Abstract
Histological subtypes of basal cell carcinoma have biological, evolutionary and distinct prognostic behavior. The analysis of characteristics of the nucleus can provide data on their cellular physiology and behavior. The authors of this study evaluated nuclear morphological parameters and textural patterns of chromatin from different subtypes of basal cell carcinoma: nodular (n=37), superficial (n=28) and sclerodermiform (n=28). The parameters were compared between neoplasms' subtypes and with unaffected adjacent basal epithelium. Nuclear area and diameter of sclerodermiform neoplasms were superior to the other subtypes. Chromatin's color intensity and fractal dimension were less intense in superficial subtypes. Nuclear roundness and chromatin's entropy presented lower values in tumors than in normal epithelium. There was significant correlation between morphological and textural variables of normal skin and tumors. Morphometric elements and textural chromatin's homogeneity of basal cell carcinomas may be related to evolutionary, biological and behavior particularities related to each histotype.
Keywords: Carcinoma, basal cell; Chromatin; Karyometry; Image processing, computer-assisted; Prognosis
INTRODUCTION
Basal cell carcinoma (BCC) is the most common malignancy among men, and its incidence is on the rise in several countries.1-3 At Faculdade de Medicina de Botucatu-SP (Brazil) more than 900 patients are operated a year.
There are few reported cases of BCCs metastasis, which leads to a survival rate close to 100%, as long as it is adequately treated. However, it has high local malignancy, growing slowly and progressively, and may expand into surface and/or invade underlying tissues such as muscle, cartilage and bones, causing destruction and deformation of the area, implying in high morbidity.1
Its histogenesis is not completely unveiled. It consists of cells similar to basaloid cells of the epidermis, however, there are elements indicating that it originates from immature pluripotent epithelial cells, unable to differentiate them and to keratinize normally.1,4
BCCs have different clinical and histopathological subtypes that present distinct evolutionary biological behavior and prognosis. The main histological subtypes are nodular (which can also present with cystic and pigmented component), sclerodermiform, superficial, infiltrative and rare variants: micronodular and fibroepithelioma of Pinkus.5,6
Evolutive differences of the various types of BCC are not well defined. The 3 subtypes that best represent the tumor are nodular, sclerodermiform and superficial. Despite more than 30% of lesions present mixed components, different kinetic characteristics, invasiveness and relapses are observed, which refer to subtypes, suggesting different biological behavior.
Sclerodermiform, infiltrative and micronodular forms are considered of infiltrative growth, with more aggressive clinical behavior and increased risk of relapse. Superficial, pigmented, and cystic forms are considered of expansive growth, with milder behavior. Superficial BCCs with multifocal characteristics tend to recur if the margin of excision is meager, and if there are neoplastic nests interspersed with health epithelium areas.1,7
Morphological analysis of cell nuclei by histology can provide data on the physiology of the cell and contribute to the study of the diagnosis and prognosis of neoplastic lesions. Similarly, changes in the cell cycle or metabolism due to pharmacological, physiological or epigenetic action are accompanied by alterations in the architecture of the nuclear chromatin. Thus, nuclear morphology and texture characteristics have been studied as prognostic factors in many neoplasms.8-11 However, to date there is no research on nuclear morphometry and chromatin heterogeneity of different subtypes of BCCs.
In this study, the authors aimed to evaluate nuclear morphometric characteristics (area, perimeter, circularity and larger diameter), intensity and heterogeneity of chromatin texture (fractal dimension, image entropy and texture-Ra estimator) between different histological subtypes of BCC, and between cancer and basal keratinocytes from normal adjacent epithelium. Also, they explored the correlation between the morphometric variables and chromatin texture.
METHODS
Cross-sectional study involving 120 BCCs selected from registered patients in the pathology department of Faculdade de Medicina da Unesp de Botucatu, from 2010 to 2012, based on histopathology reports. Initially, we had 40 nodular BCC, 40 surface BCC and 40 sclerodermiform BCC. Among them, we chose only the ones with most characteristic morphology of each subtype and better image quality, restricting the study to 37, 28 and 28 tumors in each group (N = 93). The Ethics Committee of the institution approved the study.
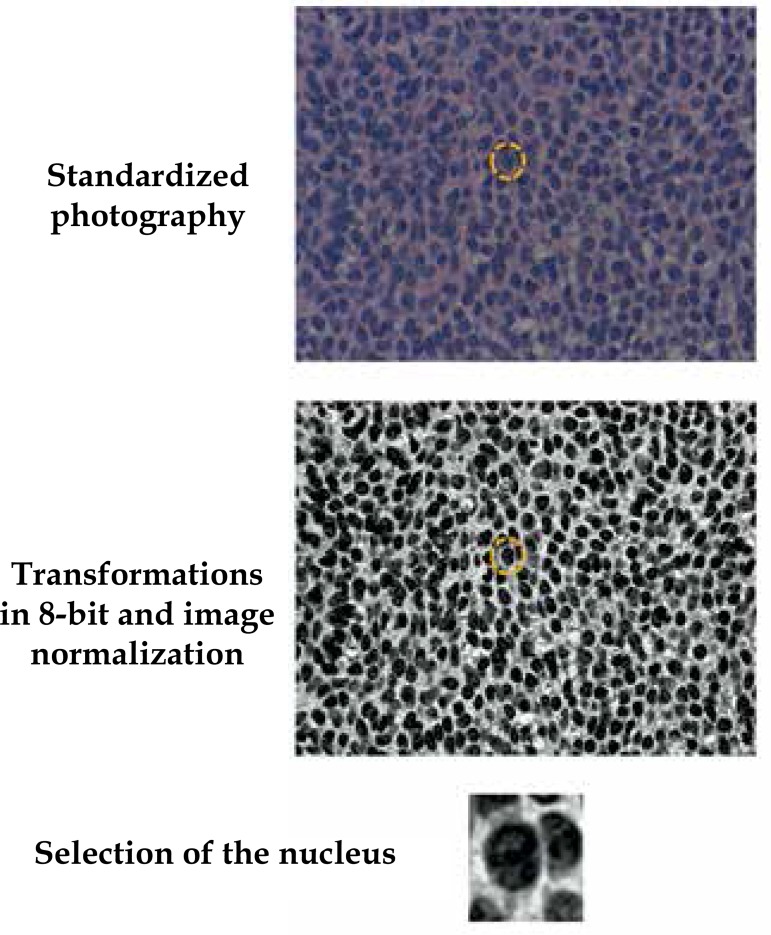
Slides were photographed in order to record 30 well-individualized nuclei of each BCC and 30 nuclei in the basal layer of the normal adjacent epithelium. After that, these nuclei were cut and the resulting images were transformed to 8-bit (256 grayscale), standardized and subjected to analysis of entropy, fractal dimension (cube box-count technique) and Ra (Figure 1).12 Morphological aspects of the nucleus, such as circularity, larger diameter, perimeter and area were also assessed. Analyses were performed by ImageJ 1.46 software and its specific plugins for each index.13
Figure 1.
Representation of the acquisition and pre-processing of nuclei
Variables were tested for normality by Shapiro-Wilk test. Variables related to tumors groups were represented by mean and standard deviations or medians and quartiles, if indicated, and compared using generalized linear mixed-effects model.14 Correlations between variables were estimated by Spearman coefficient if the distributions were not parametric.14
Data were tabulated in MSExcel and analyzed by SPSS 20.0 software.15 A two-tailed p value <0.01 was considered significant.
RESULTS
We evaluated 5580 nuclei from 93 tumors and their adjacent epithelium. The main variables evaluated are arranged in table 1.
Table 1.
Main variables evaluated in the study (mean and standard deviation) according to the cancer subtype and adjacent epithelium
| Nodular (N=37) | Sclerodermiform (N=28) | Superficial (N=28) | Epithelium (N=93) | |
|---|---|---|---|---|
| Area* | 65.4 (23.1) | 73.9 (29.6) | 66.6 (21.8) | 64.6 (17.6) |
| Perimeter** | 32.9 (7.7) | 34.3 (7.9) | 32.7 (6.1) | 33.6 (5.9) |
| Circularity | 0.77 (0.12) | 0.78 (0.12) | 0.78 (0.11) | 0.73 (0.11) |
| Larger diameter** | 11.7 (2.7) | 12.5 (2.9) | 11.9 (2.5) | 12.5 (2.2) |
| Intensity | 62.7 (24.4) | 61.5 (25.9) | 54.8 (20.9) | 43.5 (16.1) |
| Ra | 236034.4 (7663.2) | 233579.6 (12220.8) | 234991.8 (8207.5) | 234794.4 (5990.7) |
| Entropy | 5.6 (0.51) | 5.7 (0.58) | 5.6 (0.55) | 5.3 (0.53) |
| Fractal dimension | 2.48 (0.05) | 2.50 (0.05) | 2.45 (0.05) | 2.45 (0.05) |
micra2;
micra
Correlations between the evaluated nuclear variables within the epithelium and within tumors are displayed in tables 2 and 3. We highlight the significant correlation between kariometric and textural rates both in neoplastic epithelium and in adjacent epidermis.
Table 2.
Correlation (Spearman's rho) between the nuclear variables evaluated in normal epithelium
| Perimeter | Circularity | Larger diameter | Intensity | Ra | Entropy | Fractal | |
|---|---|---|---|---|---|---|---|
| Area | 0.87* | 0.12 | 0.76* | 0.43* | 0.90* | 0.23 | 0.70* |
| Perimeter | - | 0.55* | 0.90* | 0.47* | 0.73* | 0.03 | 0.03 |
| Circularity | - | - | 0.56* | 0.24 | 0.02 | 0.045* | 0.16 |
| Larger diameter | - | - | - | 0.39 | 0.65* | 0.12 | 0.47* |
| Intensity | - | - | - | - | 0.22 | 0.23 | 0.60* |
| Ra | - | - | - | - | - | 0.17 | 0.52* |
| Entropy | - | - | - | - | - | - | 0.10 |
p<0.01
Table 3.
Correlation (Spearman's rho) between the nuclear variables assessed in the tumors
| Perimeter | Circularity | Larger diameter | Intensity | Ra | Entropy | Fractal | |
|---|---|---|---|---|---|---|---|
| Area | 0.92* | 0.10 | 0.84* | 0.40* | 0.89* | 0.24 | 0.70* |
| Perimeter | - | 0.45* | 0.92* | 0.40* | 0.79* | 0.02 | 0.65* |
| Circularity | - | - | 0.45* | 0.05 | 0.05 | 0.56* | 0.09 |
| Larger diameter | - | - | - | 0.28 | 0.74* | 0.08 | 0.48* |
| Intensity | - | - | - | - | 0.26 | 0.32 | 0.67* |
| Ra | - | - | - | - | - | 0.21 | 0.56* |
| Entropy | - | - | - | - | - | - | 0.24 |
p<0.01
When we compared tumor epithelia with basal keratinocytes and different subtypes, few variables demonstrated differences between them (Table 4), especially larger area and nuclear diameter of sclerodermiform, smaller intensities of chromatin, and fractal dimension between superficial. In addition, tumors presented higher entropy of chromatin and nuclear circularity.
Table 4.
F coefficient (p value) of the regression of different variables comparison (generalized linear mixed-effects model)
| Tumor x Normal | Type of tumor | Observation | |
|---|---|---|---|
| Area | 3,30 (0,07) | 5,33 (0,01) | Sclerodermiform higher than others |
| Perimeter | 0,35 (0,55) | 4,21 (0,02) | |
| Circularity | 41,23 (0,00) | 3,57 (0,03) | Lower circularity in normal epithelium |
| Larger diameter | 6,73 (0,01) | 6,43 (0,00) | Sclerodermiform higher than others |
| Intensity | 94,90 (0,00) | 4,90 (0,01) | Superficial lower than others |
| Ra | 0,49 (0,48) | 3,44 (0,03) | |
| Entropy | 73,24 (0,00) | 0,71 (0,49) | Lower entropy in normal epithelium |
| Fractal dimension | 36,12 (0,00) | 6,91 (0,01) | Superficial lower than others |
DISCUSSION
There are nuclear morphometric elements and chromatin texture that differentiate BCC from adjacent basal epithelium and from its subtypes. Changes of nuclear morphology and chromatin texture are classically described by pathologists as criteria for tissue differentiation and tumors.8,16
Basal epidermal keratinocytes were less circular and presented lower entropy, added to the fact that the standard deviation of their measurements is lower than the same index of BCCs. All these factors point towards organization and homogeneity of normal epithelium compared with neoplastic.
Usually, nuclear alterations are observed in the characterization of neoplastic tissue (e.g.: change of shape, intensity, nucleolus and chromatin distribution), and nuclear aberrations are associated with malignancy and tumors survival. However, tissues in normal development or under epigenetic control (e.g., ultraviolet radiation, exposure to hormones) may also have measurable nuclear alterations, representing the intensity of metabolic activity.8,12,17
In this study, there was a significant correlation between various morphological variables, suggesting that the nucleus phenotypic changes are not specific to one or another morphometric marker, but occur simultaneously for characterizing a phenomenon. Multivariate models that simultaneously explore different nuclear variables may have higher discriminating behavior than the independent analysis of each variable.
In the cases studied, significant differences between normal epithelium and neoplastic tissue were identified as circularity and entropy. This suggests that there is differentiation of nuclear morphology and chromatin distribution in tumor tissue as a whole, relative to epidermis. Moreover, even among tumors subtypes we found differences in the nuclear area, larger diameter, intensity and fractal dimension of chromatin, which may represent genomic differences or metabolic activity that justify independent biological behavior among histological subtypes.8,10,17
Superficial forms, which have a lower degree of invasiveness, showed smaller fractal dimension and chromatin intensity. This can be reflected by the type of growth, more prominently horizontal and in a slurred way, of these histotypes.
Sclerodermiform type, with evident infiltrative behavior, showed higher morphometric rates of nucleus size. In contrast to the superficial forms, early and insidious invasiveness of the dermis can be represented by a greater biological activity of these histotypes.
This is a preliminary study suggesting morphometric differences in chromatin textural characteristics of BCCs and adjacent skin. Later, these rates should be estimated to study prognostic aspects of BCC, even within the same histotype.8,10,18 Authors have not had a sufficient number of BCC recurrence cases with analytical quality for this project, but this should be further investigated.
Other staining methods with stoichiometric characteristics with DNA, as silver or Feulgen, may contribute to the exploitation of kariometric variables and chromatin texture with prognostic characteristics for this study.18-20
CONCLUSION
There were characterized aspects of nuclear morphometry and textural characteristics of BCC chromatin, and identified elements that differentiate BCC from adjacent epithelia and from their subtypes, which may be related to their evolutionary biological behavior characteristics.
Funding Statement
Financial Support: FAPESP process n. 2012/21929-5
Footnotes
Financial Support: FAPESP process n. 2012/21929-5
How to cite this article: Mendaçolli PJ, Brianezi G, Schmitt JV, Marques MEA, Miot HA. Nuclear morphometry and chromatin textural characteristics of basal cell carcinoma. An Bras Dermatol. 2015;90(6):874-8.
Study performed at Departamento de Dermatologia e de Patologia da Faculdade de Medicina de Botucatu - Universidade Estadual Paulista "Júlio de Mesquita Filho" (Unesp) - Botucatu, SP, Brazil.
References
- 1.Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292–305. doi: 10.1590/s0365-05962011000200013. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt JV, Chinem VP, Marques ME, Miot HA. Increase in the incidence of basal cell carcinoma in a university hospital between 1999 and 2009. An Bras Dermatol. 2011;86:375–377. doi: 10.1590/s0365-05962011000200029. [DOI] [PubMed] [Google Scholar]
- 3.Ocanha JP1, Dias JT, Miot HA, Stolf HO, Marques ME, Abbade LP. Relapses and recurrences of basal cell face carcinomas. An Bras Dermatol. 2011;86:386–388. doi: 10.1590/s0365-05962011000200032. [DOI] [PubMed] [Google Scholar]
- 4.Roewert-Huber J, Lange-Asschenfeldt B, Stockfleth E, Kerl H. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157:47–51. doi: 10.1111/j.1365-2133.2007.08273.x. [DOI] [PubMed] [Google Scholar]
- 5.Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol. 1990;23:1118–1126. doi: 10.1016/0190-9622(90)70344-h. [DOI] [PubMed] [Google Scholar]
- 6.Kyrgidis A, Tzellos TG, Vahtsevanos K, Triaridis S. New concepts for basal cell carcinoma. Demographic, clinical, histological risk factors, and biomarkers. A systematic review of evidence regarding risk for tumor development, susceptibility for second primary and recurrence. J Surg Res. 2010;159:545–556. doi: 10.1016/j.jss.2008.11.834. [DOI] [PubMed] [Google Scholar]
- 7.Rippey JJ. Why classify basal cell carcinomas? Histopathology. 1998;32:393–398. doi: 10.1046/j.1365-2559.1998.00431.x. [DOI] [PubMed] [Google Scholar]
- 8.Metze K. Fractal dimension of chromatin: potential molecular diagnostic applications for cancer prognosis. Expert Rev Mol Diagn. 2013;13:719–735. doi: 10.1586/14737159.2013.828889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam RL, Silva RC, Pereira FG, Leite NJ, Lorand-Metze I, Metze K. The fractal dimension of nuclear chromatin as a prognostic factor in acute precursor B lymphoblastic leukemia. Cell Oncol. 2006;28:55–59. doi: 10.1155/2006/409593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedin V, Adam RL, de Sá BC, Landman G, Metze K. Fractal dimension of chromatin is an independent prognostic factor for survival in melanoma. BMC Cancer. 2010;10:260–260. doi: 10.1186/1471-2407-10-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Mello MR, Albuquerque DM, Pereira-Cunha FG, Albanez KB, Pagnano KB, Costa FF, et al. Molecular characteristics and chromatin texture features in acute promyelocytic leukemia. Diagn Pathol. 2012;7:75–75. doi: 10.1186/1746-1596-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brianezi G, Handel AC, Schmitt JV, Miot LD, Miot HA. Changes in nuclear morphology and chromatin texture of basal keratinocytes in melasma. J Eur Acad Dermatol Venereol. 2014;29(4):809–812. doi: 10.1111/jdv.12453. [DOI] [PubMed] [Google Scholar]
- 13.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman GR, Streiner DL. Biostatistics. The bare essentials. 3rd ed. Shelton, Connecticut: People's Medical Publishing House; 2008. [Google Scholar]
- 15.IBM SPSS 20.0 for Windows. 20th ed. Chicago: SPSS Incorporation; Statistical Package for Social Science; 2011. [Google Scholar]
- 16.Misteli T. Concepts in nuclear architecture. Bioessays. 2005;27:477–487. doi: 10.1002/bies.20226. [DOI] [PubMed] [Google Scholar]
- 17.Metze K, Ferreira RC, Adam RL, Leite NJ, Ward LS, de Matos PS. Chromatin texture is size dependent in follicular adenomas but not in hyperplastic nodules of the thyroid. World J Surg. 2008;32:2744–2746. doi: 10.1007/s00268-008-9736-0. [DOI] [PubMed] [Google Scholar]
- 18.Ferro DP, Falconi MA, Adam RL, Ortega MM, Lima CP, de Souza CA, et al. Fractal characteristics of May-Grunwald-Giemsa stained chromatin are independent prognostic factors for survival in multiple myeloma. PLoS One. 2011;6: doi: 10.1371/journal.pone.0020706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foucrier J, Rigaut JP, Pechinot D. A combined AgNOR-Feulgen staining technique. J Histochem Cytochem. 1990;38:1591–1597. doi: 10.1177/38.11.1698851. [DOI] [PubMed] [Google Scholar]
- 20.Merril CR. Silver staining of proteins and DNA. Nature. 1990;343:779–780. doi: 10.1038/343779a0. [DOI] [PubMed] [Google Scholar]