Supplemental Digital Content is available in the text.
Keywords: deglutition disorders, intention to treat analysis, rehabilitation, stroke, vagus nerve
Background and Purpose—
Recent animal studies demonstrate that vagus nerve stimulation (VNS) paired with movement induces movement-specific plasticity in motor cortex and improves forelimb function after stroke. We conducted a randomized controlled clinical pilot study of VNS paired with rehabilitation on upper-limb function after ischemic stroke.
Methods—
Twenty-one participants with ischemic stroke >6 months before and moderate to severe upper-limb impairment were randomized to VNS plus rehabilitation or rehabilitation alone. Rehabilitation consisted of three 2-hour sessions per week for 6 weeks, each involving >400 movement trials. In the VNS group, movements were paired with 0.5-second VNS. The primary objective was to assess safety and feasibility. Secondary end points included change in upper-limb measures (including the Fugl–Meyer Assessment-Upper Extremity).
Results—
Nine participants were randomized to VNS plus rehabilitation and 11 to rehabilitation alone. There were no serious adverse device effects. One patient had transient vocal cord palsy and dysphagia after implantation. Five had minor adverse device effects including nausea and taste disturbance on the evening of therapy. In the intention-to-treat analysis, the change in Fugl–Meyer Assessment-Upper Extremity scores was not significantly different (between-group difference, 5.7 points; 95% confidence interval, −0.4 to 11.8). In the per-protocol analysis, there was a significant difference in change in Fugl–Meyer Assessment-Upper Extremity score (between-group difference, 6.5 points; 95% confidence interval, 0.4 to 12.6).
Conclusions—
This study suggests that VNS paired with rehabilitation is feasible and has not raised safety concerns. Additional studies of VNS in adults with chronic stroke will now be performed.
Clinical Trial Registration—
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01669161.
Arm weakness is common after stroke, and its treatment is recognized as an area of considerable need.1 Approximately 85% of patients with stroke present with arm weakness,2 and 60% of stroke survivors with nonfunctional arms at 1 week do not recover function by 6 months.3 Current treatment for arm weakness typically comprises intensive, task-specific, and repetitive rehabilitative interventions or occasionally methods such as constraint-induced movement therapy and electric neurostimulation.4 A recent meta-analysis and large-scale trials show the effects of current treatments for arm weakness to be modest.5,6 Novel and more effective treatments are needed. Improvement in arm function should improve quality of life for stroke survivors, reduce comorbidities associated with loss of independence, and reduce cost to the healthcare system.7
Intensive training has been shown to facilitate a range of neuroplastic brain events.8 It is possible that augmentation of neuroplasticity to promote reorganization of neural networks is required to more fully recover motor function.9 However, no practical and effective method exists to achieve this and even if such changes occur, it is unclear whether they are clinically meaningful or long term. This study is a preliminary investigation of an intervention designed to promote specific neuroplasticity; vagus nerve stimulation (VNS) paired with movement to drive task-specific plasticity in the motor cortex.10–12 VNS activates neurons in the basal forebrain and locus coeruleus and results in release of acetylcholine and norepinephrine, respectively, which are known to facilitate reorganization of cortical networks.13 We recently demonstrated in a rat model of ischemic stroke that pairing forelimb rehabilitation with VNS significantly increases recovery of forelimb speed and strength when compared with rehabilitation alone.14,15 Our subsequent studies demonstrated that VNS paired with rehabilitative training also improves recovery in a rat model of intracerebral hemorrhage,16 and that precise timing of VNS with specific motor movements yields optimal recovery.17
We hypothesize that VNS paired with upper-limb rehabilitation therapy will result in greater recovery of arm function than rehabilitation alone in adults with chronic ischemic stroke. We performed the first-in-human evaluation of VNS paired with upper-limb rehabilitation after ischemic stroke. The main objective of the study was to evaluate the safety and feasibility of paired VNS therapy after stroke and to provide clinical data for sample size calculations for larger studies.
Methods
Study Design and Participants
We performed a randomized open active comparator study with blinded objective end point assessment. We compared VNS paired with rehabilitation with rehabilitation alone in subjects with arm weakness because of chronic ischemic stroke. Participants were enrolled at 2 stroke centers in the United Kingdom (NHS Greater Glasgow and Clyde and Newcastle upon Tyne NHS Foundation Trust). Study approval was granted by the Medicine and Healthcare Regulatory Authority and by the West of Scotland Research Ethics Committee. Participants provided voluntary, written informed consent. The trial was registered on clinicaltrials.gov (NCT01669161) and the protocol is at http://www.microtransponder.com/?page_id=972.
Patients with a history of unilateral supratentorial ischemic stroke that occurred at least 6 months before, aged ≥18 years and ≤80 years, with an Action Research Arm Test (ARAT) score of 15 to 50 (inclusive, indication moderate to severe arm impairment) were eligible for inclusion. Full inclusion and exclusion criteria are given in the Methods in the online-only Data Supplement.
Study Visits and Data Collection
Baseline Assessments
Participants had 3 visits before starting rehabilitation therapy. The first was performed soon after consent was obtained to confirm eligibility. A second was performed at least 2 weeks later at which point randomization and structural brain magnetic resonance imaging (MRI) were performed. Device implantation was then scheduled in participants randomized to the VNS group. A third baseline assessment was performed after device implantation (or before the start of rehabilitation in rehabilitation-only participants). Each assessment included a detailed medical history and details of the index stroke, assessment of eligibility, and measures of arm function. The measures of arm function used were the upper extremity Fugl–Meyer assessment (FMA-UE),18 ARAT,19 grip and pinch strength (via a hand-held dynamometer),20 Box and Block test,21 9-hole peg test,22 and robotic assessment using an InMotion Technologies robot.23 We also used the Stroke Impact Scale.24
All observers received training in these measures before performing any assessments. This included written materials, practice at an investigators meeting, and (for the FMA-UE and ARAT score) review and scoring of recorded assessments followed by discussion to agree and confirm understanding of scoring rules.
Randomization and Masking
Enrolled participants were randomized, via an interactive voice response system (Robertson Center for Biostatistics, University of Glasgow), to receive VNS plus rehabilitation or rehabilitation alone. Randomization was stratified by site, and block size was variable. Because of the nature of surgical device implantation, investigators, therapists, and participants were not blinded to treatment allocation. We attempted to ensure that outcome assessors were blinded by using assessors not involved in other aspects of the trial, by asking participants not to discuss their treatment allocation and to cover the chest and neck. We asked assessors to confirm that they were blinded before outcomes assessments.
Magnetic Resonance Imaging
MRI data were acquired on 3T MRI scanners. Full details are given in the Methods in the online-only Data Supplement. The extent of injury to the corticospinal tract (CST) was quantified as previously described.25 We defined injury to the CST by the CST fractional anisotropy ratio and mean diffusivity ratio between the stroke and the nonstroke side and the volume of the stroke lesion within the CST.
VNS Device Implantation
Implantation was performed after the second baseline assessment in the VNS and rehabilitation group. Participants had a preoperative assessment, which included anesthetic review and fiber optic laryngoscopy (Figures I–VII in the online-only Data Supplement). Participants were typically admitted on the morning of device insertion and were discharged within 24 hours. Device implantation was performed under general anesthesia by either a Consultant Ear Nose and Throat surgeon (Glasgow) or a Consultant Neurosurgeon (Newcastle). The implantation involved placement of the stimulation electrodes of the leads (Model 304; Cyberonics Inc., Houston, TX) on the left vagus nerve in the left carotid sheath. The lead is then tunnelled subcutaneously to a subcutaneous pocket created in the left pectoral region where it is attached the implantable Vivistim pulse generator. Full details are available in the Methods in the online-only Data Supplement.
Rehabilitation Training
All participants received a 6-week course of 2-hour therapy sessions 3× per week. All sessions started with 10 to 15 minutes of stretching exercises (without VNS). Thereafter, 7 standardized tasks were performed (Methods in the online-only Data Supplement). Tasks included reach and grasp, handle turning, gross movement, object flipping, simulated eating tasks, inserting objects, opening bottles, and additional tasks selected by participants as priorities for improvement. Subjects performed at least 300 to 400 movements in each session that were modified or progressed to be achievable, but challenging. Each subject spent ≈10 minutes on each of the 7 tasks per during each session. All tasks were delivered by a licensed stroke physiotherapist. Therapy sessions were video recorded to monitor the treatment delivered.
VNS Protocol
A wireless control interface was used to communicate with the VNS device and deliver stimulation during therapy sessions. A laptop with the Stroke Application Programming Software was used to program the IPG and initiate VNS. The stimulation was manually triggered by the therapist using a push button, while the patient performed a task (Figure 1). Stroke Application Programming Software allows the therapist to control the relative timing of stimulation to therapy exercises using the push button. The software also allows the physician to program the stimulation parameters (amplitude [mA], frequency [Hz], pulse width [μS], and duration [ms]), captures patient programming history, and checks lead impedance and battery status. A 500-ms burst of VNS was delivered to the VNS group during each movement (the rehabilitation-only group did not have a device implanted). Each simulation consisted of fifteen 0.8-mA, constant current, charge balanced pulses (100-μs pulse width, 30-Hz frequency). These stimulation parameters grew out of hypothesis-driven research in humans and animal models,10–14 including studies of cortical map plasticity and electroencephalogram desynchonization using VNS.12 Note stimulation was only delivered to the left vagus nerve to avoid activation of the sinoatrial node (which is innervated by the right vagus nerve), but bilateral neuronal connections mean stimulation should affect both cerebral hemispheres. Full details are given in the Methods in the online-only Data Supplement.
Figure 1.

Schematic of vagus nerve stimulation device use in a typical therapy session.
Outcome Assessments
These were performed on days 1, 7, and 30 after the conclusion of rehabilitation therapy in both groups. The FMA-UE, ARAT, grip, and pinch strength via a hand-held dynamometer, Stroke Impact Scale, Box and Block test, and 9-hole peg test were assessed. The ARAT, grip strength, Box and Block tests, and 9-hole peg test were also measured at the end of the second and fourth week of the 6-week therapy period. Assessments were always performed in the same order, with a break halfway through the visit to complete questionnaires. Long-term follow-up and postimplantation care is described in the Methods in the online-only Data Supplement.
Statistical Analysis and Study End Points
Because this was a pilot study, no formal sample size calculation was performed. An intention-to-treat and per-protocol analysis were performed. The per-protocol analysis included participants who had at least 12 of the 18 therapy sessions and who were not taking any drugs that may interfere with VNS during the study. The numbers of serious and nonserious adverse events (AEs) are described, along with the numbers who completed the therapy protocol.
The main study safety end point was the number of serious AEs related to therapy, and the main feasibility end point was the number of participants who completed therapy protocol. The main efficacy assessment was change in FMA-UE score between the main baseline assessment and the first post-therapy visit. Secondary end points were changes in ARAT, Box and Block, 9-hole peg test, and grip strength. We also assessed whether participants improved by the minimum clinically significant difference in the FMA-UE and ARAT scores. This was defined as ≥6 points in the FMA-UE score and ≥5 points in the ARAT score. The efficacy end points for change in value were compared using ANCOVA with adjustment for the baseline value. To compare the percentage of improvers in each group, we used Fisher exact test. Finally, we explored the levels of the baseline MRI measures in relation to clinical response within each group using 2-sample t tests and Pearson correlations.
Results
Study Participants
Seventy-one patients were screened, of whom 21 were enrolled between February 2013 and April 2014 (16 at the Glasgow site and 5 at the Newcastle site). Twenty participants were randomized (1 withdrew because of improvement in arm function during the baseline assessment phase and no longer wished to continue). Nine participants were randomized to VNS and rehabilitation and 11 to rehabilitation only. One patient in the VNS group received medication that may interfere with VNS so was excluded from the per-protocol analysis. A consort diagram is shown in Figure 2.
Figure 2.
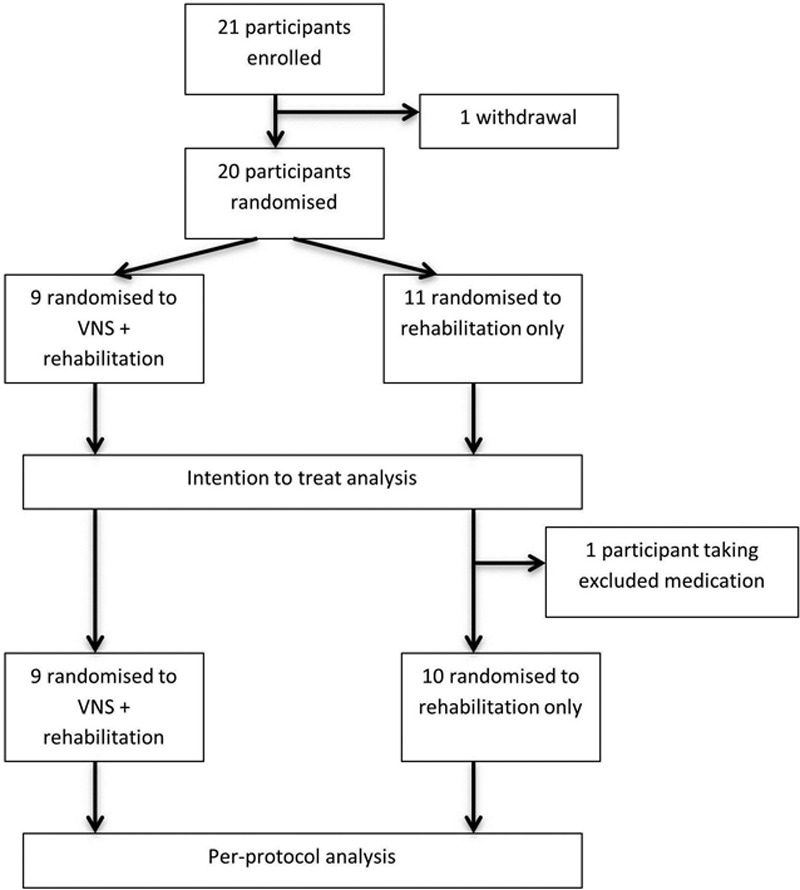
Consort diagram. VNS indicates vagus nerve stimulation.
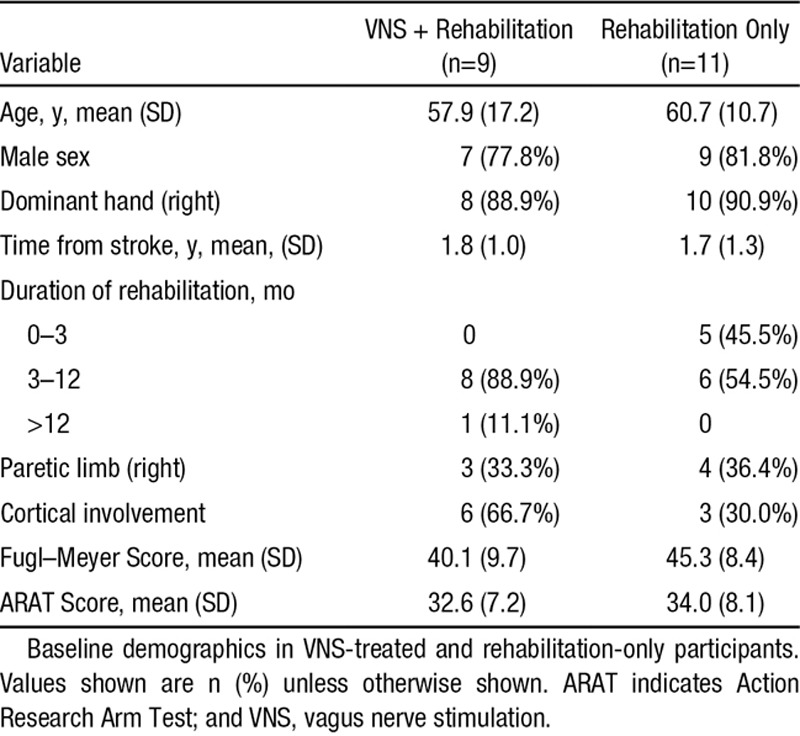
All participants were white and could ambulate independently. Baseline characteristics are shown in Table 1. Groups seemed balanced for time from stroke, age, sex, handedness, and baseline ARAT, but there was a 5-point difference in the baseline FMA-UE scores (lower in the VNS group).
Table 1.
Baseline Demographics
Feasibility
All randomized participants attended all planned therapy sessions. In the VNS group, the average duration of therapy (excluding stretching) was 72 minutes (SD, 8; range 38–90 minutes). This compared with 79 minutes (SD, 8;, range 53–113 minutes) in the rehabilitation-only group. Participants performed an average of 414 movements paired with VNS during a session in the VNS group (SD, 124; range, 104–796). This compared with 463 movements (SD, 157; range, 165–876) in the rehabilitation-only group. One participant, who had a good clinical response, opted for device removal at the end of the study. All remaining participants have the device in situ and are undergoing longer-term follow-up.
Safety
There were 22 AEs in the 8 participants in the VNS group (11 of which were adverse device effects in 5 participants) when compared with 10 AEs in 3 participants in the rehabilitation-only group. Two participants had a serious AE in the VNS group, none of which were serious device effects. One participant had 4 serious AEs in the rehabilitation group. No AEs were related to the rehabilitation therapy itself. The serious AEs are summarized in Table 2.
Table 2.
Serious Adverse Events
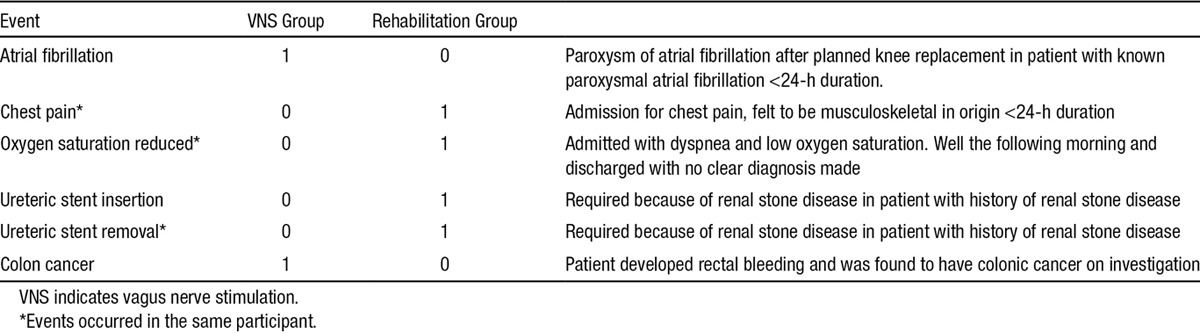
The adverse device effects were minor with the exception of one (moderate severity) and all resolved. This participant had a left vocal cord palsy and dysphagia after device implantation. Dysphagia settled over a 3-week period. This was successfully managed as an outpatient. VNS was stopped after 2 weeks in case it would hinder recovery; however, the participant continued to receive rehabilitation only. To exclude an underlying diagnosis for vocal cord palsy (the patient was an ex-smoker), computed tomographic scan of the neck and thorax was performed. This excluded any other cause but revealed left-sided phrenic nerve palsy, and the participant remained under active follow-up to exclude other causes. This vocal cord and phrenic nerve palsy had resolved at 9-month follow-up. One participant reported nausea after a single long session of VNS and rehabilitation. One participant reported a taste disturbance after surgery (metallic taste) that continued during the first 2 weeks of therapy. One participant reported mild dysphagia in the evening after therapy sessions (manifest as finding it more difficult to swallow a capsule that evening only). None of these minor symptoms required changes to the therapy protocol, and all resolved on the day of the therapy session. Six participants had a slight hoarseness or neck tingling during stimulation, 3 were not aware of stimulation.
Efficacy
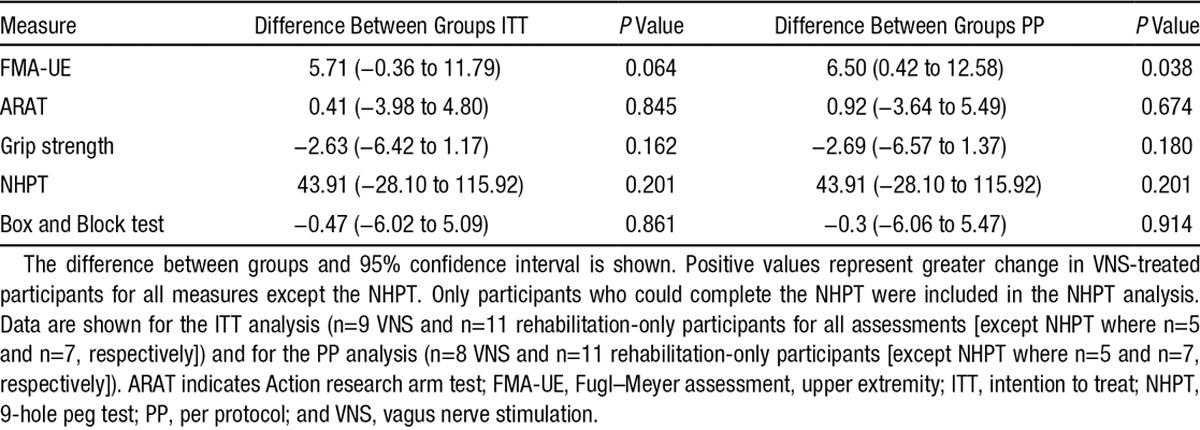
The mean change in FMA-UE scores in the VNS group was +8.7 (5.8) versus +3.0 (6.1) in the rehabilitation-only group (between-group difference, 5.7 points; 95% CI, −0.4 to 11.8; P=0.064; Figure 3). In the per-protocol analysis (n=19), the mean change in FMA-UE score was +9.6 points in the VNS group (5.3) and +3.0 points (6.1) in the rehabilitation-only group (between-group difference, 6.5 points; 95% CI, 0.4 to 12.6; P=0.038). Six (66.7%) achieved a clinically meaningful response on the FMA-UE score in the VNS group when compared with 4 (36.4%) in the rehabilitation-only group (P=0.17). There were no significant differences in the other secondary efficacy end points (Table 3) including robotic kinematic measures (data not shown).
Figure 3.
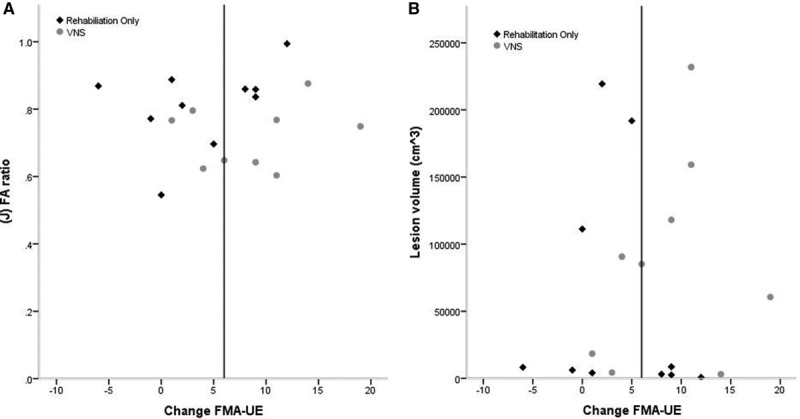
Change in upper extremity Fugl–Meyer assessment (FMA-UE) score and baseline magnetic resonance imaging variables. These figures show change in FMA-UE score plotted against the baseline infarct volume (A) and the baseline corticospinal tract fractional anisotropy (FA) ratio (B). Vagus nerve stimulation (VNS)–treated patients are shown in gray circles, and rehabilitation-only treated participants in black diamonds. Responders lie to the right of the vertical line.
Table 3.
Efficacy Measures
Response in Relation to MRI Measures
There were no significant differences in MRI measures at baseline between groups although there was a trend for baseline infarct volume, CST overlap volume, and mean diffusivity ratio to be higher and for fractional anisotropy ratio to be lower in VNS participants (Table I in the online-only Data Supplement). There was no significant correlation between baseline MRI variables and change in UEFM score (Table II in the online-only Data Supplement) in either group. However, infarct volume, CST overlap volume, fractional anisotropy ratio, and mean diffusivity ratio were significantly worse at baseline (P<0.05) in VNS responders compared with rehabilitation-only responders (Table III in the online-only Data Supplement).
Discussion
This trial provides initial evidence of safety and feasibility of a novel neurostimulation technique, which in conjunction with rehabilitation for the upper limb, aims to promote neuroplasticity specific to improved upper-limb function. The data provide no signal of harm. Our study was not designed to test clinical efficacy, and our findings are preliminary. However, the per-protocol analysis demonstrated a significantly greater improvement in FMA-UE scores in VNS-treated participants than in controls. Baseline MRI data showed a significant difference in baseline MRI variables between responders in the VNS group and responders in the rehabilitation-only group. Whether this means VNS therapy allows recovery to occur in some participants who would not respond to conventional rehabilitation techniques requires rigorous testing in further randomized clinical trials.
Our innovation is to use short bursts of VNS paired with specific movements to restore lost motor skills. To the best of our knowledge, this is an entirely novel use of VNS. This method of VNS is sharply distinct from the current clinical use of VNS for the treatment of refractory epilepsy and depression, where VNS is delivered on 30 seconds and on 5 minutes off cycle. The stimulation used in the current study delivered <1% of the charge delivered with the Food and Drug Administration–approved protocols for epilepsy and depression. VNS paired with rehabilitation has been shown to improve recovery of forelimb function in several models of experimental stroke. VNS has also shown promise in a small study of participants (n=10) with severe chronic tinnitus,26 a disorder of abnormal central auditory system plasticity although this was an open study and confirmatory data are needed. We have based our stimulation parameters on both animal and human data. We have recently found (in rat models) that pairing tones with 0.4 or 0.8 mA is sufficient to reorganize auditory cortex, whereas pairing tones with 1.2 or 1.6 mA do not result in detectable cortical plasticity.27 This observation closely mimics previous studies in epilepsy patients where moderate but not high VNS current was memory enhancing.28 However, further clinical studies will be needed to define the optimum stimulation parameters for the stroke indication.
The mechanisms by which paired VNS exerts its effects on cortical neurons are not completely understood. However, it is well known that nucleus basalis and locus coeruleus neurons are activated during VNS via activation of nucleus tractus solitarius.11 When stimulated, these neurons release acetylcholine and norepinephrine throughout the cortex.14 It is important to note that even though acetylcholine and norepinephrine are released throughout cortex, cortical plasticity is specific to the set of neurons driven by the stimulus (eg, sound or movement). Both behavioral and neurophysiologic changes can be observed when nucleus basalis or the vagus nerve is directly stimulated in short bursts and paired with sound or a movement.10–14 Short-term stimulation of locus coeruleus neurons before training also seems to enhance learning and memory.29 Thus, a reasonable mechanistic explanation exists for why pairing VNS with sensory or motor events could be an effective therapeutic approach for enhancing specific neuroplastic change.
Tolerability of surgery and stimulation was similar in our cohort when compared with that seen in current clinical applications although numbers were small. One patient developed a vocal cord palsy after device implantation. Overall, we conclude that there were no significant safety concerns that would preclude further study. This does not suggest that there are no important considerations for participants considering participation in future trials, or for patients should this treatment be introduced to clinical practice. On the basis of extensive experience of VNS in patients with epilepsy, we expect to see infection related to device implantation in ≈1% of participants,30 and other complications associated with anesthesia and surgery could arise.31 We minimized these risks by prescribing perioperative low molecular weight heparin in place of oral anticoagulants or by substituting aspirin for clopidogrel where appropriate. Some VNS devices are MRI compatible, but we excluded patients who had a condition requiring MRI surveillance and patients who would not be able to undergo contrast-based computed tomographic imaging.
Although each therapy session in the trial was participant and goal specific, the duration and intensity of therapy were higher in the rehabilitation-only group so is unlikely to have confounded the results. The improvement in function seen in the control group was similar to that demonstrated in previous clinical trials of intensive upper-limb therapy including robotic therapy and constraint induced movement therapy.6,32 However, it should be noted that participants recruited to trials of treatments such as robotic therapy had stroke symptoms that were typically more severe than those included in our study.
When interpreting the results of functional recovery from a mechanistic stand point, there is often the question of recovery of actual function versus compensation. To our knowledge, there is no measure that distinguishes the difference between compensatory and restorative change. Subtle compensatory strategies, such as accessory trunk motion, are controlled for with the choice of the Fugl–Meyer assessment tool. The nature of the Fugl–Meyer test is to measure motor impairment in stroke and because of the assessment of isolated movement of body parts, thus, typical clinical compensatory strategies are well controlled for.19 We found significant differences between responders in the VNS group and rehabilitation-only group. Infarct volume and measures of CST involvement were worse in VNS responders when compared with control responders. The CST fractional anisotropy ratio is known to be a predictor of long-term motor impairment and was lower in VNS responders.33 We hypothesize that VNS may be able to generate clinical responses in patients who would otherwise not respond to standard treatments through enhancing neuroplasticity.10–14
There are several strengths to this study. The study was randomized reducing the risk of selection bias. Blinded assessors not involved in the day-to-day treatment of participants assigned outcomes. We had 98% follow-up, and all participants completed the therapy protocol. The control group received intense rehabilitation similar to the VNS group, rather than typical standard of care.
There are limitations to consider. This study was not blinded to either the physiotherapist delivering the therapy or the participant, and there was no sham stimulation group. Although we asked participants not to discuss their treatment allocation during outcomes assessments, we cannot exclude that assessors picked up on visual cues such as a scar in the neck from lead insertion. Furthermore, the study was small leading to imprecision in some of the efficacy assessments.
In conclusion, VNS paired with rehabilitation therapy is feasible in adults with arm weakness ≥6 months after ischemic stroke. It also seems to be acceptably safe for further study.
Acknowledgments
Pamela MacKenzie, Elizabeth Colquhoun, Jen Alexander (Glasgow), and John Davis (Newcastle) for trial coordination and for assisting with recruitment.
Sources of Funding
The trial was funded entirely by MicroTransponder Inc. The funders had no responsibility for the collection, analysis, and interpretation of study data except the analysis of therapy session duration contained in the feasibility section (performed by D. Pierce). The funders had no responsibility for writing of the trial report, or in the decision to submit the article for publication.
Disclosures
Dr Dawson has received reimbursement for conference attendance where results of the study were presented from MicroTransponder Inc. Dr Cramer has served as a consultant for MicroTransponder, as well as GlaxoSmithKline, Roche, Dart Neuroscience, and RAND Corporation, and is a cofounder of personalRN. Dr Kilgard is a shareholder and consultant for MicroTransponder Inc. Drs Kilgard (R01NS085167 and R43NS084566), Rennaker, and Engineer have previously received funding from the National Institutes of Health for work involving vagus nerve stimulation. D. Pierce, B. Tarver, and Dr Engineer are employees of MicroTransponder Inc.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.115.010477/-/DC1.
References
- 1.Pollock A, St George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurol. 2012;11:209. doi: 10.1016/S1474-4422(12)70029-7. doi: 10.1016/S1474-4422(12)70029-7. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:394–398. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 3.Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry. 1983;46:521–524. doi: 10.1136/jnnp.46.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health Care and Clinical Excellence, Stroke Rehabilitation. Long term rehabilitation after stroke. CG162, London: National Institute for Health Care and Clinical Excellence; 2013. [Google Scholar]
- 5.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 6.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner TH, Lo AC, Peduzzi P, Bravata DM, Huang GD, Krebs HI, et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke. 2011;42:2630–2632. doi: 10.1161/STROKEAHA.110.606442. doi: 10.1161/STROKEAHA.110.606442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buma FE, Lindeman E, Ramsey NF, Kwakkel G. Functional neuroimaging studies of early upper limb recovery after stroke: a systematic review of the literature. Neurorehabil Neural Repair. 2010;24:589–608. doi: 10.1177/1545968310364058. doi: 10.1177/1545968310364058. [DOI] [PubMed] [Google Scholar]
- 9.Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(pt 6):1591–1609. doi: 10.1093/brain/awr039. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. 2013;207:275–299. doi: 10.1016/B978-0-444-63327-9.00010-2. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex. 2012;22:2365–2374. doi: 10.1093/cercor/bhr316. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 12.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis. 2013;60:80–88. doi: 10.1016/j.nbd.2013.08.002. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Khodaparast N, Hays SA, Sloan AM, Fayyaz T, Hulsey DR, Rennaker RL, II, et al. Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil Neural Repair. 2014;28:698–706. doi: 10.1177/1545968314521006. doi: 10.1177/1545968314521006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Rennaker RL, II, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014;45:3097–3100. doi: 10.1161/STROKEAHA.114.006654. doi: 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, II, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect poststroke recovery of forelimb strength. Neuroreport. 2014;25:676–682. doi: 10.1097/WNR.0000000000000154. doi: 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.See J, Dodakian L, Chou C, Chan V, McKenzie A, Reinkensmeyer DJ, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27:732–741. doi: 10.1177/1545968313491000. doi: 10.1177/1545968313491000. [DOI] [PubMed] [Google Scholar]
- 19.Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82:14–19. doi: 10.1053/apmr.2001.18668. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil. 1970;51:321–327. [PubMed] [Google Scholar]
- 21.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 22.Sharpless JW. The nine-hold peg test of finger hand coordination for the hemiplegic patient. In: Sharpless JW, editor. In: Mossman’s A Problem Oriented Approach to Stroke Rehabilitation. 2nd ed. Springfield, IL: Charles C Thomas Publishing; 1982. pp. 420–423. [Google Scholar]
- 23.Krebs HI, Krams M, Agrafiotis DK, DiBernardo A, Chavez JC, Littman GS, et al. Robotic measurement of arm movements after stroke establishes biomarkers of motor recovery. Stroke. 2014;45:200–204. doi: 10.1161/STROKEAHA.113.002296. doi: 10.1161/STROKEAHA.113.002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan PW, Bode RK, Min Lai S, Perera S Glycine Antagonist in Neuroprotection Americans Investigators. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil. 2003;84:950–963. doi: 10.1016/s0003-9993(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 25.Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77:132–145. doi: 10.1002/ana.24309. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Ridder D, Vanneste S, Engineer ND, Kilgard MP. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation. 2014;17:170–179. doi: 10.1111/ner.12127. doi: 10.1111/ner.12127. [DOI] [PubMed] [Google Scholar]
- 27.Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, et al. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul. doi: 10.1016/j.brs.2015.08.018. http://www.brainstimjrnl.com/article/S1935-861X(15)01125-0/pdf. Accessed October 15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 29.Sara SJ, Devauges V. Priming stimulation of locus coeruleus facilitates memory retrieval in the rat. Brain Res. 1988;438:299–303. doi: 10.1016/0006-8993(88)91351-0. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22:1260–1268. doi: 10.1111/ene.12629. doi: 10.1111/ene.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landercasper J, Merz BJ, Cogbill TH, Strutt PJ, Cochrane RH, Olson RA, et al. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125:986–989. doi: 10.1001/archsurg.1990.01410200044006. [DOI] [PubMed] [Google Scholar]
- 32.Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14:224–234. doi: 10.1016/S1474-4422(14)70160-7. doi: 10.1016/S1474-4422(14)70160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puig J, Blasco G, Daunis-I-Estadella J, Thomalla G, Castellanos M, Figueras J, et al. Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke. 2013;44:2016–2018. doi: 10.1161/STROKEAHA.111.000382. doi: 10.1161/STROKEAHA.111.000382. [DOI] [PubMed] [Google Scholar]


