Abstract
Acute generalized exanthematous pustulosis (AGEP) is a severe cutaneous adverse reaction, mostly induced by drugs. Hydroxychloroquine have been rarely reported in literature as a causative drug of this reaction. We report a case of AGEP induced by hydroxychloroquine with systemic involvement and confirmed by positive patch testing.
KEY WORDS: Hydroxychloroquine, patch test, pustulosis
Introduction
Hydroxychloroquine is used as an antimalarial drug. Immunosuppressive and anti-inflammatory properties have led to its use in the treatment of rheumatic and dermatologic diseases. Antimalarial drugs are well known to cause adverse reactions, cutaneous, and ocular side effects being the most frequently described problems. The most common cutaneous adverse reaction is skin pigmentation. Acute generalized exanthematous pustulosis (AGEP) is a severe cutaneous adverse reaction, mostly induced by drugs.[1] Hydroxychloroquine has been rarely reported in literature as the cause of this reaction.[2] We report a case of AGEP-induced by hydroxychloroquine with systemic involvement. This case was notified to the Tunisian Pharmacovigilance Center and was analyzed according to Naranjo Adverse Drug Reaction Probability scale and European group RegiSCAR in the diagnosis of AGEP.
Case Report
A 33-year-old woman was diagnosed as a case of systemic lupus erythematous in July 2013 and was prescribed chloroquine 200 mg/day. Due to persistent arthralgia, the treatment was changed to hydroxychloroquine 200 mg/day and prednisolone 40 mg/day.
Seventeen days after starting treatment, the patient developed generalized erythema and a pruritic pustular eruption. The lesions initially appeared on the face and then spread to the rest of the body, there was no fever [Figure 1].
Figure 1.
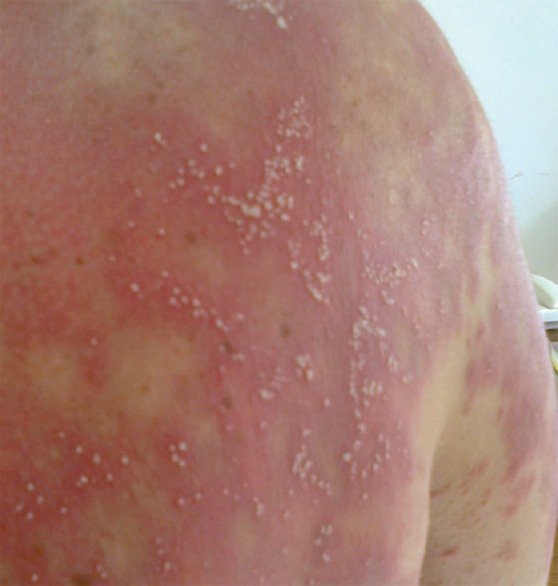
Pustules over the upper back
Five days later, she presented with fever. Hydroxychloroquine and prednisolone were stopped. The skin examination revealed a generalized erythema with a few 1–2 mm pustules over the legs and a diffuse superficial desquamation. The patient's temperature ranged from 38.5°C to 40°C. Laboratory parameters showed leukocytosis 18.370/mm3, eosinophil count 770/mm3, neutrophil count 15.750/mm3 (equivalent to 85.8%), C-reactive protein 157.7 mg/l (reference range <5), and liver enzymes: Alanine aminotransferase 78 U/l aspartate aminotransferase 74 U/l. All the data fulfilled the criteria for AGEP with a RegiScar score of 7 (probable case). A skin biopsy showed spongiform intraepidermal pustules, edema of the papillary dermis, a perivascular infiltrate with neutrophils, and some eosinophils. This was consistent with AGEP. The skin lesions cured 1 week after the drug was stopped. Laboratory parameters returned to normal level in 10 days. Patch tests were performed 4 weeks after the symptoms disappeared. They were realized with 10% hydroxychloroquine, 10% chloroquine, and 10% prednisolone in dimethyl sulfoxide (DMSO). Patch test for hydroxychloroquine was positive at 48 and 72 h, doubtful for prednisolone and negative for chloroquine. Oral provocation test with hydroxychloroquine was recommended to confirm, but the patient refused.
Discussion
AGEP is characterized by acute, extensive formation of nonfollicular sterile pustules on an erythematous and edematous substrate. It's most commonly caused by the use of drugs such as antimicrobial agents. Hydroxychloroquine has been described as a rare cause of AGEP. In fact, only 26 cases of hydroxychloroquine induced AGEP were documented following a Medline search using the keywords: Hydroxychloroquine, pustulosis.[3,4,5]
Our patient presented a systemic involvement: Elevated eosinophilia and liver disturbance. A study showed that 30% af AGEP patients had eoninophilia. In a study of 58 cases of AGEP, systemic involvements including liver, kidney, bone-marrow, and lung injury were observed in 10 cases.[6] In 2014, a study showed that among patients with AGEP, 21.6% presented with eosinophilia and 23.5% with systemic involvement, including the liver and kidney. All patients with systemic involvement showed complete recovery in renal and liver function.
Patch testing realized with 10% hydroxychloroquine in DMSO was positive in our patient. In case of severe reactions, such as AGEP, patch tests are used as the first line of investigation. Barbaud et al., in a multicenter study to determine the value and safety of drug patch tests, have reported positive results in 58% of AGEP cases.[7]
Patch tests with hydroxychloroquine in other cutaneous adverse drug reactions have been positive in a few cases. Meier et al. described a case of contact dermatitis and bronchial asthma due to hydroxychloroquine with a positive patch test at concentrations of 0.1%, 0.5%, 1%, and 2% (vehicle is not mentioned). Lisi et al. reported a positive photopatch test with 5% hydroxychloroquine sulfate in a water solution in case of photoallergic dermatitis. In a case reported in 2006 of delayed hypersensitivity to hydroxychloroquine, patch testing with 10% hydroxychloroquine in DMSO proved positive at 48 and 96 h and negative in 5 control subjects.
The responsibility of hydroxychloroquine induced AGEP was retained, according to Naranjo Adverse Drug Reaction Probability scale with a score of 5 (probable) in front of: Previous similar report, the evocative delay of outcome (17th day of treatment), the recovery within 7 days following treatment withdrawal, and the positive patch test to hydroxychloroquine.
Naranjo scale score for prednisolone was 3 (possible).
Conclusion
Hydroxychloroquine-induced AGEP is a rare disease that can be associated with systemic involvement. Immediate cessation of this drug is necessary. In case of drug association, patch testing can help identify AGEP.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP) – A clinical reaction pattern. J Cutan Pathol. 2001;28:113–9. doi: 10.1034/j.1600-0560.2001.028003113.x. [DOI] [PubMed] [Google Scholar]
- 2.Paradisi A, Bugatti L, Sisto T, Filosa G, Amerio PL, Capizzi R. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: Three cases and a review of the literature. Clin Ther. 2008;30:930–40. doi: 10.1016/j.clinthera.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Park JJ, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. A case of hydroxychloroquine induced acute generalized exanthematous pustulosis confirmed by accidental oral provocation. Ann Dermatol. 2010;22:102–5. doi: 10.5021/ad.2010.22.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey K, McKee D, Wismer J, Shear N. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: First case report in Canada and review of the literature. J Cutan Med Surg. 2013;17:414–8. doi: 10.2310/7750.2013.12105. [DOI] [PubMed] [Google Scholar]
- 5.Choi MJ, Kim HS, Park HJ, Park CJ, Lee JD, Lee JY, etal Clinicopathologic manifestations of 36 Korean patients with acute generalized exanthematous pustulosis: A case series and review of the literature. Ann Dermatol. 2010;22:163–9. doi: 10.5021/ad.2010.22.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotz C, Valeyrie-Allanore L, Haddad C, Bouvresse S, Ortonne N, Duong TA, et al. Systemic involvement of acute generalized exanthematous pustulosis: A retrospective study on 58 patients. Br J Dermatol. 2013;169:1223–32. doi: 10.1111/bjd.12502. [DOI] [PubMed] [Google Scholar]
- 7.Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, etal A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–62. doi: 10.1111/bjd.12125. [DOI] [PubMed] [Google Scholar]


