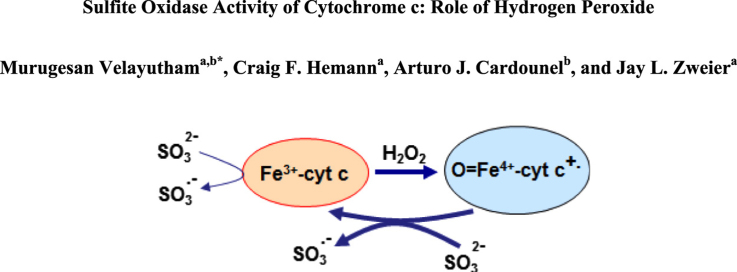
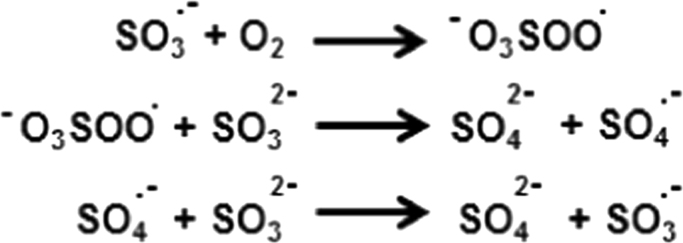Abstract
In humans, sulfite is generated endogenously by the metabolism of sulfur containing amino acids such as methionine and cysteine. Sulfite is also formed from exposure to sulfur dioxide, one of the major environmental pollutants. Sulfite is used as an antioxidant and preservative in dried fruits, vegetables, and beverages such as wine. Sulfite is also used as a stabilizer in many drugs. Sulfite toxicity has been associated with allergic reactions characterized by sulfite sensitivity, asthma, and anaphylactic shock. Sulfite is also toxic to neurons and cardiovascular cells. Recent studies suggest that the cytotoxicity of sulfite is mediated by free radicals; however, molecular mechanisms involved in sulfite toxicity are not fully understood. Cytochrome c (cyt c) is known to participate in mitochondrial respiration and has antioxidant and peroxidase activities. Studies were performed to understand the related mechanism of oxidation of sulfite and radical generation by ferric cytochrome c (Fe3+cyt c) in the absence and presence of H2O2. Electron paramagnetic resonance (EPR) spin trapping studies using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) were performed with sulfite, Fe3+cyt c, and H2O2. An EPR spectrum corresponding to the sulfite radical adducts of DMPO (DMPO-SO3-) was obtained. The amount of DMPO- formed from the oxidation of sulfite by the Fe3+cyt c increased with sulfite concentration. In addition, the amount of DMPO- formed by the peroxidase activity of Fe3+cyt c also increased with sulfite and H2O2 concentration. From these results, we propose a mechanism in which the Fe3+cyt c and its peroxidase activity oxidizes sulfite to sulfite radical. Our results suggest that Fe3+cyt c could have a novel role in the deleterious effects of sulfite in biological systems due to increased production of sulfite radical. It also shows that the increased production of sulfite radical may be responsible for neurotoxicity and some of the injuries which occur to humans born with molybdenum cofactor and sulfite oxidase deficiencies.
Keywords: Electron paramagnetic resonance (EPR), Spin trapping, Sulfite radical, Cytochrome c, Peroxidase activity, Mitochondria
Graphical abstract
Highlights
-
•
Cytochrome c oxidizes sulfite to sulfite radical.
-
•
In the presence of H2O2, sulfite radical generation from cyt c increases.
-
•
The formation of sulfite radical is sulfite concentration dependent.
-
•
This mechanism of sulfite radical formation may be important in sulfite toxicity.
1. Introduction
In humans, sulfite is generated endogenously by the metabolism of sulfur containing amino acids such as methionine and cysteine [1]. Sulfite is also formed from exposure to sulfur dioxide, one of the major environmental pollutants [2]. Sulfite is used as an antioxidant and preservative in dried fruits, vegetables, pickled onion, and beverages such as fruit juice, grape juice, beer, and wine to prevent or reduce spoilage [2], [3], [4]. Sulfite is also used as a stabilizer in many drugs and cosmetics [2], [5], [6]. For the majority of people, exposure to sulfites occurs during consumption of foods and drinks that contain sulfite preservative [2]. Sulfite toxicity has been associated with allergic reactions characterized by sulfite sensitivity, asthma, chronic airway diseases, dermatitis, anaphylactic shock, and early death [2], [7], [8]. The most frequently reported physiological response for those sensitive to sulfite is difficulty in breathing due to bronchoconstriction [2]. Steroid-dependent asthmatics and children with chronic asthma are especially vulnerable to such toxicity [2]. Sulfite is also toxic to neurons and cardiovascular system [9], [10], [11], [12], [13], [14]. The level of sulfite in serum was found to be unregulated in several disease conditions, such as pneumonia and end-stage renal failure [15], [16]. Studies have suggested that the cytotoxicity of sulfite is mediated by free radicals [9], [17], [18]. There is no specific treatment for sulfite toxicity, and the molecular mechanisms of the potentially toxic reactions of sulfite are poorly understood.
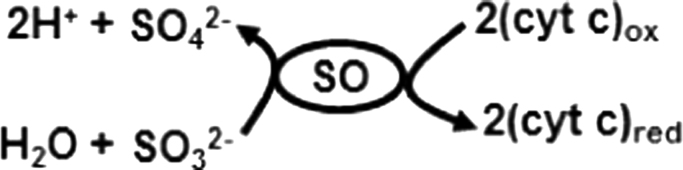
In humans, sulfite is detoxified in the liver and lung to sulfate by sulfite oxidase (SO), a molybdenum dependent mitochondrial enzyme [19]. SO ensures that intracellular levels of the sulfite ion remain at acceptably low levels. In cells, SO is localized in the intermembrane space of the mitochondria. Sulfite oxidation is the final step in the metabolism of sulfur derived from sulfur containing amino acids. SO catalyzes the oxidation of endogenous or exogenous sulfite to sulfate, which is excreted in to the urine [20]. In humans, SO deficiency is one of the most accepted causes of sulfite hypersensitivity and toxicity [21]. A congenital deficiency of SO can cause an excessive accumulation of sulfite and lead to early death in infancy (usually between 2 and 6 years of age), or in neonatal cases, neurological abnormalities, mental retardation, intractable seizures, and ocular lens dislocation [8], [20], [21], [22]. Molybdenum cofactor deficiency, which would compromise SO activity, results in profound mental retardation, brain damage, microcephaly, and spasticity [23]. It has also been suggested that hypoxic-ischemic encephalopathy is due to molybdenum cofactor deficiency [21], [24]. Importantly, in SO and molybdenum cofactor deficiency cases, the level of sulfite is increased in plasma and urine and also accumulates within the body [8], [21], [22], [24], [25], [26], [27]. Despite great advances in understanding the pathophysiology of SO and molybdenum cofactor deficiencies [22], [23], there are no available therapies to reduce mortality or to improve quality of life in survivors. Thus, a greater understanding of the mechanisms by which excess sulfite leads to pathophysiological complications could lead to the development of more effective therapies.
Under normal physiological conditions, SO catalyzes the oxidation of sulfite to sulfate with cytochrome c (cyt c) as oxidizing substrate as shown in Scheme 1 [28], [29]. Mammalian cytochrome c (cyt c) is a small, globular protein that exists in high concentrations (0.5–5 mM) in the inner membrane of mitochondria [30], [31]. At least 15% of cyt c is tightly bound to the inner membrane and the remainder is loosely attached to the inner membrane and can be readily mobilized [32]. Under physiological conditions, cyt c mediates electron shuttling between cytochrome c reductase (complex III) and cytochrome c oxidase (complex IV) during mitochondrial respiration [32]. The loosely associate cyt c also mediates superoxide removal, and prevents oxidative stress [32], [33], [34], whereas the tightly bound cyt c accounts for the peroxidase activity [35], [36], [37], [38], [39]. The peroxidase activity of cyt c increases under conditions of oxidative and nitrosative stress [31], [40], [41]. Release of cyt c from the inner mitochondrial membrane into the cytosol is a pro-apoptotic factor [42], [43]. Early in apoptosis, the redox function of cyt c in the respiratory chain switches to a peroxidase function [44], [45]. The increased peroxidase activity of cyt c is implicated in various neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS) [46]. To gain a better understanding of the role of oxidized cytochrome c (Fe3+cyt c) in oxidative sulfite toxicity, we have employed the powerful, sensitive, and specific technique of electron paramagnetic resonance (EPR) spin trapping technique to investigate the oxidation of sulfite and generation of free radicals by the Fe3+cyt c in the absence and presence of H2O2.
Scheme 1.
Sulfite oxidase catalyzes the oxidation of sulfite to sulfate and reduces oxidized cytochrome c.
2. Materials and methods
2.1. Materials
Oxidized cytochrome c (Fe3+cyt c, from horse heart), hydrogen peroxide (H2O2), and sodium sulfite (Na2SO3) were purchased from Sigma. Diethylenetriaminepentaacetic acid (DTPA) and 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) were obtained from Aldrich. Purified 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was purchased from Dojindo laboratories, Kumamoto, Japan.
2.2. Electron Paramagnetic Resonance (EPR) measurements
EPR spectra were recorded using a quartz flat cell at room temperature with a Bruker ESP 300E spectrometer operating at X-band with 100 kHz modulation frequency and a TM110 cavity. The instrument settings were as follows: microwave frequency of 9.779 GHz, modulation amplitude of 0.5 G, microwave power of 20 mW, scan time of 30 s, time constant of 82 ms, and a single scan. EPR spectral recording began two minutes after the addition of H2O2. All the experiments were carried out in phosphate buffer (50 mM and pH 7.4) containing 0.1 mM DTPA. Reactions were initiated by the addition of H2O2. Quantitation of the observed free radical signals was performed by computer simulation of the spectra with comparison of the double integral of the observed signal to that of a TEMPO standard (1 μM) measured under the identical conditions [47].
3. Results
3.1. EPR spin trapping studies of the oxidation of sulfite by Fe3+cyt c
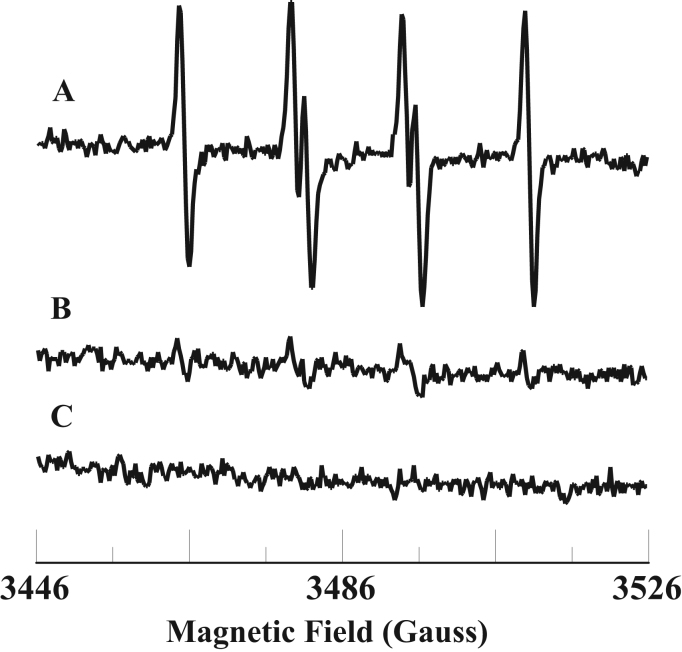
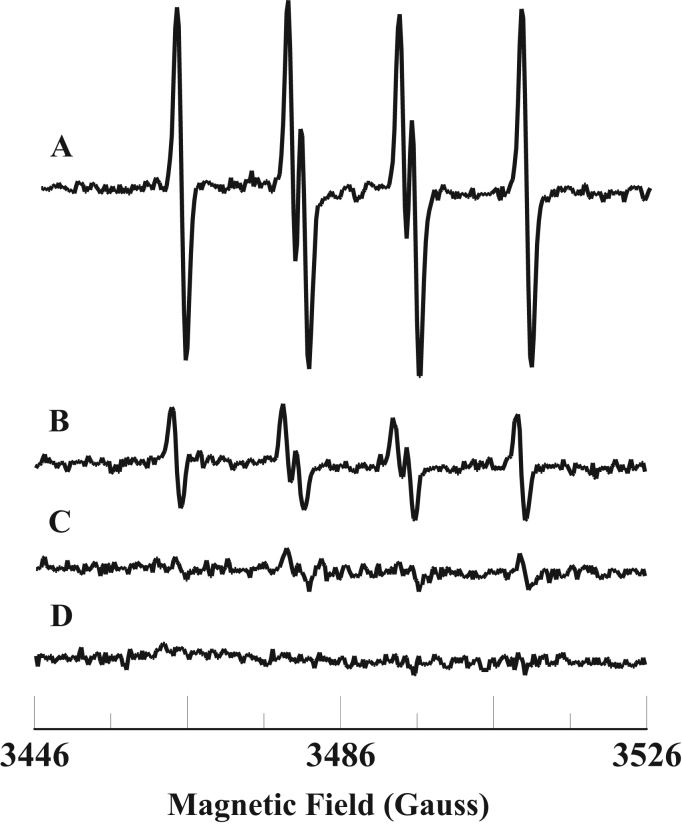
It has been demonstrated that Fe3+cyt c can oxidize various thiol compounds and superoxide radical [48], [49], [50]. To gain insight into the molecular mechanisms involved in the process of oxidation associated with sulfite toxicity, we studied the oxidation of sulfite and free radical formation by Fe3+cyt c. EPR spin trapping is a powerful technique to measure formation of free radical intermediates. EPR spin trapping studies using the spin trap DMPO were carried out to investigate the oxidation of sulfite by Fe3+cyt c. EPR spectra were recorded from the reaction mixture containing DMPO (0.1 M), Fe3+cyt c (0.1 mM), and sulfite (1 mM) in the presence of DTPA (0.1 mM). A prominent EPR signal was seen, corresponding to the sulfite radical adduct of DMPO (DMPO-), as shown in Fig. 1A. From the EPR spectrum, the calculated isotropic hyperfine coupling constants are aN=14.57 G and aH=16.09 G, which are in agreement with previous reports [51]. In the absence of Fe3+cyt c, a trace level of DMPO- signal was obtained, as shown in Fig. 1B. No EPR signal was obtained in the absence of sulfite, as shown in Fig. 1C. These results show that Fe3+cyt c oxidizes sulfite to form the sulfite radical.
Fig. 1.
Room temperature EPR spectra of the sulfite radical adduct of DMPO, DMPO-. All the reactions were performed in 50 mM phosphate buffer (pH=7.4) containing 0.1 mM DTPA. Spectrum A: DMPO (0.1 M), Fe3+cyt c (0.1 mM), and sulfite (1 mM). Spectrum B: DMPO (0.1 M) and sulfite (1 mM). Spectrum C: DMPO (0.1 M) and Fe3+cyt c (0.1 mM). EPR instrument parameters used were as described in the Materials and Methods section. EPR spectra are sum of 10 scans.
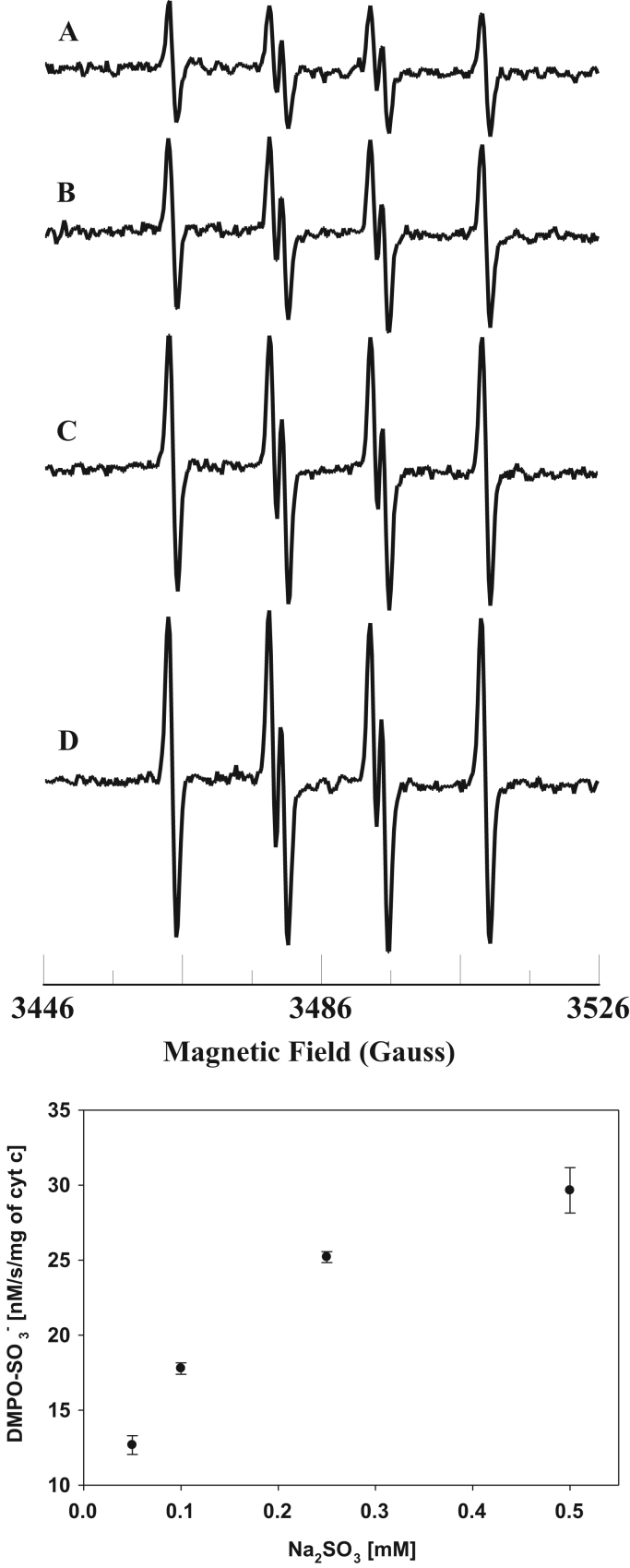
The level of sulfite is increased under various pathological conditions, including environmental exposure [2], [3], [25]. EPR spin trapping studies were carried out with varying concentrations of sulfite. The sulfite concentration dependence of sulfite radical formation is shown in Fig. 2. The EPR signal intensity increases with increasing sulfite concentration (Fig. 2).
Fig. 2.
Top: room temperature EPR spectra of the sulfite radical adduct of DMPO, DMPO-. All the reactions were performed in 50 mM phosphate buffer (pH=7.4) containing 0.1 mM DTPA. Spectrum A: DMPO (0.1 M), Fe3+cyt c (0.1 mM), and sulfite (0.25 mM). Spectrum B: DMPO (0.1 M), Fe3+cyt c (0.1 mM), and sulfite (0.5 mM). Spectrum C: DMPO (0.1 M), Fe3+cyt c (0.1 mM), and sulfite (1 mM). Spectrum D: DMPO (0.1 M), Fe3+cyt c (0.1 mM), and sulfite (2 mM). EPR instrument parameters used were as described in the Materials and Methods section. EPR spectra are sum of 10 scans. Bottom: Plot of the concentration of DMPO- vs Na2SO3. EPR spectra from the top panel were quantified by computer simulation and comparison of the double integral of the observed signal with that of a TEMPO standard (1 μM) measured under identical conditions. Data represent mean±SE (n=3).
3.2. EPR spin trapping studies of the oxidation of sulfite by Fe3+cyt c in the presence of H2O2
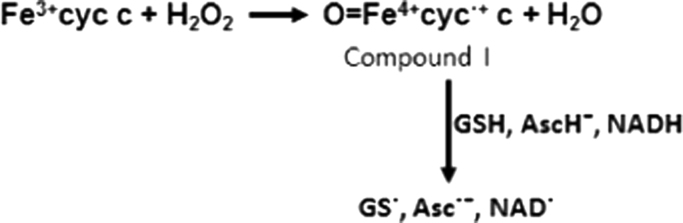
In mitochondria, ~1–2% of the oxygen consumed undergoes partial reduction to form superoxide radical and hydrogen peroxide under physiological conditions [52]. It has been demonstrated that Fe3+cyt c acts as a peroxidase and is involved in the detoxification of H2O2 [31], [38]. During peroxidase activity, Fe3+cyt c reacts with H2O2 to form the peroxidase Compound I-type intermediate, as shown in Scheme 2. The peroxidase activity of Fe3+cyt c oxidizes various endogenous antioxidants/molecules such as GSH, ascorbate, and NADH in the presence of H2O2, as shown in Scheme 2 [31], [49], [53].
Scheme 2.
Mechanism of activation of Fe3+cyt c to a peroxidase Compound I-type intermediate by H2O2 and its oxidation of substrates.
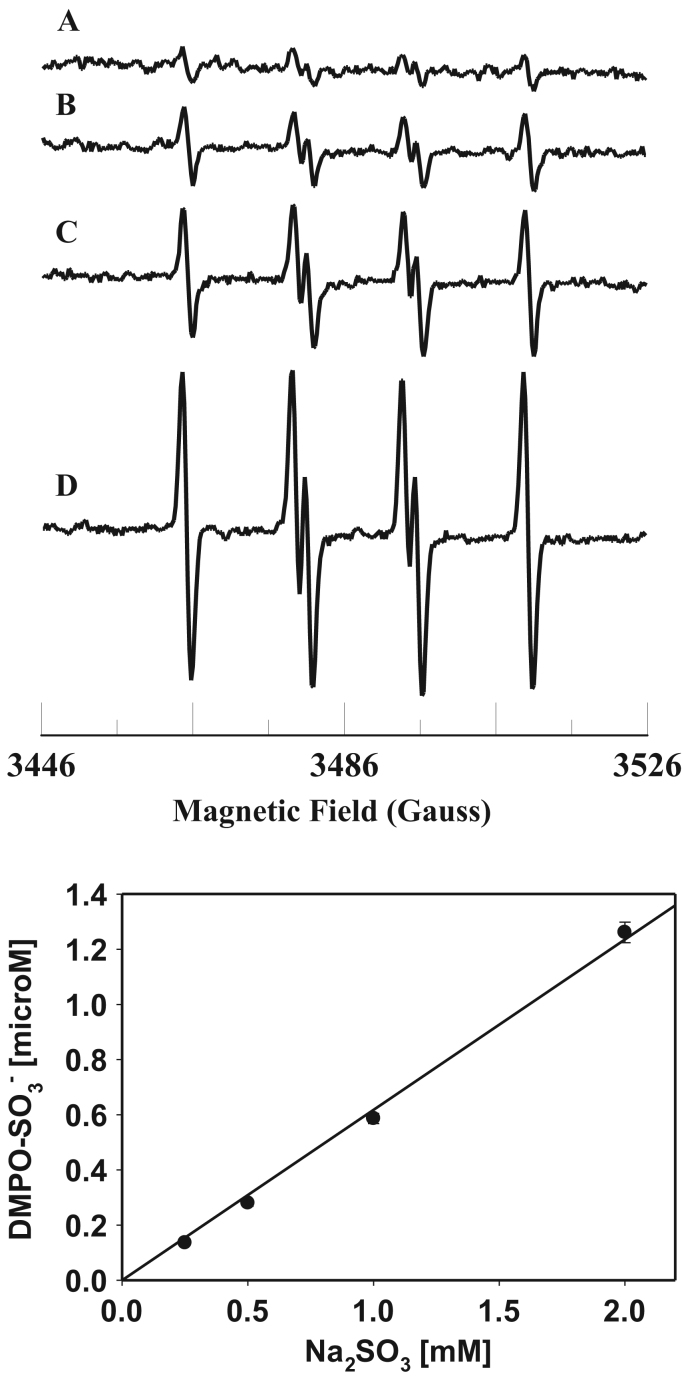
To gain further insight into the molecular mechanisms involved in sulfite toxicity, we studied the oxidation of sulfite and free radical generation by the Fe3+cyt c in the presence of H2O2. EPR spin trapping studies using the spin trap DMPO were carried out to investigate the oxidation of sulfite by Fe3+cyt c and H2O2. EPR spectra were recorded from the reaction mixture containing DMPO (0.1 M), Fe3+cyt c (0.1 mM), H2O2 (0.1 mM), and sulfite (0.5 mM) in the presence of DTPA (0.1 mM). A prominent EPR signal was seen corresponding to the sulfite radical adduct of DMPO (DMPO-), as shown in Fig. 3A. In the absence of Fe3+cyt c, a weak signal of DMPO- was obtained, as shown in Fig. 3B. A trace level of the DMPO- signal was obtained in the absence of H2O2, as shown in Fig. 3C. No EPR signal was obtained in the absence of Fe3+cyt c and H2O2, as shown in Fig. 3D. These results show that H2O2 alone oxidizes sulfite to the sulfite radical, but the oxidation of sulfite to sulfite radical is greatly increased by the combination of Fe3+cyt c and H2O2.
Fig. 3.
Room temperature EPR spectra of the sulfite radical adduct of DMPO, DMPO-. All the reactions were performed in 50 mM phosphate buffer (pH=7.4) containing 0.1 mM DTPA. Spectrum A: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.5 mM), and H2O2 (0.1 mM). Spectrum B: DMPO (0.1 mM), sulfite (0.5 mM), and H2O2 (0.1 mM). Spectrum C: DMPO (0.1 M), Fe3+cyt c (0.1 mM), and sulfite (0.5 mM). Spectrum D: DMPO (0.1 M) and sulfite (0.5 mM). EPR instrument parameters used were as described in the Materials and Methods section. Each EPR spectrum is a single scan.
The level of sulfite is increased due to consumption of food and drink containing sulfite preservatives and under pathological conditions such as SO deficiency [2], [21]. EPR spin trapping studies were carried out with varying concentrations of sulfite and H2O2. The sulfite concentration dependence of sulfite radical formation is shown in Fig. 4. The EPR signal intensity increases with increasing sulfite concentration (Fig. 4, Top). A plot of the initial rate of formation of DMPO- vs concentration of sulfite is linear up to 0.25 mM and decreases at higher concentration of sulfite (0.5 mM) as shown in Fig. 4, (Bottom).
Fig. 4.
Top: room temperature EPR spectra of the sulfite radical adduct of DMPO, DMPO-. All the reactions were performed in 50 mM phosphate buffer (pH=7.4) containing 0.1 mM DTPA. Spectrum A: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.05 mM), and H2O2 (0.1 mM). Spectrum B: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.1 mM), and H2O2 (0.1 mM). Spectrum C: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.25 mM), and H2O2 (0.1 mM). Spectrum D: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.5 mM), and H2O2 (0.1 mM). EPR instrument parameters used were as described in the Materials and Methods section. Each EPR spectrum is a single scan. Bottom: Plot of initial rate of formation of DMPO- versus sulfite concentration. Rates were obtained from the initial slope of the formation of DMPO-. EPR spectra were quantified by computer simulation of the spectra and comparison of the double integral of the observed signal with that of a TEMPO standard (1 μM) measured under the identical conditions. Data represent means±SE (n=3).
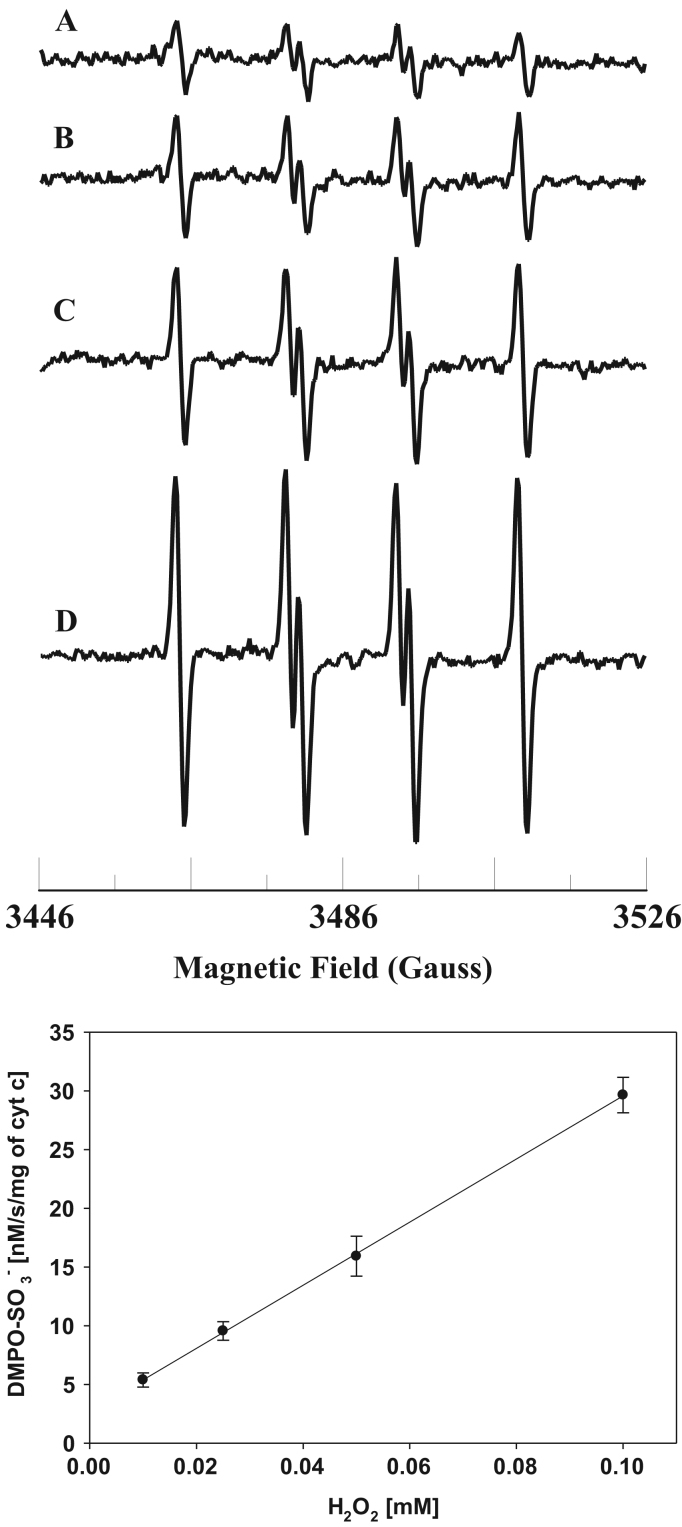
The level of reactive oxygen species (ROS) is increased under various pathological conditions such as ischemia/reperfusion and diabetes [54], [55], [56]. The H2O2 concentration dependence of sulfite radical formation is shown in Fig. 5. The EPR signal intensity increases with increasing H2O2 concentration (Fig. 5, Top). A plot of the initial rate of formation of DMPO- vs concentration of H2O2 is linear up to 0.1 mM as shown in Fig. 5 (Bottom). These results show that formation of sulfite radical increases with increasing level of H2O2.
Fig. 5.
Top: room temperature EPR spectra of the sulfite radical adduct of DMPO, DMPO-. All the reactions were performed in 50 mM phosphate buffer (pH=7.4) containing 0.1 mM DTPA. Spectrum A: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.5 mM), and H2O2 (0.01 mM). Spectrum B: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.5 mM), and H2O2 (0.025 mM). Spectrum C: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.5 mM), and H2O2 (0.05 mM). Spectrum D: DMPO (0.1 M), Fe3+cyt c (0.1 mM), sulfite (0.5 mM), and H2O2 (0.1 mM). EPR instrument parameters used were as described in the Materials and Methods section. Each EPR spectrum is a single scan. Bottom. Plot of initial rate of formation of DMPO- vs H2O2 concentration. Rates were obtained from the initial slope of the formation of DMPO-. EPR spectra were quantified by computer simulation of the spectra and comparison of the double integral of the observed signal with that of a TEMPO standard (1 μM) measured under the identical conditions. Data represent means±SE (n=3).
4. Discussion
In this study, EPR spin trapping experiments were performed to understand the molecular mechanisms involved in the sulfite toxicity. Cyt c is found in high concentrations (0.5–5 mM) in the inner membrane of mitochondria [30]. The results in Fig. 1 show that Fe3+cyt c oxidizes sulfite to the sulfite radical. In addition, the EPR spectra in Fig. 2 show that oxidation of sulfite to sulfite radical by Fe3+cyt c increases with an increasing concentration of sulfite. Studies have demonstrated that the sulfite radical further reacts with oxygen to form peroxymonosulfate and sulfate radicals, which are powerful oxidants, Scheme 3 [51]. However, the spin trap DMPO (100 mM) competes with oxygen and scavenges sulfite radical [51].
Scheme 3.
Reactions of oxidation of sulfite radical to peroxymonosulfate and sulfate radicals through chain propagation.
In cells, reactive oxygen species such as H2O2 are produced by the mitochondria under physiological and pathophysiological conditions [52]. In the mitochondrial intermembrane space, various enzymes are also involved in the generation of ROS [57], [58]. Our EPR spin trapping experiments demonstrate the oxidation of sulfite to sulfite radical by H2O2 (Fig. 3). The direct oxidation of sulfite into sulfite radical by H2O2 occurs and can cause oxidative damage to proteins, lipids, RNA, and DNA. Further, the oxidation of sulfite to sulfite radical by Fe3+cyt c increases in the presence of H2O2. The EPR results in Fig. 4 (Top) show that the oxidation of sulfite by Fe3+cyt c in the presence of H2O2 increases with increasing concentration of sulfite. A plot of the initial rate of formation of DMPO- as a function of sulfite concentration is linear up to 0.5 mM (Fig. 4, Bottom). The EPR spectra in Fig. 5 (Top) show that the oxidation of sulfite by Fe3+cyt c increasing with an increasing concentration of H2O2. A plot of the initial rate of formation of DMPO- as function of H2O2 is linear (Fig. 5, Bottom), indicating a first-order dependence on H2O2. These EPR spin trapping studies show that the increased production of ROS (H2O2) increases the formation of the Compound I intermediate of Fe3+cyt c which oxidizes sulfite to sulfite radical. These results show that the increased production of H2O2 induces increased production of sulfite radical.
Under normal physiological conditions, sulfur containing amino acids such as methionine and cysteine are metabolized to sulfite [59]. In addition, cysteine is also metabolized to H2S, which functions as a cell signaling molecule in biology [59]. Under physiological conditions, mitochondria also rapidly oxidize H2S to thiosulfate and subsequently to sulfite and sulfate [59], [60], [61]. In humans, SO is an essential protein residing in the mitochondrial intermembrane space which catalyzes the essential oxidation/degradation of endogenous or exogenous sulfite to sulfate, which is excreted into the urine [20]. Simultaneously, the reduced SO reduces Fe3+cyt c to Fe2+cyt c, Scheme 1. In the mitochondrial electron transport chain (ETC), Fe2+cyt c donates an electron to cytochrome c oxidase (complex IV), which reduces molecular oxygen to water [62]. Thus, SO and Fe3+cyt c play an important role in protecting the mitochondria/cells/tissues from sulfite toxicity.
In wine, sulfite is used as a preservative and can reach the concentration of 6 mM [3], [4], [63]. In asthmatic patients, asthma symptoms are sometimes worsened after alcohol consumption [64]. A cohort study has reported that sulfite in wine triggers asthmatic reactions [65]. Very little is known about the mechanisms involved in these reactions. EPR spin trapping studies show that oxidation of sulfite to the sulfite radical by Fe3+cyt c in the absence/presence of H2O2 increases with increasing concentration of sulfite. This shows that this reaction may play an important role in the wine-induced asthmatic responses. This also suggests that Fe3+cyt c could be involved in oxidative damage and tissue injury in sulfite-exacerbated allergic reactions.
Sulfur dioxide is one of the major air pollutants near large cities [66]. In the nasal passage and lung, sulfur dioxide is hydrated rapidly into bisulfite and sulfite [67]. In aqueous medium, bisulfite and sulfite are in equilibrium [67]. At physiological pH, sulfite predominates over bisulfite [67]. Exposure to sulfur dioxide induces accumulation of neutrophils into the airways [68]. Sulfite can also stimulate a respiratory burst and ROS production by neutrophils [69]. It has been demonstrated that sulfite is oxidized to the sulfite radical by guinea pig lung microsomes [67]. In humans, SO is expressed in the alveoli and tissues of lung and liver [70], [71]. However, the expression and activity of SO is very low in human lung tissue (135-fold lower than liver) [70]. It has been suggested that various peroxidase enzymes are involved in the oxidation of sulfite to sulfite radical in sulfite toxicity [67]. Very recently, it has been proposed that the peroxidase activity of Fe3+cyt c is involved in the oxidation of phospholipids in the lungs exposed to air pollutants [72]. Highly reactive sulfite radical can oxidize lipids. Our EPR spin trapping experiments show that oxidation of sulfite by Fe3+cyt c in the presence of H2O2 increases the generation of sulfite radical. This study shows that Fe3+cyt c may play a major role in sulfite toxicity in lungs exposed to environmental air pollutants such as sulfur dioxide.
Sulfite is one of the few sulfating agents approved by the Food and Drug Administration as a food preservative and antioxidant to prevent or reduce spoilage [3]. It is also used as an ingredient of many medications, such as antibiotics, analgesics, and anesthetics [2], [5], [6]. It has been suggested that the presence of sulfite in dexamethasone preparations has increased its neurotoxicity of the preparation [73]. However, the molecular mechanisms involved in this neurotoxicity are not known. The increased peroxidase activity of Fe3+cyt c is implicated in various neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, and ALS [46]. Exposure of rat neuronal cells to sulfite increases cell death by apoptosis [18]. The release of cyt c is an early step in apoptosis [42], [43]. Moreover, the peroxidase activity of cyt c increases during apoptosis [74], [75]. Exposure to sulfite results in DNA fragmentation, characteristic of apoptosis [18]. It has been shown that sulfite radical can damage DNA [76], [77]. However, the source of the formation of sulfite radical in neuronal cell death is not known. Based on our EPR spin trapping results, we propose that increased production of sulfite radical due to the oxidation of sulfite by Fe3+cyt c may be responsible for DNA fragmentation, apoptosis, increased neurotoxicity, and neuronal cell death.
In humans, SO is expressed in liver, kidney, skeletal muscle, heart, placenta, and brain [78]. The loss of SO is fatal in infancy or early childhood [21]. In SO-deficient and functional loss of SO patients, the main clinical symptoms are severe progressive neurologic damage, attenuated brain growth, mental retardation and alterations in muscle tone [79], [80]. SO deficiency is also one of the most accepted causes of sulfite hypersensitivity and toxicity [21]. In cases of SO deficiency and molybdenum cofactor deficiency, the levels of sulfite and sulfo derivatives of amino acids, proteins, and various compounds are increased in urine [21], [24]. SO deficiency also results in the accumulation of sulfite in various tissues, especially in the brain [81]. However, the molecular mechanisms involved are not fully understood. In clinical studies of SO and molybdenum cofactor deficiencies, it was proposed that neuronal toxicity was due to decreased ATP or energy deficit/failure [82], [83]. It has also been suggested that excess sulfite might damage mitochondrial function via disruption of membrane integrity [9], [84]. In rat brain, mitochondrial sulfite induced a decrease in ATP synthesis and disturbance of mitochondrial energy homeostasis [9], [81]. In neurons and human fetal liver cells, it has also been shown that ATP is depleted due to sulfite toxicity [9]. Similar observations were made in rat kidney mitochondria and other non-neuronal cells [85]. In addition, it has been shown that sulfite inhibits mitochondrial glutamate dehydrogenase activity [9]. However, the molecular mechanisms involved in the inactivation of mitochondria and its enzymes are still unclear.
Mitochondrial dysfunction contributes to the pathophysiology of neurologic disorders and neurodegenerative diseases [86]. Accumulation of damaged mitochondria is associated with neurodegenerative diseases [87]. The disorders of mitochondrial oxidative phosphorylation are associated with neurodegenerative diseases [88]. Mitochondrial precursor proteins (~99%) are synthesized in cytosol [89]. Precursor proteins targeted to mitochondria are imported in to the mitochondrial matrix by the protein transport machinery localized in the outer and inner membrane of mitochondria [89], [90]. Similarly, RNAs and heme are imported into mitochondria by the PNPASE protein and translocator protein (TSPO) respectively, residing in the mitochondrial intermembrane space [91], [92], [93].
During mitochondrial fission, various nuclear encoded proteins and transcription factors are transported into the preexisting mitochondria and imported before fission and subsequent incorporation into the mitochondrial network [86], [94]. Abnormalities in mitochondrial fission have been identified in several neurodegenerative diseases [86]. It has been demonstrated that sulfite toxicity is due to the increased production of ROS in the mitochondria and inactivation of mitochondrial proteins [9]. Studies have also shown that sulfite radical is capable of damaging DNA/RNA, lipids, and proteins [51], [76], [77], [95], [96], [97]. The increased production of oxidants can damage the mitochondrial proteins, lipids, DNA/RNA and this is implicated in several neurological diseases [86], [91], [98].
Our EPR spin trapping studies show that H2O2 can directly oxidize sulfite to the sulfite radical, and that Fe3+cyt c, a mitochondrial protein, oxidizes sulfite to the sulfite radical in the absence or presence of H2O2. The increased production of sulfite radical in the mitochondrial intermembrane space can damage the proteins, lipids, DNA/RNA and proteins involved in various transport machinery/processes. Moreover, the increased production of oxidants increases the oxidation of mitochondrial innermembrane cardiolipin [31], [99]. Oxidized cardiolipin translocates to the mitochondrial outer membrane and enhances apoptosis [86]. In rats, it has been shown that sulfite disrupts brain mitochondrial energy homeostasis, increases swelling, and induces mitochondrial permeability transition (MPT) pore opening [81]. MPT pore opening inhibits the ETC complex I activity, releases cyt c, and increases apoptosis [81], [100]. In the arterial system, sympathetic neurons dictate the distribution of blood flow and oxygen transport, dependent on need [101]. Defective sympathetic neurons lead to cerebral hypoperfusion [101]. Hence, the increased production of highly reactive sulfite/sulfate radical by the Fe3+cyt c and H2O2 can alter the physiological functions and dynamics of mitochondria and increase apoptosis and necrosis, which results in increased neurotoxicity, hypoperfusion, and neuronal cell death. Therefore, mitochondria are important targets for neuroprotective interventions. Mitochondria have been targeted by many experimental neuroprotective interventions [86]. Our EPR results suggest that radical metabolism may be central to the pathogenesis of SO and molybdenum cofactor deficiencies.
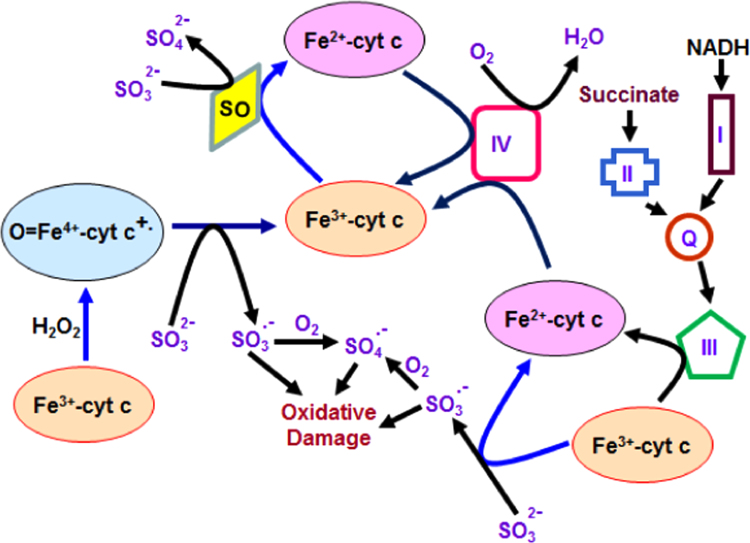
Based on our studies and reported literature, we propose that Fe3+cyt c in the absence and presence of H2O2 is involved in the oxidation of sulfite to the sulfite radical and facilitation of electron flow in the ETC as depicted in Fig. 6.
Fig. 6.
Proposed model of cytochrome c-mediated oxidation of sulfite to sulfite radical and alternative electron transfer pathway in mitochondria. The oxidation of sulfite by sulfite oxidase and cytochrome c are two and one electron processes respectively.
In conclusion, under physiological conditions, SO and Fe3+cyt c play a protective role in detoxifying sulfite in the mitochondrial intermembrane space. However, with elevated levels of sulfite and pathophysiological conditions accompanied by oxidative stress, the oxidation of sulfite by Fe3+cyt c and/or H2O2 causes potentially toxic reactions between the sulfite radical intermediate and biologically important molecules such as proteins, lipids, and DNA. The highly reactive sulfite/sulfate radical can damage the transport machinery involved in the transport of proteins, RNAs, heme, and various other biomolecules in to mitochondria and disrupts mitochondrial energy homeostasis. The oxidation of sulfite into sulfite radical by Fe3+cyt c and/or H2O2 in mitochondria may be responsible for some of the damage which occurs in humans born with SO and/or molybdenum cofactor deficiencies. Targeted clearance and replacement of damaged organelles has been identified as a neuroprotective strategy against acute neural injury [102]. Hence, sulfite-mediated mitochondrial injury may be of key importance in sulfite mediated neurological and systemic pathology.
Acknowledgment
This work was supported by National Institutes of Health Grants EB016096, HL63744, HL65608, and HL38324 (J.L.Z), and 7RO1 HL081734-11 (A.J.C).
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2015.11.025.
Appendix A. Supplementary material
Supplementary material
References
- 1.Griffith O.W. Mammalian sulfur amino acid metabolism: an overview. Methods Enzym. 1987;143:366–376. doi: 10.1016/0076-6879(87)43065-6. [DOI] [PubMed] [Google Scholar]
- 2.Vally H., Misso N.L., Madan V. Clinical effects of sulphite additives. Clin. Exp. Allergy. 2009;39:1643–1651. doi: 10.1111/j.1365-2222.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- 3.Gunnison A.F. Sulphite toxicity: a critical review of in vitro and in vivo data. Food Cosmet. Toxicol. 1981;19:667–682. doi: 10.1016/0015-6264(81)90519-8. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuhashi H., Ikeuchi H., Nojima Y. Is sulfite an antiatherogenic compound in wine? Clin. Chem. 2001;47:1872–1873. [PubMed] [Google Scholar]
- 5.Altland K., Winter P. Potential treatment of transthyretin-type amyloidoses by sulfite. Neurogenetics. 1999;2:183–188. doi: 10.1007/s100480050081. [DOI] [PubMed] [Google Scholar]
- 6.Meisel S.B., Welford P.K. Seizures associated with high-dose intravenous morphine containing sodium bisulfite preservative. Ann. Pharmacother. 1992;26:1515–1517. doi: 10.1177/106002809202601204. [DOI] [PubMed] [Google Scholar]
- 7.Komarnisky L.A., Christopherson R.J., Basu T.K. Sulfur: its clinical and toxicologic aspects. Nutrition. 2003;19:54–61. doi: 10.1016/s0899-9007(02)00833-x. [DOI] [PubMed] [Google Scholar]
- 8.Shih V.E., Abroms I.F., Johnson J.L., Carney M., Mandell R., Robb R.M., Cloherty J.P., Rajagopalan K.V. Sulfite oxidase deficiency. Biochemical and clinical investigations of a hereditary metabolic disorder in sulfur metabolism. N. Engl. J. Med. 1977;297:1022–1028. doi: 10.1056/NEJM197711102971902. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Vincent A.S., Halliwell B., Wong K.P. A mechanism of sulfite neurotoxicity: direct inhibition of glutamate dehydrogenase. J. Biol. Chem. 2004;279:43035–43045. doi: 10.1074/jbc.M402759200. [DOI] [PubMed] [Google Scholar]
- 10.Salman M.S., Ackerley C., Senger C., Becker L. New insights into the neuropathogenesis of molybdenum cofactor deficiency. Can. J. Neurol. Sci. Le. J. Can. Des. Sci. Neurol. 2002;29:91–96. doi: 10.1017/s0317167100001803. [DOI] [PubMed] [Google Scholar]
- 11.Carmi-Nawi N., Malinger G., Mandel H., Ichida K., Lerman-Sagie T., Lev D. Prenatal brain disruption in molybdenum cofactor deficiency. J. Child. Neurol. 2011;26:460–464. doi: 10.1177/0883073810383017. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S., Du J., Jin H., Li W., Liang Y., Geng B., Li S., Zhang C., Tang C. Endogenous sulfur dioxide aggravates myocardial injury in isolated rat heart with ischemia and reperfusion. Transplantation. 2009;87:517–524. doi: 10.1097/TP.0b013e318195fe82. [DOI] [PubMed] [Google Scholar]
- 13.Andersson E., Persson B., Bryngelsson I.L., Magnuson A., Toren K., Wingren G., Westberg H. Cohort mortality study of Swedish pulp and paper mill workers-nonmalignant diseases. Scand. J. Work., Environ. Health. 2007;33:470–478. doi: 10.5271/sjweh.1173. [DOI] [PubMed] [Google Scholar]
- 14.Sunyer J., Ballester F., Tertre A.L., Atkinson R., Ayres J.G., Forastiere F., Forsberg B., Vonk J.M., Bisanti L., Tenias J.M., Medina S., Schwartz J., Katsouyanni K. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study) Eur. Heart J. 2003;24:752–760. doi: 10.1016/s0195-668x(02)00808-4. [DOI] [PubMed] [Google Scholar]
- 15.Mitsuhashi H., Ikeuchi H., Yamashita S., Kuroiwa T., Kaneko Y., Hiromura K., Ueki K., Nojima Y. Increased levels of serum sulfite in patients with acute pneumonia. Shock. 2004;21:99–102. doi: 10.1097/01.shk.0000105501.75189.85. [DOI] [PubMed] [Google Scholar]
- 16.Kajiyama H., Nojima Y., Mitsuhashi H., Ueki K., Tamura S., Sekihara T., Wakamatsu R., Yano S., Naruse T. Elevated levels of serum sulfite in patients with chronic renal failure. J. Am. Soc. Nephrol. 2000;11:923–927. doi: 10.1681/ASN.V115923. [DOI] [PubMed] [Google Scholar]
- 17.Niknahad H., O’Brien P.J. Mechanism of sulfite cytotoxicity in isolated rat hepatocytes. Chem. Biol. Interact. 2008;174:147–154. doi: 10.1016/j.cbi.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Reist M., Marshall K.A., Jenner P., Halliwell B. Toxic effects of sulphite in combination with peroxynitrite on neuronal cells. J. Neurochem. 1998;71:2431–2438. doi: 10.1046/j.1471-4159.1998.71062431.x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen H.J., Fridovich I. Hepatic sulfite oxidase. Purification and properties. J. Biol. Chem. 1971;246:359–366. [PubMed] [Google Scholar]
- 20.Sardesai V.M. Molybdenum: an essential trace element. Nutr. Clin. Pr. 1993;8:277–281. doi: 10.1177/0115426593008006277. [DOI] [PubMed] [Google Scholar]
- 21.Sass J.O., Gunduz A., Araujo Rodrigues Funayama C., Korkmaz B., Dantas Pinto K.G., Tuysuz B., Yanasse Dos Santos L., Taskiran E., de Fatima Turcato M., Lam C.W., Reiss J., Walter M., Yalcinkaya C., Camelo Junior J.S. Functional deficiencies of sulfite oxidase: differential diagnoses in neonates presenting with intractable seizures and cystic encephalomalacia. Brain Dev. 2010;32:544–549. doi: 10.1016/j.braindev.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Bindu P.S., Christopher R., Mahadevan A., Bharath R.D. Clinical and imaging observations in isolated sulfite oxidase deficiency. J. Child. Neurol. 2011;26:1036–1040. doi: 10.1177/0883073811401399. [DOI] [PubMed] [Google Scholar]
- 23.Carmi-Nawi N., Malinger G., Mandel H., Ichida K., Lerman-Sagie T., Lev D. Prenatal brain disruption in molybdenum cofactor deficiency. J. Child. Neurol. 2011;26:460–464. doi: 10.1177/0883073810383017. [DOI] [PubMed] [Google Scholar]
- 24.Topcu M., Coskun T., Haliloglu G., Saatci I. Molybdenum cofactor deficiency: report of three cases presenting as hypoxic-ischemic encephalopathy. J. Child. Neurol. 2001;16:264–270. doi: 10.1177/088307380101600406. [DOI] [PubMed] [Google Scholar]
- 25.Acosta R., Granados J., Mourelle M., Perez-Alvarez V., Quezada E. Sulfite sensitivity: relationship between sulfite plasma levels and bronchospasm: case report. Ann. Allergy. 1989;62:402–405. [PubMed] [Google Scholar]
- 26.Reiss J., Bonin M., Schwegler H., Sass J.O., Garattini E., Wagner S., Lee H.J., Engel W., Riess O., Schwarz G. The pathogenesis of molybdenum cofactor deficiency, its delay by maternal clearance, and its expression pattern in microarray analysis. Mol. Genet. Metab. 2005;85:12–20. doi: 10.1016/j.ymgme.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Mendel R.R. The Molybdenum Cofactor. J. Biol. Chem. 2013;288:13165–13172. doi: 10.1074/jbc.R113.455311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis A.C., Johnson-Winters K., Arnold A.R., Tollin G., Enemark J.H. Kinetic results for mutations of conserved residues H304 and R309 of human sulfite oxidase point to mechanistic complexities. Metallomics: Integr. Biometal Sci. 2014;6:1664–1670. doi: 10.1039/c4mt00099d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hille R. The mononuclear molybdenum enzymes. Chem. Rev. 1996;96:2757–2816. doi: 10.1021/cr950061t. [DOI] [PubMed] [Google Scholar]
- 30.Forman H.J., Azzi A. On the virtual existence of superoxide anions in mitochondria: thoughts regarding its role in pathophysiology. FASEB J. 1997;11:374–375. doi: 10.1096/fasebj.11.5.9141504. [DOI] [PubMed] [Google Scholar]
- 31.Velayutham M., Hemann C., Zweier J.L. Removal of H(2)O(2) and generation of superoxide radical: role of cytochrome c and NADH. Free. Radic. Biol. Med. 2011;51:160–170. doi: 10.1016/j.freeradbiomed.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semak I., Naumova M., Korik E., Terekhovich V., Wortsman J., Slominski A. A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry. 2005;44:9300–9307. doi: 10.1021/bi050202d. [DOI] [PubMed] [Google Scholar]
- 33.Pereverzev M.O., Vygodina T.V., Konstantinov A.A., Skulachev V.P. Cytochrome c, an ideal antioxidant. Biochem. Soc. Trans. 2003;31:1312–1315. doi: 10.1042/bst0311312. [DOI] [PubMed] [Google Scholar]
- 34.Korshunov S.S., Krasnikov B.F., Pereverzev M.O., Skulachev V.P. The antioxidant functions of cytochrome c. FEBS Lett. 1999;462:192–198. doi: 10.1016/s0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- 35.Patil V.A., Fox J.L., Gohil V.M., Winge D.R., Greenberg M.L. Loss of cardiolipin leads to perturbation of mitochondrial and cellular iron homeostasis. J. Biol. Chem. 2013;288:1696–1705. doi: 10.1074/jbc.M112.428938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M., Mileykovskaya E., Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 37.Pfeiffer K., Gohil V., Stuart R.A., Hunte C., Brandt U., Greenberg M.L., Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 38.Kagan V.E., Borisenko G.G., Tyurina Y.Y., Tyurin V.A., Jiang J., Potapovich A.I., Kini V., Amoscato A.A., Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free. Radic. Biol. Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Abe M., Niibayashi R., Koubori S., Moriyama I., Miyoshi H. Molecular mechanisms for the induction of peroxidase activity of the cytochrome c-cardiolipin complex. Biochemistry. 2011;50:8383–8391. doi: 10.1021/bi2010202. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y.R., Deterding L.J., Sturgeon B.E., Tomer K.B., Mason R.P. Protein oxidation of cytochrome C by reactive halogen species enhances its peroxidase activity. J. Biol. Chem. 2002;277:29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- 41.Abriata L.A., Cassina A., Tortora V., Marin M., Souza J.M., Castro L., Vila A.J., Radi R. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J. Biol. Chem. 2009;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., Peng T.I., Jones D.P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 43.Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 44.Kagan V.E., Bayir H.A., Belikova N.A., Kapralov O., Tyurina Y.Y., Tyurin V.A., Jiang J., Stoyanovsky D.A., Wipf P., Kochanek P.M., Greenberger J.S., Pitt B., Shvedova A.A., Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free. Radic. Biol. Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Biase P.M., Paggi D.A., Doctorovich F., Hildebrandt P., Estrin D.A., Murgida D.H., Marti M.A. Molecular basis for the electric field modulation of cytochrome C structure and function. J. Am. Chem. Soc. 2009;131:16248–16256. doi: 10.1021/ja906726n. [DOI] [PubMed] [Google Scholar]
- 46.Everse J., Coates P.W. Neurodegeneration and peroxidases. Neurobiol. Aging. 2009;30:1011–1025. doi: 10.1016/j.neurobiolaging.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Zweier J.L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J. Biol. Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- 48.Creutz C., Sutin N. Reduction of ferricytochrome c by dithionite ion: electron transfer by parallel adjacent and remote pathways. Proc. Natl. Acad. Sci. USA. 1973;70:1701–1703. doi: 10.1073/pnas.70.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velayutham M., Muthukumaran R.B., Sostaric J.Z., McCraken J., Fishbein J.C., Zweier J.L. Interactions of the major metabolite of the cancer chemopreventive drug oltipraz with cytochrome c: a novel pathway for cancer chemoprevention. Free. Radic. Biol. Med. 2007;43:1076–1085. doi: 10.1016/j.freeradbiomed.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler J., Jayson G.G., Swallow A.J. he reaction between the superoxide anion radical and cytochrome c. Biochim. Biophys. Acta. 1975;408:215–222. doi: 10.1016/0005-2728(75)90124-3. [DOI] [PubMed] [Google Scholar]
- 51.Ranguelova K., Chatterjee S., Ehrenshaft M., Ramirez D.C., Summers F.A., Kadiiska M.B., Mason R.P. Protein radical formation resulting from eosinophil peroxidase-catalyzed oxidation of sulfite. J. Biol. Chem. 2010;285:24195–24205. doi: 10.1074/jbc.M109.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Rourke B., Cortassa S., Aon M.A. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawrence A., Jones C.M., Wardman P., Burkitt M.J. Evidence for the role of a peroxidase compound I-type intermediate in the oxidation of glutathione, NADH, ascorbate, and dichlorofluorescin by cytochrome c/H2O2. Implications for oxidative stress during apoptosis. J. Biol. Chem. 2003;278:29410–29419. doi: 10.1074/jbc.M300054200. [DOI] [PubMed] [Google Scholar]
- 54.Zweier J.L., Talukder M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 55.Zorov D.B., Juhaszova M., Sollott S.J. R.O.S. Mitochondrial ROS-induced release: an update and review. Biochim. Biophys. Acta. 1757;2006:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 56.Sivitz W.I., Yorek M.A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu C.A., Donald S.P., Yu J., Lin W.W., Liu Z., Steel G., Obie C., Valle D., Phang J.M. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol. Cell. Biochem. 2007;295:85–92. doi: 10.1007/s11010-006-9276-6. [DOI] [PubMed] [Google Scholar]
- 58.Orr A.L., Quinlan C.L., Perevoshchikova I.V., Brand M.D. A refined analysis of superoxide production by mitochondrial sn-Glycerol 3-phosphate dehydrogenase. J. Biol. Chem. 2012;287:42921–42935. doi: 10.1074/jbc.M112.397828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L., Rose P., Moore P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 60.Tiranti V., Viscomi C., Hildebrandt T., Di Meo I., Mineri R., Tiveron C., Levitt M.D., Prelle A., Fagiolari G., Rimoldi M., Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 61.Jackson M.R., Melideo S.L., Jorns M.S. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51:6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 62.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 63.Casella I.G., Contursi M., Desimoni E. Amperometric detection of sulfur-containing compounds in alkaline media. Analyst. 2002;127:647–652. doi: 10.1039/b111080m. [DOI] [PubMed] [Google Scholar]
- 64.Ayres J.G., Clark T.J. Alcoholic drinks and asthma: a survey. Br. J. Dis. Chest. 1983;77:370–375. [PubMed] [Google Scholar]
- 65.Vally H., de Klerk N., Thompson P.J. Alcoholic drinks: important triggers for asthma. J. Allergy Clin. Immunol. 2000;105:462–467. doi: 10.1067/mai.2000.104548. [DOI] [PubMed] [Google Scholar]
- 66.Rall D.P. Review of the health effects of sulfur oxides. Environ. Health Perspect. 1974;8:97–121. doi: 10.1289/ehp.74897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mottley C., Mason R.P., Chignell C.F., Sivarajah K., Eling T.E. The formation of sulfur trioxide radical anion during the prostaglandin hydroperoxidase-catalyzed oxidation of bisulfite (hydrated sulfur dioxide) J. Biol. Chem. 1982;257:5050–5055. [PubMed] [Google Scholar]
- 68.Shore S.A., Kariya S.T., Anderson K., Skornik W., Feldman H.A., Pennington J., Godleski J., Drazen J.M. Sulfur-dioxide-induced bronchitis in dogs. Effects on airway responsiveness to inhaled and intravenously administered methacholine. Am. Rev. Respir. Dis. 1987;135:840–847. doi: 10.1164/arrd.1987.135.4.840. [DOI] [PubMed] [Google Scholar]
- 69.Beck-Speier I., Liese J.G., Belohradsky B.H., Godleski J.J. Sulfite stimulates NADPH oxidase of human neutrophils to produce active oxygen radicals via protein kinase C and Ca2+/calmodulin pathways. Free. Radic. Biol. Med. 1993;14:661–668. doi: 10.1016/0891-5849(93)90148-n. [DOI] [PubMed] [Google Scholar]
- 70.Beck-Speier I., Hinze H., Holzer H. Effect of sulfite on the energy metabolism of mammalian tissues in correlation to sulfite oxidase activity. Biochim. Biophys. Acta. 1985;841:81–89. doi: 10.1016/0304-4165(85)90276-4. [DOI] [PubMed] [Google Scholar]
- 71.Millard J., Parsons R.B., Waring R.H., Williams A.C., Ramsden D.B. Expression of cysteine dioxygenase (EC 1.13.11.20) and sulfite oxidase in the human lung: a potential role for sulfate production in the protection from airborne xenobiotica. Mol. Pathol. 2003;56:270–274. doi: 10.1136/mp.56.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyurina Y.Y., Kisin E.R., Murray A., Tyurin V.A., Kapralova V.I., Sparvero L.J., Amoscato A.A., Samhan-Arias A.K., Swedin L., Lahesmaa R., Fadeel B., Shvedova A.A., Kagan V.E. Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes. ACS Nano. 2011;5:7342–7353. doi: 10.1021/nn202201j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baud O., Laudenbach V., Evrard P., Gressens P. Neurotoxic effects of fluorinated glucocorticoid preparations on the developing mouse brain: role of preservatives. Pediatr. Res. 2001;50:706–711. doi: 10.1203/00006450-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Godoy L.C., Munoz-Pinedo C., Castro L., Cardaci S., Schonhoff C.M., King M., Tortora V., Marin M., Miao Q., Jiang J.F., Kapralov A., Jemmerson R., Silkstone G.G., Patel J.N., Evans J.E., Wilson M.T., Green D.R., Kagan V.E., Radi R., Mannick J.B. Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proc. Natl. Acad. Sci. USA. 2009;106:2653–2658. doi: 10.1073/pnas.0809279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nur E.K.A., Gross S.R., Pan Z., Balklava Z., Ma J., Liu L.F. Nuclear translocation of cytochrome c during apoptosis. J. Biol. Chem. 2004;279:24911–24914. doi: 10.1074/jbc.C400051200. [DOI] [PubMed] [Google Scholar]
- 76.Shi X., Mao Y. 8-Hydroxy-2’-deoxyguanosine formation and DNA damage induced by sulfur trioxide anion radicals. Biochem. Biophys. Res. Commun. 1994;205:141–147. doi: 10.1006/bbrc.1994.2641. [DOI] [PubMed] [Google Scholar]
- 77.Alipazaga M.V., Cerchiaro G., Moya H.D., Coichev N. Oxidative DNA damage induced by S(IV) in the presence of Cu(II) and Cu(I) complexes. J. Braz. Chem. Soc. 2009;20:1302–1312. [Google Scholar]
- 78.Woo W.H., Yang H., Wong K.P., Halliwell B. Sulphite oxidase gene expression in human brain and in other human and rat tissues. Biochem. Biophys. Res. Comm. 2003;305:619–623. doi: 10.1016/s0006-291x(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 79.Veldman A., Santamaria-Araujo J.A., Sollazzo S., Pitt J., Gianello R., Yaplito-Lee J., Wong F., Ramsden C.A., Reiss J., Cook I., Fairweather J., Schwarz G. Successful treatment of molybdenum cofactor deficiency type A with cPMP. Pediatrics. 2010;125:e1249–e1254. doi: 10.1542/peds.2009-2192. [DOI] [PubMed] [Google Scholar]
- 80.Johnson J.L., Rajagopalan K.V. Human sulfite oxidase deficiency. Characterization of the molecular defect in a multicomponent system. J. Clin. Investig. 1976;58:551–556. doi: 10.1172/JCI108500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grings M., Moura A.P., Amaral A.U., Parmeggiani B., Gasparotto J., Moreira J.C., Gelain D.P., Wyse A.T., Wajner M., Leipnitz G. Sulfite disrupts brain mitochondrial energy homeostasis and induces mitochondrial permeability transition pore opening via thiol group modification. Biochim. Biophys. Acta. 2014;1842:1413–1422. doi: 10.1016/j.bbadis.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Salman M.S., Ackerley C., Senger C., Becker L. New insights into the neuropathogenesis of molybdenum cofactor deficiency. Can. J. Neurol. Sci. 2002;29:91–96. doi: 10.1017/s0317167100001803. [DOI] [PubMed] [Google Scholar]
- 83.Rupar C.A., Gillett J., Gordon B.A., Ramsay D.A., Johnson J.L., Garrett R.M., Rajagopalan K.V., Jung J.H., Bacheyie G.S., Sellers A.R. Isolated sulfite oxidase deficiency. Neuropediatrics. 1996;27:299–304. doi: 10.1055/s-2007-973798. [DOI] [PubMed] [Google Scholar]
- 84.Hughes E.F., Fairbanks L., Simmonds H.A., Robinson R.O. Molybdenum cofactor deficiency-phenotypic variability in a family with a late-onset variant. Dev. Med. Child. Neurol. 1998;40:57–61. doi: 10.1111/j.1469-8749.1998.tb15357.x. [DOI] [PubMed] [Google Scholar]
- 85.Vincent A.S., Lim B.G., Tan J., Whiteman M., Cheung N.S., Halliwell B., Wong K.P. Sulfite-mediated oxidative stress in kidney cells. Kidney Int. 2004;65:393–402. doi: 10.1111/j.1523-1755.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 86.Perez-Pinzon M.A., Stetler R.A., Fiskum G. Novel mitochondrial targets for neuroprotection. J. Cereb. Blood Flow. Metab. 2012;32:1362–1376. doi: 10.1038/jcbfm.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Novak I., Dikic I. Autophagy receptors in developmental clearance of mitochondria. Autophagy. 2011;7:301–303. doi: 10.4161/auto.7.3.14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koopman W.J., Distelmaier F., Smeitink J.A., Willems P.H. OXPHOS mutations and neurodegeneration. EMBO J. 2013;32:9–29. doi: 10.1038/emboj.2012.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chacinska A., Koehler C.M., Milenkovic D., Lithgow T., Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dudek J., Rehling P., van der Laan M. Mitochondrial protein import: common principles and physiological networks. Biochim. Biophys. Acta. 2013;1833:274–285. doi: 10.1016/j.bbamcr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 91.Wang G., Shimada E., Zhang J., Hong J.S., Smith G.M., Teitell M.A., Koehler C.M. Correcting human mitochondrial mutations with targeted RNA import. Proc. Natl. Acad. Sci. USA. 2012;109:4840–4845. doi: 10.1073/pnas.1116792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang G., Chen H.W., Oktay Y., Zhang J., Allen E.L., Smith G.M., Fan K.C., Hong J.S., French S.W., McCaffery J.M., Lightowlers R.N., Morse H.C., 3rd, Koehler C.M., Teitell M.A. PNPASE regulates RNA import into mitochondria. Cell. 2010;142:456–467. doi: 10.1016/j.cell.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaremko L., Jaremko M., Giller K., Becker S., Zweckstetter M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science. 2014;343:1363–1366. doi: 10.1126/science.1248725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ventura-Clapier R., Garnier A., Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc. Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 95.Shi X. Generation of SO3.- and OH radicals in SO3(2-) reactions with inorganic environmental pollutants and its implications to SO3(2-) toxicity. J. Inorg. Biochem. 1994;56:155–165. doi: 10.1016/0162-0134(94)85002-x. [DOI] [PubMed] [Google Scholar]
- 96.Ranguelova K., Bonini M.G., Mason R.P. Bi)sulfite oxidation by copper, zinc-superoxide dismutase: Sulfite-derived, radical-initiated protein radical formation. Environ. Health Perspect. 2010;118:970–975. doi: 10.1289/ehp.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pryor W.A. Free Radical Pathology. Chem. Eng. News. 1971;49:34–36. [Google Scholar]
- 98.Wallace D.C. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 99.Kagan V.E., Tyurin V.A., Jiang J., Tyurina Y.Y., Ritov V.B., Amoscato A.A., Osipov A.N., Belikova N.A., Kapralov A.A., Kini V., Vlasova Q., II, Zhao M., Zou P., Di D.A., Svistunenko I.V., Kurnikov G.G. Borisenko, Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 100.Batandier C., Leverve X., Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J. Biol. Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 101.Eichmann A., Brunet I. Arterial innervation in development and disease. Sci. Transl. Med. 2014;6:252–259. doi: 10.1126/scitranslmed.3008910. [DOI] [PubMed] [Google Scholar]
- 102.Anne Stetler R., Leak R.K., Gao Y., Chen J. The dynamics of the mitochondrial organelle as a potential therapeutic target. J. Cereb. Blood Flow. Metab. 2013;33:22–32. doi: 10.1038/jcbfm.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material