Insight from Ralf Küppers
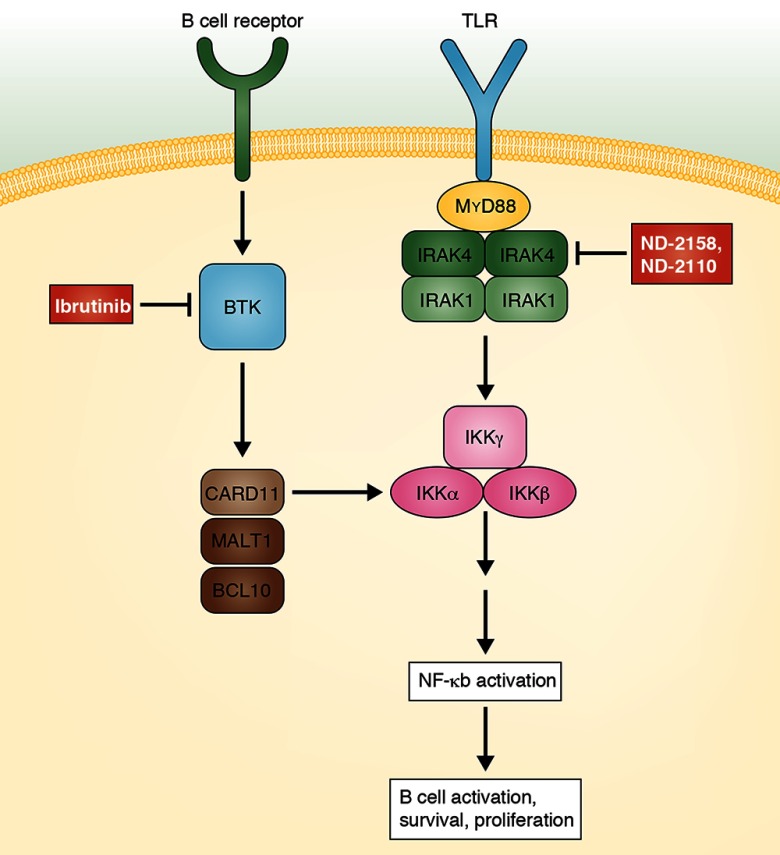
An overactive Toll-like receptor (TLR) pathway is a hallmark of several autoimmune diseases and some types of B cell lymphomas. TLR signaling is a main mediator of inflammatory signals in B cells and causes NF-κB activation, which has numerous stimulatory effects, including promotion of B cell survival and proliferation. A key factor in TLR signaling is the adaptor protein MyD88. Downstream of MyD88, the interleukin-1 receptor–associated kinase 4 (IRAK4) links TLR signaling to the NF-κB pathway. Because of the overactive TLR signaling in several autoimmune diseases, and as some B cell lymphomas carry activating mutations in MyD88, there is strong interest in developing inhibitors of this pathway. MyD88 itself is not a promising target, because it is difficult to develop inhibitors for adaptor proteins. Fortunately, IRAK4 is essential for signaling through MyD88, and numerous examples of efficient kinase inhibitors exist. In this issue, Kelly et al. now succeed in identifying two novel and very promising IRAK4 inhibitors and present for the first time exciting preclinical studies with these molecules.
The TLR pathway is overactivated in various types of inflammatory and autoimmune diseases by ligands binding to the TLR. In 30% of ABC DLBCL and in several other B cell lymphomas, MyD88, which links TLRs to the IRAK1 and IRAK4 kinases, is affected by activating L265P mutations. Activity of IRAKs leads to activation of the canonical NF-κB pathway. IRAK4 is essential for TLR signaling through MyD88. The newly developed IRAK4 inhibitors ND-2158 and ND-2110 abrogate signaling mediated by TLR receptors or mutated MyD88. In ABC DLBCL, NF-κB is also activated though chronic active B cell receptor signaling, which involves the Bruton’s tyrosine kinase (BTK). Inhibition of BTK by ibrutinib has shown clinical efficiency in first clinical studies. Notably, coapplication of ibrutininb and ND-2158 has synergistic toxic activity for ABC DLBCL cell lines in vitro and in a xenograft model. For the signaling pathways, not all components are shown.
The two inhibitors, called ND-2158 and ND-2110, bind to the ATP pocket of IRAK4, thereby inhibiting its kinase activity. These two molecules have high affinities for IRAK4 and possess good pharmacological properties. Importantly, in a screen of 334 kinases, ND-2158 and ND-2110 proved to be highly specific for IRAK4. ND-2158 and ND-2110 were efficient in reducing disease severity in mouse models of pathological inflammatory responses. In studies with cell lines from diffuse large B cell lymphoma (DLBCL), specifically those lines of the activated B cell–like (ABC) subtype of DLBCL with a specific activating mutation in MyD88 (L265P), they showed inhibition of IRAK4 and NF-κB activity upon incubation with these inhibitors. Xenograft studies with DLBCL cell lines revealed the in vivo efficiency of ND-2158 in reducing tumor growth. As it is likely that in a potential future clinical application IRAK4 inhibition alone may not be curative, the authors studied combined application of the IRAK4 inhibitors with inhibitors of other pathogenic factors in ABC DLBCL. For example, coapplication of ibrutinib, an inhibitor of B cell receptor signaling, which is chronically active in ABC DLBCL, had synergistic effects on tumor growth in mice.
The present study lays the ground for the development of clinical studies with the two IRAK4 inhibitors. It is impressive that this stage has already been reached, considering that MyD88 mutations were identified in ABC DLBCL only four years ago. As this type of DLBCL has the worst prognosis, and 30% of ABC DLBCL carries the L265P mutation, this new treatment approach may be valuable for a substantial fraction of DLBCL patients. Furthermore, patients affected by several other types of B cell lymphomas carrying this mutation, or patients with autoimmune diseases might also benefit from treatment with these inhibitors. Considering the recent clinical success with ibrutinib in the treatment of ABC DLBCL, it is even more promising that combined treatment with ND-2158 and ibrutinib shows synergistic therapeutic effects in these preclinical studies. What remains currently puzzling is why two ABC DLBCL lines with MyD88 mutations other than L265P were not sensitive to the IRAK4 inhibitors. This warrants further investigation.
References
- Kelly, P.N., et al. 2015. J. Exp. Med. 10.1084/jem.20151074 [DOI] [Google Scholar]