Abstract
Terbinafine is an allylamine antifungal agent which is widely used for the treatment of fungal infections. Cutaneous side effects have been reported in 2% of the patients on terbinafine therapy with many morphological patterns. We report a case of terbinafine induced pityriasis rosea, a very rare side effect of terbinafine. This report emphasizes the importance of counseling the patient to report immediately in the event of a cutaneous eruption.
KEY WORDS: Adverse drug reaction, drug rash, pityriasis rosea, terbinafine
Introduction
Terbinafine is an allylamine, a lipophilic compound, used for the treatment of onychomycosis and other fungal infections. Adverse effects are reported in 10% and cutaneous reactions in 2% of patients on terbinafine therapy.[1] These include pruritus, rash, urticaria, erythema multiforme, cutaneous lupus, and psoriasis. Terbinafine induced pityriasis rosea-like eruption is not common.
Case Report
A 25-year-old male with mycologically confirmed fingernail onychomycosis, who was prescribed oral terbinafine 250 mg daily for 18 days, presented with an itchy rash on the body for 4 days. The rash started on the chest and then progressed to involve the rest of the body within 4 days [Figure 1]. There was no history of fever or preceding upper respiratory illness. He was not on any other medication simultaneously and never had a similar rash in the past. There was no history of previous drug allergy. He had taken terbinafine for 1 month for the same indication, which he discontinued 2 weeks before restarting the present course of this antifungal. There was no family history of similar illness.
Figure 1.
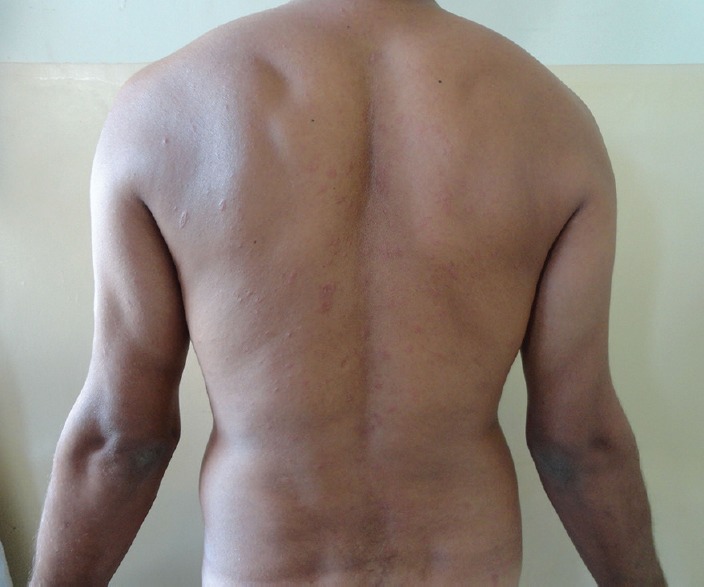
Erythematous plaques with elevated borders and collarettes of fine scaling on the back. Most of the lesions have their long axis parallel to the lines of cleavage giving a “Christmas tree” distribution
General physical examination was noncontributory. Cutaneous examination revealed scattered erythematous papules and plaques on the trunk, upper limbs, and gluteal region. Few plaques were noted to have peripheral collarettes of scaling. Erythematous tumid plaques were noted on the cheeks. There was a single erythematous papule on the penile shaft. Scalp and oral mucosa were normal. Few fingernails were dystrophic.
He was clinically diagnosed to have terbinafine induced pityriasis rosea-like eruption and admitted in the dermatology ward. Investigations revealed a total leukocyte count of 5.2 × 109/L with a differential of 67% neutrophils, 30% lymphocytes, and 2% eosinophils. Liver and renal function tests were within normal limits. The Venereal disease research laboratory (VDRL) test was carried out to rule out secondary syphilis, which was negative. However, a skin biopsy was not performed.
The offending drug, terbinafine, was withdrawn. The patient was given supportive management in the form of antihistamines and topical steroids. The rash began to resolve quickly, and the patient was discharged from the hospital after 4 days.
The common cutaneous adverse effects associated with terbinafine therapy are urticaria, rash, and pruritus, usually occurring within a month of therapy. The other less common adverse effects are erythema multiforme, toxic epidermal necrolysis, Stevens–Johnson syndrome, erythema annulare-like eruption, fixed drug eruption, alopecia areata, flare up of dermatitis,[1] induction and exacerbation of psoriasis,[2,3] and subacute cutaneous lupus-like eruption.[4] Pityriasis rosea is a rare adverse effect of terbinafine therapy.[5]
Pityriasis rosea is an inflammatory skin disease characterized by erythematous papulosquamous eruptions localized mainly to the trunk. Viral agents, especially human herpes virus 6 and 7, autoimmunity, drugs such as captopril, nonsteroidal anti-inflammatory drugs, barbiturates, metronidazole, isotretinoin, griseofulvin, terbinafine, and psychogenic factors have been proposed as possible etiological factors.[6] The initial lesion is larger than subsequent lesions and is called the herald patch. This herald patch is usually absent in drug induced pityriasis rosea; in our patient too, this feature was conspicuously absent. Itching was a prominent feature in our patient which is consistent with a drug reaction. Gupta et al.[5] have reported pityriasis rosea as an itchy rash beginning on the trunk after 4 weeks of regular treatment with terbinafine. The patient developed lesions after 18 days of regular treatment; however, he had been previously sensitized during the prior treatment with the drug.
Secondary syphilis can a have similar cutaneous presentation but is usually asymptomatic. The patient denied a history of unprotected sexual intercourse and VDRL was negative. Pityriasiform variant of seborrhoeic dermatitis was ruled out because other seborrhoeic sites were spared, and there were no previous episodes of such eruptions. The other supportive evidence in favor of a drug-induced reaction was the improvement noted in the lesions on stopping the offending drug. Pityriasis rosea typically occurs in the spring season with a history of preceding upper respiratory infection. The patient presented in winter without any prodrome. The Naranjo causality assessment score for this case was 6, thereby suggesting probable causal association of the adverse drug reaction with terbinafine.
It is recommended that all patients who receive terbinafine therapy should be counseled about the possible adverse effects and instructed to discontinue the drug and report to the physician if a cutaneous eruption develops.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Amichai B, Grunwald MH. Adverse drug reactions of the new oral antifungal agents – Terbinafine, fluconazole, and itraconazole. Int J Dermatol. 1998;37:410–5. doi: 10.1046/j.1365-4362.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Sibbald RG, Knowles SR, Lynde CW, Shear NH. Terbinafine therapy may be associated with the development of psoriasis de novo or its exacerbation: Four case reports and a review of drug-induced psoriasis. J Am Acad Dermatol. 1997;36(5 Pt 2):858–62. doi: 10.1016/s0190-9622(97)70041-0. [DOI] [PubMed] [Google Scholar]
- 3.Verros CD, Rallis E. The role of terbinafine in induction and/or exacerbation of psoriasis. Int J Dermatol. 2013;52:1155–6. doi: 10.1111/j.1365-4632.2011.05117.x. [DOI] [PubMed] [Google Scholar]
- 4.Hill VA, Chow J, Cowley N, Marsden RA. Subacute lupus erythematosus-like eruption due to terbinafine: Report of three cases. Br J Dermatol. 2003;148:1056. doi: 10.1046/j.1365-2133.2003.05235.x. [DOI] [PubMed] [Google Scholar]
- 5.Gupta AK, Lynde CW, Lauzon GJ, Mehlmauer MA, Braddock SW, Miller CA, et al. Cutaneous adverse effects associated with terbinafine therapy: 10 case reports and a review of the literature. Br J Dermatol. 1998;138:529–32. doi: 10.1046/j.1365-2133.1998.02140.x. [DOI] [PubMed] [Google Scholar]
- 6.González LM, Allen R, Janniger CK, Schwartz RA. Pityriasis rosea: An important papulosquamous disorder. Int J Dermatol. 2005;44:757–64. doi: 10.1111/j.1365-4632.2005.02635.x. [DOI] [PubMed] [Google Scholar]


