Phong et al. show that depending on the expression of p-Lyn, mast cell activation by antigen can result in dichotomous effects on mast cell function and signaling that can be accentuated by Tim-3 ligation.
Abstract
T cell (or transmembrane) immunoglobulin and mucin domain protein 3 (Tim-3) has attracted significant attention as a novel immune checkpoint receptor (ICR) on chronically stimulated, often dysfunctional, T cells. Antibodies to Tim-3 can enhance antiviral and antitumor immune responses. Tim-3 is also constitutively expressed by mast cells, NK cells and specific subsets of macrophages and dendritic cells. There is ample evidence for a positive role for Tim-3 in these latter cell types, which is at odds with the model of Tim-3 as an inhibitory molecule on T cells. At this point, little is known about the molecular mechanisms by which Tim-3 regulates the function of T cells or other cell types. We have focused on defining the effects of Tim-3 ligation on mast cell activation, as these cells constitutively express Tim-3 and are activated through an ITAM-containing receptor for IgE (FcεRI), using signaling pathways analogous to those in T cells. Using a variety of gain- and loss-of-function approaches, we find that Tim-3 acts at a receptor-proximal point to enhance Lyn kinase-dependent signaling pathways that modulate both immediate-phase degranulation and late-phase cytokine production downstream of FcεRI ligation.
T cell, or transmembrane, immunoglobulin domain and mucin domain (Tim-3) is a type I membrane protein expressed on a variety of innate and adaptive immune cell types. Tim-3 is often referred to as a checkpoint receptor due to its apparent inhibitory function on T cells and its association with activation-induced T cell exhaustion in tumors and chronic viral infection (Sánchez-Fueyo et al., 2003; Jones et al., 2008; Fourcade et al., 2010; Jin et al., 2010; Sakuishi et al., 2010). Recent studies, however, suggest a more nuanced picture of Tim-3 function in T cells, depending on the setting, e.g., acute versus chronic stimulation (Ferris et al., 2014; Gorman and Colgan, 2014). In addition to CD4 and CD8 T cells, Tim-3 is also expressed on other immune cell types, such as NK cells, macrophages, DCs, and mast cells, but its function on these cell types is less clear. Tim-3 blockade was shown to enhance macrophage function in response to sepsis (Yang et al., 2013), and also to regulate antigen (Ag) presentation by DCs, partly through Btk and c-Src (Maurya et al., 2014). On the other hand, Tim-3 expression on monocytes infiltrating the CNS during EAE was shown to promote inflammation (Anderson et al., 2007).
Mast cells are first-line defenders against allergens and invading pathogens as a result of their proximity to the external environment. Cross-linking of IgE bound to the high-affinity IgE receptor FcεRI by Ag leads to the release of preformed mediators and de novo synthesis of proinflammatory and antiinflammatory mediators and cytokines, which together serve to regulate hypersensitivity, autoimmunity, cardiovascular disease, and tumor progression (Kalesnikoff and Galli, 2008). In addition to their well-known pathological roles in allergic responses, mast cells also contribute to defense against bacteria, helminthes, and tumors (Abraham and St John, 2010). It was reported that mast cells constitutively express cell surface Tim-3, and that cross-linking of Tim-3 could enhance cytokine production of IgE-sensitized and Ag-stimulated BM-derived mast cells (BMMCs) and peritoneal mast cells (pMCs) without affecting degranulation (Nakae et al., 2007). TGF-β has been shown to up-regulate expression of Tim-3 in tumor-infiltrating mast cells and a human mast cell line, through a mitogen-activated protein kinase Erk-kinase (MEK)–dependent pathway (Wiener et al., 2006; Yoon et al., 2011). Although previous data suggest that Tim-3 is a positive regulator of mast cell activation, the molecular mechanisms behind the contribution of Tim-3 to mast cell function are still unknown. Importantly, there was until now no genetic evidence addressing the function of Tim-3 in these cells. Given the important role of mast cells as sentinels in both allergic and nonallergic diseases, it is of interest to explore Tim-3 activity on this cell type and how antibody (Ab) modulation can affect its function.
Here, we demonstrate through multiple approaches that Tim-3 functions to enhance proximal FcεRI signaling in mast cells. Cross-linking of Tim-3 with multiple independent antibodies enhanced mast cell degranulation and cytokine release in a dose-dependent manner. Acute knock-down or genetic deficiency of Tim-3 rendered mast cells less responsive to Ag cross-linking of FcεRI, resulting in decreased degranulation and cytokine production. The cytoplasmic tail of Tim-3 was required for co-stimulatory signal transduction in mast cells, together with FcεRI signaling pathways. This was shown in part with the use of recently reported Nur77-GFP transgenic models, which have not previously been used for the study of FcεRI signaling. Collectively, our data demonstrate that Tim-3 acts at a receptor-proximal level to intensify activation of FcεRI-dependent signaling pathways upon Ag cross-linking, while maintaining the threshold for negative signaling of Lyn.
RESULTS
Tim-3 cross-linking enhances cytokine production in IgE/Ag-stimulated BMMCs
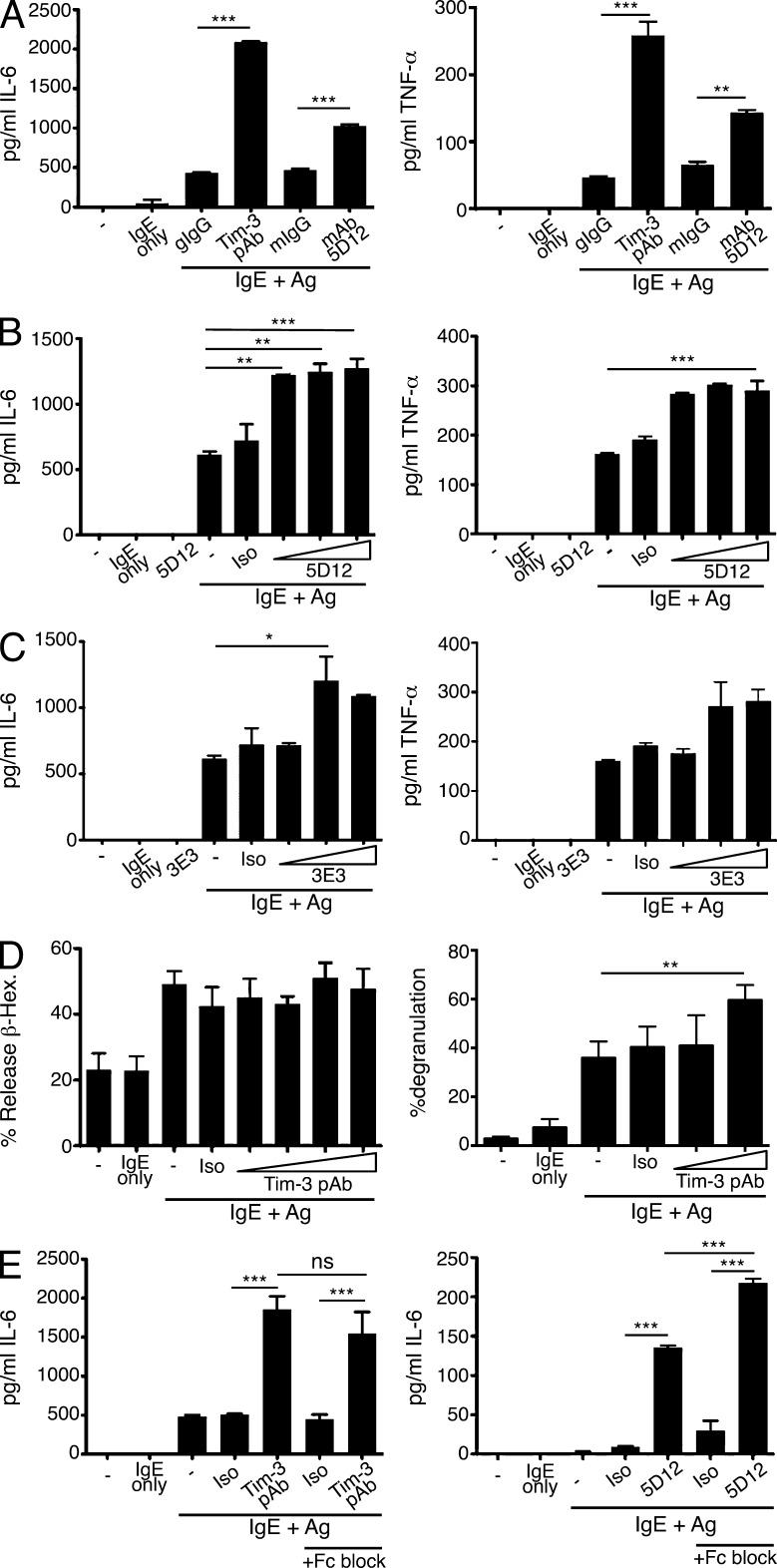
At least one Tim-3 Ab has been shown to enhance cytokine production in Ag-stimulated mast cells (Nakae et al., 2007). We examined the ability of other anti-Tim3 antibodies to co-stimulate FcεRI-mediated mast cell activation. In addition, we wanted to determine whether the effects of Tim-3 antibodies on mast cells might be the result of cross-linking–induced co-stimulatory activity or, as postulated in most T cell studies, an effect of Tim-3 blockade. Thus, 5D12, an Ab that is thought to antagonize the inhibitory activity of Tim-3 in T cells (Anderson et al., 2007; Lee et al., 2011), was able to enhance mast cell IL-6 and TNF cytokine release in BMMCs, albeit not to the same extent as a polyclonal Tim-3 Ab (pAb; Fig. 1 A). We also determined that the 5D12 Ab alone does not induce cytokine production by mast cells (Fig. 1 B). Another, less well-characterized, monoclonal Ab (3E3) also exhibited co-stimulatory activity when combined with IgE/Ag treatment (Fig. 1 C). As previously demonstrated (Nakae et al., 2007), Tim-3 antibodies did not affect Ag-induced mast cell degranulation as read-out by β-hexosaminidase release 30 min after stimulation. However, we were able to detect a small, but significant, increase in the percentage of mast cells degranulating at 90 min after stimulation, using a flow cytometry-based assay (Fig. 1 D). In addition to yielding a higher signal-to-noise ratio that allowed for more accurate assessment of IgE/Ag-induced degranulation, this assay revealed that engagement of Tim-3 may need to be maintained for an extended period of time to mediate this particular co-stimulatory activity. Fcγ receptors, including FcγRIIB, are expressed abundantly on mast cells and have been shown to inhibit the induction of cellular activation programs in an ITIM-dependent manner (Malbec and Daëron, 2007). Therefore, we compared IL-6 release by BMMCs as above, with or without addition of an FcγR-blocking mAb. Although FcγR binding was not a factor at the concentrations of Tim-3 pAb used throughout this study (5 µg/ml), blocking Fcγ receptor binding to the Tim-3 mAb 5D12 actually further enhanced the agonistic effect of this Ab (Fig. 1 E). Thus, the ability of Tim-3 antibodies to enhance mast cell activation was not a result of FcγR binding.
Figure 1.
Tim-3 antibodies enhance IgE/Ag-mediated IL-6 and TNF production in BMMCs with a modest effect on degranulation. (A–C) BM mast cells (BMMCs) were generated from C57BL/6 mice, sensitized with IgE overnight, and then stimulated with either Ag alone, Ag with Tim-3 pAb, mAbs 5D12, 3E3, or the respective isotype controls, for 6 h. Culture supernatants were collected and analyzed for IL-6 (left) or TNF (right) by ELISA. Results shown are representative of three independent experiments performed in duplicates. (D) BMMCs were sensitized overnight with IgE, stimulated with DNP32-HSA alone or together with isotype control, or Tim-3 pAb, for 30 min. Degranulation was assessed by measurement of β-hexosaminidase release (left); alternatively, cells were labeled with LysoTracker Deep Red, sensitized with IgE for 1 h, and stimulated with Ag in the presence of isotype control or indicated amount of Tim-3 pAb for 90 min before Annexin V staining and flow cytometry analysis (right). Results shown are average of three independent experiments performed in triplicates. (E) BMMCs were sensitized and stimulated for 6 h with Ag in the presence of isotype control, Tim-3 pAb (left), or 5D12 (right), with or without Fc block (2.4G2). Supernatants were harvested and analyzed by ELISA for IL-6. Results shown are based on duplicate samples and are representative of two independent experiments. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Tim-3 knockdown or KO attenuates FcεRI-mediated cytokine production in BMMCs
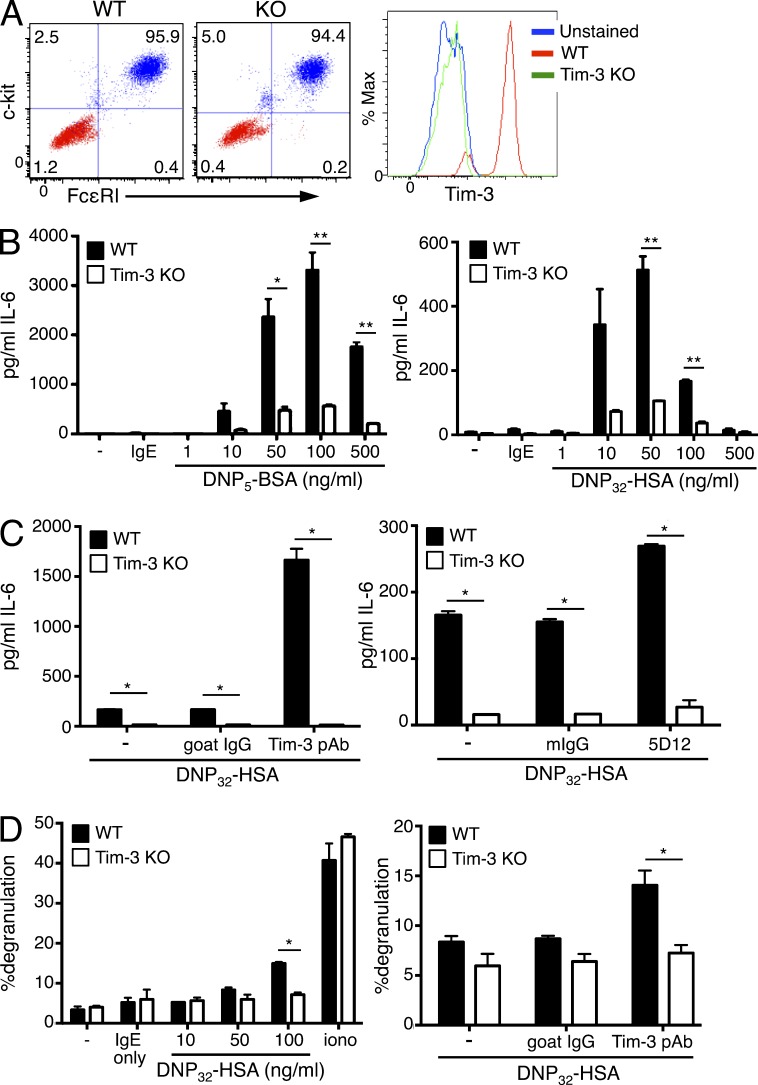
We next determined whether Tim-3 deficiency could impact the maturation and/or function of BMMCs, using Tim-3 KO mice (Gorman et al., 2014). Tim-3 deficient BMMCs exhibited normal development and maturation compared with WT controls, as judged by similar levels of c-kit and FcεRI expression at various times during their in vitro development (Fig. 2 A). Strikingly however, Tim-3 KO BMMCs displayed a significant defect in cytokine production in response to FcεRI activation, using either low (DNP5) or high (DNP32) valency Ag (Fig. 2 B). Of note, we observed significantly stronger stimulation with low valency Ag, likely due to the delayed engagement of negative feedback pathways that serve to down-regulate FcεRI signaling (Xiao et al., 2005; Monu and Frey, 2007; Poderycki et al., 2010; da Silva et al., 2014). Thus, previous studies have noted the preferential engagement of positive or negative signaling pathways upon stimulation with low or high valency (or concentration) of Ag, respectively (Xiao et al., 2005; Mahajan et al., 2014). Importantly, the co-stimulatory effects of Tim-3 pAb or mAb 5D12 were severely impaired in Tim-3 KO BMMCs, which further reinforced the specificity of these antibodies for Tim-3 (Fig. 2 C). In agreement with our findings (Fig. 1 D) that Tim-3 antibodies modestly enhance mast cell degranulation, Tim-3 deficiency impaired Ag-induced degranulation of BMMCs, using the flow cytometry–based assay (Fig. 2 D). Thus, Tim-3 deficiency impaired the ability of BMMCs to generate robust responses to IgE receptor cross-linking, demonstrating that endogenous Tim-3 modulates the intensity of FcεRI signaling upon Ag challenge.
Figure 2.
Tim-3 regulates cytokine production by BMMCs. (A) BMMCs from WT or Tim-3 KO mice were differentiated as described in Materials and methods. Maturity of BMMCs was determined by FcεRI and c-kit staining. Tim-3 surface expression was compared between WT and Tim-3 KO BMMCs, which were sensitized with IgE overnight and stimulated with the indicated amount of Ag and Tim-3 pAb or 5D12 mAb. (B-C) Supernatants were collected after 6 h and analyzed by ELISA for IL-6. (B) BMMCs were stimulated with low valency (DNP5; left) or high valency (DNP32; right) Ag. (C) BMMCs were stimulated with high valency Ag, plus isotype control, Tim-3 pAb (left), or Tim-3 mAb (right). (D) BMMCs were stimulated with high valency Ag alone (left), or with isotype control or Tim-3 pAb (right), followed by quantitation of degranulation by flow cytometry. Results are representative of three independent experiments performed in duplicate. *, P < 0.05; **, P < 0.005.
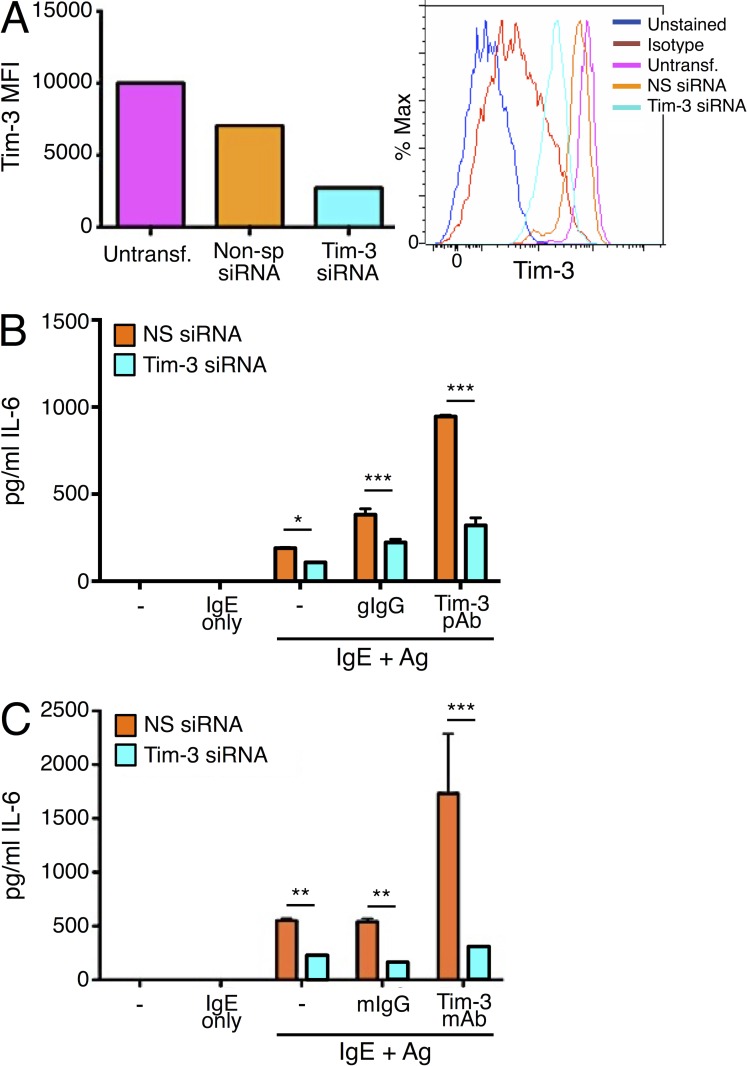
Multiple cis- and trans-acting Tim-3 ligands have been described, and thus far include phosphatidylserine (PS), galectin-9, high mobility group protein B1 (HMGB1), and CEACAM1, none of which is specific to Tim-3 (Han et al., 2013; Anderson, 2014; Gorman and Colgan, 2014; Huang et al., 2015). Furthermore, antibodies targeting Tim-3 have been shown to deliver either agonistic or antagonistic actions, as interpreted in the context of positive or negative effects that Tim-3 may exert on the specific cell type or disease model (Monney et al., 2002; Anderson et al., 2007; Kearley et al., 2007; Fourcade et al., 2010; da Silva et al., 2014; Maurya et al., 2014). It was important to confirm that the effects of Tim-3 deficiency observed in the knockout mice were not caused by secondary effects on mast cell development. Thus, we next determined whether acute Tim-3 deficiency would have a direct impact on mast cell activation, by siRNA knockdown, while taking an agnostic view toward specific Tim-3 ligands. Consistent with a positive role of Tim-3, as demonstrated in Tim-3 KO BMMCs, even incomplete knockdown of Tim-3 (∼80% knockdown efficiency) resulted in a reduction of IL-6 production in Ag-stimulated BMMCs, a defect that was further emphasized when BMMCs were co-stimulated with Ag and Tim-3 pAb (Fig. 3, A and B). Similar results were obtained across multiple batches of BMMCs, as well as when the anti-Tim3 mAb 5D12 was used, providing further evidence that Tim-3 functions as a positive regulator of FcεRI-induced cytokine production in mast cells (Fig. 3 C). Furthermore, our experiments with Tim-3 KO BMMC confirmed that Tim-3 pAb and 5D12 are indeed specific to Tim-3.
Figure 3.
Tim-3 knockdown attenuates Ag-induced cytokine production by BMMC. BMMCs generated from C57BL/6 mice were transfected with control (nonsp) or Tim3-specific siRNA. (A) 48 h later, cells were analyzed by flow cytometry for Tim-3 expression. (B and C) BMMCs were sensitized overnight with IgE and stimulated with DNP32-HSA for 6 h. IL-6 secretion was assessed by ELISA, using either Tim-3 pAb (B) or 5D12 (C) for co-stimulation. Results are representative of three independent experiments, performed in duplicate. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Tim-3 requires tyrosine phosphorylation of its cytoplasmic tail to augment mast cell activation
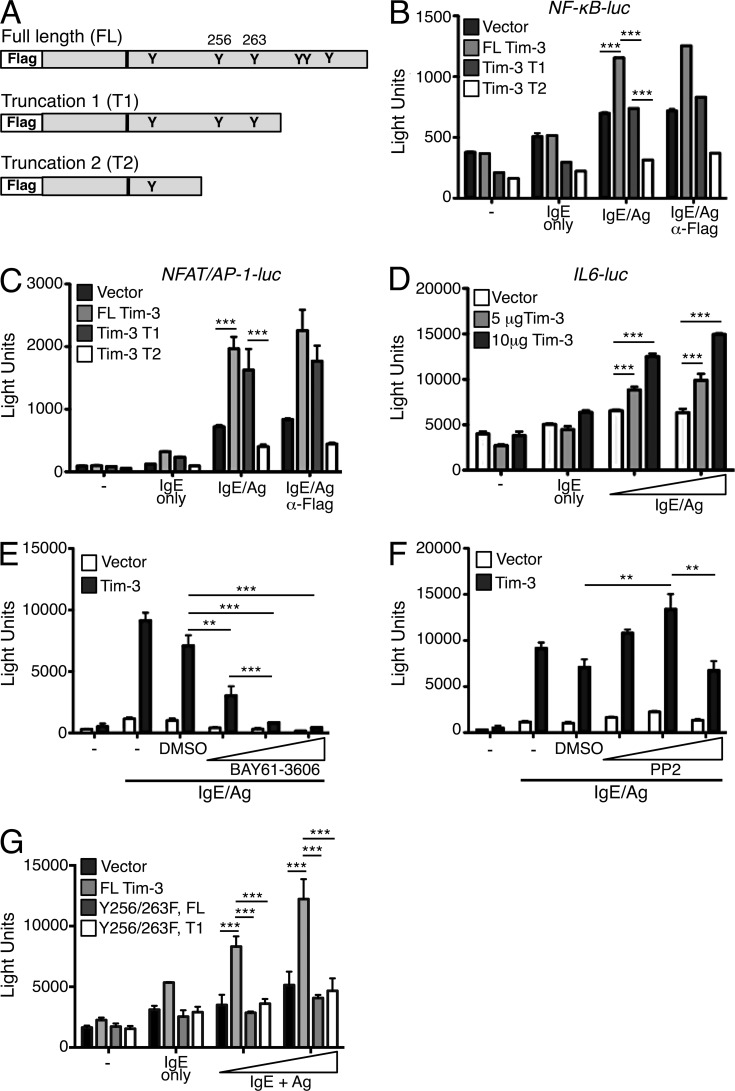
The cytoplasmic tail of Tim-3 contains six conserved tyrosine residues, at least some of which have been shown to couple to the intracellular signaling network downstream of TCR/CD3, based on our previous study (Lee et al., 2011). Contrary to its apparent inhibitory role in vivo, ectopic expression of Tim-3 in human and murine T cell lines augmented CD3/CD28-stimulated NF-κB and NFAT/AP1 activation in a manner that was dependent on the Tim-3 cytoplasmic tail, particularly residues Y256 and Y263 (Lee et al., 2011). Therefore, we asked whether overexpression of Tim-3 in a mast cell line could also augment transcriptional activation by Ag receptor signaling. We expressed our previously described full-length (FL) murine Tim-3, a truncation (T1) lacking the three most distal C-terminal tyrosines, and a truncation (T2) lacking all tyrosines but one membrane-proximal tyrosine, in the mouse mast cell line MC/9 (Fig. 4 A). Similar to our findings in T cells, FL Tim-3 efficiently augmented NF-κB, NFAT, AP1, and NFAT/AP1 composite reporter activity, compared with empty vector control, in IgE/Ag-stimulated mast cells (Fig. 4, B and C; and not depicted). The cytoplasmic tail was important for Tim-3 signal transduction in mast cells, as all reporter co-stimulatory activity was abrogated when the T2 Tim-3 construct was expressed. Although induction of NFAT/AP1 activity required mainly the more N-terminal tyrosines (Fig. 4 C), NF-κB transcriptional activity was sensitive to deletion of both N- and C-terminal tyrosines (Fig. 4, B and C). Intriguingly, the T2 construct appeared to possess dominant-negative activity in these assays. Because Tim-3 cross-linking enhanced IL-6 secretion, and there are binding sites for NF-κB, AP1, and C/EBP in the IL-6 promoter (Akira et al., 1990, 1992; Isshiki et al., 1990; Libermann and Baltimore, 1990; Betts et al., 1993), we examined the IL-6 promoter to determine if it was impacted by Tim-3 overexpression. As expected, FL Tim-3 augmented IL-6 promoter activity in a dose-dependent manner (Fig. 4 D). These results suggest that Tim-3 mediates its co-stimulatory effect on IL-6 production through transcriptional activation of NF-κB and AP1, which in turns drives IL-6 promoter activation and cytokine production.
Figure 4.
Tim-3 cytoplasmic tail tyrosines are required to augment NF-κB, NF-AT/AP1, and IL-6 transcriptional activation in a Syk-dependent manner. (A) MC/9 mouse mast cells were transfected with empty vector (pCDEF3) or one of the indicated Flag-tagged Tim-3 constructs. Transfected MC/9 mast cells were stimulated with IgE and 50 or 100 ng/ml of DNP32-HSA, with or without addition of anti-Flag Ab for 6 h. (B and C) MC/9 mast cells were cotransfected with luciferase reporters for NF-κB (B) or NFAT/AP-1 (C). (D–G) MC/9 mast cells were cotransfected with empty vector (pCDEF3) or full-length Flag-tagged Tim-3 and an IL-6 promoter luciferase reporter. Transfected cells were stimulated with IgE and Ag in the presence or vehicle control (DMSO), BAY61-3606 (E), or PP2 compounds (F) for 6 h. (G) MC/9 mast cells were transfected with the indicated Tim-3 constructs and IL-6 promoter luciferase reporter and stimulated as described above. Results are representative of three independent experiments performed in triplicate. **, P < 0.005; ***, P < 0.0005.
Next, we explored the role of known FcεRI signaling intermediates in Tim-3 function in mast cells. Using IL-6 reporter activity as a read-out, we treated FL Tim-3–transfected MC/9 mast cells with the Src family tyrosine kinases (SFK) inhibitor PP2 or the Syk kinase inhibitor BAY61-3606, which demonstrated potent inhibition of Src phosphorylation and total tyrosine phosphorylation, respectively (unpublished data). IL-6 reporter activity was severely impaired in the presence of the Syk inhibitor, consistent with a strict requirement for Syk in FcεRI signaling (Rivera and Gilfillan, 2006; Gilfillan and Rivera, 2009) and overexpression of Tim-3 did not rescue this inhibition (Fig. 4 E). In contrast, PP2 inhibited IL-6 reporter activity only at high concentrations (10 µM), where pSrc was severely diminished (not depicted), whereas lower concentrations of PP2 actually enhanced IL-6 promoter responses (Fig. 4 F). Tim-3 co-stimulatory activity was also similarly modulated by high versus low concentrations of PP2 (Fig. 4 F). Several SFK family members are expressed in mast cells, including Lyn, Fyn, and Hck, whose positive and/or negative regulatory function can be differentially modulated using either high or low dose PP2 (Poderycki et al., 2010). Thus, low-dose PP2 appears to selectively block the negative regulatory functions of SFKs, whereas high-dose PP2 preferentially affects the positive function. Returning to the tyrosines within the Tim-3 cytoplasmic tail, we previously showed that phosphorylation of tyrosines 256 and 263 in the Tim-3 cytoplasmic tail is essential for its co-stimulatory activity in T cells (Lee et al., 2011). Consistent with this finding, a full-length Tim-3 construct harboring tyrosine to phenylalanine mutation at these sites (Y256/263F) also abrogated Tim-3–augmented IL-6 reporter activation in mast cells. Phosphorylation of tyrosines 256 and 263 appears to be the dominant mode of activation, as additional deletion of the three C-terminal tyrosines did not result in further reduction of IL-6 reporter activity (Fig. 4 G).
Tim-3 shares signaling components with FcεRI pathway
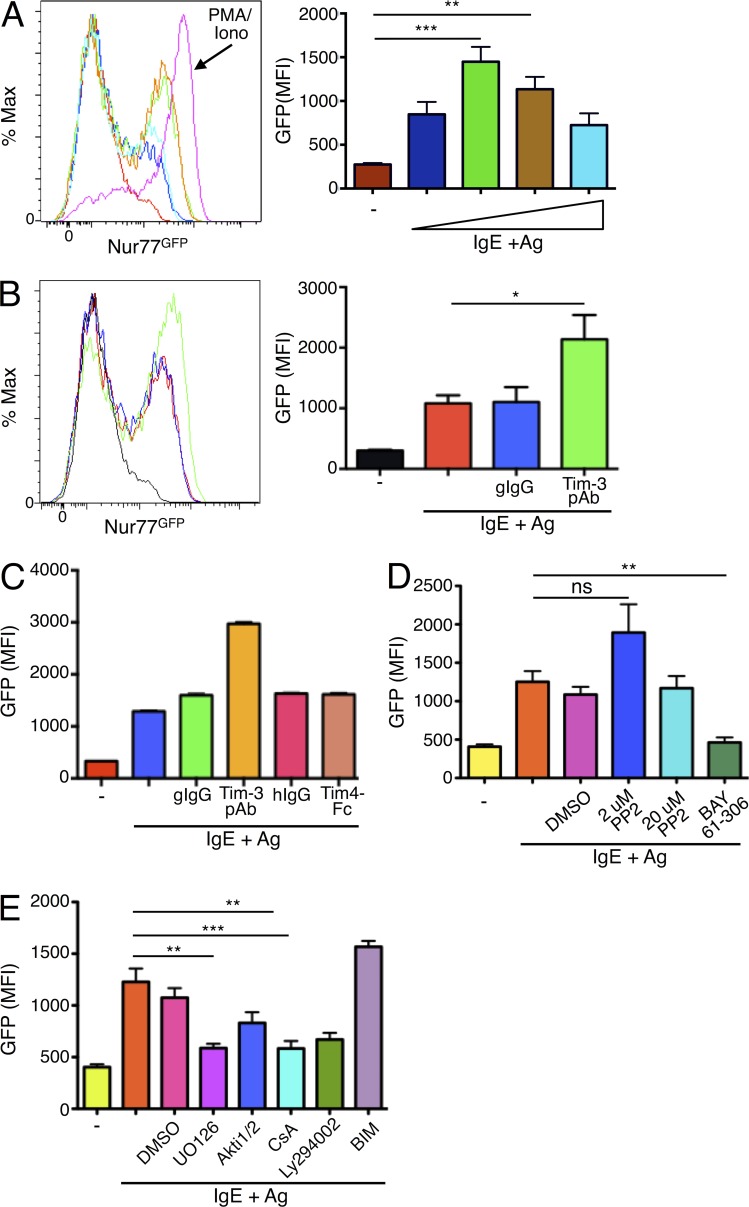
We wanted to further explore the possibility that Tim-3 could augment FcεRI signaling, as opposed to acting through a parallel signaling pathway. We generated BMMCs from transgenic Nur77GFP mice (Zikherman et al., 2012) to determine if Ab-modulated Tim-3 activity could supplement Ag receptor activation. Nur77, also known as NR4A1, is a member of the orphan nuclear receptor subfamily NR4A, and is an immediate early gene that can be induced upon a variety of stimuli (Zhao and Bruemmer, 2010; Mohan et al., 2012; Kurakula et al., 2014). Nur77GFP reporter mice have been useful tools to study development and activation of lymphocytes, as the intensity of T cell and B cell Ag receptor activation is proportional to GFP expression (Moran et al., 2011; Zikherman et al., 2012). We found that Nur77GFP expression could be induced in Ag-stimulated BMMCs in a dose-dependent manner (Fig. 5 A), and its expression was maintained for at least 48 h (not depicted). Nur77GFP induction was further augmented in Tim-3 pAb co-stimulated cells, but was not induced by Tim-3 pAb alone (Fig. 5 B and not depicted). In addition, Tim-3 cross-linking did not augment Nur77GFP induction when administered with a low dose of either the phorbol ester PMA or ionomycin (unpublished data). Our results further revealed that the co-stimulatory effect observed with Tim-3 pAb is unique to Tim-3, as engagement of the related family member Tim-1 (also expressed on BMMCS) by its ligand Tim-4 did not yield similar GFP up-regulation (Fig. 5 C). Thus, our results demonstrate that Tim-3 acts at a point proximal to FcεRI to up-regulate the magnitude of IgE/Ag-dependent signaling, rather than signaling through a parallel pathway.
Figure 5.
Nur77-GFP reporter expression is induced by IgE/Ag and enhanced by coengagement of Tim-3. BMMCs were generated from Nur77GFP reporter mice, sensitized with IgE overnight, and stimulated with Ag. In A, Ag (DNP32-HSA) was titrated over a range of 10–500 ng/ml. PMA plus ionomycin stimulation was used as a positive control. GFP expression was determined after stimulation for 6 h. (B) Nur77GFP BMMCs were stimulated with a fixed concentration of Ag, with or without Tim-3 pAb for 6 h. (C) Nur77GFP BMMCs were stimulated with IgE/Ag and either Tim-3 pAb or Tim4-Fc (which binds to Tim-1), or appropriate isotype controls. (D) Nur77GFP BMMCs were stimulated with IgE/Ag plus either inhibitor to Src kinases (PP2) or Syk (BAY). (E) Nur77GFP BMMCs were stimulated with IgE/Ag and the indicated inhibitors to MEK (UO126), Akt (Akti1/2), calcineurin (CsA), PI3K (LY294002), or PKC (BIM). GFP signal was quantified by flow cytometry. Results are the average of three independent experiments performed in duplicate. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Nur77GFP has been demonstrated to be a faithful reporter for Ag receptor signaling in T and B cells, and now from our findings, in mast cells as well. Given the limited knowledge of how Nur77 expression is regulated in mast cells, we explored the contribution of various signaling pathways downstream of FcεRI to Nur77GFP expression using pharmacologic inhibitors. Similar to our observations with IL-6 reporter activity (Fig. 4), low doses of the Src kinase inhibitor PP2 actually led to an Ag-dependent increase in Nur77GFP induction, whereas high dose PP2 had no effect (Fig. 5 D). In contrast, the Syk inhibitor completely abrogated Ag-stimulated Nur77 expression, a phenomenon comparable to the effects of SFK and Syk inhibitors on Nur77GFP induction in B cells (Fig. 5 D; Zikherman et al., 2012). In addition, Nur77 expression was also regulated by MEK, PI3K/Akt, calcineurin/NFAT, and to a lesser extent, PKC (Fig. 5 E). To confirm our findings, we generated BMMCs and assessed Nur77GFP induction using an independently generated Nur77GFP reporter mouse model (Moran et al., 2011). Although we observed somewhat less robust overall Nur77GFP expression in response to Ag stimulation in mast cells from this strain, Tim-3 cross-linking also induced significant co-stimulation of FcεRI-mediated Nur77GFP expression in this model (unpublished data). Thus, based on results obtained with two independent Nur77GFP reporter strains, we propose that Tim-3 functions to strengthen FcεRI-dependent signaling itself, and not through a parallel pathway.
Bat3 and CEACAM1 are expressed by mast cells and associate with Tim-3
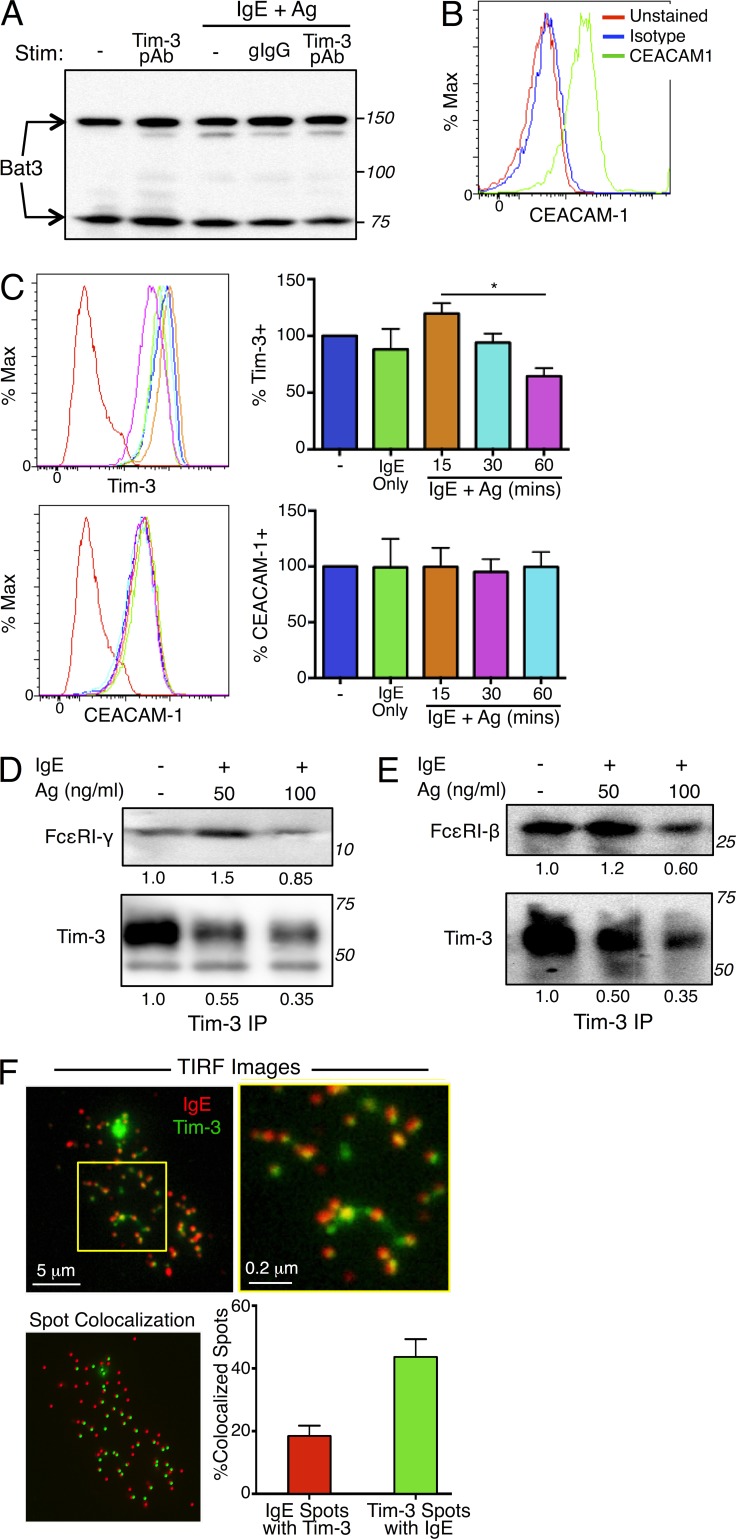
The human leukocyte Ag B (HLA-B)–associated transcript 3 (Bat3) has been proposed to be a negative regulator of Tim-3 and to protect Th1 T cells from Tim-3–mediated cell death and exhaustion (Rangachari et al., 2012). We therefore determined whether the co-stimulatory activity of Tim-3 in mast cells might be caused by a lack of Bat3 expression. We found that Bat3 was expressed in both primary BMMCs and the MC/9 mast cell line and that its expression was not affected by FcεRI cross-linking, either alone or in conjunction with Tim-3 engagement (Fig. 6 A and not depicted). Recently, carcinoembryonic Ag cell adhesion molecule 1 (CEACAM1), a molecule inhibitory activity, was described as a facilitator of Tim-3 surface expression and its subsequent inhibitory function in CD4+ T cells by forming heterodimers with Tim-3 (Huang et al., 2015). As with Bat3, we wanted to determine whether lack of CEACAM1 expression might explain the co-stimulatory activity of Tim-3 on mast cells. We found that CEACAM1 was indeed expressed at a high level on both the MC/9 mast cell line and primary BMMCs (Fig. 6 B). Although Tim-3 expression was reduced upon Ag stimulation, CEACAM1 levels remained relatively unchanged (Fig. 6 C). Thus, the ability of Tim-3 to act as a co-stimulatory molecule to enhance FcεRI-mediated mast cell activation is not caused by an absence of Bat3 and/or CEACAM1 expression in these cells.
Figure 6.
Tim-3 associates with subunits of FcεRI and is comodulated with FcεRI after stimulation with Ag and IgE. (A–C) Tim-3–interacting proteins Bat3 and CEACAM1 are expressed in mast cells. BMMCs were stimulated with IgE/Ag in the presence of isotype control or Tim-3 pAb. Bat3 expression was determined by Western blotting (A). CEACAM1 expression was determined by flow cytometry in the resting state (B) or after stimulation with IgE/Ag (C); Tim-3 modulation was also assessed by flow cytometry. (D and E) Tim-3 IPs were analyzed for the presence of FcεRI γ chain (D; top) or β chain (E; top), or Tim-3 (bottom). (F) BMMCs were transfected with Tim3-mYFP, sensitized with IgE-Alexa Fluor 647 for 1 h, and settled onto poly-D-lysine–treated glass-bottom dishes coated with 1 µg/ml DNP32-HSA. Cells were fixed with 2% PFA after 1 h, and TIRF images were collected. Results are representative of three independent experiments (A–C) or two independent experiments (D–E). *, P < 0.05. The percentage of colocalized spots was derived from the average of 21 cells both labeled with anti-IgE-Alexa 647 and expressing Tim3-mYFP, in three independent experiments.
Cell surface Tim-3 is comodulated with FcεRI after IgE and Ag stimulation
Consistent with the aforementioned findings, which suggest that Tim-3 partners closely with the FcεRI, we observed by coimmunoprecipitation constitutive interaction between Tim-3 and the FcεRI β and γ subunits (Fig. 6, D and E). These interactions were diminished somewhat after Ag stimulation, possibly because of turnover in the total amount of Tim-3 protein. Next, we used total internal reflection fluorescence (TIRF) microscopy to obtain further evidence that Tim-3 localizes with, or proximal to, FcεRI. Thus, we were able to observe partial co-localization of Tim-3 and the FcεRI receptor at the cell surface when a Tim-3-mYFP construct was expressed in either WT or Tim-3 KO BMMCs (Fig. 6 F, yellow clusters; and not depicted). Ligation of FcεRI by IgE/Ag complexes has been shown to trigger receptor endocytosis and subsequent ubiquitin-mediated degradation, resulting in signal termination (Molfetta et al., 2010). Because we observed a reduction of surface Tim-3 upon IgE/Ag stimulation (Fig. 6 C), our data suggested that surface Tim-3 may be internalized by a similar mechanism, perhaps even as part of the same FcεRI complex.
Tim-3 enhances Ag-triggered phosphorylation of PLC-γ1 and ribosomal protein S6
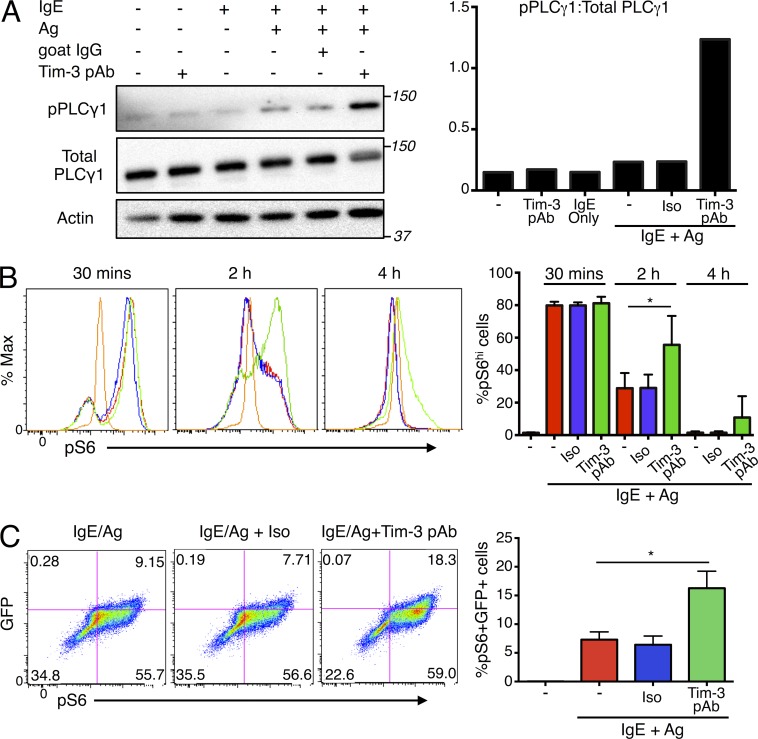
Ag stimulation of FcεRI triggers activation of the Src kinases Lyn and Fyn, which control the primary and complementary pathways downstream of FcεRI, respectively. Receptor aggregation by Ag stimulation leads to Lyn-mediated phosphorylation of the FcεRI β chain, as well as phosphorylation of the γ subunit of the receptor, which promotes Syk recruitment and activation. Syk mediates phosphorylation of downstream adaptor molecules, such as LAT and Slp76, and the enzyme PLC-γ1 (Rivera and Gilfillan, 2006; Gilfillan et al., 2009; Gilfillan and Rivera, 2009). Consistent with enhanced mast cell activation by Tim-3 Abs, the phosphorylation of PLC-γ1 (at Y783) was more robust in BMMCs co-stimulated with Ag and Tim-3 pAb (Fig. 7 A), compared with cells stimulated with Ag alone. One of the major signaling pathways that regulate cell growth, protein synthesis, and metabolism in mast cell is the PI3K–mTOR pathway (Kim et al., 2008). We observed robust phosphorylation of ribosomal protein S6 after 30 min of IgE/Ag stimulation, when 80% of BMMCs exhibited high levels of pS6 and Tim-3 pAb did not have any additive effect (Fig. 7 B). However, Tim-3 cross-linking was able to maintain a significantly larger proportion of pS6high cells for as long as 4 h after Ag stimulation, when pS6 levels had returned to baseline (Fig. 7 B). More importantly, the increase in pS6 correlated with the intensity of FcεRI engagement, as shown with Nur77GFP BMMCs (Fig. 7 C). Overall, these results are consistent with a close physical and functional association between Tim-3 and the FcεRI.
Figure 7.
Tim-3 cross-linking enhances PLC-γ1 and ribosomal protein S6 phosphorylation. (A) BMMCs were sensitized with IgE overnight and stimulated as indicated for 10 min, lysed, and analyzed by Western blotting for phospho-PLC-γ1 (top), total PLC-γ1 (middle), and actin. (right) Quantification of relative proportions of PLC-γ1 phosphorylation. (B and C) Nur77GFP BMMCs were stimulated as indicated and analyzed for pS6 (S235/236) by flow cytometry. Results are quantitated as %pS6hi (B) and %pS6+GFP+ cells (C). Results are representative of three independent experiments. *, P < 0.05.
Tim-3 functions through Lyn kinase to regulate FcεRI activating versus inhibitory signaling
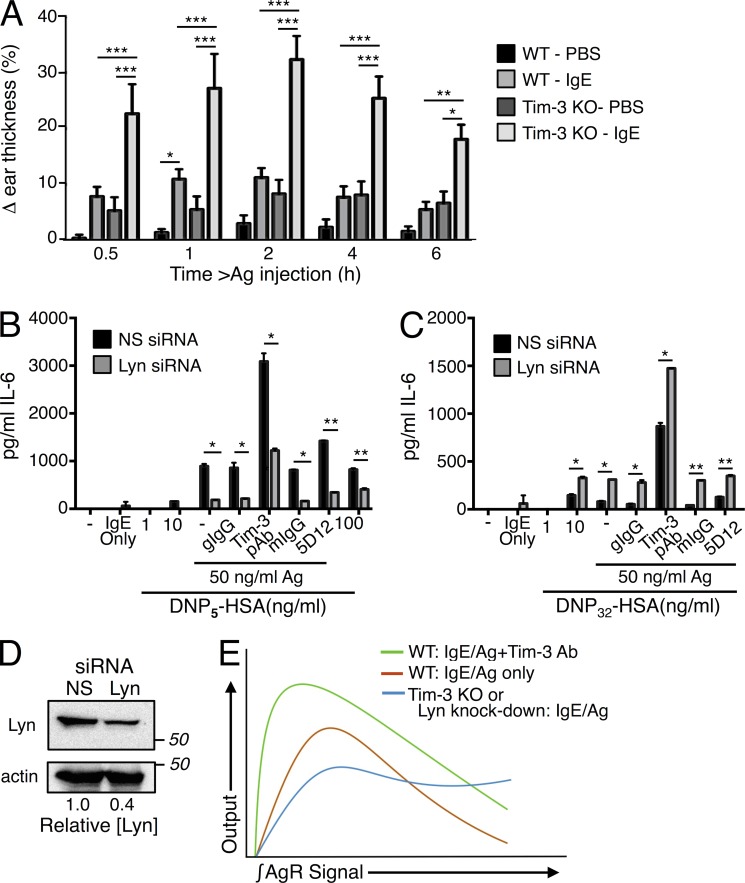
Our in vitro studies thus far showed that Tim-3 signaling can positively regulate IgE/Ag-mediated mast cell degranulation and cytokine production. We next explored whether a similar function for Tim-3 could be observed in an in vivo model of mast cell–dependent anaphylaxis, with the commonly used passive cutaneous anaphylaxis (PCA) model. To our surprise, Tim-3 KO mice displayed a heightened immediate-phase PCA response compared with WT littermates, as measured by increased ear thickness (Fig. 8 A). We did find higher levels serum IgE in some, but not all, naive Tim-3 KO mice (two to three times; unpublished data), consistent with the slightly higher swelling observed in PBS-treated ears of Tim-3 KO mice (Fig. 8 A). However, this would not explain the higher PCA responses, as previous studies indicated that increased basal levels of IgE should result in higher receptor occupancy at baseline and less efficient priming with the exogenously administered IgE used in the PCA model (Odom et al., 2004; Poderycki et al., 2010).
Figure 8.
Tim-3 regulates FcεRI positive versus negative signaling. (A). PCA responses in WT (n = 13) and Tim-3 KO (n = 11) mice were measured as described in the Materials and methods. Results are representative of three independent experiments totaling 19 WT and 14 Tim-3 KO mice. Outliers were removed using QuickCalcs software (GraphPad). Lyn siRNA-treated cells were sensitized with IgE and stimulated with low valency (B) or high valency (C) Ag, plus isotype control, Tim-3 pAb, or Tim-3 mAb. (D) Efficiency of siRNA-mediated Lyn knockdown in BMMCs. Results shown are representative of three independent experiments performed in duplicate. (E) Proposed model for the effects of Tim-3 engagement or deficiency on the functional outputs downstream of FcεRI signaling. Note that the effects of Tim-3 deficiency closely mirror those of partial loss-of-function of Lyn kinase activity.
The PCA response of Tim-3 KO mice is reminiscent of enhanced anaphylaxis observed in the WeeB mouse strain, which expresses a mutant form of Lyn with reduced catalytic activity (Poderycki et al., 2010). Studies using WeeB mice and mast cells revealed that the majority of Lyn kinase activity is required for negative regulation of mast cell signaling, whereas only a small fraction of Lyn activity is needed to induce mast cell activation (Poderycki et al., 2010). Given the stronger anaphylactic response in the absence of Tim-3, it seemed possible that Tim-3 might be involved in tuning receptor signal strength through Lyn kinase, and subsequently its negative effects, leading to enhanced mast cell responses in vivo. To further investigate a possible intimate role for Tim-3 in regulating the relative activity of Lyn, we performed siRNA-mediated knockdown of Lyn in WT BMMCs. We then activated these cells with high or low valency Ag to engage its positive and negative functions, respectively, in the presence of Tim-3 cross-linking. Thus, under conditions of weaker stimulation, where Lyn primarily acts to enhance FcεRI signaling, knocking down Lyn expression resulted in decreased cytokine release that could be enhanced by Tim-3 Ab co-stimulation (Fig. 8 B). Strikingly, under conditions of high intensity stimulation, where the negatively regulatory role of Lyn is revealed, Tim-3 cross-linking was still able to maintain, or even enhance, cytokine secretion (Fig. 8 C). The degree of Lyn knock-down (∼60%) is shown in Fig. 8 D. It should be noted that even with in vitro experiments using BMMC, we occasionally observed enhanced cytokine production by Tim-3–deficient mast cells, compared with WT mast cells, when they were stimulated with very high concentrations of Ag (unpublished data). Thus, collectively, our results suggest an intimate role for Tim-3 in regulating the intensity of FcεRI signaling delivered through Lyn. Furthermore, the effects of Lyn knockdown allowed us to clarify the apparently paradoxical effects of Tim-3 knockout in the PCA model, where it is more difficult to control the strength of Ag receptor stimulation.
DISCUSSION
We have demonstrated here that Tim-3 enhances mast cell activation by IgE/Ag. Similar to our findings in T cells under conditions of ectopic Tim-3 expression (Lee et al., 2011), Tim-3 exerts this effect without the addition of cross-linking antibodies or exogenous ligands, although galectin-9 is present on mast cells, as is the cis-interacting ligand CEACAM1. To determine whether Tim-3 plays a positive or negative role in mast cell activation, we used both siRNA knockdown of endogenous Tim-3 in BMMCs, as well as a Tim-3–deficient mouse strain. Tim-3 knockdown and KO BMMCs exhibited defective degranulation and cytokine production at both high and low intensity IgE/Ag stimulation. Thus, Tim-3 amplifies the intensity and duration of signaling downstream of FcεRI, leading to cytokine production.
Using BMMCs generated from Nur77GFP reporter mice, we show for the first time that Nur77GFP expression can be used as a readout for FcεRI signaling intensity. Similar to T and B cell receptor signaling (Zikherman et al., 2012), we observed a bimodal distribution of GFP in Ag-stimulated BMMCs, with increasing Ag dosage favoring an increase in the frequency of mast cells expressing Nur77GFP, rather than the amount of GFP itself. The same trend was also observed after co-stimulation with Tim-3 Ab. Thus, Tim-3 engagement acts in a similar manner to increasing Ag dose, leading to intensified IgE receptor activation and an increase in the number of cells reaching a critical threshold for activation. To better define which Ag receptor-induced signaling pathways were driving Nur77GFP expression in mast cells, we used various pharmacologic inhibitors. Our results revealed that, similar to Ag receptor signaling in B cells, Syk activity is required for Nur77 transcription in mast cells, whereas calcineurin, PI3K–Akt, and MAPK are less essential. In contrast to T cells where PKC is required for Nur77GFP expression, treatment of mast cells with the pan-PKC inhibitor BIM actually up-regulated GFP expression. Given the differential expression of various PKC species in T cells and mast cells, BIM may have prevented the activation of a particular PKC isoform that is normally involved in negative regulation of Nur77 expression. One candidate for this activity is PKC δ, which was previously shown to act as a negative regulator of mast cell activation (Poderycki et al., 2010).
Another focus of this study was to determine how Tim-3 engagement by antibodies modulates mast cell activation and mediator release. This is a relevant issue, as various Tim-3 antibodies have been used in preclinical models, and are being considered for clinical use as immunotherapeutic agents (Anderson, 2014; Perez-Gracia et al., 2014). Consistent with a previous report that Tim-3 pAb could co-stimulate FcεRI-stimulated IL-4, IL-5, and IL-13 (Nakae et al., 2007), we showed that this co-stimulation occurred in a dose-dependent fashion. We also identified other Tim-3 antibodies that could up-regulate Ag-stimulated cytokine production to varying degrees, notably the monoclonal Ab 5D12, which has often been used as an antagonist in studies of Tim-3 function in T cells (Dardalhon et al., 2010; Veenstra et al., 2012; Huang et al., 2015). Tim-3 Ab co-stimulation did not increase the amount of IL-6 produced by Tim-3 knockdown or KO BMMCs, demonstrating the specificity of the antibodies for Tim-3. In a previous study of dendritic cell regulation, Tim-3 pAb pretreatment was able to induce Tim-3 phosphorylation and block LPS-mediated NF-κB activation through recruitment and activation of Btk and c-Src (Maurya et al., 2014). We have not observed any significant change in Nur77GFP expression or phosphorylation of downstream signaling intermediates after treatment with Tim-3 pAb alone. Thus, our results suggest that the sensitivity of Tim-3 to Ab cross-linking may differ among cell types.
Our decision to focus on Tim-3 engagement by antibodies was based in part on the nonspecific binding of known Tim-3 ligands to other molecules on mast cells. Thus, phosphatidylserine (PS) can bind to several Tim family members besides Tim-3, including Tim-1 and Tim-4, through a conserved binding pocket in the Ig domain (Freeman et al., 2010), in addition to binding to other cell surface receptors (Ravichandran, 2011; Hochreiter-Hufford and Ravichandran, 2013). Galectin-9 has been shown to bind to Tim-3 and mediate down-regulation of Th1 immunity (Zhu et al., 2005). However, galectin-9 is also an IgE-binding lectin and can down-regulate allergic responses by disrupting IgE/Ag complex formation (Niki et al., 2009). Galectin-9 was also shown to bind to the receptor CD44, through which it can regulate inflammation at several levels (Katoh et al., 2007; Nobumoto et al., 2008; Tanikawa et al., 2010; Wu et al., 2014). Tim-3 expressed on tumor-infiltrating DCs interacts with the alarmin HMGB1, a DNA-binding protein associated with cellular injury, and interferes with an HMGB1-activated nucleic acid sensing system in the tumor microenvironment (Chiba et al., 2012). However, HMGB1 can also bind to TLR4, which is expressed on mast cells, and the cellular receptor RAGE (Sims et al., 2010). Thus, engaging Tim-3 through Ab treatment offers a mechanism to more specifically modulate Tim-3 activity. Understanding how various antibodies can enhance or antagonize Tim-3 signaling will be important for the development of more effective Ab-mediated Tim-3 immunotherapies.
In addition to the previously described in-trans ligands of Tim-3, we also examined other effector molecules that have been described as regulators of Tim-3 function. We found that the chaperone Bat3, a putative negative regulator of Tim-3 function in T cells (Rangachari et al., 2012), is expressed in mast cells, but mechanisms of its function are relatively unknown. CEACAM1 was recently described as a binding partner of Tim-3 and was observed to be coexpressed with Tim-3 during the induction of T cell tolerance (Huang et al., 2015). We detected CEACAM1 expression in both the MC/9 mast cell line and primary BMMCs, although its role in mast cell function is unclear. Thus, the potential roles that Bat3 and CEACAM1 may play in regulating mast cell function via Tim-3 require further investigation. Nonetheless, the enhancement of mast cell activation by Tim-3 cannot be solely attributed to the lack of expression of either Bat3 or CEACAM1.
FcεRI is a multichain receptor lacking intrinsic kinase activity and, as such, uses Src family tyrosine kinases (SFKs) for signal initiation and propagation. We showed that the tyrosine-containing cytoplasmic tail of Tim-3, which is indispensable for Tim-3 co-stimulation of T cell activation (Lee et al., 2011), was also required for its co-stimulatory activity in mast cells. NF-κB and NFAT/AP1 promoter activity were most affected when five of the six tyrosines were removed. Although it is possible that Tim-3 might signal through a tyrosine-independent mechanism, we consider this unlikely, given the lack of any other obvious signaling motifs in this region and our previous findings that Fyn kinase could associate and phosphorylate Tim-3 at these residues. We explored the contribution of SFKs involved in mast cell activation in an IL-6 reporter assay using the pan-SFK inhibitor PP2. Surprisingly, Tim-3–mediated IL-6 reporter activity was not affected by high concentrations of PP2, and was in fact enhanced at low concentrations of PP2. In contrast, pharmacologic inhibition of Syk drastically diminished Tim-3–mediated IL-6 reporter activity. These results indicate that Tim-3 activity converges on FcεRI-mediated intracellular pathways that require Syk for both of its catalytic and adaptor functions. Given the complex hierarchical network of multiple SFKs in mast cell regulation, their variable sensitivity to PP2, and the existence of both positive and negative regulatory activities depending on Ag affinity and concentration (Xiao et al., 2005; Hong et al., 2007; da Silva et al., 2014), it will be important to determine the prominent kinases that cooperate with Tim-3 to amplify responses to Ag stimulation. Lyn is a particularly appealing candidate for this activity. Thus, Lyn has been shown to possess both positive and negative regulatory functions in FcεRI activation in response to intensity of stimulus (Xiao et al., 2005), and recruitment and activation of Syk requires phosphorylation of FcεRIγ ITAMs by Lyn (Pribluda et al., 1994; El-Hillal et al., 1997). Previous studies also showed that Lyn is constitutively associated with FcεRIβ in both soluble and lipid raft fractions and that this association is increased upon FcεRI aggregation (Hong et al., 2007). Thus, the majority of the evidence points to a close association between FcεRIβ, Lyn, and Tim-3 under basal conditions. Upon high intensity Ag stimulation, FcεRI is recruited into the lipid rafts, where enhanced β phosphorylation by Lyn occurs to facilitate binding of phosphatases, leading to down-regulation of signaling (Hong et al., 2007).
We also examined the potential signaling pathways leading to enhanced degranulation and cytokine production Tim-3 pAb and Ag co-stimulation. Specifically, Tim-3 cross-linking led to increased Ag-mediated PLC-γ1 phosphorylation, which is necessary for mast cell degranulation, yet failed to enhance Ag-activated phosphorylation of Erk, JNK, or p38. PLCγ1 is a substrate of Syk and increased PLCγ1 activity implies that Syk activity may also be positively regulated by Tim-3 engagement. However, we did not reliably observe increased Syk phosphorylation after Tim-3 co-stimulation (unpublished data). Syk is phosphorylated on several tyrosines, which are differentially regulated by either autophosphorylation or transphosphorylation by SFKs, such as Lyn (Sanderson et al., 2010). Because we only examined phosphorylation of Y519 and Y520, indicators of Syk autophosphorylation, it is possible that Lyn-mediated phosphorylation of other activating tyrosines could be promoted by Tim-3 cross-linking. PI3K–mTOR signaling pathways are crucial to mast cell survival, proliferation, protein synthesis, and metabolism. Here, we showed that Tim-3 enhancement of Ag-activated cytokine secretion correlates with sustained S6 phosphorylation. Tim-3 pAb co-stimulation was previously shown to rescue IL-3 withdrawal-mediated apoptosis of mast cells, through induction of Ag-mediated IL-3 release (Nakae et al., 2007). Our data provide additional evidence to support a direct role for Tim-3 in mast cell survival, proliferation, and cytokine production.
Studies using pharmacologic inhibitors and genetic models have unraveled a complex network of positive and negative regulation of mast cell activation by SFKs, and Lyn in particular. Our studies suggest that Tim-3 can fine-tune FcεRI signal strength and modulate the negative and positive aspects of Lyn kinase activity. We found that Tim-3 colocalizes with FcεRI complex at the cell surface and associates with FcεRI β and γ subunits. Given the involvement of the FcεRI β subunit ITAM in mediating negative regulatory activity of Lyn, it is possible that Tim-3 cross-linking controls this negative signal by competing with Lyn for association with FcεRIβ. In addition, further studies are needed to determine whether Tim-3 engagement or deficiency affects activation or localization of Lyn. Although Lyn is the major SFK in mast cells, other members such as Fyn, Hck, and Fgr play more straightforward roles as positive regulators of mast cell activation. Lyn is known to negatively control Fyn and Fgr activity (Odom et al., 2004; Lee et al., 2011). Therefore, Tim-3 cross-linking may preferentially enhance Fyn, Hck, or Fgr kinase activity, bringing the net SFK signal to a positive one.
We propose a model in which Tim-3 cross-linking provides a co-stimulatory signal (or set of signals) that integrates into the stimulatory signals that regulate IgE/Ag-mediated mast cell activation. In the absence of Tim-3, there is reduced functional output as a result of decreased Ag receptor intensity. However, Ag receptor signaling at high concentrations (or valency) of Ag is more complex, and the altered output may be attributed to reduced Lyn activity, most of which is involved in negative feedback signaling (Poderycki et al., 2010), leaving the residual kinase activity to enhance biological functions (Fig. 8 E). Performing the PCA assays with weaker Ag stimulation (i.e., varying either the dose or valency) may help to confirm or refine this model. However, with the standard PCA model, the response rapidly declines to undetectable levels below the amount of Ag that we and others have used (Poderycki et al., 2010). Further testing of this model in vivo will require more sensitive assays for mast cell function, as well as more sophisticated models of conditional Tim-3 deficiency.
Collectively, our findings suggest that Tim-3 promotes mast cell activation and cytokine production by closely associating with FcεRI and its proximal signaling pathways. Contrary to the negative regulatory role that has been described in other innate and adaptive immune cells, Tim-3 activity in mast cells is unequivocally positive, despite the presence on mast cells of multiple Tim-3 ligands, which have been previously described to mediate inhibition of cellular activation through Tim-3. Aside from their well-known role in hypersensitivity, mast cell involvement in inflammation-associated with cancer, and other nonallergic diseases has been increasingly appreciated (Bischoff, 2007). Tim-3 is detected on tumor infiltrating human mast cells and its expression is increased upon TGF-β treatment (Wiener et al., 2006). Increased numbers of Tim-3/chymase double-positive mast cells have been shown to correlate with the severity of chronic inflammatory periodontitis (Huang et al., 2014). Manipulation of Tim-3 activity on mast cells could be a promising target for development of novel therapeutic modalities to combat cancer and allergic and autoimmune disease.
MATERIALS AND METHODS
Antibodies and reagents
Monoclonal anti-dinitrophenyl (DNP) Ab, IgE isotype, clone SPE-7, anti-Flag Ab M2, DNP32 –HSA, 4-Nitrophenyl N-acetyl-β-D-glucosaminide (pNAG), and cyclosporin A (CsA) were obtained from Sigma-Aldrich. DNP5–BSA was purchased from Biosearch Technologies. Purified polyclonal Ab (Tim-3 pAb), directly fluorescence-conjugated Ab to murine Tim-3, and normal goat IgG control were purchased from R&D Systems. Purified mouse IgG control and mouse Fc block (clone 2.4G2) were obtained from BD. Ab to FcεRI β chain was purchased from Santa Cruz Biotechnology. Antibodies to Bat3 and FcεRI γ chain were purchased from EMD Millipore. Monoclonal antibodies to murine Tim-3 (5D12, 3E3) and purified Tim4-Fc were obtained from V. Kuchroo (Harvard Medical School, Cambridge, MA). Phosphospecific Abs to ERK (T202/Y204), PLC-γ1 (Y783), and total PLC-γ1 were obtained from BD. Phosphospecific antibodies to p38 MAPK (T180/Y182), SAPK/JNK (T183/Y185), and total Lyn were purchased from Cell Signaling Technology. Fluorescent antibodies to CEACAM1/2 (CD66a) and mouse IgG isotype control were obtained from eBioscience.
Phorbol myristate acetate (PMA), ionomycin, aprotinin, leupeptin, pepstatin, sodium orthovanadate, 4-(2-aminoethyl)benzene sulfonyl fluoride (AEBSF), inhibitors to Src-family kinases (PP2), Syk (BAY61-3606), PI3K (LY294002), MEK (UO126), and Akt (Akti 1/2) were purchased from EMD Biosciences. Inhibitor to PKC (Bisindolylmaleimide [BIM] VIII) was purchased from Cayman Chemical. IL-6 luciferase reporter constructs (full length, mutants lacking binding sites for NF-κB, AP1, C/EBP, and NF-κB/C/EBP) were obtained from S. Gaffen (University of Pittsburgh, Pittsburgh, PA), and were originally obtained from O. Eickelberg (Helmholtz Zentrum Munchen, Munchen, Germany).
Mice
Tim-3 KO mice were originally obtained from John Colgan (University of Iowa, Iowa City, IA). The Nur77GFP reporter mouse, C57BL/6-Tg(Nr4a1-EGFP/cre)820Khog/J, was purchased from The Jackson Laboratory. Additional Nur77GFP mice were obtained from A. Weiss and J. Zikherman (University of California, San Francisco, CA)All mouse strains had been backcrossed for at least 10 generations to C57BL/6 and were maintained in the University of Pittsburgh Animal Facility. Age-matched female WT C57BL/6 mice (7–8 wk old) were purchased from The Jackson Laboratory and housed for 7 d before use as control for PCA experiments. All studies were performed in accordance with University of Pittsburgh Institutional Animal Care and Use Committee procedures.
BMMC and mast cell line culture
BM cells from C57BL/6 WT and KO mice were cultured in RPMI-1640 supplemented with 10% BGS, nonessential amino acid, 2-ME, Hepes, penicillin/ streptomycin with glutamine, and 20% IL-3–conditioned media for 4–6 wk, after which >95% of viable cells are mature mast cells (c-kit+ FcεRI+ by flow cytometry). MC/9 mast cells were maintained in DMEM supplemented with 10% BGS, 2-ME, penicillin/streptomycin with glutamine, and 10% IL-3–conditioned media.
BMMC stimulation and cytokine measurement
BMMCs were sensitized with 1 µg/ml IgE overnight in complete media without IL-3. Cells were then stimulated with either anti-IgE DNP32-HSA or DNP5-BSA in IL-3–free media for indicated times. Supernatants were assayed for murine IL-6 and TNF by ELISA (BioLegend) 6 or 24 h after stimulation.
β-hexosaminidase release and flow cytometry assays for mast cell degranulation
BMMCs (2.5 × 105 cells) were stimulated in Tyrode’s buffer (135 mM NaCl, 5 mM KCl, 5.6 mM glucose, 1.8 mM CaCl2, 1 mM MgCl2, 20 mM Hepes, and 0.5 mg/ml BSA). 30 min after stimulation, supernatants (stimulated release) were collected and cells were lysed with 200 µl of 0.5% Triton X-100 in PBS for 15 min on ice. 20 µl each of lysate (content) and stimulated release were mixed with 20 µl of 1 mM pNAG substrate for 1 h at 37°C. Reaction was stopped by addition of 200 µl of carbonate buffer (0.1 M, pH 9.0) and absorbance was read at 405 nm. Percentage of β-hexosaminidase release was calculated using the following equation: % release = (releasestimulated/contenttotal) × 100.
Measurement of degranulation by flow cytometry was conducted as previously described (Demo et al., 1999; Sumpter et al., 2015). In brief, BMMCs were loaded with 0.1 µM of LysoTracker Deep Red (Invitrogen) for 30 min at 37°C and then sensitized with 1 µg/ml IgE for 1 h. Receptor cross-linking was induced by addition of DNP32-HSA at indicated concentration or 2 µM ionomycin as positive control for 90 min before Annexin V staining (BioLegend). Percent of degranulation was determined as percentage of BMMCs that was AnnexinV+Lysotrackerlo.
Transcriptional reporter assays
MC/9 mast cells (15 × 106) were transfected with 15 µg of NF-κB-luc, NFAT/AP1-luc, NFAT-luc, AP1-luc, or IL-6-luc together with the indicated amount of empty vector (pCDEF3), or FLAG-tagged Tim-3: full length (FL), truncation 1, or truncation 2 mutants (T1 or T2). Transfection was by electroporation at 290 V, 950 μF using a Gene Pulser II apparatus (Bio-Rad Laboratories). 24 h later, transfected cells were collected and stimulated with 0.5 µg/ml IgE and the indicated amount of DNP32-HSA Ag for 6 h. Luciferase assays were conducted as described previously (Kane et al., 1999).
siRNA knock-down of Tim-3 and Lyn in BMMC by nucleofection
BMMCs (3 × 106) were transfected with 100 pmol of nonspecific, Tim-3, or Lyn siRNA (GE Healthcare) using the mouse macrophage nucleofector kit (Lonza), Y-001 program, and the Nucleofector II/2b device (Lonza). Transfected cells were collected after 48 h and efficiency of knockdown was determined by Tim-3 staining, followed by flow cytometry. Efficiency of Lyn knockdown was determined by Western blotting.
Immunoprecipitation and Western blotting
BMMCs or MC/9 were stimulated as described above. Cells were lysed in 1% Nonidet-P-40 lysis buffer (20 mM Tris-HCl, pH 7.5, and 150 mM NaCl) supplemented with AEBSF, aprotinin, leupeptin, pepstatin, sodium fluoride, sodium orthovanadate, and β-glycerophosphate on ice for 20 min. Lysates were centrifuged at 4°C for 15 min. For Western blotting analysis, SDS-containing sample buffer was added to lysates before loading onto 10 or 12% SDS-PAGE gels when analyzing for FcεRI β and γ chains. For IPs, lysates were incubated overnight at 4°C with appropriate antibodies. Immune complexes were precipitated at 4°C for 2 h with protein A or G agarose beads (Thermo Fisher Scientific) for rabbit or mouse Abs, respectively, then washed with NP-40 lysis buffer three times before analysis by SDS-PAGE gels. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes using the PierceG2 fast blotter (Thermo Fisher Scientific), probed with appropriate antibodies, and imaged on a ProteinSimple Fluochem M cooled charge-coupled device imager (ProteinSimple).
TIRF microscopy
Anti-DNP IgE was labeled with Alexa Fluor 647 (Life Technologies). BMMCs were transfected with Tim-3-mYFP construct by nucleofection (Lonza). 24 h after transfection, BMMCs were sensitized with 1 µg/ml IgE-Alexa Fluor 647 for 1 h at 37°C. 3 × 105 cells were loaded into poly-D-lysine–treated glass bottom dishes (Thermo Fisher Scientific) coated with 1 mg/ml DNP32-HSA Ag in PBS for 1 h and fixed with 2% PFA. Images were captured in TIRF mode using Nikon Eclipse Ti Live Cell Microscope equipped with Andor Zyla VSC-00311 camera and Apo TIRF 100× oil DIC N2 objective, and analyzed with NIS-Elements Ar 4.20 software (Nikon). Colocalization analysis was performed using the Spots Colocalize function of the Imaris Scientific 3D/4D Imaging Processing and Analysis software (Bitplane).
Passive cutaneous anaphylaxis (PCA)
Mice were sensitized by intradermal injection of anti-DNP IgE (50 ng/50 µl) or equal volume of vehicle (PBS) in each ear. 24 h later, mice were challenged i.v. with DNP32-HSA (100 µg/200 µl in PBS). Mouse ear edema was evaluated by measuring ear swelling at indicated time points. The percent change in ear thickness of PBS- or IgE-treated ears was determined as changes above baseline before Ag challenge. Results are represented as the mean ± SEM. Two-way ANOVA analysis was performed with Bonferroni post hoc multiple comparison test. Outliers were removed using Outlier calculator (GraphPad software).
Statistical analysis
All statistical analysis was performed using Prism software (GraphPad Software). Paired, two-tailed Student’s t test, one-way and two-way ANOVA with Bonferroni post hoc test were used for data analysis and determination of p-values, as appropriate.
Acknowledgments
We thank A. Weiss and J. Zikherman for providing Nur77GFP mice, and A. Weiss for critical reading of the manuscript. We also thank C. Carey for assistance with the PCA experiments.
This work was supported by Public Health Service grants R56AI067544 and P01AI073748 to L.P. Kane, R01AI093737 and R01AI054821 to J.D. Colgan, and 1K01AR067250-01A1 to T.L. Sumpter.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- Ab
- antibody
- Ag
- antigen
- BMMC
- BM-derived mast cell
- CEACAM1
- carcinoembryonic Ag cell adhesion molecule 1
- DNP
- 2,4-Dinitrophenyl
- HAS
- human serum albumin
- HMGB1
- high mobility group protein B1
- NR4A1
- nuclear receptor subfamily 4, group A, member 1
- Nur77
- nuclear hormone receptor 77
- PCA
- passive cutaneous anaphylaxis
- PKC
- protein kinase C
- PLC-γ1
- phospholipase C-γ1
- PS
- phosphatidylserine
- SFK
- Src family tyrosine kinase
- Tim-3
- T cell immunoglobulin and mucin domain protein 3
References
- Abraham S.N., and St John A.L.. 2010. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10:440–452. 10.1038/nri2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., and Kishimoto T.. 1990. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 9:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Isshiki H., Nakajima T., Kinoshita S., Nishio Y., Natsuka S., and Kishimoto T.. 1992. Regulation of expression of the interleukin 6 gene: structure and function of the transcription factor NF-IL6. Ciba Found. Symp. 167:47–62, discussion :62–67. [DOI] [PubMed] [Google Scholar]
- Anderson A.C. 2014. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2:393–398. 10.1158/2326-6066.CIR-14-0039 [DOI] [PubMed] [Google Scholar]
- Anderson A.C., Anderson D.E., Bregoli L., Hastings W.D., Kassam N., Lei C., Chandwaskar R., Karman J., Su E.W., Hirashima M., et al. 2007. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 318:1141–1143. 10.1126/science.1148536 [DOI] [PubMed] [Google Scholar]
- Betts J.C., Cheshire J.K., Akira S., Kishimoto T., and Woo P.. 1993. The role of NF-kappa B and NF-IL6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by interleukin-1 and interleukin-6. J. Biol. Chem. 268:25624–25631. [PubMed] [Google Scholar]
- Bischoff S.C. 2007. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 7:93–104. 10.1038/nri2018 [DOI] [PubMed] [Google Scholar]
- Chiba S., Baghdadi M., Akiba H., Yoshiyama H., Kinoshita I., Dosaka-Akita H., Fujioka Y., Ohba Y., Gorman J.V., Colgan J.D., et al. 2012. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 13:832–842. 10.1038/ni.2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V., Anderson A.C., Karman J., Apetoh L., Chandwaskar R., Lee D.H., Cornejo M., Nishi N., Yamauchi A., Quintana F.J., et al. 2010. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J. Immunol. 185:1383–1392. 10.4049/jimmunol.0903275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva I.P., Gallois A., Jimenez-Baranda S., Khan S., Anderson A.C., Kuchroo V.K., Osman I., and Bhardwaj N.. 2014. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2:410–422. 10.1158/2326-6066.CIR-13-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo S.D., Masuda E., Rossi A.B., Throndset B.T., Gerard A.L., Chan E.H., Armstrong R.J., Fox B.P., Lorens J.B., Payan D.G., et al. 1999. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry. 36:340–348. [DOI] [PubMed] [Google Scholar]
- El-Hillal O., Kurosaki T., Yamamura H., Kinet J.P., and Scharenberg A.M.. 1997. syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc. Natl. Acad. Sci. USA. 94:1919–1924. 10.1073/pnas.94.5.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris R.L., Lu B., and Kane L.P.. 2014. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J. Immunol. 193:1525–1530. 10.4049/jimmunol.1400557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade J., Sun Z., Benallaoua M., Guillaume P., Luescher I.F., Sander C., Kirkwood J.M., Kuchroo V., and Zarour H.M.. 2010. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 207:2175–2186. 10.1084/jem.20100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G.J., Casasnovas J.M., Umetsu D.T., and DeKruyff R.H.. 2010. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 235:172–189. 10.1111/j.0105-2896.2010.00903.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan A.M., and Rivera J.. 2009. The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 228:149–169. 10.1111/j.1600-065X.2008.00742.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan A.M., Peavy R.D., and Metcalfe D.D.. 2009. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol. Res. 43:15–24. 10.1007/s12026-008-8046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J.V., and Colgan J.D.. 2014. Regulation of T cell responses by the receptor molecule Tim-3. Immunol. Res. 59:56–65. 10.1007/s12026-014-8524-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman J.V., Starbeck-Miller G., Pham N.L., Traver G.L., Rothman P.B., Harty J.T., and Colgan J.D.. 2014. Tim-3 directly enhances CD8 T cell responses to acute Listeria monocytogenes infection. J. Immunol. 192:3133–3142. 10.4049/jimmunol.1302290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G., Chen G., Shen B., and Li Y.. 2013. Tim-3: an activation marker and activation limiter of innate immune cells. Front. Immunol. 4:449 10.3389/fimmu.2013.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter-Hufford A., and Ravichandran K.S.. 2013. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 5:a008748 10.1101/cshperspect.a008748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H., Kitaura J., Xiao W., Horejsi V., Ra C., Lowell C.A., Kawakami Y., and Kawakami T.. 2007. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 110:2511–2519. 10.1182/blood-2007-01-066092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Lu F., Li J., Lan T., Huang B., Yin X., and Jin H.. 2014. Quantification of tryptase-TIM-3 double-positive mast cells in human chronic periodontitis. Arch. Oral Biol. 59:654–661. 10.1016/j.archoralbio.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Huang Y.H., Zhu C., Kondo Y., Anderson A.C., Gandhi A., Russell A., Dougan S.K., Petersen B.S., Melum E., Pertel T., et al. 2015. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 517:386–390. 10.1038/nature13848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Tanabe O., Nakajima T., Shimamoto T., Hirano T., and Kishimoto T.. 1990. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol. Cell. Biol. 10:2757–2764. 10.1128/MCB.10.6.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.T., Anderson A.C., Tan W.G., West E.E., Ha S.J., Araki K., Freeman G.J., Kuchroo V.K., and Ahmed R.. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 107:14733–14738. 10.1073/pnas.1009731107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.B., Ndhlovu L.C., Barbour J.D., Sheth P.M., Jha A.R., Long B.R., Wong J.C., Satkunarajah M., Schweneker M., Chapman J.M., et al. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205:2763–2779. 10.1084/jem.20081398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnikoff J., and Galli S.J.. 2008. New developments in mast cell biology. Nat. Immunol. 9:1215–1223. 10.1038/ni.f.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane L.P., Shapiro V.S.S., Stokoe D., and Weiss A.. 1999. Induction of NF-kappaB by the Akt/PKB kinase. Curr. Biol. 9:601–604. 10.1016/S0960-9822(99)80265-6 [DOI] [PubMed] [Google Scholar]
- Katoh S., Ishii N., Nobumoto A., Takeshita K., Dai S.Y., Shinonaga R., Niki T., Nishi N., Tominaga A., Yamauchi A., and Hirashima M.. 2007. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am. J. Respir. Crit. Care Med. 176:27–35. 10.1164/rccm.200608-1243OC [DOI] [PubMed] [Google Scholar]
- Kearley J., McMillan S.J., and Lloyd C.M.. 2007. Th2-driven, allergen-induced airway inflammation is reduced after treatment with anti-Tim-3 antibody in vivo. J. Exp. Med. 204:1289–1294. 10.1084/jem.20062093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Rådinger M., and Gilfillan A.M.. 2008. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 29:493–501. 10.1016/j.it.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakula K., Koenis D.S., van Tiel C.M., and de Vries C.J.. 2014. NR4A nuclear receptors are orphans but not lonesome. Biochim. Biophys. Acta. 1843:2543–2555. 10.1016/j.bbamcr.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Lee J., Su E.W., Zhu C., Hainline S., Phuah J., Moroco J.A., Smithgall T.E., Kuchroo V.K., and Kane L.P.. 2011. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol. Cell. Biol. 31:3963–3974. 10.1128/MCB.05297-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T.A., and Baltimore D.. 1990. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 10:2327–2334. 10.1128/MCB.10.5.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A., Barua D., Cutler P., Lidke D.S., Espinoza F.A., Pehlke C., Grattan R., Kawakami Y., Tung C.S., Bradbury A.R., et al. 2014. Optimal aggregation of FcεRI with a structurally defined trivalent ligand overrides negative regulation driven by phosphatases. ACS Chem. Biol. 9:1508–1519. 10.1021/cb500134t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbec O., and Daëron M.. 2007. The mast cell IgG receptors and their roles in tissue inflammation. Immunol. Rev. 217:206–221. 10.1111/j.1600-065X.2007.00510.x [DOI] [PubMed] [Google Scholar]
- Maurya N., Gujar R., Gupta M., Yadav V., Verma S., and Sen P.. 2014. Immunoregulation of dendritic cells by the receptor T cell Ig and mucin protein-3 via Bruton’s tyrosine kinase and c-Src. J. Immunol. 193:3417–3425. 10.4049/jimmunol.1400395 [DOI] [PubMed] [Google Scholar]
- Mohan H.M., Aherne C.M., Rogers A.C., Baird A.W., Winter D.C., and Murphy E.P.. 2012. Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clin. Cancer Res. 18:3223–3228. 10.1158/1078-0432.CCR-11-2953 [DOI] [PubMed] [Google Scholar]
- Molfetta R., Gasparrini F., Santoni A., and Paolini R.. 2010. Ubiquitination and endocytosis of the high affinity receptor for IgE. Mol. Immunol. 47:2427–2434. 10.1016/j.molimm.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Monney L., Sabatos C.A., Gaglia J.L., Ryu A., Waldner H., Chernova T., Manning S., Greenfield E.A., Coyle A.J., Sobel R.A., et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 415:536–541. 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- Monu N., and Frey A.B.. 2007. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 67:11447–11454. 10.1158/0008-5472.CAN-07-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A.E., Holzapfel K.L., Xing Y., Cunningham N.R., Maltzman J.S., Punt J., and Hogquist K.A.. 2011. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208:1279–1289. 10.1084/jem.20110308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S., Iikura M., Suto H., Akiba H., Umetsu D.T., Dekruyff R.H., Saito H., and Galli S.J.. 2007. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 110:2565–2568. 10.1182/blood-2006-11-058800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki T., Tsutsui S., Hirose S., Aradono S., Sugimoto Y., Takeshita K., Nishi N., and Hirashima M.. 2009. Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-antigen complex formation. J. Biol. Chem. 284:32344–32352. 10.1074/jbc.M109.035196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobumoto A., Nagahara K., Oomizu S., Katoh S., Nishi N., Takeshita K., Niki T., Tominaga A., Yamauchi A., and Hirashima M.. 2008. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology. 18:735–744. 10.1093/glycob/cwn062 [DOI] [PubMed] [Google Scholar]
- Odom S., Gomez G., Kovarova M., Furumoto Y., Ryan J.J., Wright H.V., Gonzalez-Espinosa C., Hibbs M.L., Harder K.W., and Rivera J.. 2004. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J. Exp. Med. 199:1491–1502. 10.1084/jem.20040382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gracia J.L., Labiano S., Rodriguez-Ruiz M.E., Sanmamed M.F., and Melero I.. 2014. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr. Opin. Immunol. 27:89–97. 10.1016/j.coi.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Poderycki M., Tomimori Y., Ando T., Xiao W., Maeda-Yamamoto M., Sauer K., Kawakami Y., and Kawakami T.. 2010. A minor catalytic activity of Src family kinases is sufficient for maximal activation of mast cells via the high-affinity IgE receptor. J. Immunol. 184:84–93. 10.4049/jimmunol.0901590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribluda V.S., Pribluda C., and Metzger H.. 1994. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc. Natl. Acad. Sci. USA. 91:11246–11250. 10.1073/pnas.91.23.11246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari M., Zhu C., Sakuishi K., Xiao S., Karman J., Chen A., Angin M., Wakeham A., Greenfield E.A., Sobel R.A., et al. 2012. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3–mediated cell death and exhaustion. Nat. Med. 18:1394–1400. 10.1038/nm.2871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K.S. 2011. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 35:445–455. 10.1016/j.immuni.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., and Gilfillan A.M.. 2006. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 117:1214–1225, quiz :1226. 10.1016/j.jaci.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., and Anderson A.C.. 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207:2187–2194. 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Fueyo A., Tian J., Picarella D., Domenig C., Zheng X.X., Sabatos C.A., Manlongat N., Bender O., Kamradt T., Kuchroo V.K., et al. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093–1101. 10.1038/ni987 [DOI] [PubMed] [Google Scholar]
- Sanderson M.P., Wex E., Kono T., Uto K., and Schnapp A.. 2010. Syk and Lyn mediate distinct Syk phosphorylation events in FcɛRI-signal transduction: implications for regulation of IgE-mediated degranulation. Mol. Immunol. 48:171–178. 10.1016/j.molimm.2010.08.012 [DOI] [PubMed] [Google Scholar]
- Sims G.P., Rowe D.C., Rietdijk S.T., Herbst R., and Coyle A.J.. 2010. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28:367–388. 10.1146/annurev.immunol.021908.132603 [DOI] [PubMed] [Google Scholar]
- Sumpter T.L., Ho C.H., Pleet A.R., Tkacheva O.A., Shufesky W.J., Rojas-Canales D.M., Morelli A.E., and Larregina A.T.. 2015. Autocrine hemokinin-1 functions as an endogenous adjuvant for IgE-mediated mast cell inflammatory responses. The Journal of allergy and clinical immunology 135:1019-1030 e1018. 10.1016/j.jaci.2014.07.036 [DOI] [PMC free article] [PubMed]
- Tanikawa R., Tanikawa T., Hirashima M., Yamauchi A., and Tanaka Y.. 2010. Galectin-9 induces osteoblast differentiation through the CD44/Smad signaling pathway. Biochem. Biophys. Res. Commun. 394:317–322. 10.1016/j.bbrc.2010.02.175 [DOI] [PubMed] [Google Scholar]
- Veenstra R.G., Taylor P.A., Zhou Q., Panoskaltsis-Mortari A., Hirashima M., Flynn R., Liu D., Anderson A.C., Strom T.B., Kuchroo V.K., and Blazar B.R.. 2012. Contrasting acute graft-versus-host disease effects of Tim-3/galectin-9 pathway blockade dependent upon the presence of donor regulatory T cells. Blood. 120:682–690. 10.1182/blood-2011-10-387977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener Z., Kohalmi B., Pocza P., Jeager J., Tolgyesi G., Toth S., Gorbe E., Papp Z., and Falus A.. 2006. TIM-3 Is Expressed in Melanoma Cells and Is Upregulated in TGF-Beta Stimulated Mast Cells. J. Invest. Dermatol. 21:21–22. [DOI] [PubMed] [Google Scholar]
- Wu C., Thalhamer T., Franca R.F., Xiao S., Wang C., Hotta C., Zhu C., Hirashima M., Anderson A.C., and Kuchroo V.K.. 2014. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 41:270–282. 10.1016/j.immuni.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Nishimoto H., Hong H., Kitaura J., Nunomura S., Maeda-Yamamoto M., Kawakami Y., Lowell C.A., Ra C., and Kawakami T.. 2005. Positive and negative regulation of mast cell activation by Lyn via the FcepsilonRI. J. Immunol. 175:6885–6892. 10.4049/jimmunol.175.10.6885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Jiang X., Chen G., Xiao Y., Geng S., Kang C., Zhou T., Li Y., Guo X., Xiao H., et al. 2013. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J. Immunol. 190:2068–2079. 10.4049/jimmunol.1202661 [DOI] [PubMed] [Google Scholar]
- Yoon S.J., Lee M.J., Shin D.C., Kim J.S., Chwae Y.J., Kwon M.H., Kim K., and Park S.. 2011. Activation of mitogen activated protein kinase-Erk kinase (MEK) increases T cell immunoglobulin mucin domain-3 (TIM-3) transcription in human T lymphocytes and a human mast cell line. Mol. Immunol. 48:1778–1783. 10.1016/j.molimm.2011.05.004 [DOI] [PubMed] [Google Scholar]
- Zhao Y., and Bruemmer D.. 2010. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler. Thromb. Vasc. Biol. 30:1535–1541. 10.1161/ATVBAHA.109.191163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Anderson A.C., Schubart A., Xiong H., Imitola J., Khoury S.J., Zheng X.X., Strom T.B., and Kuchroo V.K.. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6:1245–1252. 10.1038/ni1271 [DOI] [PubMed] [Google Scholar]
- Zikherman J., Parameswaran R., and Weiss A.. 2012. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 489:160–164. 10.1038/nature11311 [DOI] [PMC free article] [PubMed] [Google Scholar]