Abstract
Introduction
Outside the intensive care unit (ICU), clinically important deep vein thrombosis (DVT) is usually defined as a symptomatic event that leads to objective radiologic confirmation and subsequent treatment. The objective of the present survey is to identify the patient factors and radiologic features of lower limb DVT that intensivists consider more or less likely to make a DVT clinically important in ICU patients.
Methods
Our definition of clinically important DVT was a DVT likely to result in short-term or long-term morbidity or mortality if left untreated, as opposed to a DVT that is unlikely to have important consequences. We asked respondents to indicate the likelihood that patient factors and ultrasonographic features make a DVT clinically important using a five-point scale (from 1 = much less likely to 5 = much more likely).
Results
Of the 71 Canadian intensivists who responded, 70 (99%) rated three patient factors as most likely to make a DVT clinically important: clinical suspicion of pulmonary embolism (mean score 4.6), acute or chronic cardiopulmonary morbidity that might limit a patient's ability to tolerate pulmonary embolism (score 4.5), and leg symptoms (score 4.2). Of the ultrasound features, proximal (score 4.7), large (score 4.2), and totally occlusive (score 3.9) thrombi were considered the three most important.
Conclusion
When labeling a DVT as clinically important, intensivists rely on different patient specific factors and thrombus characteristics than are used to assess the clinical importance of DVT outside the ICU. The clinical importance of DVT is influenced by unique factors such as cardiopulmonary reserve among mechanically ventilated patients.
Keywords: deep venous thrombosis, pulmonary embolism, ultrasound
Introduction
Venous thromboembolism (VTE), which includes both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication of critical illness [1,2]. Critically ill patients harbor many coincident risk factors for DVT, such as the need for surgery, catheters, immobility, and use of sedatives and paralytic agents [3-5]. As in the noncritically ill population, it is likely that most episodes of DVT are asymptomatic and confined to the deep veins of the calf. However, with time, 20–30% of untreated calf vein thrombi extend proximally into the thigh, where they pose a 40–50% risk for PE [6]. Early studies of the natural history of PE suggest that untreated PE has a mortality rate of at least 25% [7].
One large study found PE at autopsy in 59 out of 404 hospitalized patients (14.6%); among the 20 patients who died from PE, this diagnosis was not suspected in 14 (70)% [8]. In a 25-year longitudinal study, 9% of patients had autopsy evidence of PE; again, the diagnosis of PE was not suspected in 84% of patients before autopsy [9]. In a landmark study highlighting this problem in critical care practice, 13 out of 34 (38%) intensive care unit (ICU) patients with known DVT who had no symptoms of PE were diagnosed with PE by ventilation-perfusion scans [10]. It is possible that many mechanically ventilated patients with sudden episodes of hypotension, tachycardia, or hypoxia may have undetected PE [11]. Unsuspected PE may also contribute to difficulty in weaning patients from mechanical ventilation [12]. We posit that in critically ill patients with impaired cardiopulmonary reserve, a small PE, which might be of minimal clinical importance in patients who are less ill, might have severe or fatal consequences [13]. Thus, the clinical consequences of DVT have the potential to be particularly serious, but may be unrecognized in the ICU. It is also possible that ICU patients undergoing treatment for DVT are more likely than other patients to suffer serious complications from anticoagulant therapy.
Among ambulatory patients, most clinicians consider symptomatic DVT to be more clinically important than asymptomatic DVT. Some clinicians may consider clinically important DVT to be proximal (thigh DVT) rather than distal (calf DVT), based on the greater propensity of untreated proximal DVT to cause PE. Thus, clinically important episodes of DVT are usually defined as symptomatic events that lead to objective radiologic confirmation and subsequent treatment [2,14]. However, critically ill patients cannot reliably communicate their symptoms, and intensivists rarely use the reference standard of venography to diagnose DVT [15]. Furthermore, physical signs such as unilateral leg edema, which are important clinical features of DVT outside the ICU, are uncommon in the ICU because many critically ill patients are supine and frequently have severe bilateral edema [16]. These observations suggest that the classic definition of a clinically important DVT is not suitable in the ICU setting.
There is no objective reference standard for or definition of clinically important DVT in critically ill patients because, to our knowledge, no studies have systematically correlated clinical or radiographic characteristics of acute venous thrombosis with patient outcomes. Despite the lack of such data, the concept of a clinically important DVT in the critically ill is important because such an assessment may be used to determine whether a DVT should be treated; in addition, a definition of clinically important DVT could be used in future clinical research. Therefore, as a first step toward defining a 'clinically important DVT', we surveyed critical care physicians to identify those patient factors and radiologic features of lower limb DVT that influence their perceptions about the clinical importance of DVT. Our primary hypothesis was that intensivists will consider the following patient factors more likely to make a DVT clinically important: the DVT is symptomatic, is detected by physical examination, is found in a patient with risk factors, occurs in a patient who has been receiving DVT prophylaxis or therapeutic anticoagulation, and occurs in a patient with either chronic cardiopulmonary comorbidity or acute cardiopulmonary illness that might limit tolerance to a PE. Our secondary hypothesis was that intensivists will consider the following compression ultrasound features more likely to make a DVT clinically important: the DVT is proximal, large, occlusive, and persists 1 week later.
Methods
Instrument development
We developed a survey for members of the Canadian Critical Care Trials Group who care for critically ill adults [17]. We generated items for this instrument based on a review of the literature, our prior work [15,18,19], and discussion among the investigators. We reduced items for this instrument by interviews with five intensivists who did not participate in the survey.
Instrument formatting
In the survey we provided the rationale for the questionnaire, defined the concept of clinically important DVT, and requested basic demographic information about respondents and their ICU settings. We then surveyed respondents about the extent to which various factors influence their assessment of the clinical importance of DVT in critically ill patients. A self-administered rather than interviewer-administered format was used to maximize the validity of self-reported information [20].
We used the following conceptual definition of clinically important DVT: a DVT that, if untreated, would will lead to increased short-term morbidity (e.g. cardiopulmonary consequences that may delay weaning from mechanical ventilation), long-term morbidity (e.g. patient-centered consequences such as chronic venous insufficiency), or mortality. We listed potential characteristics of clinically important DVTs in two domains: patient factors and ultrasonographic features. For an ICU patient with a DVT we asked respondents to indicate, using a Likert scale, whether each of these characteristics was more or less likely to make a DVT clinically important (1 = much less likely to make the DVT clinically important, 2 = somewhat less likely to make the DVT clinically important, 3 = neither more nor less likely to make the DVT clinically important, 4 = somewhat more likely to make the DVT clinically important, and 5 = much more likely to make the DVT clinically important; see Appendix 1).
Demographic questions included year of graduation from medical school and clinical discipline (internal medicine or its subspecialities, surgery, or anesthesia). We also requested information about the number of ICU beds in which the respondent works, the type of ICU (medical, surgical or mixed medical–surgical), and the presence of a thrombosis consult service. We used closed-ended questions for the ICU demographic data to maximize the accuracy and completeness of responses [21].
Instrument administration
We administered the survey instrument by e-mail to all members of the Canadian Critical Care Trial Group who care for critically ill adults. We contacted nonrespondents by facsimile with a second copy of the questionnaire [22], and then by e-mail or telephone contact. The survey was conducted in September and October 2003.
Research ethics
Participation was voluntary, individual responses were kept confidential, and data were anonymized upon receipt. The study was approved by the Research Ethics Board of St Joseph's Healthcare in Hamilton, Ontario.
Analysis
Descriptive statistics were used, including proportions with associated confidence intervals, as well as measures of central tendency and dispersion (mean and standard deviation [SD]). Within each domain (patient factors and ultrasonographic features), we ranked the characteristics according to which were most likely to make the DVT clinically important.
Results
The response rate was 70 out of 71 (99%). Sixty-six (93%) of the surveys were returned without a reminder e-mail; five (7%) required a reminder. Clinical discipline backgrounds were internal medicine in 52 (75%), anesthesia in eight (12%), surgery in six (9%), and other in three (4%). Respondents had 16.2 (SD 8.2) years of ICU experience, and worked in ICUs with a mean of 21.5 (SD 8.0) beds. ICUs represented were from 31 hospitals in 16 cities in Canada; 67 (94%) of respondents were university affiliated. Twelve out of 31 hospitals (39%) had a thrombosis consultation service.
Patient factors considered to increase the clinical importance of a DVT, ranked according to the mean score, are shown in Table 1. A clinical suspicion of PE (mean score 4.6 [SD 0.7]), acute or chronic cardiopulmonary morbidity that might limit a patient's ability to tolerate PE (score 4.5 [0.7]), and the presence of leg symptoms (score 4.2 [0.8]) were most likely to make a DVT clinically important. In Fig. 1 we present the distribution of responses from 1 to 5 for patient factors associated with clinically important DVT. The absence of risk factors, symptoms, or physical signs of DVT, DVT associated with central venous catheterization, and DVT in the absence of prophylaxis were all considered to be less important than the other factors in determining the clinical relevance of an ICU-acquired DVT.
Table 1.
Patient factors affecting the clinical importance of deep venous thrombosis in intensive care unit patients
| Patient factor | Mean (SD) rating |
| Clinical suspicion of pulmonary embolism | 4.6 (0.7) |
| Chronic/acute cardiopulmonary comorbidity | 4.5 (0.7) |
| Leg symptoms | 4.2 (0.8) |
| Occurring while receiving therapeutic anticoagulation | 4.0 (1.2) |
| Detected by physical examination | 3.8 (0.7) |
| Occurring while receiving thromboprophylaxis | 3.6 (0.8) |
| At least one risk factor for venous thromboembolism | 3.6 (0.6) |
| Associated with central venous catheterization | 3.3 (0.9) |
| Not receiving prophylaxis | 3.2 (0.7) |
| Not associated with central venous catheterization | 3.1 (0.5) |
| Not receiving therapeutic anticoagulation | 3.1 (0.3) |
| No risk factors | 3.0 (0.4) |
| Asymptomatic | 2.8 (0.5) |
| Not detected by physical examination | 2.8 (0.5) |
According to mean scores we ranked the patient factors considered by intensivists as likely to make a deep venous thrombosis clinically important. SD, standard deviation
Figure 1.
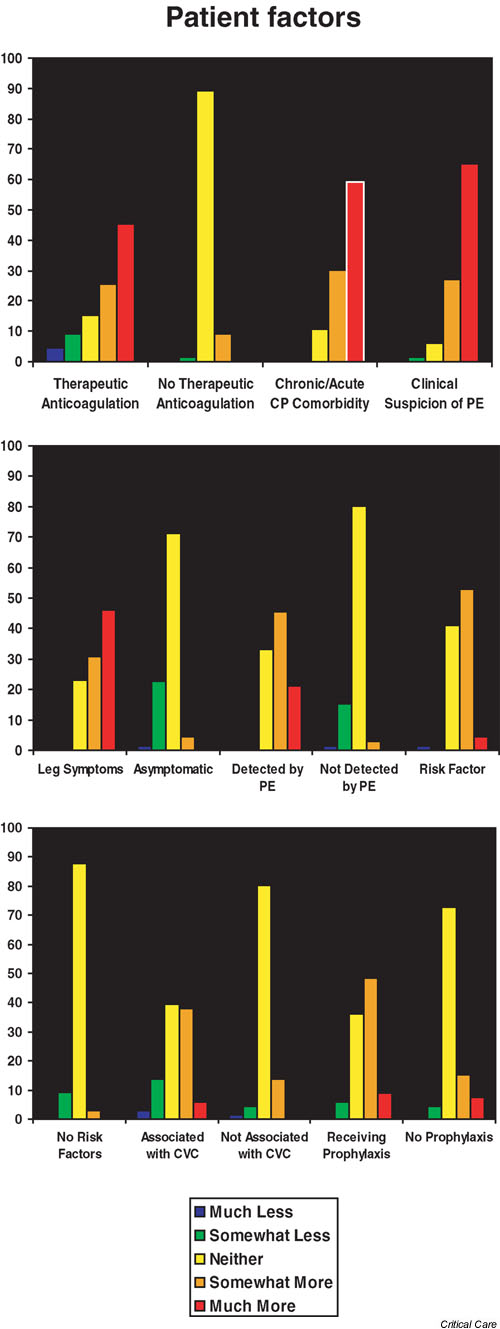
Patient factors considered by respondents to make a deep venous thrombosis (DVT) clinically important in the intensive care unit setting. The scale used was as follows: 1 = much less likely to make the DVT clinically important; 2 = somewhat less likely to make the DVT clinically important; 3 = neither more nor less likely to make the DVT clinically important; 4 = somewhat more likely to make the DVT clinically important; and 5 = much more likely to make the DVT clinically important.
In Table 2 we present the ultrasonographic features considered likely to make a DVT clinically important, ranked according to the mean score. Proximal location of the DVT was rated as most likely to make a DVT clinically important (score 4.7 [SD 0.5]). Of the other ultrasound features, large (score 4.2 [0.6]) and totally occlusive (score 3.9 [0.8]) thrombi were considered most important. In Fig. 2 we present the distribution of responses from 1 to 5 for ultrasonographic features associated with clinically important DVT. A DVT that is small, partially occlusive, or has flow around it did not make the DVT unlikely to be considered clinically important.
Table 2.
Ultrasonographic features affecting the clinical importance of deep venous thrombosis in intensive care unit patients
| Ultrasonographic feature | Mean (SD) Rating |
| Proximal DVT | 4.7 (0.5) |
| Large DVT | 4.2 (0.6) |
| Totally occlusive DVT | 3.9 (0.8) |
| No flow on color Doppler ultrasound | 3.6 (0.7) |
| DVT still present 1 week after diagnosis | 3.6 (0.7) |
| Partially occlusive DVT | 3.3 (0.7) |
| Flow on color Doppler ultrasound | 3.0 (0.6) |
| DVT not extending into deep system | 2.7 (1.0) |
| Localized DVT | 2.7 (0.7) |
| Small DVT | 2.6 (0.6) |
| DVT not present 1 week after diagnosis | 2.3 (0.8) |
| Distal DVT | 2.0 (0.7) |
According to mean scores we ranked the ultrasonographic features considered by intensivists as likely to make a deep venous thrombosis (DVT) clinically important. SD, standard deviation
Figure 2.
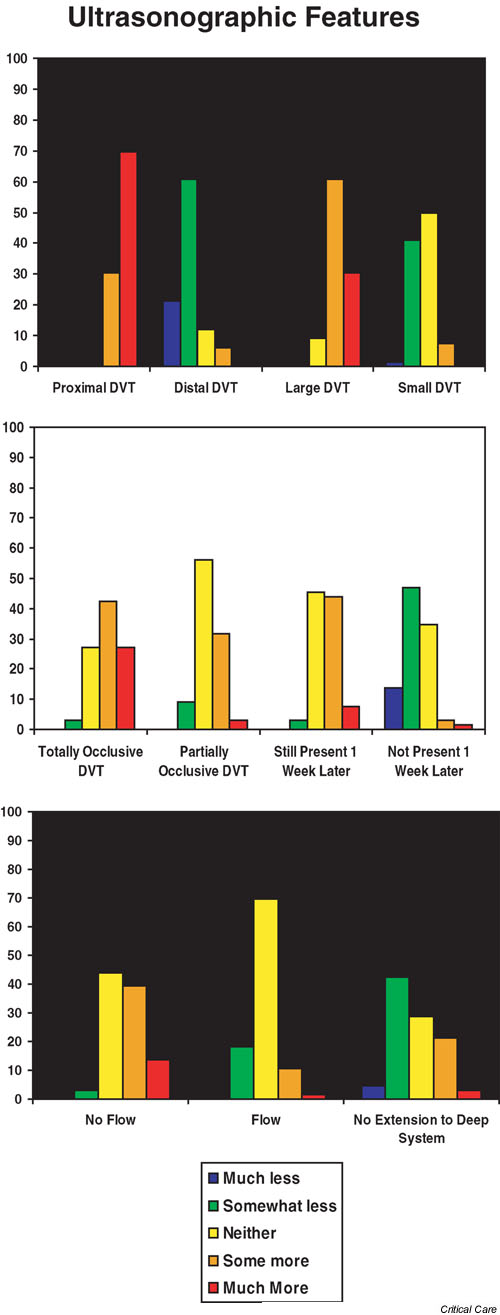
Ultrasonographic factors considered by respondents to make a deep venous thrombosis (DVT) clinically important in the intensive care unit setting. The scale used was as follows: 1 = much less likely to make the DVT clinically important; 2 = somewhat less likely to make the DVT clinically important; 3 = neither more nor less likely to make the DVT clinically important; 4 = somewhat more likely to make the DVT clinically important; and 5 = much more likely to make the DVT clinically important.
Discussion
DVT is underdiagnosed and probably undertreated in critically ill patients. This occurs despite the high likelihood that PE is clinically more important in the critically ill, who often have impaired cardiopulmonary reserve [13]. We conducted the present survey to determine which characteristics of DVT are considered to be clinically important by intensivists. Determining which characteristics impart clinical significance is important because the thrombi that are considered to be clinically important are likely to be treated, and these treatments have adverse effects such as bleeding, inconvenience, and cost [23].
Outside the ICU setting, many clinicians consider DVT clinically significant primarily when patients have referable symptoms and evidence of occlusive thrombosis on ultrasonography. However, critically ill patients are generally unable to report symptoms and uncommonly have unilateral leg edema because they are recumbent. Therefore, 'typical' clinical symptoms and signs cannot be used to establish the clinical significance of a DVT in the ICU. Our survey confirmed that intensivists use some different criteria to assign clinical significance than are used by nonintensivists, particularly thrombosis experts [24-26]. For example, asymptomatic DVT detected on screening ultrasonography in critically ill patients with severely impaired cardiorespiratory reserve are accorded substantial clinical importance in the ICU, whereas the significance of these thrombi may be debated by clinicians working outside the ICU setting.
Strengths of the survey include comprehensive lists of both patient factors and ultrasonographic features that may influence clinicians' interpretation of the importance of a DVT in the ICU setting. Ideally, empiric data would inform us about which DVT characteristics are most likely to be associated with short-term and long-term morbidity and mortality in critically ill patients. In the absence of such data, we defined clinically important DVT as a DVT that is likely, in the opinion of the intensivists, to result in short-term or long-term morbidity or mortality if left untreated, as opposed to a DVT that will probably not have important consequences. We used evidence from three randomized trials to conduct this survey; these suggested that a self-administered format yields more valid self-reports than interviewer-administered questionnaires [20], that closed-ended formats yield more complete and valid demographic data about the respondents than do open-ended formats [21], and that appending second questionnaires to reminders maximizes response rates [22]. Our respondents represented largely university affiliated ICUs from coast to coast in Canada, and our response rate was 99%. We believe that our results are generalizable to similar multidisciplinary secondary and tertiary care settings in which the majority of mechanically ventilated, critically ill patients are cared for.
However, in focusing on the short-term consequences of VTE, there are three major limitations of the survey. The first is that we did not evaluate thresholds for DVT treatment in the ICU setting, which is a more complex phenomenon. Treatment thresholds are also influenced by other factors such as the natural history of the ICU patient's underlying illness, the type of therapeutic options available and suitable for each patient, intensivists' knowledge about the realistic magnitude of therapeutic benefits and harms, patient-specific and dynamic balance between bleeding and thrombosis risks, and alternative options such as surveillance monitoring. Evaluating treatment thresholds is also interesting, although it is a more complex issue and would require different survey questions (or an alternative study design altogether). Second, we did not elicit views on the risks, costs, and consequences of investigating and managing treatment complications such as bleeding. Third, we did not consider the long-term consequences of DVT such as postphlebitic syndrome, which may be associated with disabling symptoms and/or ulceration among survivors.
Experts express varying views on the appropriate end-points for studies of thromboprophylaxis [27-31]. Over the years, the majority of thromboprophylaxis trials have used DVT detected by sensitive screening methods such as venography as the primary efficacy outcome. Only one of two randomized trials in the ICU has employed ultrasonographic followed by venographic screening for DVT [32], and venograms are rarely performed in critical care practice today [15]. The clinical importance of DVT identified by screening tests outside the ICU is controversial. Two lines of evidence support the hypothesis that DVT detected by screening tests is clinically unimportant. The first line of evidence is the fact that even with use of appropriate VTE prophylaxis, 15–30% of patients undergoing major orthopedic surgery will be discharged from the hospital with venographically detectable calf or more proximal DVT; fewer (about 3% of such patients) ultimately return with a symptomatic DVT [33].
The second line of evidence is that, even when clinicians perform screening ultrasonography at the time of hospital discharge and then treat patients who have DVT with antithrombotic therapy, there is no evidence that the subsequent risk for thrombosis is decreased. For example, Robinson and colleagues [34] randomized patients after orthopedic surgery to undergo screening ultrasonography or sham ultrasonography. After hospital discharge the risk for patients returning with clinical symptoms of venous thrombosis was the same, irrespective of whether patients underwent ultrasonography, suggesting that thrombi identified by screening ultrasonography in hospitalized patients are not necessarily destined to produce clinical symptoms. As a result of these observations, routine screening of such patients for DVT at the time of hospital discharge has effectively been abandoned [2].
Caution is needed, however, in extrapolating this practice pattern to critically ill patients. For example, patients in the ICU frequently have significantly impaired cardiorespiratory reserve. Therefore, they may be unable to tolerate a small PE that an otherwise healthy outpatient might tolerate easily. Better quality evidence on the clinical impact of DVT diagnosed by screening ultrasound in the critically ill is needed. Meanwhile, most such patients with lower limb DVT diagnosed by screening ultrasonography appear to be treated, based on recent studies [5]. Interpreting studies about VTE in the ICU requires consideration of the clinical importance of the DVT end-points. In future randomized trials testing the efficacy of thromboprophylaxis interventions, we recommend that validated and feasible screening tests, such as Doppler ultrasonography or serial Doppler ultrasonography, be used [31]. Large, simple randomized trials that use clinically important VTE end-points should follow, consisting of events such as symptomatic or fatal PE, and ultrasonographically diagnosed proximal DVT [28-31].
The results of this survey can inform our research program on VTE in the ICU. We conclude that most forms of VTE are considered to be clinically serious, perhaps more so than in patients outside the critical care setting, supporting the need for ongoing research in this area. From this survey we generated new information showing how the critical care context is important to intensivists caring for patients with DVT. Development of DVT in the critically ill patient may have unique consequences, particularly in patients requiring mechanical ventilation, vasopressors, or inotropes, because intensivists report that cardiopulmonary reserve is crucial in interpreting the features of a DVT that make it clinically important. Our results also suggest that treatment thresholds used by intensivists may be lower than the treatment thresholds employed by other clinicians, although this hypothesis can be formally tested. On the other hand, the treatment threshold may be higher for intensivists who take into account other complications of critical illness. For example, dynamic bleeding and thrombotic risks in ICU patients must be traded off when considering whether to treat a DVT, and with what to treat it. Such factors will influence the associated morbidity and mortality of VTE in the ICU setting, which need to be more rigorously evaluated.
Conclusion
We demonstrated that intensivists define clinically important DVT using criteria that are relevant to critical care patients in addition to those suitable for general DVT patients. Pending the availability of data that correlate clinical outcomes with the criteria we studied in this survey, these concepts could be used in future studies of the prevention and treatment of DVT in the critically ill.
Competing interests
MM received grants for investigator initiated studies from Pharmica, Aventis and Leo Pharmaceuticals (latter for this survey). JG as a co-investigator, indirectly received funding from Pharmacia and Leo Pharmaceuticals for the DVT study. GG received grants for investigator initiated studies from Pharmica, Aventis and Leo Pharmaceuticals (latter for this survey). MC received honoraria and research grants from Pfizer, Pharmica, Aventis, Leo Pharmaceuticals and Novo Nordisk. LG received a grant from Leo Pharmaceuticals for this survey. DC has received a grant for this survey from Leo Pharmaceuticals, and additional grants for investigator initiated research from Pharmacia and Aventis.
Abbreviations
DVT = deep vein thrombosis; ICU = intensive care unit; PE = pulmonary embolism; SD = standard deviation; VTE = venous thromboembolism.
Key messages
1. Determining which DVT characteristics impart clinical significance to intensivists is important because DVT considered to be clinically important are likely to be treated, and treatments have adverse effects such as bleeding, inconvenience and cost.
2. In this survey of intensivists, we defined a clinically important DVT as one that, if untreated, would will lead to increased short term morbidity, long term morbidity, or mortality.
3. The 3 patient factors reported as most likely to make a DVT clinically important were clinical suspicion of PE, acute or chronic cardiopulmonary morbidity which might limit the tolerability of a PE, and leg symptoms.
4. The 3 ultrasound features reported as most likely to make a DVT clinically important were proximal location, large size, and total vessel occlusion.
5. The clinical importance of DVT according to intensivists is influenced by unique ICU factors such as cardiopulmonary reserve among mechanically ventilated patients.
Supplementary Material
Appendix 1
Acknowledgments
Acknowledgements
We appreciate the data management assistance of Nicole Zytaruk. We are grateful to the Canadian Critical Care Trials Group for their support of the present study.
This study was funded by an unrestricted grant in aid from Leo Pharmaceuticals. Dr D Cook is a Research Chair of the Canadian Institutes for Health Research. Drs Crowther and Meade are Investigators of the Canadian Institutes for Health Research.
The authors’ contributions were as follows: conception: D Cook, G Guyatt, and M Meade; design: D Cook, M Crowther, M Meade, G Guyatt, W Geerts, and L Griffith; data collection: D Cook; analysis: L Griffith; interpretation: D Cook, M Crowther, M Meade, G Guyatt, J Granton, and W Geerts; drafting of manuscript: D Cook, M Crowther, and L Griffith; and critical revision of manuscript: M Meade, G Guyatt, J Granton, and W Geerts.
References
- Attia J, Cook DJ, Douketis J, Ginsberg JS, Geerts W. Deep vein thrombosis and its prevention in critically ill patients. Arch Intern Med. 2001;161:1268–1279. doi: 10.1001/archinte.161.10.1268. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Heit JA, Clagett P, Pineo GF, Colwell CW, Anderson FA, Wheeler HB. Prevention of venous thromboembolism. Sixth ACCP Antithrombotic Consensus Conference on Antithrombotic Therapy. Chest. 2001;119(1 suppl):132S–175S. doi: 10.1378/chest.119.1_suppl.132S. [DOI] [PubMed] [Google Scholar]
- Geerts W, Cook DJ, Selby R, Etchells E. Venous thromboembolism and its prevention in critical care. J Crit Care. 2002;17:95–104. doi: 10.1053/jcrc.2002.33941. [DOI] [PubMed] [Google Scholar]
- Ibrahim EH, Iregui M, Prentice D, Sherman G, Kollef M, Shannon W. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med. 2002;30:771–774. doi: 10.1097/00003246-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Crowther M, Meade M, Rabbat C, Schiff D, Geerts W, Griffith L, Guyatt GH. Deep venous thrombosis in medical-surgical ICU patients: prevalence, incidence and risk factors. Crit Care. 2003;Suppl 2:S54. doi: 10.1097/01.ccm.0000171207.95319.b2. [DOI] [PubMed] [Google Scholar]
- Kakkar VV, Howe CT, Flanc C, Clarke MB. Natural history of postoperative deep vein thrombosis. Lancet. 1969;2:230–232. doi: 10.1016/S0140-6736(69)90002-6. [DOI] [PubMed] [Google Scholar]
- Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism: A controlled trial. Lancet. 1960;i:1309–1312. doi: 10.1016/S0140-6736(60)92299-6. [DOI] [PubMed] [Google Scholar]
- Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995;108:978–981. doi: 10.1378/chest.108.4.978. [DOI] [PubMed] [Google Scholar]
- Karwinski B, Svendsen E. Comparison of clinical and postmortem diagnosis of pulmonary embolism. J Clin Pathol. 1989;42:135–139. doi: 10.1136/jcp.42.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser KM, Fedullo PF, LittleJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271:223–225. doi: 10.1001/jama.271.3.223. [DOI] [PubMed] [Google Scholar]
- McKelvie PA. Autopsy evidence of pulmonary thromboembolism. Med J Aust. 1994;160:127–128. [PubMed] [Google Scholar]
- Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274:335–337. doi: 10.1001/jama.274.4.335. [DOI] [PubMed] [Google Scholar]
- Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279:458–462. doi: 10.1001/jama.279.6.458. [DOI] [PubMed] [Google Scholar]
- Warwick D, Samama MM. The contrast between venographic and clinical endpoints in trials of thromboprophylaxis in hip replacement. J Bone Joint Surg. 2000;82:480–482. doi: 10.1302/0301-620X.82B4.9876. [DOI] [PubMed] [Google Scholar]
- Cook D, McMullin J, Hodder R, Heule M, Pinilla J, Dodek P, Stewart T, for the Canadian ICU Directors Group Prevention and diagnosis of venous thromboembolism in critically ill patients: a Canadian survey. Crit Care. 2001;5:336–342. doi: 10.1186/cc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke FJ, Griffith L, McDonald E, Hoad N, Rabbat C, Meade M, Guyatt GH, Devereaux PJ, Geerts W, Wells P, Crowther M, Cook DJ, for the Canadian Critical Care Trials Group Deep vein thrombosis: Clinically silent in the ICU. Am J Resp Crit Care Med. 2004.
- Cook DJ, Todd TRJ. The Canadian Critical Care Trials Group: a collaborative educational organization for the advancement of adult clinical ICU research. Intensive Care World. 1997;14:68–70. [Google Scholar]
- Cook D, Attia J, Weaver B, McDonald E, Meade M, Crowther M. Venous thromboembolic disease: An observational study in medical-surgical intensive care unit patients. J Crit Care. 2000;15:127–132. doi: 10.1053/jcrc.2000.19224. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Laporta D, Skrobik Y, Peters S, Sharpe M, Murphy P, Chin D, Crowther V, for the Canadian ICU Directors Group Prevention of venous thromboembolism in critically ill surgery patients. J Crit Care. 2001;16(4):161–166. doi: 10.1053/jcrc.2001.30665. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Guyatt GH, Juniper E, Griffith LE, McIlroy W, Willan A, Jaeschke R, Epstein R. Interviewer versus self-administered questionnaires in developing a disease-specific, health related quality of life instrument for asthma. J Clin Epidemiol. 1993;46(6):529–534. doi: 10.1016/0895-4356(93)90125-K. [DOI] [PubMed] [Google Scholar]
- Griffith LE, Cook DJ, Guyatt GH, Charles C. Comparison of open versus closed questionnaire formats in obtaining demographic information from Canadian general internists. J Clin Epidemiol. 1999;52:997–1005. doi: 10.1016/S0895-4356(99)00106-7. [DOI] [PubMed] [Google Scholar]
- Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–1136. doi: 10.1016/S0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- Linkins LA, Choi P, Douketis J. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, Clement C, Robinson KS, Lewandowski B. Application of a diagnostic clinical model for the management of hospitalized patients with suspected deep-vein thrombosis. Thromb Haemost. 1999;81:493–497. [PubMed] [Google Scholar]
- Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, Clement C, Robinson KS, Lewandowski B. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- Wells PS, Hirsh J, Anderson DR, Lensing AW, Foster G, Kearon C, Weitz J, D'Ovidio R, Cogo A, Prandoni P. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345:1326–1330. doi: 10.1016/S0140-6736(95)92535-X. [DOI] [PubMed] [Google Scholar]
- Kearon C, Salzman EW, Hirsh J. Epidemiology, pathogenesis, and natural history of venous thrombosis. In: Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editor. In Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 4. Philadelphia, PA: JB Lippincott; 2001. pp. 1153–1177. [Google Scholar]
- Kearon C. Noninvasive diagnosis of deep venous thrombosis in postoperative patients. Semin Thromb Hemost. 2001;27:3–8. doi: 10.1055/s-2001-12842. [DOI] [PubMed] [Google Scholar]
- Sonaglia F, Rossi R, Agnelli G. End points in studies on the prevention of deep vein thrombosis. Semin Thromb Hemost. 2001;27:41–46. doi: 10.1055/s-2001-12850. [DOI] [PubMed] [Google Scholar]
- Leizorovicz A, Kassai B, Becker F, Cucherat M. The assessment of deep vein thromboses for therapeutic trials. Angiology. 2003;54:19–24. doi: 10.1177/000331970305400103. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW. Prevention of venous thromboembolism. Chest. 2004. [DOI] [PubMed]
- Fraisse F, Holzapfel L, Couland JM, Simonneau G, Bedock B, Feissel H, Herbecq P, Pordes R, Poussel JF, Roux L, for the Association of Non-University Affiliated Intensive Care Specialist Physicians in France Nadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. Am Rev Resp Crit Care Med. 2000;161:1109–1114. doi: 10.1164/ajrccm.161.4.9807025. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet. 2001;358:9–15. doi: 10.1016/S0140-6736(00)05249-1. [DOI] [PubMed] [Google Scholar]
- Robinson SK, Anderson DR, Gross M, Petrie D, Leighton R, Stanish W, Alexander D, Mitchell M, Flemming B, Gent M. Ultrasonographic screening before hospital discharge for deep venous thrombosis after arthroplasty. Ann Intern Med. 1997;127:439–445. doi: 10.7326/0003-4819-127-6-199709150-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1


