Abstract
Phosphodiesterases (PDE) are exciting new targets in medical sciences. These enzymes are some of the key mediators of cellular functions in the body and hence are attractive sites for drug-induced modulations. With the finding that Tofisopam, a new anxiolytic, inhibits PDEs, the authors were inspired to look into the role of PDE and drugs acting on them in psychiatry. Hence, the review was undertaken. We found several research materials available highlighting the role of PDE in cellular functions and the possible newer etiological mechanisms of neuropsychiatric illnesses such as schizophrenia, depression/anxiety disorders, and cognitive dysfunction involving PDEs. We also found that there are many molecules acting on PDEs, which have the potential to alter the way we treat mental illnesses today. This article is intended to provide an in-depth look at these enzymes so that more cost-effective therapeutic molecules may be synthesized and marketed in India for managing mental illnesses.
KEY WORDS: Affective disorders, anxiety, cognition, phosphodiesterase, phosphodiesterase inhibitor, schizophrenia
Introduction
Phosphodiesterases (PDE) are exciting new targets in medical sciences. These enzymes are some of the key mediators of cellular functions in the body and hence are attractive sites for drug-induced modulations. Research into molecules, which alter PDE actions, has expanded to the field of psychiatry and neurosciences in recent times. The introduction of the drug Tofisopam in India as a novel nonsedating anxiolytic has inspired the authors to embark on a brief review of PDE in psychiatry. Tofisopam is a 2,3-benzodiazepine, which does not act on the benzodiazepine site of the gamma amino butyric acid (GABA) receptor but has anxiolytic properties without having sedative, anticonvulsant, amnestic, or muscle-relaxant actions.[1] It is widely gaining popularity for treatment of anxiety disorders even for long-term use. However, what is more interesting and relevant to this review is the newer research showing that Tofisopam has multiple PDE-inhibiting actions which are being actively evaluated for managing negative and cognitive symptoms of schizophrenia.[2] It selectively inhibits PDE 4A1, PDE 10A1, PDE 3, and PDE 2A3.[1]
PDE inhibitors have been popularized by Sildenafil used widely for erectile dysfunction. However, there is more to PDE inhibitors than their sexual benefits alone, and we would like to highlight the important research in psychiatry that has happened and is happening around the world in this field. With this review, we aim to generate interest among Indian researchers into PDE as novel therapeutic targets which could help treat several neuropsychiatric disorders as it appears to have been largely ignored here. This would be the first review paper of the psychiatric implications of PDE to the best of the authors’ knowledge in India.
Cellular communication involves electrical and chemical signal transmissions. Important to this are chemical messengers, which transmit signals for downstream action. When a ligand binds to the cell surface receptor, the concentration of intracellular molecules called second messengers is altered, which then results in signal transmission within the cell. These include adenosine 3’,5’ cyclic monophosphate (cAMP), calcium ions (Ca2+), diacylglycerol, and inositol 1,4,5-triphosphate.[3] Cyclic nucleotide PDEs are a family of ubiquitous enzymes, which selectively hydrolyze the 3’ cyclic phosphate bond in cAMP and/or guanosine 3’,5’ cyclic monophosphate (cGMP).[4] cAMP and cGMP are known as the second messengers in the intra-cellular signaling cascade. They are active molecules which are produced by the action of adenylyl or guanylyl cyclase enzymes on AMP or GMP, respectively. cAMP/cGMP most often activate specific protein kinases, which further regulate downstream signaling. Conversely, inactivation of these second messengers whenever termination of intracellular signaling is required is done by hydrolyzing the cyclic phosphate bonds by PDE. This causes a reduction in the intracellular signal transmission. The role of cAMP and cGMP in the tissues vary from cellular metabolism, modulation of synapse physiology to inter-cellular electrical signaling [Figure 1].
Figure 1.
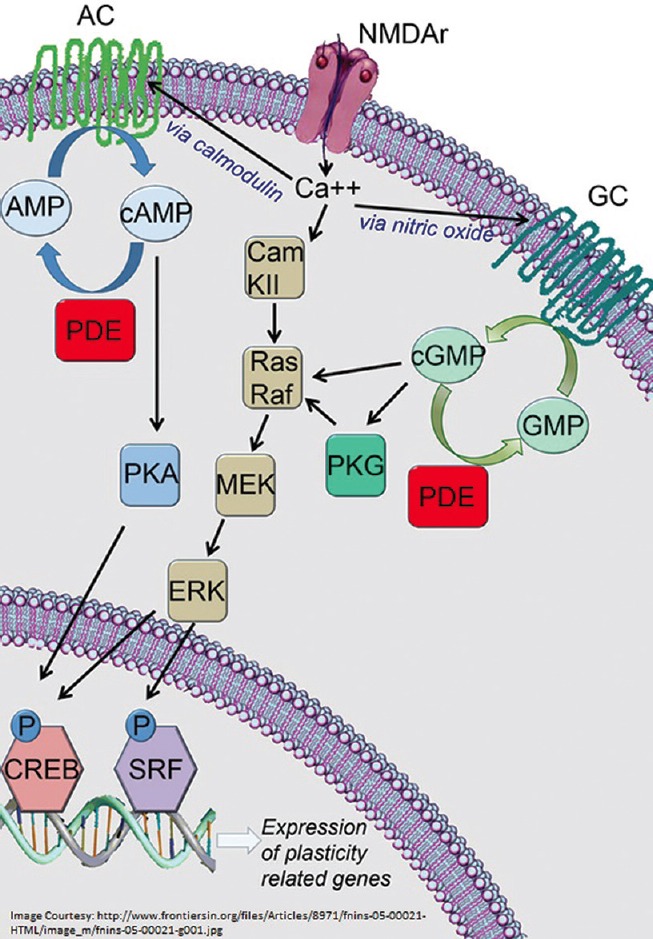
Phosphodiesterases and second messengers
11 PDE isoenzymes have been identified until date. Each isoenzyme has several isoforms. PDEs are distributed in almost all cells, but it is their various isoenzymes, and isoforms of these, that are expressed differentially in various tissues. Hence, it is possible to deliver selective therapy with decreased risk of adverse effects, using selective PDE modulators. With respect to this, the specific sites of expression of PDEs in the body and their substrates have also been identified[5] [Figure 2]. Hence, PDEs form excellent targets for modification of intracellular signal transduction. Drugs inhibiting PDEs have already been successfully tried in the management of many illnesses. For example, PDE 5 inhibitors such as Sildenafil, tadalafil, and vardenafil decrease intracellular signal transduction in vascular smooth muscles consequently leading to reduced intracellular calcium concentration. This serves to produce smooth muscle relaxation and hence, vasodilatation. This effect is used therapeutically in conditions like are erectile dysfunction as well as patent ductus arteriosus. Other known PDE inhibitors include milrinone (PDE 3) and dipyridamole (PDE 5). New research has also shown the potentially anticancer effects of PDE 4 inhibition.[6] Hence, we see that PDE modulation is now gaining wide-spread cognizance as an important modality of treating varied medical conditions. In the following pages, we discuss the role of PDEs in neuropsychiatric disorders and drugs modulating them.
Figure 2.
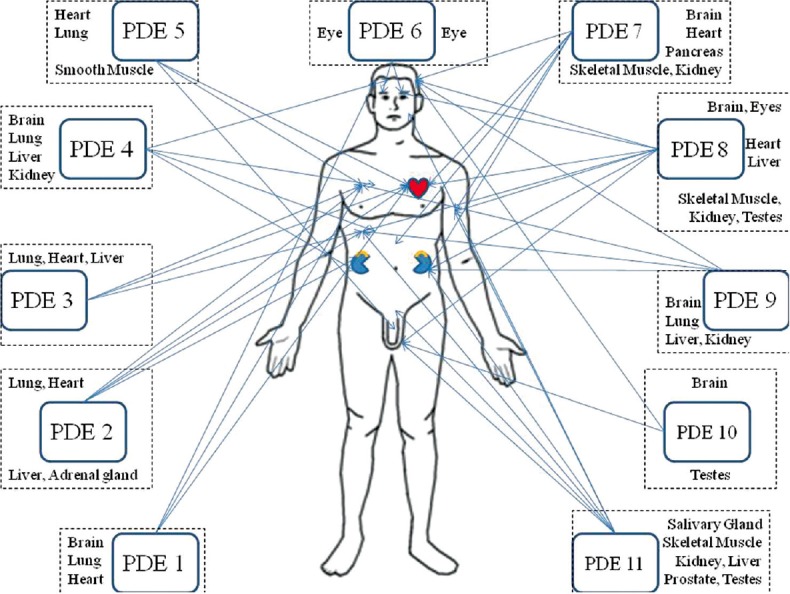
Differential expression of phosphodiesterases in human body
Phosphodiesterases and Schizophrenia
The genetic etiology of schizophrenia is now an established fact, and a number of potentially putative chromosomes have already been identified. Important ones among them are the Disrupted in Schizophrenia-1 and 2 (DISC-1 and 2) genes located on chromosome 1 which have been found to be associated not only with schizophrenia, but also bipolar disorder and unipolar depression.[7] DISC-1 is believed to be a scaffolding protein involved in normal neuronal migration and DISC-2 regulates gene expression. An important binding partner of the DISC proteins is PDE 4B, encoded by a gene on chromosome 1. PDE 4B hydrolyses cAMP and stops cAMP-stimulated signaling cascades. It has a complex interaction with DISC-1. It is hypothesized that the DISC-1 protein sequesters PDE 4B in a low activity form until there is a need to stop cAMP signaling, at which time it releases PDE 4B in a highly active form.[8]
PDE 4B itself has been implicated as an independent risk factor for schizophrenia.[9] A Scottish family with a chromosomal translocation - t(1,16) has been described, and at least 2 persons carrying this were found to have psychotic disorders. t(1,16) involves genes for Cadherin 8 encoded on chromosome 16 and PDE 4B encoded on chromosome 1.[8]
Antipsychotic drugs block dopamine receptors, predominantly D2 receptors, which leads to increased cAMP levels and activity in the brain.[10] It is then rationalized that the same effect can be obtained by inhibiting PDE 4. This has several potential advantages, like bypassing the dopamine receptors. This may reduce the likelihood of adverse effects which arise due to the nonspecific blockade of D2 receptors - such as hyperprolactinemia and extrapyramidal symptoms. A dysregulation of PDE 4 activity is believed to have a role in schizophrenia. Hence, trials of PDE 4 inhibitors in the management of psychosis have been conducted, as discussed further.
Rolipram was the first specific PDE 4 inhibitor which was first studied for its likely antipsychotic effect. It has shown to increase both, the intensity and duration of cAMP-mediated signaling.[11] Kanes et al. in 2007 published their study of the antipsychotic potential of rolipram.[12] They used mice models of psychoses. Psychosis was induced using D-amphetamine and acoustic startle, and prepulse inhibition (PPI) of the startle response was evaluated. As PPI is found to be DISC, the ability of a drug to increase PPI in experimental mice is considered to be indicative of its antipsychotic efficacy. Similar to traditional antipsychotic drugs, rolipram was found to increase PPI without altering the acoustic startle response in the mice at lower doses (0.66 mg/kg). Rolipram also reversed the disruption of PPI-induced by D-amphetamine. Higher doses (1.0 mg/kg) were found to induce catalepsy in the mice. Rolipram has also been found to produce favorable results in cognitive symptoms associated with schizophrenia-like memory impairment through its action of enhancing long-term potentiation (LTP).[13,14,15] Depressive symptoms are also commonly noted in schizophrenia though the prevalence varies between studies. Anywhere between 10% and 50% patients with schizophrenia experience significant depressive symptoms at some point during the course of their illness.[16] Rolipram been found to have antidepressant effects as detailed below. Prolonged use of antipsychotics, especially the typical or first generation dopamine blockers has been associated with disabling dyskinetic movements. In a study Sasaki et al. treated laboratory rats with haloperidol to induce involuntary chewing movements and tongue protrusions (a model of drug-induced dyskinesias in humans). When this was followed by administering rolipram, they found that rolipram suppresses the abnormal movements. They hypothesized that drug-induced striatal dopamine receptor supersensitivity leads to reduced cAMP levels within the neurons. This effect could be reversed by increasing cAMP levels using PDE 4 inhibitors like rolipram.[17] The above research shows that rolipram and other compounds with actions on PDE are potentially viable alternative treatments to typical and atypical antipsychotics currently in use. In keeping with the positive results obtained with rolipram, researchers have begun exploring other drugs inhibiting PDEs in schizophrenia. Halene and Siegel in 2008 have published a study conducted on assessing the antipsychotic properties of a compound- RO-20-1724- a PDE inhibitor. They used 2 models of psychoses in laboratory mice - event-related potentials (ERP) and PPI. ERP is a sensory model and PPI is a sensori-motor model. They used D-amphetamine to induce psychosis. D-amphetamine disrupts sensory gating as measured by ERPs - especially the amplitudes of P20 and N40. RO-20-1724 was found to restore the sensory gating to normal. D-amphetamine is also known to disrupt PPI and clinical antipsychotic efficacy of a drug is predicted by reversal of this effect. Here, RO-20-1724 was not found to have any significant antipsychotic efficacy as per this model.[18]
Tofisopam, a new nonsedative anxiolytic drug is a 2,3-benzodiazepine which does not act on the benzodiazepine site of the GABA receptor as mentioned in the introduction of the review. It has also been found to have an inhibitory action on PDE with potential therapeutic benefits in negative symptoms of schizophrenia. Rundfeldt et al. evaluated this angle in Albino Swiss mice which were treated with N-methyl-D-aspartate-receptor antagonist dizocilpine to induce the experimental model of psychosis, especially negative symptoms in them. The forced swim test (FST) was used to evaluate and quantify the negative symptoms. The team found that Tofisopam reduced the increased immobility times on the FST in the dizocilpine treated mice. They also found that the drug predominantly inhibits PDE 4 and PDE 10, which may mediate this antipsychotic (mainly against negative symptoms) effect in their sample.[1] Studies evaluating PDEs in cognitive dysfunction have been discussed further. Thus, we see that PDE inhibition offers a window of opportunity to treat the various symptoms of psychotic disorders with an added bonus of reduced rates of extrapyramidal adverse events.
Other than PDE 4 and PDE 10 inhibitors as mentioned above, very recently a novel brain penetrant PDE 2A inhibitor has been identified with potential relevance to cognitive deficits in schizophrenia. It was found to attenuate sub-chronic phencyclidine-induced deficits in novel object exploration in rats, blocked early postnatal phencyclidine-induced deficits in the intradimensional/extradimensional shift task in rats and attenuated spontaneous P20-N40 auditory gating deficits in dilute brown non-agouti (DBA/2) mice. In contrast, it failed to attenuate phencyclidine-induced hyperactivity in mice, and was devoid of antipsychotic-like activity in the conditioned avoidance response paradigm in rats, probably suggesting its specificity to cognitive processes only.[19]
Phosphodiesterases and Depression/Anxiety
Since the 1980's, rolipram has been investigated for its potential antidepressant effects. There are several possible mechanisms by which this effect is achieved. For example, stimulation of tyrosine hydroxylase, increasing 3,4-dihydroxyphenylalanine (DOPA) availability, increase in synthesis and release of norepinephrine, and induction of brain-derived neurotrophic factor as an outcome of PDE inhibition among others.[20] It has been found to be comparable to tricyclic antidepressants in efficacy without the troubling anticholinergic adverse effects which have largely reduced the popularity of tricyclic antidepressants today.[21]
Shifting focus from the neuro-biological models of depression, pro-inflammatory cytokines have also been implicated in depressive disorders. Medical conditions such as heart diseases, autoimmune disorders, and multiple sclerosis are known to be associated with increased rates of depression. Disease worsening is often accompanied by worsened depressive symptoms.[22] Rolpiram and other PDEs have been shown to suppress cytokine production, especially tumor necrosis factor (TNF)-alpha, TNF-gamma, interleukin-5 and 13. This effect is seen at various levels - gene down-regulation, inhibition of mRNA, and protein production.[20]
The clinical antidepressant/anxiolytic efficacy of PDE inhibition has been studied as highlighted below. A study in Gujarat, India studied the efficacy of Cilostazol in laboratory mice models of depression and anxiety.[23] Cilostazol is a PDE 3 inhibitor which has been to decrease hamilton depression rating scale (HAM-D) scores in poststroke depression. In this study, FST and tail suspension test were the models of depression and the time of immobility measured indicated the antidepressant effect. Interventions used were Fluoxetine, Cilostazol and a control vehicle. Fluoxetine and Cilostazol significantly reduced immobility time compared to control. Furthermore, mice treated with Cilostazol had significantly lower immobility time compared to those treated with Fluoxetine. Anxiety model used was the marble-burying one in the mice. A larger number of marbles buried by the mice in the sand-bed in the specified period of time is suggestive of anxiety/defensiveness or obsessive-compulsiveness. The Cilostazol and Fluoxetine-treated mice were again found to bury significantly less number of marbles compared to controls, and Cilostazol mice performed significantly better than Fluoxetine mice. Cilostazol acts by inhibiting PDE 3 in the hippocampus (where PDE 3 is present in higher densities) and hence appears to have antidepressant/anxiolytic effect.
Another target is PDE 2, inhibition of which may be associated with anxiolysis as found with the drug Tofisopam.[24] Stress plays a very important role in precipitating/worsening anxiety and depressive disorders. Dysfunctional hypothalamo-pituitary axis or antioxidant/oxidation balance may play a role. A very recently identified PDE 2 inhibitor “Bay 60–7550” regulated Cu/Zn superoxide dismutase levels differentially in hippocampus and amygdala, when administered before chronic stress. It also regulated abnormalities of Bax, Caspase 3, and Bcl-2, which play roles in apoptosis. Thus, inhibition of PDE 2 may be an innovative treatment strategy for psychiatric disorders, such as depression and anxiety by alleviating oxidative stress and modulating the apoptotic machinery.[25]
Phosphodiesterases and Cognition
PDE inhibitors have now aroused the interests of scientists working on cognitive enhancement in patients with dementia, especially Alzheimer's Disease. This is because of the finding that Sildenafil, a PDE 5 inhibitor used for erectile dysfunction has shown beneficial effects on attention, memory and visual word recognition tasks. LTP is an important process in memory formation, which is primarily done by glutamatergic stimulation of neurons. It is proposed that Sildenafil aids LTP by the following mechanism. Nitric oxide synthase-1 (NOS-1) is one of a family of 3 types of NOS which synthesizes NO from L-arginine. NO diffuses into the excitatory presynaptic nerve terminal and increases synthesis of cGMP. cGMP, in turn causes vesicles containing the excitatory neurotransmitter glutamate to fuse with the cell membrane and release glutamate into the synapse. cGMP, as discussed above, is degraded by PDE 5. Sildenafil inhibits PDE 5 and hence increases net available cGMP, which then increases glutamate release. Several scientists have studied the effects of Sildenafil on learning and memory in laboratory animals.[26] Another important mechanism by which PDE 5 inhibition may have beneficial effects on cognitive functioning includes neuroprotection from glutamate-induced toxicity and promotion of neurogenesis.[26]
PDE 2 and 9 inhibition too have been found to show cognitive benefits in laboratory experiments.[27] Another potential mechanism by which memory improvement could be explained is improved vascular perfusion in the hippocampi. However, a study by Rutten et al. in rats failed to find any conclusive evidence showing improved cerebral blood flow and cerebral glucose metabolism resulting in cognitive improvements.[28]
HT-0712, a PDE 4 inhibitor has also been found to improve hippocampus-dependent memory in aged mice.[29] There are several on-going human trials of this molecule for the treatment of age-related memory impairment. It has also shown promise in poststroke recovery in animal models.[30] Previously described PDE 3 inhibitor, cilostazol, which was found to have beneficial effects in depression is also being tested as co-treatment to the acetylcholinesterase inhibitor donepezil. However, the results have been inconclusive. Similarly, PDE 7 inhibitors have been found to improve memory performance in mouse models of Alzheimer's dementia.[31]
Conclusion
This review thus, highlighted some of the important research work that has been done in testing new etiological theories of mental illnesses involving the PDE family of enzymes which are ubiquitously found in most of the tissues in the body. We have compiled a list of the most recent studies on molecules targeting specific PDE isoenzymes [Table 1]. More importantly, we have pointed out the important role the PDEs play in cellular functioning and inter/intracellular communication. They have a role that goes far beyond vasodilatation alone and encompasses the possibility of affecting neurotransmission in psychiatric disorders and cognitive dysfunction. Molecules which have been around for several years, for example, rolipram, are now being found to possess the ability to improve disabling symptoms of mental illnesses. We hope to have generated an interest of the scientific community, especially in India, to take up more targeted, and systematic research into the potential PDE modulation holds for the treatment of neuropsychiatric diseases. This can bring additional indigenous more effective, less expensive, and relatively safer molecules, which can then be used in India.
Table 1.
The role of PDE in psychiatric disorders: Studies on molecules targeting specific PDE isoenzymes

Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Srivastava S, Bhatia MS, Gupta K, Rajdev K. Current update on evidence based literature of tofisopam. Delhi Psychiatry J. 2014;17:154–9. [Google Scholar]
- 2.Rundfeldt C, Socala K, Wlaz P. The atypical anxiolytic drug, tofisopam, selectively blocks phosphodiesterase isoenzymes and is active in the mouse model of negative symptoms of psychosis. J Neural Transm. 2010;117:1319–25. doi: 10.1007/s00702-010-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. 4th ed. Sec. 20.1. New York: W. H. Freeman; 2000. [Last accessed on 2015 Sep 20]. Overview of Extracellular Signaling. Available from: http://www.ncbi.nlm.nih.gov/books/NBK21517/ [Google Scholar]
- 4.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 5.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl 1):S252–7. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta R, Sun T, Warrington NM, Rubin JB. Treating brain tumors with PDE4 inhibitors. Trends Pharmacol Sci. 2011;32:337–44. doi: 10.1016/j.tips.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millar JK, James R, Brandon NJ, Thomson PA. DISC1 and DISC2: Discovering and dissecting molecular mechanisms underlying psychiatric illness. Ann Med. 2004;36:367–78. doi: 10.1080/07853890410033603. [DOI] [PubMed] [Google Scholar]
- 8.Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–91. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 9.Pickard BS, Thomson PA, Christoforou A, Evans KL, Morris SW, Porteous DJ, et al. The PDE4B gene confers sex-specific protection against schizophrenia. Psychiatr Genet. 2007;17:129–33. doi: 10.1097/YPG.0b013e328014492b. [DOI] [PubMed] [Google Scholar]
- 10.Kelly MP, Isiegas C, Cheung YF, Tokarczyk J, Yang X, Esposito MF, et al. Constitutive activation of Galphas within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2007;32:577–88. doi: 10.1038/sj.npp.1301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scuvée-Moreau J, Giesbers I, Dresse A. Effect of rolipram, a phosphodiesterase inhibitor and potential antidepressant, on the firing rate of central monoaminergic neurons in the rat. Arch Int Pharmacodyn Ther. 1987;288:43–9. [PubMed] [Google Scholar]
- 12.Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: A specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. 2007;144:239–46. doi: 10.1016/j.neuroscience.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randt CT, Judge ME, Bonnet KA, Quartermain D. Brain cyclic AMP and memory in mice. Pharmacol Biochem Behav. 1982;17:677–80. doi: 10.1016/0091-3057(82)90344-6. [DOI] [PubMed] [Google Scholar]
- 14.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–5. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, et al. A mouse model of Rubinstein-Taybi syndrome: Defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A. 2003;100:10518–22. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki H, Hashimoto K, Maeda Y, Inada T, Kitao Y, Fukui S, et al. Rolipram, a selective c-AMP phosphodiesterase inhibitor suppresses oro-facial dyskinetic movements in rats. Life Sci. 1995;56:PL443–7. doi: 10.1016/0024-3205(95)00218-u. [DOI] [PubMed] [Google Scholar]
- 18.Halene TB, Siegel SJ. Antipsychotic-like properties of phosphodiesterase 4 inhibitors: Evaluation of 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (RO-20-1724) with auditory event-related potentials and prepulse inhibition of startle. J Pharmacol Exp Ther. 2008;326:230–9. doi: 10.1124/jpet.108.138586. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001;7:387–98. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redrobe JP, Jørgensen M, Christoffersen CT, Montezinho LP, Bastlund JF, Carnerup M, et al. In vitro and in vivo characterisation of Lu AF64280, a novel, brain penetrant phosphodiesterase (PDE) 2A inhibitor: Potential relevance to cognitive deficits in schizophrenia. Psychopharmacology (Berl) 2014;231:3151–67. doi: 10.1007/s00213-014-3492-7. [DOI] [PubMed] [Google Scholar]
- 21.Bobon D, Breulet M, Gerard-Vandenhove MA, Guiot-Goffioul F, Plomteux G, Sastre-y-Hernández M, et al. Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur Arch Psychiatry Neurol Sci. 1988;238:2–6. doi: 10.1007/BF00381071. [DOI] [PubMed] [Google Scholar]
- 22.Cavanagh J, Mathias C. Inflammation and its relevance to psychiatry. Adv Psychiatr Treat. 2008;14:248–55. [Google Scholar]
- 23.Patel DS, Anand IS, Bhatt PA. Evaluation of antidepressant and anxiolytic activity of phosphodiesterase 3 inhibitor-cilostazol. Indian J Psychol Med. 2012;34:124–8. doi: 10.4103/0253-7176.101776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masood A, Huang Y, Hajjhussein H, Xiao L, Li H, Wang W, et al. Anxiolytic effects of phosphodiesterase-2 inhibitors associated with increased cGMP signaling. J Pharmacol Exp Ther. 2009;331:690–9. doi: 10.1124/jpet.109.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devan BD, Duffy KB, Bowker JL, Bharati IS, Nelson CM, Daffin LW, Jr, et al. Phosphodiesterase type 5 (PDE5) inhibition and cognitive enhancement. Drugs Future. 2005;30:725. [Google Scholar]
- 26.Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: A promising target for cognition enhancement. Psychopharmacology. 2009;202:419–43. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding L, Zhang C, Masood A, Li J, Sun J, Nadeem A, et al. Protective effects of phosphodiesterase 2 inhibitor on depression-and anxiety-like behaviors: Involvement of antioxidant and anti-apoptotic mechanisms. Behav Brain Res. 2014;268:150–8. doi: 10.1016/j.bbr.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutten K, Van Donkelaar EL, Ferrington L, Blokland A, Bollen E, Steinbusch HW, et al. Phosphodiesterase inhibitors enhance object memory independent of cerebral blood flow and glucose utilization in rats. Neuropsychopharmacology. 2009;34:1914–25. doi: 10.1038/npp.2009.24. [DOI] [PubMed] [Google Scholar]
- 29.Peters M, Bletsch M, Stanley J, Wheeler D, Scott R, Tully T. The PDE4 inhibitor HT-0712 improves hippocampus-dependent memory in aged mice. Neuropsychopharmacology. 2014;39:2938–48. doi: 10.1038/npp.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald E, Van der Lee H, Pocock D, Cole C, Thomas N, VandenBerg PM, et al. A novel phosphodiesterase type 4 inhibitor, HT-0712, enhances rehabilitation-dependent motor recovery and cortical reorganization after focal cortical ischemia. Neurorehabil Neural Repair. 2007;21:486–96. doi: 10.1177/1545968307305521. [DOI] [PubMed] [Google Scholar]
- 31.Heckman PR, Wouters C, Prickaerts J. Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer's disease: A translational overview. Curr Pharm Des. 2015;21:317–31. doi: 10.2174/1381612820666140826114601. [DOI] [PubMed] [Google Scholar]


