Abstract
Objective:
The study aims to evaluate the protective effects of coenzyme Q10 (CoQ10) and Cynara scolymus L (CS) on doxorubicin (dox)-induced toxicity.
Materials and Methods:
Sixty male rats were divided into six groups. Group 1 as a control. Group 2 received dox (10 mg/kg) intraperitoneally. Group 3 received CoQ10 (200 mg/kg). Group 4 received CS (500 mg/kg). Group 5 received CoQ10 (200 mg/kg) and dox (10 mg/kg). Group 6 received CS (500 mg/kg) and dox (10 mg/kg). The rats were then evaluated biochemically and immunohistochemically.
Results:
Dox produced a significant deterioration of hepatic and renal functional parameters. Moreover, an upsurge of oxidative stress and nitrosative stress markers. The expression of alpha-smooth muscle actin (α-SMA) was increased and proliferating cell nuclear antigen (PCNA) expression was decreased. Administration of CoQ10 and CS resulted in a significant improvement of hepatic and renal functional parameters, and an improvement of both α-SMA and PCNA.
Conclusion:
It is concluded that pretreatment with CoQ10 and CS is associated with up-regulation of favorable protective enzymes and down-regulation of oxidative stress. That can be advised as a supplement to dox-treated patients.
KEY WORDS: Alpha-smooth muscle actin, doxorubicin, nitrosative, oxidative, proliferating cell nuclear antigen
Introduction
One of the major problems is the severe adverse effects of anticancer drugs, which can harm organs and systems such as the immune system, liver, kidney, and heart. Hence, it is necessary to find effective methods to reduce the toxic effects of those drugs.[1]
Doxorubicin (dox) is an anthracycline antibiotic drug that is linked to the natural product daunomycin, which previously had been referred to as adriamycin. Dox is produced by variety of wild strains of Streptomyces and is usually utilized as chemotherapy in malignant neoplastic disease.[2] Pharmacological actions of dox induced through triggering include DNA damage and the suppression of macromolecule synthesis.[3] The molecular mechanism that explains the multiorgan toxicity induced by dox needs to be clarified, but the appropriate explanation for dox multiorgan toxicity is oxidative stress, the genesis of an inflammatory cascade, and apoptosis.[4]
Coenzyme Q10 (CoQ10), or ubiquinone, is considered one of the natural antioxidants that can be synthesized endogenously or supplied through food. It is present in the biological membranes of cellular organelles, such as peroxisomes and lysosomes, and is principally located in the inner mitochondrial membrane as part of the electron transport chain, which is responsible for adenosine triphosphate synthesis.[5] CoQ10 is considered a potent lipophilic antioxidant; it acts directly with free radicals or as a reducing agent for regenerating Vitamins E and C from their oxidized forms.[6] CoQ10 inhibits the generation of reactive oxygen species and lipid peroxidation products during free-radical scavenging, suppresses excess nitric oxide (NO) production, and prevents nitrative stress in tissues.[7] In addition, CoQ10 exhibits anti-inflammatory properties by reducing the release of proinflammatory cytokines during inflammatory injury.[8]
Cynara scolymus L. (CS) (artichoke) is rich in minerals, phenolics, and fiber, and low in lipids. It is considered as one of the most famous Mediterranean vegetables. It has been used from ancient times in traditional medicines as a diuretic.[9] CS has antioxidant properties due to its content of hydroxycinnamates and flavonoid glycosides.[10] Various studies are ongoing to evaluate the role of CS as a hepatoprotective and anticarcinogenic plant.[9]
Proliferating cell nuclear antigen (PCNA) is a nuclear protein that engages in the coordination of DNA replication and the regulation of the cellular cycle.[11] Studies have found that PCNA protein can be used to quantitatively measuring hepatic regenerative activity.[12]
In normal condition, hepatic stellate cells (HSCs) are quiescent cells that generate an extracellular matrix (ECM) in the space of Disse.[13] HSCs perform a crucial role in the pathogenesis of fibrosis by excessive deposition of ECM materials.[14] Furthermore, hepatic injury leads to activation of HSCs, recognized by proliferation and myofibroblastic transformation.[15] Activated HSCs display a strong immunoreactivity of cytoplasmic alpha-smooth muscle actin (α-SMA).[14]
The aim of this study was to evaluate the possible ameliorative potential of CoQ10 and CS on dox-induced toxicity.
Materials and Methods
Animals and Treatment
Adult male albino Sprague-Dawely rats, weighing 130–160 g, 10–12 weeks’ age, were obtained from the animal house of the National Research Center, Giza, Egypt. The rats were subjected to controlled conditions of temperature (25°C ± 3°C), humidity (50–60%) and illumination (12-h light, 12-h dark cycle, lights on at 08:00 h) and were provided with standard pellet diet and water ad libitum for 1 week before starting the experiment. All animal care and procedures were in accordance with the European Communities Council Directive of November 24, 1986 (86/609/ECC) and the R.D. 223/1988 and were approved by Ethics Committee of National Research Centre, Egypt. The rats were exposed to dox (Pharmacia Italia Spa, Italy) and CoQ10 (Sigma Chemical Co., St Louis, Mo., USA), which was prepared in a 1% aqueous solution of Tween 80; they were also exposed to CS purchased from Biover (Bruges, Belgium), which was prepared in a 1% aqueous solution of Tween 80.
The chemical composition of the CS leaf extract was determined by analyzing its chlorogenic acid (CGA), cynarin, and luteolin-7-O-glucoside content in triplicate by an high performance liquid chromatography (HPLC) method adapted from European Pharmacopoeia.[8]
CS leaf extract (30 mg) was dissolved in 25 ml 30% methanol. Calibration curves were composed for the three standards (CGA, cynarin and luteolin-7-O-glucoside). All samples were analyzed on a Gilson HPLC system with ultraviolet detector at wavelength 330 nm. As mobile phase, solvent A: 0.5% phosphoric acid in 5% methanol and solvent B: 0.5% phosphoric acid in acetonitrile were used. The gradient profile started with a linear increase of 5–25% B in 30 min, followed by a 5 min linear increase to 100% B, after which the initial conditions were reinstalled. A flow rate of 1.0 ml/min was used. A Licrospher 100 C18 reversed-phase analytical column (250 mm × 4 mm, 5 µl) with a Licrospher guard column (4 mm × 4 mm, 5 µm) from Merck (Germany) was used for the separation of the phenolic compounds.
Experimental Design
Sixty rats were randomly allocated into six groups (10 rats each). Groups were treated with the drugs as follows:
Group 1 received 1% aqueous solution of Tween 80 and served as a normal control. Group 2 received dox (10 mg/kg, single dose intraperitoneally [i.p.]) on the 6th day and served as positive control. Group 3 received CoQ10 (200 mg/kg) for 21 days. Group 4 received CS (500 mg/kg) for 21 days. Group 5 received CoQ10 (200 mg/kg) for 6 days, dox (10 mg/kg, single dose i.p.) on the 6th day and continued administration of CoQ10 until day 21. Group 6 received CS (500 mg/kg) for 6 days, dox (10 mg/kg, single dose i.p.) on the 6th day and continued administration of CS day 21. On day 21, blood samples were collected, animals were sacrificed, and organs (liver and kidney) were excised.
Serum and Tissue Preparation
Preparation of serum and tissue homogenates
All rats were sacrificed under anesthesia 24 h after the last treatment and overnight fasting. Before sacrifice, blood samples were collected from the retro-orbital venous plexus. These samples were kept at room temperature for 30 min and centrifuged at 3000 rpm for 10 min. Serum samples obtained in this way were aliquoted and stored in a freezer (−20°C) for use in biochemical analyses that included aspartate transaminase (AST), alanine transaminase (ALT), albumin, total protein, total bilirubin, urea, and creatinine.
After sacrifice, the abdomen was opened wide and the liver and kidneys were removed and washed 3 times with cold physiological saline (0.9% NaCl). Then, the liver and kidneys were weighed and part of them was homogenized for the measurement of oxidative stress markers. Kidneys and livers were homogenized (GlasCol homogenizer), and a 20% w/v homogenate was prepared in ice-cold phosphate buffer (0.01 M, pH 7.4). The homogenate was centrifuged at 3000 rpm for 20 min and the supernatant was then divided over several containers to avoid sample thawing and refreezing, and was kept at −80°C until analysis.
Serum parameters
Serum ALT and AST were determined using ELISA kits supplied by the Bio Diagnostic Company (Cairo, Egypt). Serum albumin, total protein, total bilirubin, urea, and creatinine were determined by spectrophotometer using the corresponding colorimetric kits supplied by Bio Diagnostic Company (Cairo, Egypt).
Hepatic and renal oxidative markers
After homogenization, the supernatant was obtained and used to determine malondialdehyde (MDA) and reduced glutathione (GSH) levels in liver and kidney using colorimetric assay kits in accordance with the manufacturer's instructions (Bio Diagnostic, Cairo, Egypt). NO levels were assayed in liver and kidney using a colorimetric assay kit as directed by the manufacturer (Cayman Chemical Co., USA).
Activities of antioxidant enzymes in liver tissue homogenate
The activities of antioxidant enzymes, such as catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx), in the liver tissue homogenates of all experimental groups were measured using a Cayman (USA) assay kit in accordance with the manufacturer's instructions.
Histological examination
Liver samples were fixed in 10% neutral buffered formalin and embedded in paraffin. All tissue samples were sectioned at four µm and stained with hematoxylin and eosin and periodic acid-Schiff (PAS). For each specimen, at least three to five slides were examined using an Olympus BX53 microscope equipped with DP73 camera (Olympus, Tokyo, Japan).
Histopathological evaluation
The sections were analyzed for hepatocyte degeneration, parenchymal necrosis, central vein congestion and thrombosis, bile duct proliferation and leukocyte infiltrations. At the end of the analyses, the findings were presented in a table in which the degree of degeneration was scored. Score levels of 0, +1, +2, +3 were equivalent to no, mild, moderate, and severe levels, respectively. The scores represented values obtained from tissue sections of six animals of each group, five fields/section.
Immunohistochemical examination
Using the streptavidine-biotin-peroxidase technique, the endogenous peroxidase activity was eliminated using 10% H2O2 for 15 min. Sections were then incubated for 1 h with the primary antibody against α-SMA (a mouse monoclonal antibody; Dako, Carpinteria, California, USA; dilution 1:50; cellular site was cytoplasmic) as a marker of activated HSCs. They were similarly incubated with the primary antibody against PCNA (a mouse monoclonal antibody; Dako, Carpinteria, California, USA; dilution 1:200; cellular site was nuclear) as a marker of hepatocyte regeneration. All sections were counter-stained with Mayer's hematoxylin. Negative control sections were prepared by omitting the primary antibody. Positive control standard laboratory slides were used for all stains to prove the success of the technique. All slides were examined under light microscopy at ×400 magnification and the presence of labelled cells was documented. Absence of staining was recognized as a negative result (−), while the presence of brown staining was recognized as positive result (+).
Morphometry
Ten nonoverlapping fields for each animal at a magnification of ×400 were selected indiscriminately and analyzed. The measurements were done with the use of Image-Pro Plus v6.0 (Media Cybernetics, Maryland, USA) and NIH ImageJ (v1.49) (http://rsb.info.nih.gov/ij/) associated with an Olympus BX53 microscope. The area percentage of α-SMA immunopositive cells and the optical density (OD) of PCNA immunostaining was evaluated. OD was estimated by the following formula:
OD = log (max intensity/mean intensity), where max intensity = 255 for 8-bit images.
Semithin sections
Liver samples of approximately 1 mm3 were obtained and immersed in 2.5% glutaraldehyde in 0.1 M phosphate buffer at 4°C for 3 h and postfixed in 1% osmium tetraoxide. After dehydration in ascending grades of ethanol, the tissues were embedded in Epon 812. Semithin sections were prepared from the blocks, stained using toluidine blue, and observed with the light microscope.
Statistical Analysis
The data analysis was carried out using the Statistical Package for Social Science (SPSS software version 20, Chicago, Illinois, USA). All numeric variables were expressed as mean ± standard deviation. Statistical comparisons were performed using the one-way analysis of variance (ANOVA) test, followed by post-hoc least significant difference multigroup comparison. Homogeneity of variance was assessed using the one-way ANOVA test and Levene's statistic test. For all tests, a probability (P < 0.05) was considered significant.
Results
Body Weights and Organ Weights
During the study, three cases of animal mortality in dox-treated group were recorded. No significant changes were observed between the study groups and the control in terms of food and water consumption or body weight gain/loss.
Biochemical Markers
This study showed deterioration of liver function in the dox group, which was represented by significant elevation of serum ALT, AST and total bilirubin in comparison to the control, CoQ10 and CS groups regarding ALT (P = 0.000, P = 0.0001, P = 0.001) while for AST and total bilirubin (P = 0.0001 for all groups). Treatment with CoQ10 and CS led to significant decrease in the serum levels of ALT, AST and total bilirubin in comparison to the dox group (P = 0.0001 for all groups). However, regarding total bilirubin levels still significantly higher in treated groups with CoQ10 and CS in comparison to the control group (P = 0.022; P = 0.03) respectively. Neither CoQ10 nor CS alone, without dox treatment, had any effect on these liver biomarkers compared to the control group [Table 1].
Table 1.
Effect of CoQ10 and CS on serum levels of liver and kidney biomarkers in doxorubicin induced toxicity in rats
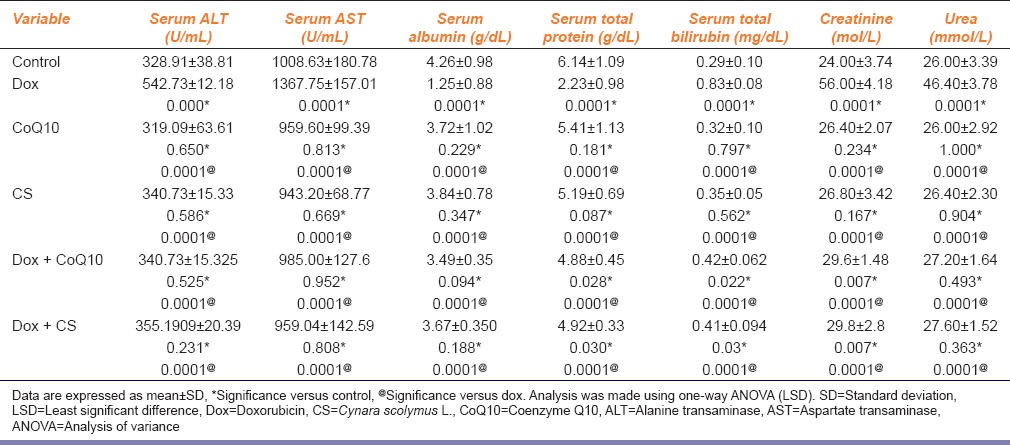
Moreover, dox group experienced significant decrease in serum albumin and total protein in comparison to the control, CoQ10 and CS groups (P = 0.0001 for all groups). Treatment with CoQ10 and CS led to significant increase of both serum albumin and total protein in the dox-CoQ10 and dox-CS groups, as compared to the dox group (P = 0.0001 for all groups). However, serum total protein levels still significantly higher in treated groups with CoQ10 and CS in comparison to the control group (P = 0.023; P = 0.03), respectively. Neither CoQ10 nor CS alone, without dox treatment, had any effect on these liver biomarkers compared to the control group [Table 1].
In addition, administration of dox resulted in the deterioration of kidney function, as shown by an increase in the serum levels of creatinine and urea in the dox group compared to the control, CoQ10 and CS groups (P = 0.0001 for all groups). Treatment with CoQ10 and CS led to a significant decrease in the serum levels of creatinine and urea in the treated groups compared to the dox group (P = 0.0001 for all groups). Serum creatinine in both treated groups, however, were significantly higher than the control groups (P = 0.007). Neither CoQ10 nor CS alone, without dox treatment, had any effect on these two markers of renal function compared to the control [Table 1].
As regard the oxidative biomarkers in liver, the results of this study showed that the administration of dox increased lipid peroxidation, as shown by a significant increase in the level of MDA in the dox group compared to the control, CoQ10 and CS groups (P = 0.0001 for all groups). Administration of CoQ10 and CS led to a significant decrease in the levels of MDA in the treated groups (P = 0.0001 for all groups) but still significantly higher when compared to the control groups (P = 0.041; P = 0.0001), respectively [Table 2].
Table 2.
Comparison of serum levels of oxidative stress markers in the liver of the study groups versus control and dox groups
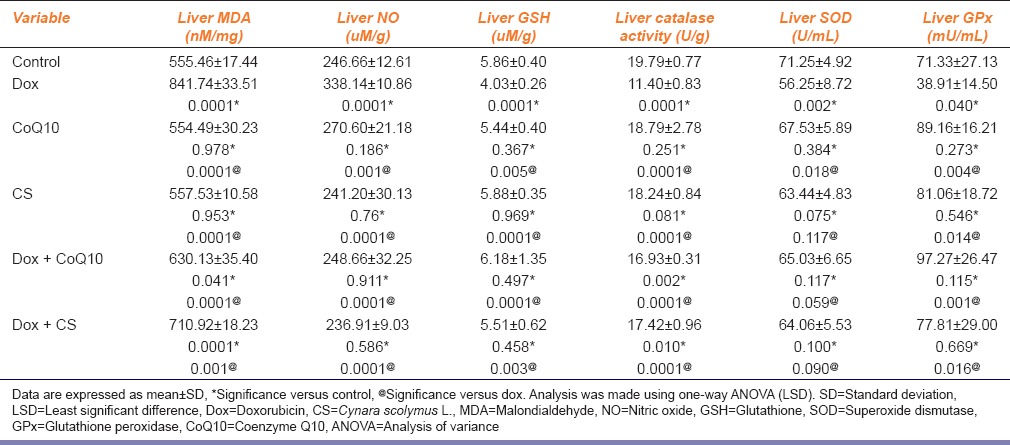
In addition, liver nitrate (NO) levels were increased significantly in dox group compared to the control, CoQ10 and CS groups (P = 0.0001, P = 0.001, P = 0.0001), respectively. Treatment with CoQ10 and CS led to a significant decrease in the levels of NO in the treated groups and their levels were higher than the control group (P = 0.0001 for all groups) [Table 2].
Moreover, administration of dox resulted in a significant decrease in the level of reduced liver GSH and a significant decrease in the activity of antioxidant enzymes in the liver, including catalase and GPx, as compared to the control, CoQ10 and CS groups [Table 2].
Treatment with CoQ10 and CS led to an elevation of GSH levels and an increase in the activity of antioxidant liver GPx to normal levels, for GSH (P = 0.0001, P = 0.003), and for GPx (P = 0. 0.001, P = 0.016), moreover treatment with CoQ10 and CS led to significant elevation liver catalase levels compared to dox group (P = 0.0001 for both groups); however, their levels were still significantly higher than the control group (P = 0.002, P = 0.01), while regarding SOD levels were increased in treated groups but not reaching significant levels (P = 0.059, P = 0.090) [Table 2].
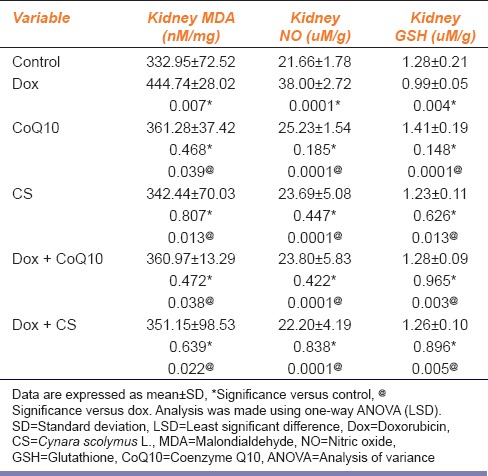
Regarding the results of the oxidative biomarkers in the kidney, the results of this study showed that the administration of dox resulted in a significant increase in the levels of both MDA and NO in the dox group compared to the control, CoQ10 and CS groups for MDA (P = 0.007, P = 0.039, P = 0.013), while for NO (P = 0.0001 for all groups), respectively. Administration of CoQ10 and CS led to a significant decrease in the levels of both MDA and NO in the treated groups compared to the dox group for MDA (P = 0.038, P = 0.022), while for NO (P = 0.0001 for all groups) [Table 3]. Moreover, administration of dox resulted in a significant decrease in the levels of GSH compared to the control, CoQ10 and CS groups (P = 0.004, P = 0.0001, P = 0.013). Treatment with CoQ10 and CS led to a significant increase in the levels of GSH in the treated groups compared to the dox group (P = 0.003, P = 0.005) [Table 3].
Table 3.
Comparison of oxidative stress markers in the kidney of the study groups versus control and dox groups
Histological Results of Hematoxylin and Eosin and Periodic Acid-Schiff Stained Sections
In all treated groups, an apparent parenchymatous degeneration and disseminated eosinophilic degeneration were noticed, especially in the middle and central zones of the hepatic lobules. Moreover, a noticeable vacuolar degeneration with ballooning and pyknotic nuclei were observed in the treated groups. Few necrosis were found in the examined groups and some inflammatory infiltrations in the middle and central zones were seen. The occurrence of hepatic changes was reduced among the dox-CoQ10 and dox-CS groups and clearly apparent in the group treated only with dox [Table 4].
Table 4.
Histopathological findings in dox-induced hepatotoxicity
Controls showed normal appearance with a strong PAS positivity corresponding to the accumulation of glycogen. Those exposed to CoQ10 and CS were similar to the control, while the dox-administered group showed reduced PAS positivity. Those treated with dox and CoQ10 exhibited improvement in the cellular damage, with visible PAS positivity. The group treated with dox and CS exhibited a decrease the cellular damage, with apparent PAS positivity [Table 4].
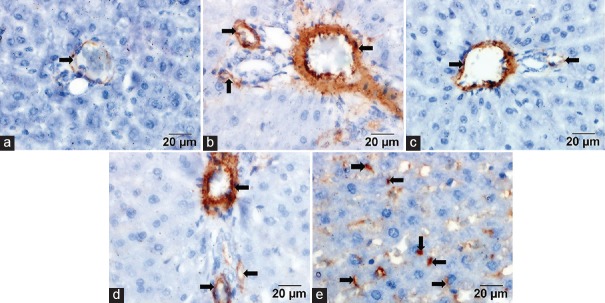
Immunoreaction to Alpha-smooth Muscle Actin
Controls showed faint α-SMA staining in the vascular media, especially in those of the portal area [Figure 1a]. The dox group showed positive α-SMA immunoreactivity in the portal vessels media, the periportal area and along the perisinusoidal spaces [Figure 1b and e]. Sections treated with dox and CoQ10 showed mild α-SMA immunoreactivity in the vascular media [Figure 1c]. Sections treated with dox and CS showed mild α-SMA immunoreactivity observed in the vascular media [Figure 1d].
Figure 1.
(a) Control group showed faint immunoexpression. (b) Doxorubicin group showed positive immunoexpression. (c) Doxorubicin and coenzyme Q10 showed mild expression. (d) Doxorubicin and Cynara scolymus showed mild expression. (e) Doxorubicin group showed positive immunoreactivity of spindle-shaped stromal cells (arrows) (alpha-smooth muscle actin, scale bar = 20 μm)
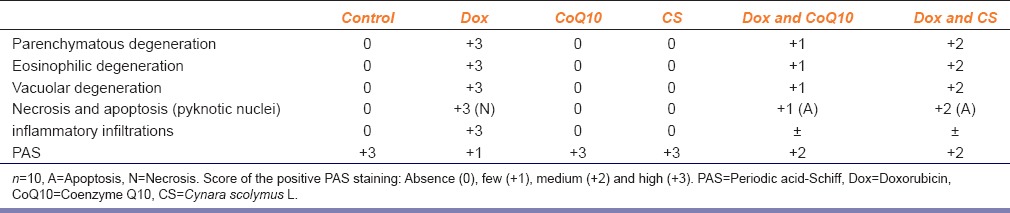
Immunoreaction to Proliferating Cell Nuclear Antigen
Controls showed moderate immunoreactivity in some hepatocyte nuclei [Figure 2a]. The dox group showed weak immunoreactivity in the hepatocyte nuclei [Figure 2b]. Sections treated with dox and CoQ10 showed intense immunoreactivity in most of the hepatocyte nuclei [Figure 2c]. Sections treated with dox and CS showed intense immunoreactivity in most of the hepatocyte nuclei [Figure 2d].
Figure 2.
(a) Control group showed moderate immunoreactivity (arrows). (b) Doxorubicin group showed minimal or no immunoreactivity in the nuclei (arrows). (c) Doxorubicin and coenzyme Q10 groups showed intense expression (arrows). (d) Doxorubicin and Cynara scolymus groups showed intense expression (proliferating cell nuclear antigen, scale bar = 20 μm)
Morphometric Results
The proportion of α-SMA-positive area in cells was significantly higher in the groups treated with dox and either CoQ10 or CS as compared to the dox group (P = 0.001, P = 0.001) [Table 5]. Groups treated with dox and either CoQ10 or CS were significantly improved OD of PCNA expression as compared to the dox group (P = 0.001, P = 0.001) [Table 5].
Table 5.
Area percentage of α-SMA-positive cells (means±SD) and PCNA OD
Toluidine Blue Stained Sections
Control group showed normal architecture [Figure 3a]. The dox group showed intensive vacuolar degeneration and necrosis, and hepatocytes with abnormal metaphase chromatin and dilated sinusoids [Figure 3b]. The dox-CoQ10 groups showed microvacuoles [Figure 3c], while the dox-CS groups showed variable-sized vacuoles [Figure 3d].
Figure 3.
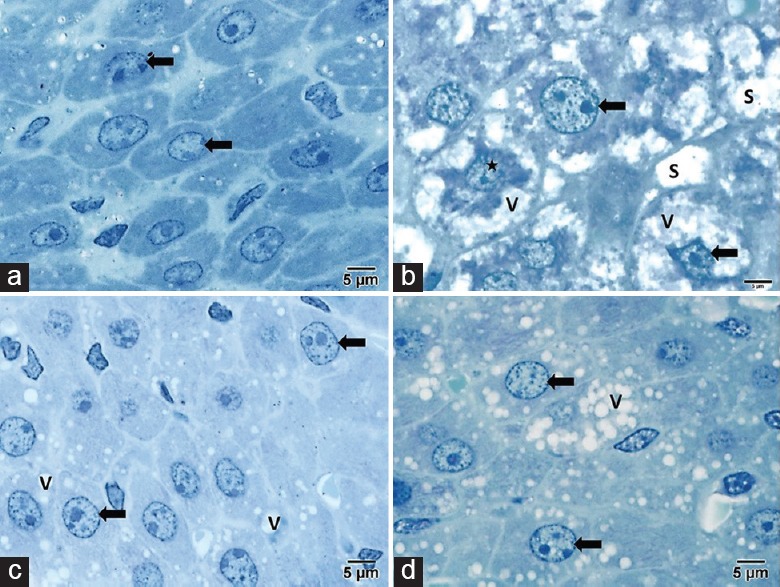
(a) Control group showed normal architecture. (b) The doxorubicin group showed hepatocytes (arrow) with markedly foamy vacuolated cytoplasm (V), abnormal metaphase chromatin (star) and dilated sinusoids (S). (c) The doxorubicin and coenzyme Q10 groups showed intense microvacuoles (V). (d) The doxorubicin and Cynara scolymus L. groups showed variable-sized vacuoles (toluidine blue, scale bar = 5 μm)
Discussion
Endogenous CoQ10 is increased following dox treatment, might be a cellular defense related to CoQ10 gene expression.[16] In this study, dox group showed a deterioration in liver parameters. These results agreed with other study.[17] Moreover, dox elevates ALT because of lipid peroxidation and activation of phospholipases.[16] Dox decreased serum albumin and total protein due to deterioration of liver synthetic functions resulting from hepatocytes necrosis. Furthermore, total bilirubin rise is due to partial bile duct obstruction because of hepatocytes inflammation and fibrosis.[17]
In the present study, dox resulted in deterioration of renal functions. This is explained by increased capillary permeability and glomerular atrophy that resulted in albumin loss.[18]
Dox elevated oxidative stress markers that agreed with others.[19] Nitrosative stress produced by dox was consistent with the finding that NO generation as inducible NOS form was related to dox cytotoxicity.[18] Dox administration decreased GSH level. These results might lead to peroxidative injury and destruction of cellular defense toward ROS.[20] Dox administration decreased liver antioxidant enzymes. That is attributed to free-radical formation and oxidative stress.[21] Dox produces free radicals through transformation into semiquinone, which reacts with molecular oxygen to form superoxide radicals. And through an iron-dox complex that is effective in reducing oxygen to H2O2.[20]
In this study, co-administration of CoQ10 and CS with dox led to a decrease in oxidative stress. These results agreed with other study by Heidarian and Rafieian-Kopaei.[22] Further, the decrease in MDA was associated with increased concentrations of reduced GSH, catalase activity and caspase 3 expression.[23]
The mechanisms whereby CoQ10 acts as an antioxidant include removal of free radicals, such as lipid peroxyl and alkoxyl radicals.[24] In addition, NO bioactivity may be improved by decreasing superoxide generation.[25] It may be improved by increasing the expression of mitochondrial uncoupling proteins; this is an anti-apoptotic effect that leads to a reduction in free-radical generation.[24] CS has a high percentage of minerals and polyphenolic compounds and inulin.[9] The antioxidant effect of CS is attributed to interference with the gene expression of inflammatory pathways and the induction of antioxidant enzyme synthesis, which results in oxidative stress reduction.[25]
In current work, dox histological changes are explained by fatty infiltration[20] and oxidative damage that leads to mitochondrial DNA damage (mtDNA).[23] Those changes might attribute to DNA fragmentation and apoptosis initiation.[25] Sinusoidal congestion might explain tissue damage and blood coagulation.[24] Disruption of sinusoidal wall integrity was indicated by extravasation of erythrocytes in space of Disse.[18] Hepatic fibrogenesisis initiated by hepatocytes injury, which leads to inflammatory cells recruitment, activation of von Kupffer cells and cytokines release.[17]
CoQ10 and CS protect against dox toxicity through upregulating anti-reactive oxygen species, prevention of mtDNA injury, promotion of replication, suppression of membrane-active lipases, and defend the electron transport chain.[12]
In the current study, increased PCNA expression by administration of CoQ10 and CS with dox that is a consequence to the ability of HSCs to engraft damaged cells.[15] α-SMA expression observed in periportal tract refer to myofibroblasts. The portal fibroblasts might be the source of myofibroblasts and activated HSC.[14] Apoptosis following the injury initiates HSCs through a process mediated by Fas death receptor.[15] Activated HSCs proliferate and produce collagen through free-radical construction and activation of mitogen-activated protein kinase.[14]
The limitations of the study are the number of animals that might be larger and the fixed dose of the dox that might be variant to facilitate the statistical analysis and to broaden the feedback. Furthermore, different doses of CoQ10 and CS are advised. Quantitative measures as stereology and immune Biomarkers of liver cells are recommended to support the hypothesis of the study.
Conclusion
The present findings demonstrate that the renal toxicity and hepatotoxicity induced by dox may be related to imbalance of the oxidative stress. Moreover, pretreatment with CoQ10 and CS effectively improve the toxic effects of dox in kidney and liver, so that it was associated with up-regulation of favorable protective enzymes and down-regulation of oxidative stress. Overall, the study suggests that administration of CoQ10 and CS limit renal and hepatotoxicity of dox.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Wang B, Ma Y, Kong X, Ding X, Gu H, Chu T, et al. NAD(+) administration decreases doxorubicin-induced liver damage of mice by enhancing antioxidation capacity and decreasing DNA damage. Chem Biol Interact. 2014;212:65–71. doi: 10.1016/j.cbi.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Ganash MA, Mujallid MI, Al-Robai AA, Bazzaz AA. Cytoprotectivity of the natural honey against the toxic effects of Doxorubicin in mice. Adv Biosci Biotechnol. 2014;05:252–60. [Google Scholar]
- 3.Swarnakar NK, Thanki K, Jain S. Enhanced antitumor efficacy and counterfeited cardiotoxicity of combinatorial oral therapy using Doxorubicin- and Coenzyme Q10-liquid crystalline nanoparticles in comparison with intravenous Adriamycin. Nanomedicine. 2014;10:1231–41. doi: 10.1016/j.nano.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 4.El-Moselhy MA, El-Sheikh AA. Protective mechanisms of atorvastatin against doxorubicin-induced hepato-renal toxicity. Biomed Pharmacother. 2014;68:101–10. doi: 10.1016/j.biopha.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Machado C, Mendonça LM, Venancio VP, Bianchi ML, Antunes LM. Coenzyme Q10 protects Pc12 cells from cisplatin-induced DNA damage and neurotoxicity. Neurotoxicology. 2013;36:10–6. doi: 10.1016/j.neuro.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez-Santos MA, Juárez-Rojop IE, Tovilla-Zárate CA, Espinosa-García MT, Juárez-Oropeza MA, Ramón-Frías T, et al. Coenzyme Q10 supplementation improves metabolic parameters, liver function and mitochondrial respiration in rats with high doses of atorvastatin and a cholesterol-rich diet. Lipids Health Dis. 2014;13:22. doi: 10.1186/1476-511X-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohet FM, Neyrinck AM, Pachikian BD, de Backer FC, Bindels LB, Niklowitz P, et al. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol. 2009;78:1391–400. doi: 10.1016/j.bcp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Salama AA, El-Baz FK. Antioxidant and antiproliferativeeffects on human liver HePG2Epithelial cells from artichoke (Cynara scolymus L.) By-products. J Nat Sci Res. 2013;3:17–24. [Google Scholar]
- 9.Magielse J, Verlaet A, Breynaert A, Keenoy BM, Apers S, Pieters L, et al. Investigation of thein vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol Nutr Food Res. 2014;58:211–5. doi: 10.1002/mnfr.201300282. [DOI] [PubMed] [Google Scholar]
- 10.Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann Bot. 2011;107:1127–40. doi: 10.1093/aob/mcq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalopoulos GK. Liver regeneration after partial hepatectomy: Critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellerbrand C. Hepatic stellate cells – The pericytes in the liver. Pflugers Arch. 2013;465:775–8. doi: 10.1007/s00424-012-1209-5. [DOI] [PubMed] [Google Scholar]
- 13.Carpino G, Morini S, Ginanni Corradini S, Franchitto A, Merli M, Siciliano M, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37:349–56. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Chen R, Song Z, Ye S, Sun R, Xue Q, et al. Gene expression profiles during activation of cultured rat hepatic stellate cells by tumoral hepatocytes and fetal bovine serum. J Cancer Res Clin Oncol. 2010;136:309–21. doi: 10.1007/s00432-009-0666-5. [DOI] [PubMed] [Google Scholar]
- 15.El-Sheikh AA, Morsy MA, Mahmoud MM, Rifaai RA, Abdelrahman AM. Effect of coenzyme-q10 on Doxorubicin-induced nephrotoxicity in rats. Adv Pharmacol Sci 2012. 2012 doi: 10.1155/2012/981461. 981461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soliman HA, Ahmed RR, Ali AT. Assessment of the chemo-preventive effects of various plant constituents against doxorubicin-induced toxicity in rats. J Am Sci. 2014;10:153–64. [Google Scholar]
- 17.Rashid S, Ali N, Nafees S, Ahmad ST, Arjumand W, Hasan SK, et al. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol Mech Methods. 2013;23:337–45. doi: 10.3109/15376516.2012.759306. [DOI] [PubMed] [Google Scholar]
- 18.Koçkar MC, Naziroglu M, Celik O, Tola HT, Bayram D, Koyu A. N-acetylcysteine modulates doxorubicin-induced oxidative stress and antioxidant Vitamin concentrations in liver of rats. Cell Biochem Funct. 2010;28:673–7. doi: 10.1002/cbf.1707. [DOI] [PubMed] [Google Scholar]
- 19.Abd El-Aziz TA, Mohamed RH, Pasha HF, Abdel-Aziz HR. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med. 2012;12:233–40. doi: 10.1007/s10238-011-0165-2. [DOI] [PubMed] [Google Scholar]
- 20.Ali ZY. Neurotoxic effect of lambda-cyhalothrin, a synthetic pyrethroid pesticide: Involvement of oxidative stress and protective role of antioxidant mixture. N Y Sci J. 2012;9:93–103. [Google Scholar]
- 21.Tawfik MK. Combination of coenzyme Q10 with methotrexate suppresses Freund's complete adjuvant-induced synovial inflammation with reduced hepatotoxicity in rats: Effect on oxidative stress and inflammation. Int Immunopharmacol. 2015;24:80–7. doi: 10.1016/j.intimp.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Heidarian E, Rafieian-Kopaei M. Protective effect of artichoke (Cynara scolymus) leaf extract against lead toxicity in rat. Pharm Biol. 2013;51:1104–9. doi: 10.3109/13880209.2013.777931. [DOI] [PubMed] [Google Scholar]
- 23.Berthiaume JM, Wallace KB. Persistent alterations to the gene expression profile of the heart subsequent to chronic Doxorubicin treatment. Cardiovasc Toxicol. 2007;7:178–91. doi: 10.1007/s12012-007-0026-0. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed MA. The protective effect of ginger (Zingiber officinale) against adriamycin – Induced hepatotoxicity in rats: Histological study. Life Sci J. 2013;10:1412–22. [Google Scholar]
- 25.Sugimoto R, Enjoji M, Kohjima M, Tsuruta S, Fukushima M, Iwao M, et al. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver Int. 2005;25:1018–26. doi: 10.1111/j.1478-3231.2005.01130.x. [DOI] [PubMed] [Google Scholar]