minutes to hours (5, 6). Secondly, intratumor heterogeneity tends to increase as tumors grow (7, 13). Thirdly, established spatial heterogeneity frequently indicates poor clinical prognosis (14), in part due to resistant subpopulations of cells driving resistance to therapy (3, 15). Finally, intratumor heterogeneity may increase or decrease following efficacious anti-cancer therapy (11, 16), depending on the imaging test used and the underlying tumor biology (17).
Imaging depicts spatial heterogeneity in tumors. However, while imaging is central to diagnosis, staging, response assessment and recurrence detection in routine oncological practice, most clinical radiology and research studies only measure tumor size or average parameter values, such as median blood flow (18). In doing so, spatially rich information is discarded. There has been considerable effort to use more sophisticated analyses to either quantify overall tumor spatial complexity or identify the tumor sub-regions that may drive disease transformation, progression and drug resistance (11, 19).
In this review we highlight the strengths and weaknesses of methods that measure intratumor spatial heterogeneity (Fig. 1 and Table 1). We evaluate evidence that heterogeneity analyses provide any clinical benefit over simple ‘average value’ measurements. We discuss how imaging, genomic and pathology biomarkers of intratumor heterogeneity relate to one another. Finally, we identify the hurdles to translating image biomarkers of spatial heterogeneity into clinical practice.
Figure 1.
Quantifying intratumoral heterogeneity: The example liver metastasis from a patient with a colonic primary tumor can be measured in several different ways. (A) Most clinical assessment of tumors is size-based. (B) Functional imaging methods can measure tumor pathophysiology but tend to derive average parameter values, such as median Ktrans. (C) Some intratumoral heterogeneity methods quantify overall complexity of a distribution (histograms) or spatial arrangement of data (texture analysis). Other methods identify tumor sub-regions using a priori assumptions (partitioning) or data driven approaches (multispectral analysis).
Table 1.
Examples of beneficial information from analyzing tumor heterogeneity
| Indication | Beneficial information from assessment of spatial heterogeneity | |
|---|---|---|
| Examples of current clinical use | Examples of research application | |
| Screening | Subjective morphology (mammography): Lesion spiculation used in BI-RADS in breast cancer (22) | Texture analysis: (mammography): improves sensitivity in distinguishing benign and malignant lesions (31) |
| Diagnosis | Morphology (CT attenuation): Lung nodule speculation (20) | - |
| Staging (TNM) | Hot spot analysis (SUVmax): 18F-FDG PET improves N and M staging in multiple cancers (25) | - |
| Grading | Hot spot analysis (rCBV): DSC-MRI maps targeted biopsy to accurately grade glioma (27) | Histogram analysis (rCBV): improves sensitivity and specificity of grading HGG (38) |
| Hot spot analysis (SUVmax): 18F-FDG PET and 11C-Methionine maps targeted biopsy to accurately grade glioma (26) | ||
| Early Change | - | Histogram analysis (rCBV): detects early transformation of low grade glioma to HGG (40) |
| Partitioning data (ETV and EF): assessment of response, reveals subtle changes in tumor biology (48, 72) | ||
| A priori segmentation (Ktrans): demonstrates differential response in tumor rim and core (62) | ||
| Data-driven segmentation (Ktrans): demonstrates differential response in viable and necrotic tumor regions (79) | ||
| Outcome | - | Histogram analysis (ADC): data relates to OS (42) |
| Feature analysis (CT and MRI): data relates to PFS and OS in various tumor types (55-59) | ||
| Partitioning data (ETV and EF): baseline values prognostic of outcome in HGG (67-69) and cervical cancer (70, 71) | ||
| Partitioning data (SUVmax): persistent values above a threshold indicate poor PFS in GIST or renal cancer treated with TKI (73-75) | ||
Qualitative Assessment of Heterogeneity
Radiologists use qualitative descriptors to describe adverse spatial features and functional heterogeneity on clinical scans. For example, when assessing pulmonary nodules on CT (20) and breast lumps on x-ray mammography (21), spiculation implies greater risk of malignancy compared with well circumscribed lesions. Indeed, spiculate morphology is part of the BI-RADS lexicon that classifies breast lesions as ‘radiologically malignant’ (22).
Identifying a tumor ‘hot spot’ is also commonplace in cancer radiology. The maximum standardized uptake value (SUVmax) derived from 18F-FDG PET imaging is an established proxy for identifying abnormal glucose metabolism, based on identifying the one or more voxels with greatest abnormality. Measuring SUVmax is simple, reproducible (23, 24) and can be performed on clinical workstations. The presence of abnormal glycolysis in part of a tumor using SUVmax is used widely to stage and monitor response in several malignant tumors (25). In glioma, regional high values of tracer uptake (18F-FDG and 11C-methionine) have been used to grade tumors using targeted biopsy (26).
Perfusion CT and MRI methods employ ‘hot spot’ analysis to identify tumor regions with the most abnormal vascular features. Specialist neuro-oncology centres use dynamic contrast-enhanced MRI (DCE-MRI) or dynamic susceptibility contrast MRI (DSC-MRI) in patients with high grade glioma (HGG) to map relative cerebral blood volume (rCBV) (27) based on the rationale that ‘more vascular’ regions correspond to highest malignant grade and that this improves prognostic assessment in patients. It is important to note that hot spot analyses are subjective, only identify regions that are maximum (or minimum when gray scale is inverted) and that observer evaluation of heterogeneity is highly influenced by display ranges and color schemes (28) unless objective algorithms are employed.
Voxels: Considerations for Quantifying Heterogeneity
Imaging modalities measure biophysical signals in tissues and spatially encode these signals to create clinical images or parameter maps composed of three dimensional picture elements called voxels. Heterogeneity analyses combine data from many voxels. Several issues arise when interpreting these data.
Firstly, some voxels suffer partial volume averaging (typically at interface with non-tumor tissue). Secondly, there is inevitable compromise between having sufficient numbers of voxels to perform the analysis versus sufficiently large voxels to overcome noise and keep imaging times practical (29). Most analysis methods require hundreds to thousands of voxels for robust application. Thirdly, many studies of tumor spatial heterogeneity have used standard clinical data from protocols dictated by clinical rather than research needs. In some cases sections of tumor were omitted when non-contiguous tumor sampling was used (30) which may confound 3D spatial analyses (31). Fourthly, some calculated voxel values, such as apparent diffusion coefficient (ADC), contrast transfer coefficient (Ktrans) and blood flow are derived from multiple images obtained over time. The estimation errors associated with motion vary for different parameters and for different voxels, which should be considered when assessing tumor heterogeneity.
Finally, CT, MRI or PET voxels are usually non-isotropic (slice thickness exceeds in plane resolution). Dimensions are typically 200-2000 μm for rodent models and 750-5000 μm for clinical tumors. Compared with genomic and histopathology biomarkers, this represents many orders of magnitude difference in scale (32), making it difficult to validate image heterogeneity biomarkers against pathology. However, imaging methods have the distinct advantage of whole tumor sampling, ensuring that no region of genetic and pathological variation within tumors is omitted (33).
Measuring Degree of Heterogeneity: Quantifying Parameter Distributions
Voxel values can be plotted as histograms, from which many simple descriptors can be extracted as potential biomarkers. These include simple descriptors of image heterogeneity such as standard deviation, interquartile range, nth centile(s), skew and kurtosis (Supplementary Fig. S1), as well as mean and median values (34). In these approaches the inherent spatial relationship between voxels is discarded and data are treated as a list of continuous variables.
Histogram analysis
Histograms can be generated using widely available software and have proved popular methods for characterizing intratumoral heterogeneity, accounting for approximately half of all published studies (35). Several important points should be considered. Histogram analyses have high dimensionality and generate many parameters, so require correction for multiple comparisons (36). The repeatability and reproducibility of many histogram-derived parameters are uncertain and have not yet been evaluated in multi-centre studies (29). Further, many parameters, such as 5th centile or kurtosis, have no clear biological correlate making biological validation difficult.
Evidence for clinical benefit
Over 200 histogram-based studies have analyzed imaging data of tumor response or outcome (PubMed search performed 22nd October 2014), with rapid rise in numbers recently (Supplementary Fig. S2). Unfortunately, many of these studies were retrospective, performed in small numbers of patients and did not demonstrate added benefit over simpler measurements of tumor structure and function. For example, in HGG, various histogram parameters have related to overall survival (OS) (p<0.05), but relationships were generally equivalent or weaker than that seen with median rCBV values (37). Other studies have shown marginal benefits. Measurements such as peak height of the CBV histogram had superior sensitivity and negative predictive value over hot spot analysis for distinguishing low grade glioma from HGG (histogram 90% sensitivity; hot spot 55-76% sensitivity) (38).
The real value in histogram analysis appears twofold. Firstly, changed heterogeneity in the data distribution relates to clinical outcome. In cervical cancer, more heterogeneous distributions of FDG-PET voxel values related to greater risk of lymph node metastases, risk of local recurrence and worse progression free survival (PFS) (39). In low grade glioma, changes in just the top few centiles of voxel enhancement were prognostic for subsequent early transformation into HGG (p=0.011) (40). Preclinical data has shown that regional response to therapy may result in unimodal histograms becoming bimodal (41) (Fig. 2).
Figure 2.
A GH3 prolactinoma in a rat shows a heterogeneous response to 50mg/kg ZD6126. Histograms analysis quantifies this effect and demonstrates transformation from a unimodal distribution to a bimodal one. Adapted from Robinson et al. (41).
Secondly, histograms can quantify data with complex distributions, where average values may mask important information within the data. For example, in patients with HGG receiving bevacizumab and cytotoxic therapies, pre-treatment ADC values had a bimodal distribution. Overall mean ADC did not relate to subsequent PFS (p=0.14), but the mean ADC value of the lower mode related significantly to PFS (p=0.004) (30), suggesting MRI identified two distinct tumor sub-regions (Supplementary Fig. S1). These findings have been replicated in a multicentre study (42), suggesting possible clinical translation although when histograms from different tumors differ in both location and degree of stretch along the x axis, this can hinder analysis of cohorts of histogram data. Attempts to model for distribution shape may address this problem (43, 44) but remain in their infancy.
Measuring Degree of Heterogeneity: Quantifying Spatial Complexity
Feature analyses measure the spatial complexity of objects. Unlike histogram analysis, the spatial arrangement of voxel values is retained. Related methods including texture analysis, fractal techniques and Minkowski functionals have been applied to tumor data and account for around half of studies measuring tumour heterogeneity (35). Initial reports suggest that feature based metrics derived from 18F-FDG PET (45), 18F-FLT PET (46), CT (47) and MRI (48) have good limits of agreement, with co-efficients of variation comparable to summary statistics derived from the same data.
Texture analysis
There is a large literature of texture analysis in breast cancer. Many studies use the Haralick method (49) where a co-occurrence matrix element denoted Pd, θ (i, j) measures the probability of starting from any image voxel with designated value i, moving d voxels along the image in direction θ, and then arriving at another voxel with value j. This co-occurrence matrix is a two dimensional histogram describing a joint distribution of all the possible moves with step size d and direction θ on the image. From this, various extracted features, including lesion contrast and correlation, can estimate shape and/or spatial complexity.
Fractal analysis and Minkowski functionals
Fractal dimensions estimate the complexity of geometrical patterns resulting from abstract recursive procedures (50). The simplest fractal dimension is the box-counting dimension (d0), computed by imposing regular grids of a range of scales on a binary object in question and then counting the number of grid elements (boxes) that are occupied by the object at each scale (Supplementary Fig. S3). The box counting dimension is the slope of the line of best fit when plotting the number of occupied boxes against the reciprocal of the scale on log-log axes (51). Increasingly complex variants can incorporate continuous scale values of parameters such as Hounsfield unit density or Ktrans. Minkowski functionals analyse binarized images over a range of thresholds and also quantify space-filling properties of tumors.
Evidence for clinical benefit
Texture analyses have been used extensively in x-ray mammography (52). Early applications included discrimination of glandular and fatty regions in mammograms (53) and distinguishing benign and malignant lesions (31). Minkowski functionals analysis can improve stratification of risk for developing breast cancer (receiver operating curve area under curve of >0.9) (54). Here, more heterogeneous images indicated more aggressive tumors.
Feature analyses show greatest promise as prognostic indicators. CT based feature analysis parameters predicted OS independent of tumor stage in colorectal cancer patients treated with cytotoxic therapy (p<0.01) (55), predicted time to progression in renal cancer patients treated with various anti-vascular therapies (p=0.005) (56) and predicted OS in NSCLC (p=0.046) (57). Similarly, MRI fractal biomarkers have shown prognostic relationships in colorectal cancer patients treated with bevacizumab (58) (p<0.00005) and in sarcoma patients treated with cytotoxic therapy (59) (p<0.005). These approaches are now being explored and validated in large populations using an approach termed ‘radiomics’, where existing clinical imaging data are mined to identify heterogeneity features that predict clinical outcome (60).
Identifying Tumor Subregions
Tumor images contain hundreds-thousands of voxels. Grouping ‘similar’ voxels together (parcellation) may define multiple sub-regions with common biology that respond differentially to therapy, or drive progression. Parcellation techniques differ in their underlying assumptions and methodology. Some use prior knowledge whereas others rely solely on information contained within the image(s) (Fig. 3).
Figure 3.
Quantifying tumor sub-regions: A priori methods parcellate tumor regions based on binary classifiers (e.g. non-enhancing (=0) or enhancing (=1) voxels from a Ktrans map or threshold classification using a ‘cut point’ defined from previous data, or an arbitrary value such as the median (e.g. of an ADC distribution). Multispectral classification generates clusters based on data driven segmentation from multiple signals (e.g. Ktrans and ADC), for example using principal components (PC) analysis (graph shows PC1, PC2 and PC3). The volume or proportional fraction of each region is measured in these methods. Geographical methods are distinct since they define sub-regions based on the location of a pixel rather than its parameter value(s).
Parcellation using a priori assumptions
Voxels can be categorized by presence or absence of a hallmark, such as enhancement. Alternatively, tumor signals (such as CT density, 18F-FDG PET SUVmax or Ktrans) can be parcellated using one or more thresholds. In distinction, tumor regions may be defined geographically, for example by modeling tumors as spheres with concentric radial sub-regions (61) or by labelling voxels as ‘rim’ or ‘core’ based on relative voxel position in histogram distributions (62).
These simple approaches have important caveats. ‘Binary’ features such as enhancement are not absolute but depend on how images are acquired and analyzed. Threshold values for continuous data based on ‘cut points’ selected to enhance statistical separation in studies are arbitrary. A priori methods may have misleading biological basis. The Macdonald criteria in HGG illustrate this point, since measuring contrast-enhancing tumor only has been superseded by the Response Assessment in Neuro-Oncology criteria which incorporate measures of non-enhancing tissue (with likely microscopic foci of infiltrative neoplastic cells) into response criteria (63).
Data-driven segmentation
Some studies acquire multiple imaging parameters (for example Ktrans, ADC and 18F-FDG SUVmax) and analyse each signal independently (64). An alternative strategy parcellates voxels with similar signals (or ‘spectra’) into functionally coherent regions within a lesion (65). Most multi-spectral analyses use pattern recognition techniques that simultaneously analyze images to identify voxel clusters in a multi-dimensional feature space. A classifier then groups individual voxels together based on their similarities and differences (66).
Evidence for clinical benefit
Multiple studies of HGG patients have shown lower baseline lesion enhancing tumor volume (ETV) had beneficial OS (p=0.0026) (67) or PFS (p=0.0309) (68) and that early reduction in ETV after bevacizumab related to OS (p=0.0008) (69), whereas WTV and Ktrans did not. Studies of solid tumors outside the brain have shown that ETV or the proportion of enhancing voxels (EF) prior to treatment relate to PFS in cervical cancer (70, 71). Trials of anti-angiogenic drugs suggest that EF provides pharmacodynamic information, independent from other DCE-MRI parameters (48, 72). These data support the hypothesis that measuring tumor regions may be a more useful biomarker than average values of whole tumors in some clinical scenarios.
Threshold-derived partitioning has shown value in GIST patients imaged with 18F-FDG PET. Here, tumor SUV below 8 g/ml four weeks after treatment with sunitinib was associated with markedly longer PFS than those with SUV above the same level (29 v 4 weeks; p<0.0001) (73). Similarly, SUV thresholds have distinguished good and poor time to treatment failure in GIST patients treated with imatinib (p<0.0001) (74). Further, there is some evidence that the presence of high SUVmax values may relate to OS in some cancers (75). In these studies cut-points have been chosen using post hoc criteria. Prospective studies are required to validate these thresholds in specific patient-therapy combinations if these techniques are to have clinical translation.
Data driven approaches have successfully distinguished viable tumor from non-viable tumor using multiparametric MRI and validated the method against H&E histology (76, 77). Moreover, image-defined regions of viable and non-viable tumor show differential response to radiotherapy (78) and to anti-VEGF antibody (79) (Fig. 4). Multispectral analyses of baseline data that identify tumor sub-regions with distinct biology, responsible for driving tumor response, resistance and progression are highly attractive, although performed rarely. Algorithms to perform this are commercially available (80).
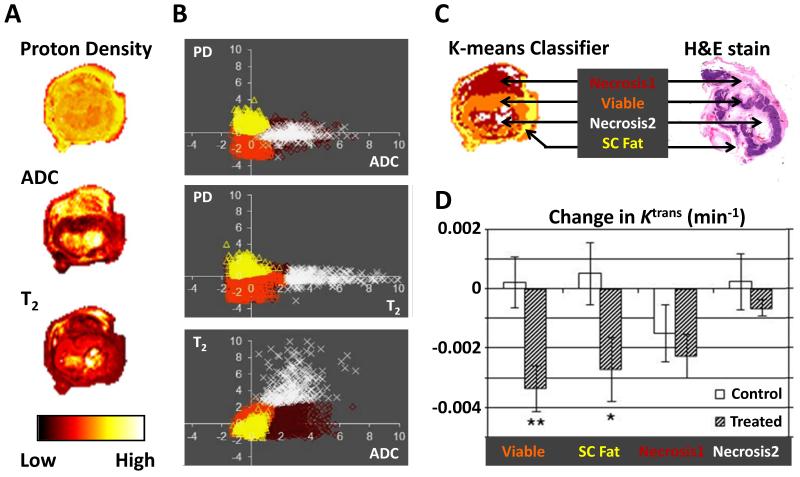
Figure 4.
Multispectral analysis: (A) ADC, T2 values and proton density (PD) maps shown for a colorectal cancer murine xenograft. (B) K-means clustering is performed and decision boundaries are formed. (C) Four tissue categories are produced (viable tumor, subcutaneous (SC) fat and two necrotic regions (differentiated from each other by the T2 signal) that relate to pathology as seen on the H&E section. (D) The anti-vascular effect of G6-31 in treated animals is significant viable tumor tissue but not significant in necrotic regions. Adapted from Carano et al. (76) and Berry et al. (79).
In distinction, it is very difficult to track changes in individual voxels as tumors change shape and size. Some progress has been made in studies of HGG using a method termed ’response parametric mapping’ where rCBV and ADC maps have been categorized as showing no change or increasing/decreasing by greater than 20% following therapy. The proportion of tumor with reduced rCBV related to OS (p=0.019), where mean rCBV did not (81). However, it is extremely difficult to extend this approach to accommodate changes in tumor size, orientation and deformation, which make translation of these methods from specialist laboratories to healthcare systems extremely challenging.
Integrating Imaging, Genomics, and Histopathology
Recent work highlights the intratumor variation in gene mutation and expression (33), but few studies have explored the spatial relationship between imaging, genomics and histopathology. Pre-clinical data have reported differential levels of gene activation and protein transcription that relate to regional perfusion as measured with DCE-MRI (82) and related differential gene expression to regional PET signals (83). Pilot data has associated heterogeneous enhancement patterns with genetic subtypes of breast cancer (84). The ADC characteristics at tumor margins, rather than overall mean values, have been shown to correctly categorize oligodendrogliomas by their 1p/19q loss status (85).
These data highlight the need for larger prospective studies to elucidate how and when integration of imaging, genomic and pathology data may be useful. These studies must evaluate large complex data across a range of biological different scales (15, 32). Two questions require urgent answers. Firstly, do imaging, genomics and histopathology show spatial correspondence because they measure the same biology in different ways and if so, can imaging reliably identify important sub-regions non-invasively? If so, imaging could extend personalized medicine to tumors that cannot be biopsied safely. Alternatively, do imaging, genomics and histopathology measure different biology, providing complementary data? If so, this would open up new multidisciplinary strategies for advancing personalized medicine focussing phenotype and genome together, rather than genome in isolation.
Future Directions and Conclusions
Diverse philosophical and mathematical approaches can quantify intratumor heterogeneity. Most techniques can be applied across all imaging modalities, while some require datasets with multiple signals. Each method has strengths and limitations (Table 2). Advances in hardware such as simultaneous PET-MRI and whole body imaging with MRI diffusion will further stimulate imaging research into analysis of both intratumor heterogeneity and in differences between multiple lesions within individuals.
Table 2.
Image analysis methods for quantifying spatial heterogeneity: comparison of strengths and weaknesses.
| Analysis | Availability | Literature | Spatial information | Single centre reproducibility | Potential statistical and design issues | Biological correlate |
|---|---|---|---|---|---|---|
| Qualitative interpretation by radiologist | ||||||
| Subjective Morphology | Worldwide | Extensive | Evaluated qualitatively | Largely unknown | Subjective and difficult to apply in quantitative study | Measure of overall complexity |
| Hot Spot | Worldwide | Extensive | Ignored outside hot spot | Very good * | Subjective | Difficult to evaluate |
| Quantitative measurement by scientist | ||||||
| Histogram Analysis | Extensive | Extensive | Discarded | Largely unknown | Large number of parameters derived: multiple comparisons | Difficult to evaluate |
| Feature Analysis | Specialist technique | Moderate | Used to compute spatial complexity | Very good * | Large number of parameters derived: multiple comparisons | Measure of overall complexity |
| A priori Segmentation | Specialist technique | Small | Used to define sub-regions | Largely unknown | Various methods; category definition may be arbitrary | Sub-region identified: can match to histology |
| Multi-spectral Analysis | Specialist technique | Minimal | Used to define sub-regions | Unknown | Multi-dimension datasets | Sub-region identified: can match to histology |
Note: indicates in some studies.
All methods must overcome significant hurdles before cementing a role in cancer radiology. Firstly, clear patient benefit must be demonstrated from heterogeneity analyses, above and beyond that found with simple imaging (and non-imaging) biomarkers. Secondly, promising data must be replicated in other institutions and biomarkers must be validated (35, 86). Thirdly, relationships between heterogeneity parameters and underlying biology must be established. Finally, the development of large imaging datasets with multiple candidate ‘probes’ has become an area of intense interest, attempting to parallel advances in next generation sequencing. This ‘radiomic’ approach (60) places heterogeneity parameters at its centre, but the sheer number of parameters under investigation incurs statistical dangers due to large numbers of multiple comparisons. As these challenges are addressed, heterogeneity analyses have great potential to translate from the research arena to play a role in clinical decision making.
Supplementary Material
Acknowledgments
Grant Support
J.P.B. O’Connor is supported by a Cancer Research UK Clinician Scientist Fellowship (C19221/A15267). J.P.B. O’Connor, G.J.M. Parker, and A. Jackson are supported by a Cancer Research UK & EPSRC Comprehensive Imaging Centre grant (C8742/A018097).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
J.C. Waterton is an employee of and has ownership interest (including patents) in AstraZeneca. R.A.D. Carano is an employee of Genentech and has ownership interest (including patents) in Roche. G.J.M. Parker is CEO of and has ownership interest (including patents) in Bioxydyn Limited; reports receiving commercial research grants from AstraZeneca, Merck Serono, and Roche; and is consultant/advisory board member for GlaxoSmithKline. No potential conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012;72:4875–82. doi: 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–65. [PubMed] [Google Scholar]
- 3.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder T, Yuan H, Viglianti BL, Peltz C, Asopa S, Vujaskovic Z, et al. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Res. 2005;65:5163–71. doi: 10.1158/0008-5472.CAN-04-3900. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas-Navia LI, Mace D, Richardson RA, Wilson DF, Shan S, Dewhirst MW. The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 2008;68:5812–9. doi: 10.1158/0008-5472.CAN-07-6387. [DOI] [PubMed] [Google Scholar]
- 6.Serganova I, Doubrovin M, Vider J, Ponomarev V, Soghomonyan S, Beresten T, et al. Molecular imaging of temporal dynamics and spatial heterogeneity of hypoxia-inducible factor-1 signal transduction activity in tumors in living mice. Cancer Res. 2004;64:6101–8. doi: 10.1158/0008-5472.CAN-04-0842. [DOI] [PubMed] [Google Scholar]
- 7.Eskey CJ, Koretsky AP, Domach MM, Jain RK. 2H-nuclear magnetic resonance imaging of tumor blood flow: spatial and temporal heterogeneity in a tissue-isolated mammary adenocarcinoma. Cancer Res. 1992;52:6010–9. [PubMed] [Google Scholar]
- 8.Hamberg LM, Kristjansen PE, Hunter GJ, Wolf GL, Jain RK. Spatial heterogeneity in tumor perfusion measured with functional computed tomography at 0.05 microliter resolution. Cancer Res. 1994;54:6032–6. [PubMed] [Google Scholar]
- 9.Degani H, Gusis V, Weinstein D, Fields S, Strano S. Mapping pathophysiological features of breast tumors by MRI at high spatial resolution. Nat Med. 1997;3:780–2. doi: 10.1038/nm0797-780. [DOI] [PubMed] [Google Scholar]
- 10.Choi YP, Shim HS, Gao MQ, Kang S, Cho NH. Molecular portraits of intratumoral heterogeneity in human ovarian cancer. Cancer Lett. 2011;307:62–71. doi: 10.1016/j.canlet.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69:4894–903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–37. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brurberg KG, Gaustad JV, Mollatt CS, Rofstad EK. Temporal heterogeneity in blood supply in human tumor xenografts. Neoplasia. 2008;10:727–35. doi: 10.1593/neo.08388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Gatenby RA, Grove O, Gillies RJ. Quantitative imaging in cancer evolution and ecology. Radiology. 2013;269:8–15. doi: 10.1148/radiol.13122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson-Herren L, Noker PE, Wagoner SD. Variability of tumor response to chemotherapy. II. Contribution of tumor heterogeneity. Cancer Chemother Pharmacol. 1988;22:131–6. doi: 10.1007/BF00257310. [DOI] [PubMed] [Google Scholar]
- 17.Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014 Sep 30; doi: 10.1038/bjc.2014.512. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor JP, Jackson A, Asselin MC, Buckley DL, Parker GJ, Jayson GC. Quantitative imaging biomarkers in the clinical development of targeted therapeutics: current and future perspectives. Lancet Oncol. 2008;9:766–76. doi: 10.1016/S1470-2045(08)70196-7. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Garcia I, Sole RV, Costa J. Metapopulation dynamics and spatial heterogeneity in cancer. Proc Natl Acad Sci U S A. 2002;99:13085–9. doi: 10.1073/pnas.202139299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu DM, van Klaveren RJ, de Bock GH, Leusveld A, Zhao Y, Wang Y, et al. Limited value of shape, margin and CT density in the discrimination between benign and malignant screen detected solid pulmonary nodules of the NELSON trial. Eur J Radiol. 2008;68:347–52. doi: 10.1016/j.ejrad.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Burrell HC, Pinder SE, Wilson AR, Evans AJ, Yeoman LJ, Elston CW, et al. The positive predictive value of mammographic signs: a review of 425 non-palpable breast lesions. Clin Radiol. 1996;51:277–81. doi: 10.1016/s0009-9260(96)80346-1. [DOI] [PubMed] [Google Scholar]
- 22.Orel SG, Kay N, Reynolds C, Sullivan DC. BI-RADS categorization as a predictor of malignancy. Radiology. 1999;211:845–50. doi: 10.1148/radiology.211.3.r99jn31845. [DOI] [PubMed] [Google Scholar]
- 23.Huang YE, Chen CF, Huang YJ, Konda SD, Appelbaum DE, Pu Y. Interobserver variability among measurements of the maximum and mean standardized uptake values on (18)F-FDG PET/CT and measurements of tumor size on diagnostic CT in patients with pulmonary tumors. Acta Radiol. 2010;51:782–8. doi: 10.3109/02841851.2010.497772. [DOI] [PubMed] [Google Scholar]
- 24.Jackson T, Chung MK, Mercier G, Ozonoff A, Subramaniam RM. FDG PET/CT interobserver agreement in head and neck cancer: FDG and CT measurements of the primary tumor site. Nucl Med Commun. 2012;33:305–12. doi: 10.1097/MNM.0b013e32834e5397. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 26.Pirotte B, Goldman S, Massager N, David P, Wikler D, Vandesteene A, et al. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med. 2004;45:1293–8. [PubMed] [Google Scholar]
- 27.Knopp EA, Cha S, Johnson G, Mazumdar A, Golfinos JG, Zagzag D, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology. 1999;211:791–8. doi: 10.1148/radiology.211.3.r99jn46791. [DOI] [PubMed] [Google Scholar]
- 28.Giesel FL, Choyke PL, Mehndiratta A, Zechmann CM, von Tengg-Kobligk H, Kayser K, et al. Pharmacokinetic analysis of malignant pleural mesothelioma-initial results of tumor microcirculation and its correlation to microvessel density (CD-34) Acad Radiol. 2008;15:563–70. doi: 10.1016/j.acra.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Jackson A, O’Connor JP, Parker GJ, Jayson GC. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res. 2007;13:3449–59. doi: 10.1158/1078-0432.CCR-07-0238. [DOI] [PubMed] [Google Scholar]
- 30.Pope WB, Kim HJ, Huo J, Alger J, Brown MS, Gjertson D, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–9. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Giger ML, Li H, Bick U, Newstead GM. Volumetric texture analysis of breast lesions on contrast-enhanced magnetic resonance images. Magn Reson Med. 2007;58:562–71. doi: 10.1002/mrm.21347. [DOI] [PubMed] [Google Scholar]
- 32.Cebulla J, Kim E, Rhie K, Zhang J, Pathak AP. Multiscale and multi-modality visualization of angiogenesis in a human breast cancer model. Angiogenesis. 2014;17:695–709. doi: 10.1007/s10456-014-9429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Issa B, Buckley DL, Turnbull LW. Heterogeneity analysis of Gd-DTPA uptake: improvement in breast lesion differentiation. J Comput Assist Tomogr. 1999;23:615–21. doi: 10.1097/00004728-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 35.Alic L, Niessen WJ, Veenland JF. Quantification of heterogeneity as a biomarker in tumor imaging: a systematic review. PLoS One. 2014;9:e110300. doi: 10.1371/journal.pone.0110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Knopp MV. Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol. 2011;2011:732848. doi: 10.1155/2011/732848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Law M, Young R, Babb J, Pollack E, Johnson G. Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol. 2007;28:761–6. [PMC free article] [PubMed] [Google Scholar]
- 38.Emblem KE, Nedregaard B, Nome T, Due-Tonnessen P, Hald JK, Scheie D, et al. Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology. 2008;247:808–17. doi: 10.1148/radiol.2473070571. [DOI] [PubMed] [Google Scholar]
- 39.Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res. 2008;14:5236–41. doi: 10.1158/1078-0432.CCR-07-5252. [DOI] [PubMed] [Google Scholar]
- 40.Tofts PS, Benton CE, Weil RS, Tozer DJ, Altmann DR, Jager HR, et al. Quantitative analysis of whole-tumor Gd enhancement histograms predicts malignant transformation in low-grade gliomas. J Magn Reson Imaging. 2007;25:208–14. doi: 10.1002/jmri.20800. [DOI] [PubMed] [Google Scholar]
- 41.Robinson SP, McIntyre DJ, Checkley D, Tessier JJ, Howe FA, Griffiths JR, et al. Tumour dose response to the antivascular agent ZD6126 assessed by magnetic resonance imaging. Br J Cancer. 2003;88:1592–7. doi: 10.1038/sj.bjc.6600926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pope WB, Qiao XJ, Kim HJ, Lai A, Nghiemphu P, Xue X, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108:491–8. doi: 10.1007/s11060-012-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor E, Fieller N, Holmes A, Waterton JC, Ainscow E. Functional principal component analyses of biomedical images as outcome measures. J R Stat Soc (Ser C Appl Stat) 2010;59:57–76. [Google Scholar]
- 44.Rose CJ, O’Connor JPB, Cootes TF, Taylor CJ, Jayson GC, Parker GJM, et al. Indexed distribution analysis for improved signicance testing of spatially heterogeneous parameter maps: application to dynamic contrast-enhanced MRI biomarkers. Magn Reson Med. 2014;71:1299–311. doi: 10.1002/mrm.24755. [DOI] [PubMed] [Google Scholar]
- 45.Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med. 2012;53:693–700. doi: 10.2967/jnumed.111.099127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willaime JM, Turkheimer FE, Kenny LM, Aboagye EO. Quantification of intra-tumour cell proliferation heterogeneity using imaging descriptors of 18F fluorothymidine-positron emission tomography. Phys Med Biol. 2013;58:187–203. doi: 10.1088/0031-9155/58/2/187. [DOI] [PubMed] [Google Scholar]
- 47.Sanghera B, Banerjee D, Khan A, Simcock I, Stirling JJ, Glynne-Jones R, et al. Reproducibility of 2D and 3D fractal analysis techniques for the assessment of spatial heterogeneity of regional blood flow in rectal cancer. Radiology. 2012;263:865–73. doi: 10.1148/radiol.12111316. [DOI] [PubMed] [Google Scholar]
- 48.O’Connor JP, Carano RA, Clamp AR, Ross J, Ho CC, Jackson A, et al. Quantifying antivascular effects of monoclonal antibodies to vascular endothelial growth factor: insights from imaging. Clin Cancer Res. 2009;15:6674–82. doi: 10.1158/1078-0432.CCR-09-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haralick RM, Shanmuga K, Dinstein I. Textural features for image classification. IEEE Trans Systems Man Cybernetics. 1973;6:610–21. [Google Scholar]
- 50.Peitgen H-O, Jurgens H, Saupe D. Chaos and fractals. Springer; Berlin: 2004. [Google Scholar]
- 51.Rose CJ, Mills SJ, O’Connor JP, Buonaccorsi GA, Roberts C, Watson Y, et al. Quantifying spatial heterogeneity in dynamic contrast-enhanced MRI parameter maps. Magn Reson Med. 2009;62:488–99. doi: 10.1002/mrm.22003. [DOI] [PubMed] [Google Scholar]
- 52.Oliver A, Freixenet J, Marti J, Perez E, Pont J, Denton ER, et al. A review of automatic mass detection and segmentation in mammographic images. Medical image analysis. 2010;14:87–110. doi: 10.1016/j.media.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Miller P, Astley S. Classification of breast tissue by texture analysis. Image Vis Comput. 1992;10:277–82. [Google Scholar]
- 54.Li H, Giger ML, Olopade OI, Lan L. Fractal analysis of mammographic parenchymal patterns in breast cancer risk assessment. Acad Radiol. 2007;14:513–21. doi: 10.1016/j.acra.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology. 2013;266:177–84. doi: 10.1148/radiol.12120254. [DOI] [PubMed] [Google Scholar]
- 56.Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165–71. doi: 10.1148/radiol.11110264. [DOI] [PubMed] [Google Scholar]
- 57.Balagurunathan Y, Gu Y, Wang H, Kumar V, Grove O, Hawkins S, et al. Reproducibility and prognosis of quantitative features extracted from CT images. Transl Oncol. 2014;7:72–87. doi: 10.1593/tlo.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Connor JP, Rose CJ, Jackson A, Watson Y, Cheung S, Maders F, et al. DCE-MRI biomarkers of tumour heterogeneity predict CRC liver metastasis shrinkage following bevacizumab and FOLFOX-6. Br J Cancer. 2011;105:139–45. doi: 10.1038/bjc.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alic L, van Vliet M, van Dijke CF, Eggermont AM, Veenland JF, Niessen WJ. Heterogeneity in DCE-MRI parametric maps: a biomarker for treatment response? Phys Med Biol. 2011;56:1601–16. doi: 10.1088/0031-9155/56/6/006. [DOI] [PubMed] [Google Scholar]
- 60.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Cavalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaustad JV, Benjaminsen IC, Graff BA, Brurberg KG, Ruud EB, Rofstad EK. Intratumor heterogeneity in blood perfusion in orthotopic human melanoma xenografts assessed by dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2005;21:792–800. doi: 10.1002/jmri.20321. [DOI] [PubMed] [Google Scholar]
- 62.Checkley D, Tessier JJ, Kendrew J, Waterton JC, Wedge SR. Use of dynamic contrast-enhanced MRI to evaluate acute treatment with ZD6474, a VEGF signalling inhibitor, in PC-3 prostate tumours. Br J Cancer. 2003;89:1889–95. doi: 10.1038/sj.bjc.6601386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 64.Dingemans AM, de Langen AJ, van den Boogaart V, Marcus JT, Backes WH, Scholtens HT, et al. First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging. Ann Oncol. 2011;22:559–66. doi: 10.1093/annonc/mdq391. [DOI] [PubMed] [Google Scholar]
- 65.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256:348–64. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 66.Vannier MW, Butterfield RL, Jordan D, Murphy WA, Levitt RG, Gado M. Multispectral analysis of magnetic resonance images. Radiology. 1985;154:221–4. doi: 10.1148/radiology.154.1.3964938. [DOI] [PubMed] [Google Scholar]
- 67.Zhang W, Kreisl TN, Solomon J, Reynolds RC, Glen DR, Cox RW, et al. Acute effects of bevacizumab on glioblastoma vascularity assessed with DCE-MRI and relation to patient survival. Proceedings ISMRM. 2009;17:282. [Google Scholar]
- 68.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13:401–9. doi: 10.1093/neuonc/noq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kreisl TN, Zhang W, Odia Y, Shih JH, Butman JA, Hammoud D, et al. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011;13:1143–50. doi: 10.1093/neuonc/nor091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donaldson SB, Buckley DL, O’Connor JP, Davidson SE, Carrington BM, Jones AP, et al. Enhancing fraction measured using dynamic contrast-enhanced MRI predicts disease-free survival in patients with carcinoma of the cervix. Br J Cancer. 2010;102:23–6. doi: 10.1038/sj.bjc.6605415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mannelli L, Patterson AJ, Zahra M, Priest AN, Graves MJ, Lomas DJ, et al. Evaluation of nonenhancing tumor fraction assessed by dynamic contrast-enhanced MRI subtraction as a predictor of decrease in tumor volume in response to chemoradiotherapy in advanced cervical cancer. AJR Am J Roentgenol. 2010;195:524–7. doi: 10.2214/AJR.09.3437. [DOI] [PubMed] [Google Scholar]
- 72.Messiou C, Orton M, Ang JE, Collins DJ, Morgan VA, Mears D, et al. Advanced solid tumors treated with cediranib: comparison of dynamic contrast-enhanced MR imaging and CT as markers of vascular activity. Radiology. 2012;265:426–36. doi: 10.1148/radiol.12112565. [DOI] [PubMed] [Google Scholar]
- 73.Prior JO, Montemurro M, Orcurto MV, Michielin O, Luthi F, Benhattar J, et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol. 2009;27:439–45. doi: 10.1200/JCO.2008.17.2742. [DOI] [PubMed] [Google Scholar]
- 74.Holdsworth CH, Badawi RD, Manola JB, Kijewski MF, Israel DA, Demetri GD, et al. CT and PET: early prognostic indicators of response to imatinib mesylate in patients with gastrointestinal stromal tumor. AJR Am J Roentgenol. 2007;189:W324–30. doi: 10.2214/AJR.07.2496. [DOI] [PubMed] [Google Scholar]
- 75.Kayani I, Avril N, Bomanji J, Chowdhury S, Rockall A, Sahdev A, et al. Sequential FDG-PET/CT as a biomarker of response to Sunitinib in metastatic clear cell renal cancer. Clin Cancer Res. 2011;17:6021–8. doi: 10.1158/1078-0432.CCR-10-3309. [DOI] [PubMed] [Google Scholar]
- 76.Carano RA, Ross AL, Ross J, Williams SP, Koeppen H, Schwall RH, et al. Quantification of tumor tissue populations by multispectral analysis. Magn Reson Med. 2004;51:542–51. doi: 10.1002/mrm.10731. [DOI] [PubMed] [Google Scholar]
- 77.Henning EC, Azuma C, Sotak CH, Helmer KG. Multispectral quantification of tissue types in a RIF-1 tumor model with histological validation. Part I. Magn Reson Med. 2007;57:501–12. doi: 10.1002/mrm.21161. [DOI] [PubMed] [Google Scholar]
- 78.Henning EC, Azuma C, Sotak CH, Helmer KG. Multispectral tissue characterization in a RIF-1 tumor model: monitoring the ADC and T2 responses to single-dose radiotherapy. Part II. Magn Reson Med. 2007;57:513–9. doi: 10.1002/mrm.21178. [DOI] [PubMed] [Google Scholar]
- 79.Berry LR, Barck KH, Go MA, Ross J, Wu X, Williams SP, et al. Quantification of viable tumor microvascular characteristics by multispectral analysis. Magn Reson Med. 2008;60:64–72. doi: 10.1002/mrm.21470. [DOI] [PubMed] [Google Scholar]
- 80.Chen L, Choyke PL, Chan TH, Chi CY, Wang G, Wang Y. Tissue-specific compartmental analysis for dynamic contrast-enhanced MR imaging of complex tumors. IEEE Trans Med Imaging. 2011;30:2044–58. doi: 10.1109/TMI.2011.2160276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galban CJ, Chenevert TL, Meyer CR, Tsien C, Lawrence TS, Hamstra DA, et al. Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res. 2011;17:4751–60. doi: 10.1158/1078-0432.CCR-10-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costouros NG, Lorang D, Zhang Y, Miller MS, Diehn FE, Hewitt SM, et al. Microarray gene expression analysis of murine tumor heterogeneity defined by dynamic contrast-enhanced MRI. Mol Imaging. 2002;1:301–8. doi: 10.1162/15353500200202124. [DOI] [PubMed] [Google Scholar]
- 83.von Forstner C, Egberts JH, Ammerpohl O, Niedzielska D, Buchert R, Mikecz P, et al. Gene expression patterns and tumor uptake of 18F-FDG, 18F-FLT, and 18F-FEC in PET/MRI of an orthotopic mouse xenotransplantation model of pancreatic cancer. J Nucl Med. 2008;49:1362–70. doi: 10.2967/jnumed.107.050021. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto S, Maki DD, Korn RL, Kuo MD. Radiogenomic analysis of breast cancer using MRI: a preliminary study to define the landscape. AJR Am J Roentgenol. 2012;199:654–63. doi: 10.2214/AJR.11.7824. [DOI] [PubMed] [Google Scholar]
- 85.Jenkinson MD, Smith TS, Brodbelt AR, Joyce KA, Warnke PC, Walker C. Apparent diffusion coefficients in oligodendroglial tumors characterized by genotype. J Magn Reson Imaging. 2007;26:1405–12. doi: 10.1002/jmri.21062. [DOI] [PubMed] [Google Scholar]
- 86.Waterton JC, Pylkkanen L. Qualification of imaging biomarkers for oncology drug development. Eur J Cancer. 2012;48:409–15. doi: 10.1016/j.ejca.2011.11.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.