SUMMARY
Colonization of the fetal and infant gut microbiome results in dynamic changes in diversity, which can impact disease susceptibility. To examine the relationship between human gut microbiome dynamics throughout infancy and type 1 diabetes (T1D), we examined a cohort of 33 infants genetically predisposed to T1D. Modeling trajectories of microbial abundances through infancy revealed a subset of microbial relationships shared across most subjects. Although strain composition of a given species was highly variable between individuals, it was stable within individuals throughout infancy. Metabolic composition and metabolic pathway abundance remained constant across time. A marked drop in alpha-diversity was observed in T1D progressors in the time-window between seroconversion and T1D diagnosis, accompanied by spikes in inflammation-favoring organisms, gene functions, and serum and stool metabolites. This work identifies trends in the development of the human infant gut microbiome along with specific alterations that precede T1D onset and distinguish T1D progressors from non-progressors.
INTRODUCTION
The initial colonization of the human gut microbiota begins in utero (Aagaard et al., 2014) and is strongly influenced by microbial exposures at birth (Dominguez-Bello et al., 2010). The initial seeding and development of this community may have long-term physiological consequences. Low resolution longitudinal studies in 14 infants (Palmer et al., 2007) and higher resolution studies in a single infant (Koenig et al., 2011) have documented the gradual increase in phylogenetic diversity, nonrandom community assembly, the effects of introducing table foods, and the large taxonomic shifts that can occur during infancy. High resolution multi’omic studies that examine the dynamics of infant gut microbiome development in a large, longitudinal cohort have been lacking, though one recent study has shown that children with severe acute malnutrition exhibit decreased ‘microbiota maturity’ using such a cohort (Subramanian et al., 2014). Events in early microbiome development may have a role in promoting susceptibility to or protection from disease later in life; this has been demonstrated in mice (Cho et al., 2012; Cox et al., 2014), and it may also be true for type 1 diabetes (T1D) (Brown et al., 2011; Giongo et al., 2011; de Goffau et al., 2013).
T1D is an autoimmune disorder that results from T cell-mediated destruction of the insulin-producing beta cells of the pancreatic islets. Although approximately 70% of T1D cases carry predisposing HLA risk alleles, only 3–7% of children with those alleles develop T1D (Achenbach et al., 2005), suggesting a significant non-genetic component to the disease. The incidence of T1D has been increasing rapidly over the past few decades, particularly in the youngest age groups (0–4 years) (Harjutsalo et al., 2008), suggesting a significant non-genetic component to the disease. The incidence of T1D is particularly high in Finland, where 1 in 120 children develop T1D before 15 years of age (Knip et al., 2005).
Although there have been limited human studies of the microbiome in T1D to date, the notion that T1D pathogenesis may be influenced by microbial exposures has been well established in murine models. The knockout of MyD88, an adapter downstream of multiple Toll-like receptors involved in microbial sensing, in the NOD mouse results in complete protection from diabetes (Wen et al., 2008). Further, heterozygous MyD88KO/+ NOD mice, which normally develop robust disease, are protected from diabetes when exposed from birth to the gut microbiota of a MyD88-KO NOD donor (Wen et al., 2008). Therefore, disease progression in the NOD mouse is driven in part by an exaggerated innate immune response to symbiotic microbiota, and altering the composition of the microbiota can curtail this response and prevent disease. Prospective studies are required to assess whether the microbiota is similarly involved in human T1D progression, however such cohorts are exceedingly difficult to build (Brown et al., 2011; Giongo et al., 2011).
Here, we assess the composition of the gut microbiota in a densely sampled, prospective, longitudinal cohort of 33 HLA-matched infants followed from birth until 3 years of age. We use this unprecedented sample resolution to describe the dynamics and stability of the developing microbiome in the infant gut of an at-risk T1D cohort. We show that although there are significant shifts in taxonomic composition over time, the relative abundance of metabolic pathways within individuals remains remarkably constant throughout infancy. We identify a 25% drop in alpha-diversity in children who progress to T1D compared to controls that occurs after seroconversion but before disease diagnosis, and identify alterations to both the phylogenetic and metabolic pathway composition of the microbiome during this time that is characteristic of a pro-inflammatory environment. Our results demonstrate significant alterations to the gut microbiome in T1D progressors prior to disease onset.
RESULTS
Extensive Characterization of the Infant Gut Microbiota in a Longitudinal Cohort
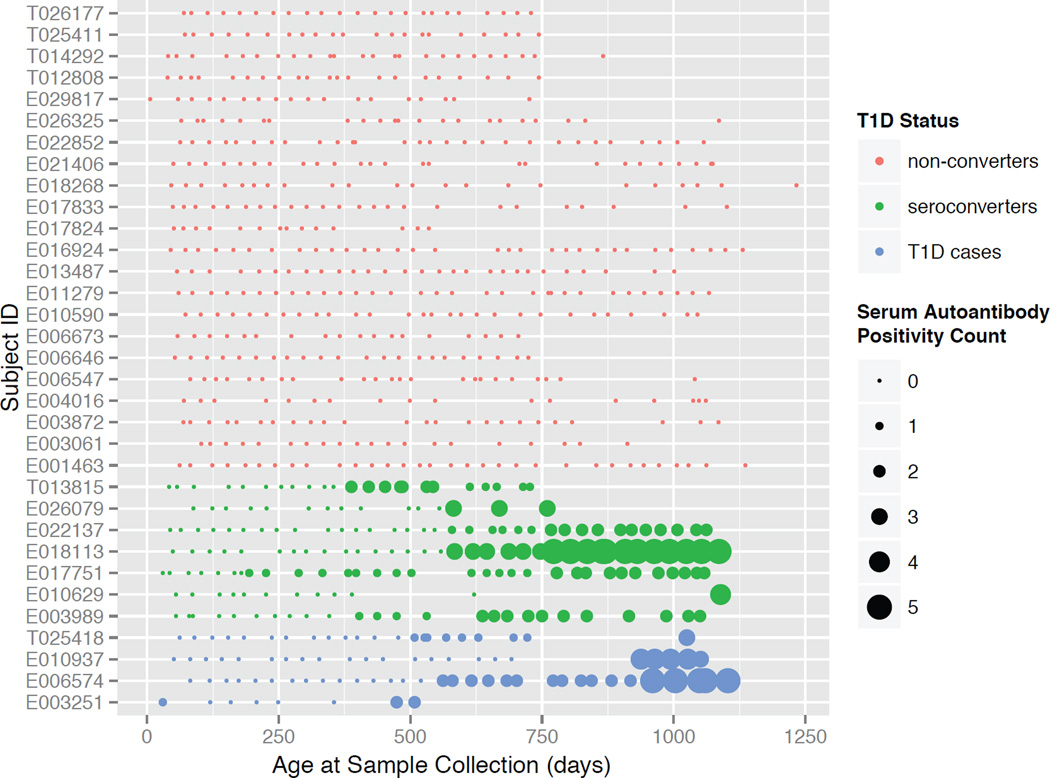
To characterize the development of the infant gut microbiome and the relationship between the gut microbiota and islet autoimmunity and progression to T1D, we assembled a prospective, longitudinal collection of stool samples from infants at risk for disease (Figure 1). Infants from Finland and Estonia were recruited at birth based on HLA risk genotyping (Table 1 and Table S1). Parents collected their infants’ stool at approximately monthly intervals. The cohort was comprised of 33 infants, 11 of whom seroconverted to serum autoantibody positivity (referred to hereafter as ‘seroconverters’; defined as being positive for at least two of the five autoantibodies analyzed in this study; see Experimental Procedures) and of those, four developed T1D within the time frame of this study (referred to as ‘T1D cases’; see Table 1 and Figure 1). The 11 seroconverters were matched with the 22 controls for gender, HLA genotype and country.
Figure 1. A cohort to assess the dynamics of the developing human gut microbiota in infancy.
Individuals are represented in rows and each point is a stool sample. The size of the points represents the number of serum autoantibodies (0–5) that were positive at the time of the sample collection. See also Figure S1.
Table 1.
Summary of patient cohort.
| Subject ID | Country of Origin |
T1D Status | HLA Type | Autoantibody Positive | Age at Seroconversion (days) |
Age at T1D Diagnosis (days) |
|---|---|---|---|---|---|---|
| T026177 | Estonia | non-converter | DQB1*0302/*0501-DRB1*0401 | N/A | N/A | N/A |
| T025411 | Estonia | non-converter | DQB1*0302/*0501-DRB1*0404 | N/A | N/A | N/A |
| T014292 | Estonia | non-converter | DQA1*05/*03-DQB1*02/*0301 | N/A | N/A | N/A |
| T012808 | Estonia | non-converter | DQA1*05/*0201-DQB1*02/*02 | N/A | N/A | N/A |
| E029817 | Finland | non-converter | DQB1*0302/*04-DRB1*0404 | N/A | N/A | N/A |
| E026325 | Finland | non-converter | DQB1*0302/*0501-DRB1*0404 | N/A | N/A | N/A |
| E022852 | Finland | non-converter | DQB1*0302/*0501-DRB1*0404 | N/A | N/A | N/A |
| E021406 | Finland | non-converter | DQB1*0302/*0302-DRB1*0401/*0401 | N/A | N/A | N/A |
| E018268 | Finland | non-converter | DQB1*0302/*0604-DRB1*0404 | N/A | N/A | N/A |
| E017833 | Finland | non-converter | DQA1*05-DQB1*02/*04 | N/A | N/A | N/A |
| E017824 | Finland | non-converter | DQB1*0302/*0604-DRB1*0404 | N/A | N/A | N/A |
| E016924 | Finland | non-converter | DQA1*05-DQB1*02/*04 | N/A | N/A | N/A |
| E013487 | Finland | non-converter | DQA1*05/*03-DQB1*02/*0302-DRB1*0401 | N/A | N/A | N/A |
| E011279 | Finland | non-converter | DQB1*0302/*04-DRB1*0404 | N/A | N/A | N/A |
| E010590 | Finland | non-converter | DQB1*0302/*04-DRB1*0401 | N/A | N/A | N/A |
| E006673 | Finland | non-converter | DQA1*05/*03-DQB1*02/*0302-DRB1*0401 | N/A | N/A | N/A |
| E006646 | Finland | non-converter | DQB1*0302/*0501-DRB1*0401 | N/A | N/A | N/A |
| E006547 | Finland | non-converter | DQB1*0302/*0501-DRB1*0404 | N/A | N/A | N/A |
| E004016 | Finland | non-converter | DQB1*0302/*0502-DRB1*0404 | N/A | N/A | N/A |
| E003872 | Finland | non-converter | DQB1*0302/*0501-DRB1*0404 | N/A | N/A | N/A |
| E003061 | Finland | non-converter | DQB1*0302/*0502-DRB1*0405 | N/A | N/A | N/A |
| E001463 | Finland | non-converter | DQB1*0302/*04-DRB1*0401 | N/A | N/A | N/A |
| T013815 | Estonia | seroconverter | DQA1*05/*0201-DQB1*02/*02 | IAA, GADA | 350.4 | N/A |
| E026079 | Finland | seroconverter | DQB1*0302/*04-DRB1*0401 | IAA, GADA | 580.35 | N/A |
| E022137 | Finland | seroconverter | DQB1*0302/*0501-DRB1*0401 | IAA, GADA, IA-2A, ZNT8A, ICA | 562.1 | N/A |
| E018113 | Finland | seroconverter | DQB1*0302/*04-DRB1*0401 | IAA, GADA, IA-2A, ZNT8A, ICA | 587.65 | N/A |
| E017751 | Finland | seroconverter | DQA1*05-DQB1*02/*0604 | IAA, ICA | 175.2 | N/A |
| E010629 | Finland | seroconverter | DQB1*0302/*0501-DRB1*0401 | IAA, GADA, ZNT8A, ICA | 945.35 | N/A |
| E003989 | Finland | seroconverter | DQB1*0302/*04-DRB1*0401 | IAA, GADA, ZNT8A, ICA | 346.75 | N/A |
| T025418 | Estonia | T1D case | DQA1*0201/*03-DQB1*02/*0302-DRB1*0404 | IAA, GADA, IA-2A, ICA | 540.2 | 879.65 |
| E010937 | Finland | T1D case | DQA1*05/*03-DQB1*02/*0302-DRB1*0401 | IAA, IA-2A, ZNT8A, ICA | 905.2 | 959.95 |
| E006574 | Finland | T1D case | DQB1*0302/*0501-DRB1*0401 | IAA, GADA, IA-2A, ZNT8A, ICA | 532.9 | 1339.55 |
| E003251 | Finland | T1D case | DQB1*0302/*0501-DRB1*0401 | IAA, GADA, IA-2A, ZNT8A, ICA | 357.7 | 1168 |
Sequencing of the V4 region of the 16S rDNA gene was carried out on a total of 989 samples using paired-end, partially overlapping reads on the Illumina MiSeq V2 platform as previously described (Caporaso et al., 2012), yielding a very high depth of sequencing with a mean of 65,076 reads per sample. Taxonomic profiling was performed using QIIME (Caporaso et al., 2010), and functional profiling of microbial pathways was inferred from 16S sequences with PICRUSt (Langille et al., 2013). In total, there were 777 unique samples sequenced by 16S, with a median of 23 unique samples per individual (minimum 8, maximum 34); the full OTU table is available in Table S2. Shotgun metagenomic sequencing was performed on a subset of 124 samples from 19 individuals, including all 11 seroconverters, with a median of 6 samples per individual (minimum 3, maximum 11) (Figure S1). The median depth of sequencing was 2.5 gigabases per sample. Phylogenetic community profiling of metagenomic data was performed using MetaPhlAn (Segata et al., 2012a) and functional profiling of microbial pathways was characterized with HUMAnN (Abubucker et al., 2012).
In addition to 16S and metagenomic sequencing, serum and stool metabolomics were performed on the cohort. For each of the 33 participants, 7 serum samples taken throughout the experimental time frame (Table S1) were subject to metabolomics and lipidomics, and all samples that were used for shotgun metagenomics were also analyzed by stool metabolomics and lipidomics (see Experimental Procedures).
Gut Microbial Metabolites and Functional Pathways but not Taxonomies are Stable throughout Infant Development
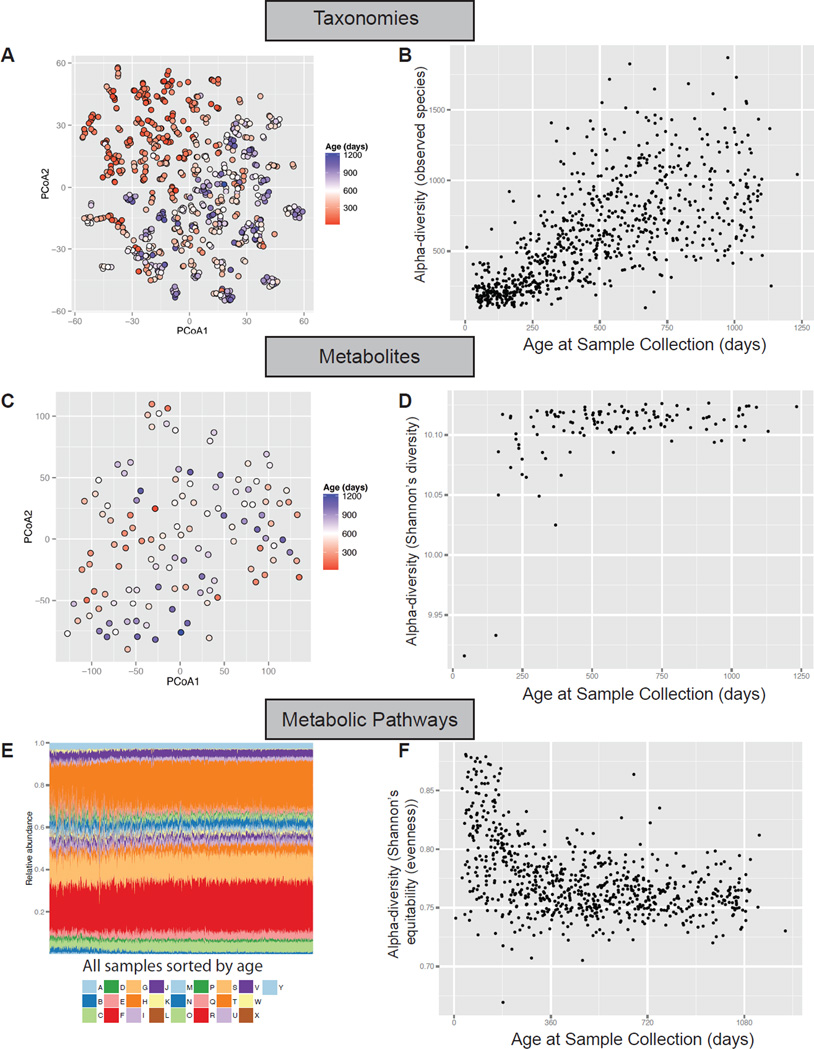
Principal coordinates analysis of the Bray-Curtis dissimilarity between all 777 16S-sequenced samples revealed that age is the strongest driver of the composition of the infant gut microbiome (Figure 2A). Age accounted for 18% of the variation between samples, and showed a nearly linear gradient diagonally along the first and second principal coordinates. Similarly, the Chao1 alpha-diversity, a measure of the number of distinct microbes in a community, exponentially increased in early development until reaching a maximum at three years of age (Figure 2B).
Figure 2. Gut microbial taxonomies shift dramatically whereas microbial metabolites and metabolic pathways remain relatively stable throughout infant development.
(A) Principal coordinates analysis on the unweighted UniFrac distances between samples based on 16S sequencing. Samples are colored by age at stool collection. (B) Alpha-diversity using the QIIME ‘observed species’ metric on 16S sequencing. (C) Principal coordinates analysis on stool metabolomics data. (D) Shannon’s diversity measured on stool metabolomics data. (E) Bars indicate relative abundances of KEGG metabolic modules: A Aminoacyl tRNA; B Arginine and proline metabolism; C Aromatic amino acid metabolism; D Branched chain amino acid metabolism; E Carbon fixation; F Central carbohydrate metabolism; G Cofactor and vitamin biosynthesis; H Cysteine and methionine metabolism; I Fatty acid metabolism; J Glycosaminoglycan metabolism; K Histidine metabolism; L Lipid metabolism; M Lipopolysaccharide metabolism; N Lysine metabolism; O Methane metabolism; P Nitrogen metabolism; Q Nucleotide sugar; R Other amino acid metabolism; S Other carbohydrate metabolism; T Polyamine biosynthesis; U Purine metabolism; V Pyrimidine metabolism; W Serine and threonine metabolism; X Sulfur metabolism; Y Terpenoid backbone biosynthesis. (F) A measure of evenness of KEGG metabolic modules.
We hypothesized that with increasing taxonomic diversity in the developing gut comes an equivalent change in the metabolic composition of the gut community; however, this was not the case. The stool metabolomics beta-diversity distances between samples did not have as strong of an age trend as did taxonomies (Figure 2C), and the alpha-diversity of stool metabolites was nearly flat across time, with the exception of a few outlier very-early-timepoint samples that had a much lower diversity (Figure 2D). More strikingly, the relative abundance of metabolic modules in the microbiome remained approximately constant throughout time and across individuals (Figure 2E shows all samples sorted by age across all individuals). Essentially all pathways are encoded by all individuals from the earliest to the latest time points. The evenness is higher in the first few months until it stabilizes (Figure 2F). This may be because the composition of the microbiome requires time to “settle” into its most optimal abundance of functional pathways, which is less evenly distributed than in the earliest timepoints.
These results demonstrate a remarkable stability in the metabolic pathway coding potential – and the metabolic content – of the microbiome despite dramatic shifts of taxonomic composition throughout human infancy.
A Model of the Dynamics of the Developing Gut Microbiome
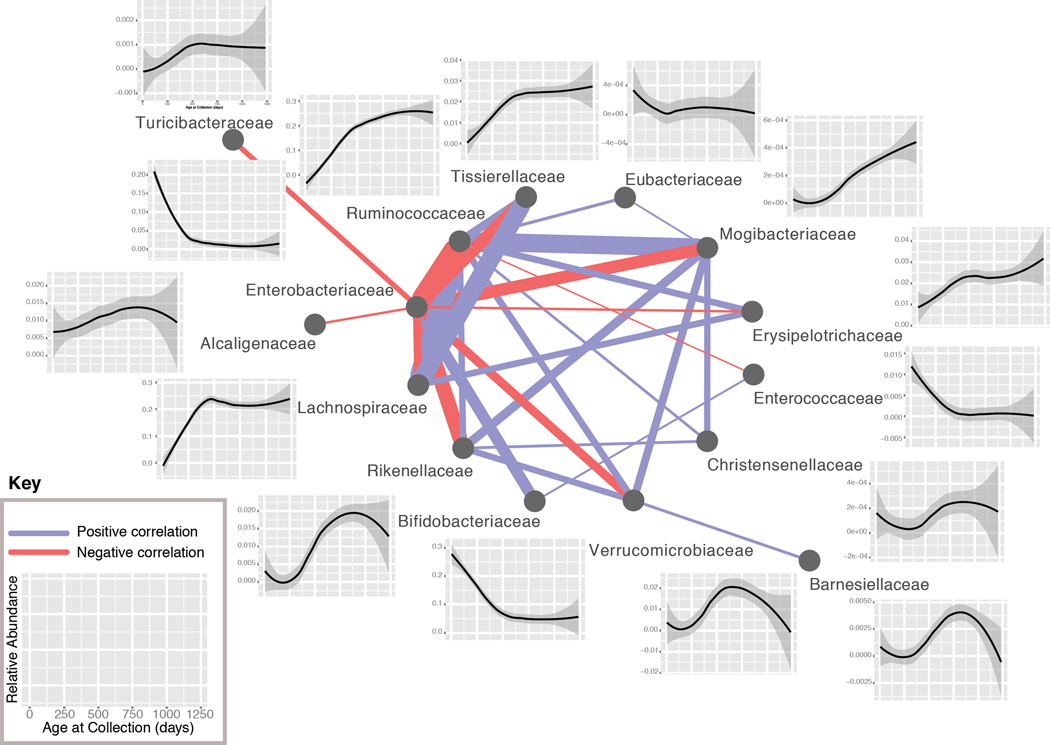
The strength of the age effect in taxonomies and its consistency across individuals suggested that there may be closely shared phylogenetic trajectories that define the development of the gut microbiome, in agreement with previous cross-sectional studies in older children (Yatsunenko et al., 2012). To investigate the driving forces behind this effect, we performed pairwise correlations of the trajectories of abundance between all clades across time on a per-individual basis, excluding T1D cases. Correlations were determined using CCREPE (Faust et al., 2012), a tool designed to find significant correlations in sparse, compositional data such as 16S sequencing data, which are prone to spurious correlations. The Z-score for each clade-clade pair was summed across all individuals (excluding T1D cases), revealing a small set of pairs with very strong correlations that were consistent across most subjects. This allowed us to produce a network of the dynamics of the developing gut microbiome (Figure 3). Plotting the corresponding clade abundances over time demonstrates a strongly shared dynamic relationship across time, and across nearly all individuals, for many specific clades in this developmental process. The resulting network at the family level is shown in Figure 3; see Figure S2 for other phylogenetic levels.
Figure 3. Temporal dynamics of microbial taxonomies in infant gut development.
Family-level network diagram of the correlation between clades in their trajectories across time, excluding individuals with T1D. Positive correlations are in blue, negative correlations are in red, and the line thickness is proportional to the strength of the correlation (cumulative CCREPE Z-statistic). The plots show the abundance of the indicated family as a smoothing spline across all healthy individuals with a 95% confidence interval (shaded region). See also Figure S2.
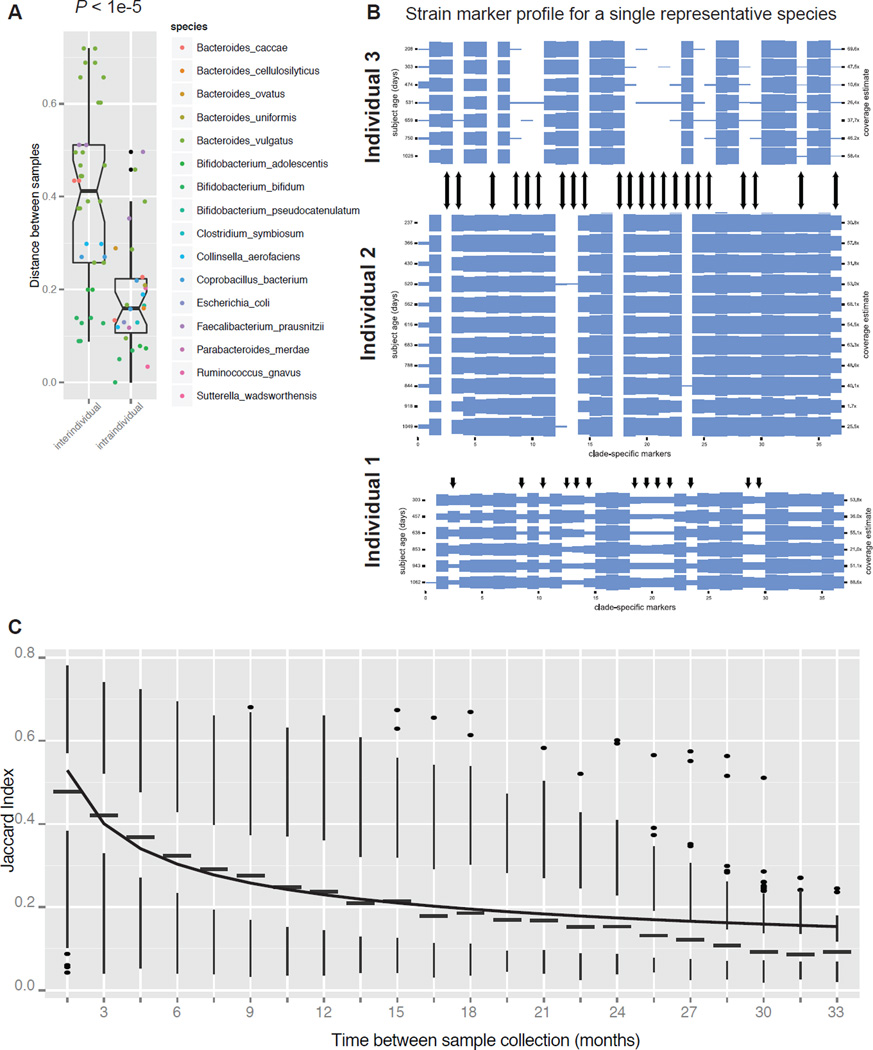
The Infant Gut Microbiome Remains Stable at the Strain Level
Having investigated the dynamics of the infant gut microbiome, we examined its strain-level stability, i.e. the retention of microbial strains across time. Using the shotgun metagenomics data available on 124 samples, we analyzed strain-level markers on a per-species and per-individual basis using MetaPhlAn (Segata et al., 2012b). Analysis was restricted to species that have a mean of at least 1× coverage across all timepoints per individual; 21 species and 12 individuals met this requirement. Using an unweighted discordant marker distance metric (see Supplemental Experimental Procedures), we found that samples were more similar in the intraindividual versus interindividual comparison (P < 1e-5; Figure 4A), suggesting that the strain profile for a given species is more similar between samples in a single individual than between samples in two people. Surprisingly, the strain profile remained essentially constant over time for almost all species and in almost all individuals (Figure 4B shows a representative example in which the marker abundance is constant over time (individuals #2 and #3). In a rare case we observed a shift in the strain signature at a specific timepoint (individual #1). Table S3 shows marker abundances for all individuals with sufficient marker coverage.
Figure 4. Bacterial strains are stably maintained in the infant gut throughout development.
(A) Distance between samples between subjects (interindividual) and within subjects (intraindividual) based on MetaPhlAn cladespecific strain marker analysis. (B) Shown is the MetaPhlAn clade-specific strain marker profile for a single representative species (Bacteroides ovatus) for three separate individuals. Columns represent the 37 markers for this species, rows represent samples, and arrows indicate discordant markers between individuals 2 and 3, and indicate markers that undergo a change in abundance in individual 1. (C) The Jaccard index (fraction of shared OTUs) between pairs of samples from the same individual within the indicated time window (i.e. 1.5 indicates 0 to 1.5 months, 6 indicates 4.5 to 6 months). The Jaccard index is shown for all pairs of samples across all subjects. A power-law curve was fitted to the medians of the boxplots (line). The box represents the first and third quartiles and error bars indicate 95% confidence of median. See also Table S3.
We investigated community stability by calculating the Jaccard index, which is defined as the fraction of shared operational taxonomic units (OTUs), between all pairs of samples within an individual in specified time windows (Figure 4C). For instance, we calculated the fraction of shared OTUs within a subject between 2 samples collected approximately 3 months apart, and found that the Jaccard index is significantly higher than for 2 samples from the same individual collected 6 months apart. As has been observed in an adult population (Faith et al., 2013), we found that the Jaccard index followed a power-law function (Figure 4C, line). The curve reached an asymptote at a value of approximately 0.1, suggesting that about 10% of bacterial strains (observed here at an OTU-level resolution) were maintained in the infant gut from birth until 3 years of age (Figure 4C). This surprising result demonstrates that although there is tremendous variability in the gut microbiome through infancy, the community at 3 years of age retained a non-negligible fraction of members that it acquired just after birth.
Correlations between the Gut Microbiome and Diet and Environmental Factors
Extensive metadata relating to both clinical and non-clinical factors were collected for each participant in the study (Table S1), allowing us to assess the association between the gut microbiome and environmental factors in our cohort. To avoid the potential confounding effects of age, multiple sampling from the same individual, and each of the other metadata, all analyses were performed on a reduced set of samples in a limited timeframe, using age and other metadata as fixed effects and subject identity as a random effect. This analysis was performed using MaAsLin (Morgan et al., 2012)), an additive general linear model with boosting that can capture the effects of a parameter of interest while deconfounding the effects of other metadata. This is particularly important in the current study, as age, diet, and other factors are expected to have strong influences on community composition (Table S1; see Experimental Procedures for the metadata included in the MaAsLin analysis). With MaAsLin, we focused our analysis on a single variable of interest, and systematically “subtracted-out” the effect of each of the other potentially confounding metadata variables. A series of five samples from each breastfed subject taken during and after cessation of breastfeeding revealed an increase in Bifidobacterium and Lactobacillus species during breastfeeding; however, we found that the reduction in Lachnospiraceae was an even stronger effect (Figure S3A). We observed substantial differences between Estonian and Finnish infants including significantly higher levels of Bacteroides and Streptococcus species, which contain a number of potential pathobionts, in the Estonians (Figure S3B). Additionally, we observed specific shifts in phylogenetic abundance with several other dietary parameters: eggs, barley, soy, and fish (nonsignificant) (Figure S3C). Notably, these shifts are less significant than those associated with geography or breastfeeding. Although we included antibiotic usage as a fixed effect for all MaAsLin analyses in this study, we did not have sufficient annotation on the timing of antibiotic usage to identify community shifts associated with antibiotics. We did not find differences in community composition between cesarean section- versus vaginally delivered infants, perhaps because our cohort included only 3 cesarean-delivered subjects.
Gut Microbiota Composition Distinguishes T1D Status
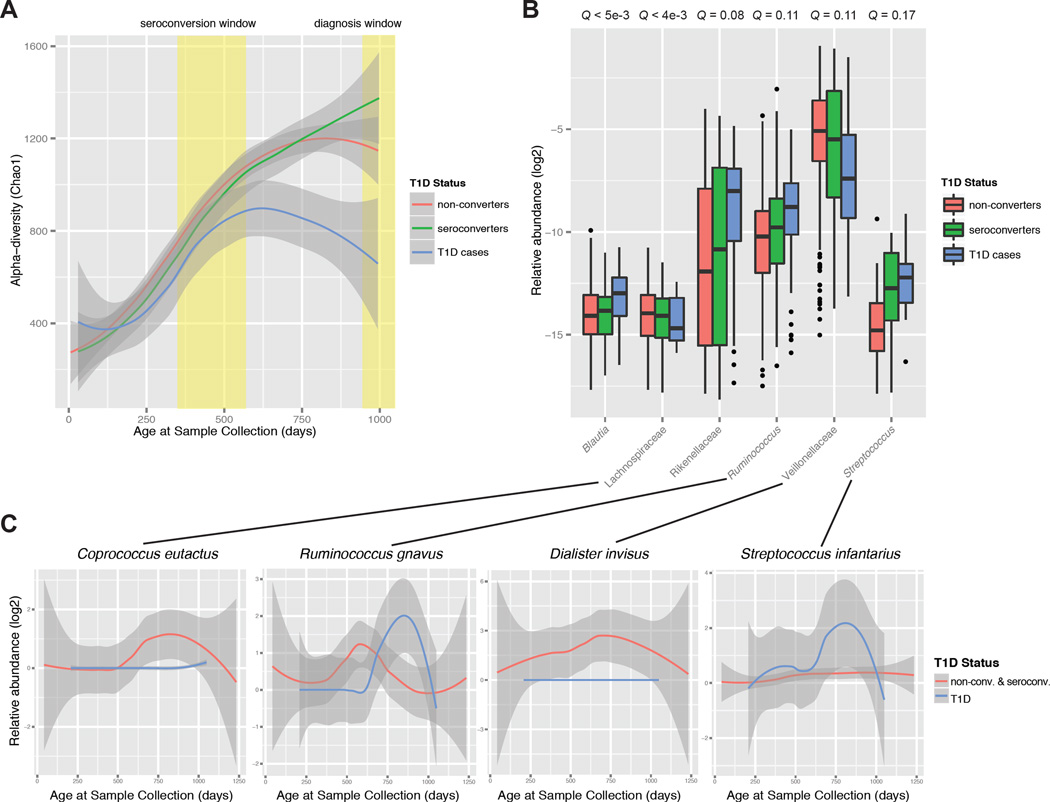
We next examined whether there were features of the microbial community that could distinguish T1D disease state. We assessed Chao1 alpha-diversity across time in non-converter (not seroconverted), seroconverted (not diagnosed with T1D), and T1D cases (seroconverted subjects also diagnosed with T1D). We observed a pronounced flattening of the alpha-diversity in T1D subjects at a time when the gut communities of the non-converter and seroconverted individuals continued to rise in alpha-diversity (Figure 5A). This result was significant by permutation test on subject labels (P < 0.025; Figure S3D). Intriguingly, this divergence in alpha-diversity occurred after the time period in which most subjects seroconverted, but before the progressors presented with clinical disease.
Figure 5. The gut microbiota distinguishes disease status in T1D prior to diagnosis.
(A) Plot of Chao1 alpha-diversity across time, represented as a smoothing spline with a 95% confidence interval (shaded region). The seroconversion window indicates the first and third quartiles for age at seroconversion for all seroconverted and T1D-diagnosed individuals, and the diagnosis window indicates the first quartile of time at T1D diagnosis (third quartile is 1,211 days). (B) Abundances of the significantly differentially abundant taxa between T1D versus non-converter and seroconverted individuals, including only samples between the seroconversion and diagnosis windows. FDR-corrected P-values (Q-values) were calculated using MaAsLin. The box represents the first and third quartiles; error bars indicate 95% confidence of median. (C) Plots of the relative abundance of representative species from shotgun metagenomics data, represented as a smoothing spline with a 95% confidence interval (shaded region). See also Figure S3 and Figure S5.
To investigate the specific changes to the community that accounted for the decreased alpha-diversity in T1D subjects, we used MaAsLin analysis to focus on the time of the alpha-diversity divergence, after 600 days of age, and observed a number of significant alterations that distinguish T1D cases from non-converters and seroconverters (Figure 5B). After correcting for potential confounding variables, we found that the drop in alpha-diversity in T1D cases could be accounted for by the relative overabundance of a few groups: Blautia, the Rikenellaceae, and the Ruminococcus and Streptococcus genera (not statistically significant). These groups of bacteria contain species that have been characterized as ‘pathobionts’ (Chow and Mazmanian, 2009), which are members of the commensal microbiota that have the capacity to behave as pathogens. Our shotgun metagenomic sequencing revealed that specific pathobiont-like species within these groups, such as Ruminococcus gnavus and Streptococcus infantarius, showed a spike in relative abundance within the T1D cases at the time of alpha-diversity divergence (Figure 5C). Conversely, we saw a relative under-abundance of a few groups of bacteria that are commonly depleted in the inflammatory state, namely the Lachnospiraceae and Veillonellaceae (not statistically significant) (Figure 5B), and metagenomic sequencing showed the complete absence of a number of these species, such as Coprococcus eutactus and Dialister invisus, in T1D cases (Figure 5C). Remarkably, seroconverters had an intermediate abundance of all of these groups of organisms between non-converters and T1D cases (Figure 5B), providing further evidence that this shift in microbiome composition is linked to the T1D disease state.
Gut Microbial Gene Content is Altered Prior to Clinical Onset of T1D
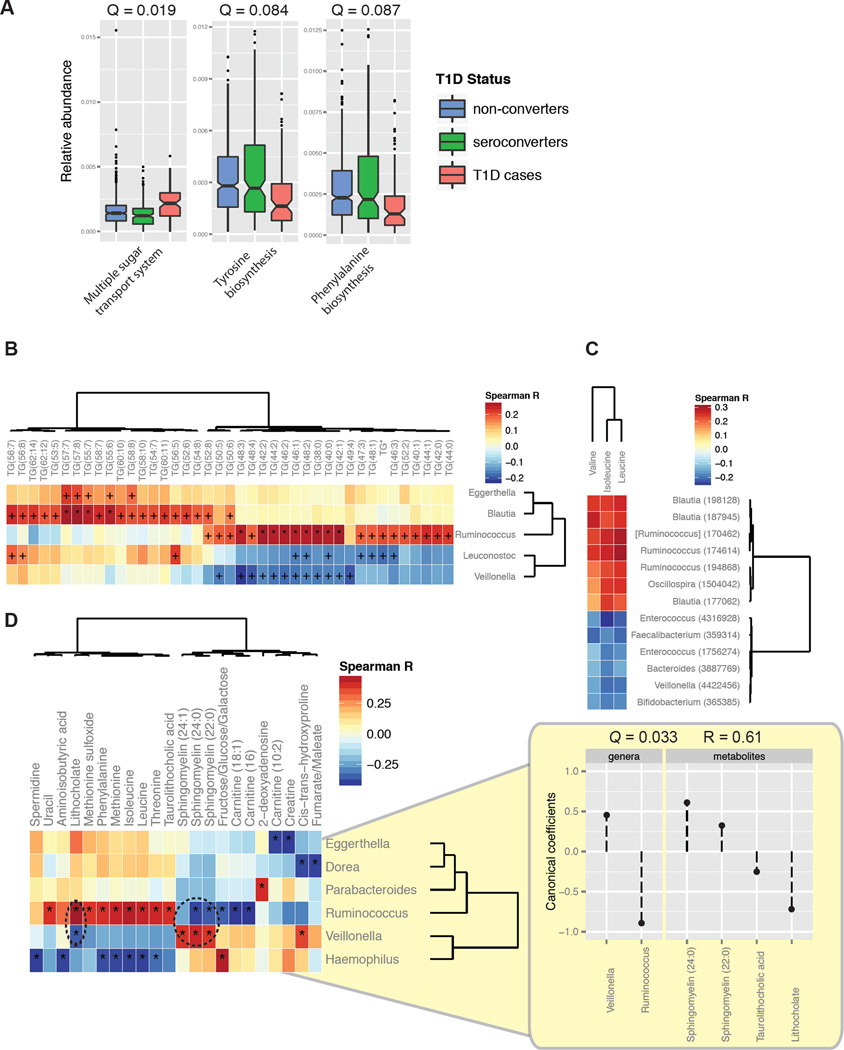
The NIH Human Microbiome Project has shown significant stability in microbial metabolic pathways across individuals despite high variability in taxonomic composition (Consortium, 2012). We investigated whether changes in the abundance of specific metabolic pathways correlated with T1D status. After correcting for the effects of confounding variables such as age and diet using MaAsLin, we found significant shifts that occured within T1D cases including an increase in the multiple sugar transport system, which is involved in the utilization of D-galactose, D-xylose, L-arabinose, D-glucose, and D-mannose, and a decrease in the biosynthesis of a number of amino acids (Figure 6A). A shift in functional potential from the synthesis of nutrients to the passive transporting-in of nutrients is characteristic of auxotrophic organisms. Auxotrophs thrive in inflammatory environments where dead tissue provides easy access to many nutrients that are less available in the healthy gut (Morgan et al., 2012). As was found for T1D-associated phylogenies, seroconverters had an intermediate level of abundance between non-converters and T1D cases in metabolic pathway carriage (Figure 6A), and were more similar to non-converters than T1D cases.
Figure 6. Gut microbial gene content and serum and gut metabolites are altered prior to T1D onset.
(A) Abundances of the significantly differentially abundant KEGG modules between T1D versus seroconverted individuals, including only samples between the seroconversion and diagnosis windows. FDR-corrected P-values (Q-values) were calculated using MaAsLin. The box represents the first and third quartiles; error bars indicate 95% confidence of median. (B) Spearman correlations between serum triglycerides and the five most correlated genera, and (C) between the branched-chain amino acids and OTUs using a cutoff of P < 0.001. +, correlations with P < 0.05; *, correlations with Q < 0.05. “TG*” represents TG(14:0/18:1/18:1)+TG(16:0/16:1/18:1). (D) Spearman correlations between stool metabolites and lipids and the most correlated genera. Coefficients of the canonical variates including Ruminococcus, Veillonella, and correlated metabolites obtained using penalized canonical correlation analysis. See also Figure S4.
Serum and Gut Lipids and Metabolites Relevant to Disease are Correlated with T1D-associated Microbial Taxa
The physiological effects of the gut microbiota extend beyond the gut; there is an interplay between both host and microbial enzymes and their metabolites which impacts host metabolism (Velagapudi et al., 2010), mucosal immunity (Smith et al., 2013), as well as diseases including cardiovascular disease (Koeth et al., 2013; Wang et al., 2011). We assessed the correlation between serum polar metabolites and lipids with the gut microbiota. A Spearman correlation between absolute abundances of metabolites and microbial relative abundances yielded a number of clusters related groups of metabolites and microbes clustered together (Figures S4). Most significantly, we observed a clustering of triglycerides with a number of microbes, including a positive correlation between Blautia and long-chain triglycerides and Ruminococcus with short-chain triglycerides, and a negative correlation between Veillonella and short-chain triglycerides (Figure 6B). At the OTU level, we also observed correlations between members of these genera with branched-chain amino acids, specifically a positive correlation with Blautia and Ruminococcus members and a negative correlation with a Veillonella member (Figure 6C). Altered levels of serum triglycerides are a common feature of obesity and type 2 diabetes, and hypertriglyceridemia is associated with poor glycemic control and nephropathy in T1D (Alcantara et al., 2011; Vergès, 2009). Additionally, elevated branch-chain amino acids have been shown in both patients (Vannini et al., 1982) and in mouse models (Mochida et al., 2011; Sailer et al., 2013) of diabetes, as well as preceding islet autoimmunity in children who later progress to T1D (Orešič et al., 2008). We found a positive correlation between Blautia and Ruminococcus, both of which have increased abundance in T1D cases, with triglycerides and branch-chain amino acids, possibly indicating that these microbe-metabolite relationships cooperatively impact T1D progression.
To conduct an integrative analysis of the correlations that exist between the gut microbiome and the gut (stool) metabolome, we performed penalized canonical correlation analysis (Figures 6D; see Experimental Procedures). This analysis identified a canonical variate that associates increased Ruminococcus and decreased Veillonella abundance with increased sphingomyelin and decreased lithocholic acid levels (Pearson R = 0.61; Q = 0.03). Sphingomyelin is a member of the sphingolipids, which inhibit intestinal natural killer T cell function and protect against oxazolone-induced colitis (An et al., 2014). Lithocholic acid, similar to deoxycholic acid, is a secondary bile acid that promotes intestinal inflammation by eliciting reactive oxygen and nitrogen species and activating NF-κB activity in intestinal epithelial cells (Lee et al., 2004; Mühlbauer et al., 2004; Payne et al., 2007; Sears and Garrett, 2014; Da Silva et al., 2012). Although the alterations to the microbiota that we observed may be related to impaired glucose metabolism in the prediabetic stage, these results suggest that the T1D-associated microbiota that becomes established prior to disease onset may actively promote a metabolic environment in the gut that is permissive to inflammation and promotes pathogenesis.
DISCUSSION
To identify and understand alterations to the gut microbial community composition that may contribute to childhood disease, we must first investigate the normal dynamics of the community in the developing infant. Here, we identify a set of principles that describe microbiome development in the infant gut. We note as a caveat that all children in this cohort carry T1D-predisposing HLA alleles and are restricted to the countries of Finland and Estonia, and are therefore not necessarily representative of genetically ‘normal’ infants in other regions of the world. First, although there is great variation in overall taxonomic composition between and within individuals over time, there is significantly less variation in the metabolic composition of the microbiome, and almost no variation in its metabolic pathway coding potential. This result provides a variation on the finding made in the NIH Human Microbiome Project regarding the stability of metabolic pathways in the microbiome between healthy adults (Consortium, 2012) and suggests that the relative proportions of bacterial functional pathways remains the same from soon after birth until three years of age. We speculate that because the taxonomic composition of the microbiome stabilizes at approximately three years, functional pathways likely remain stable for long after this age as well.
Second, we identified shared taxonomic trajectories, remarkably consistent across individuals, that indicate general changes in abundance, the timing of these shifts, and the relationships between community members. For example, we saw a strong positive correlation between the Lachnospiraceae and Ruminococcaceae, both Gram-positive anaerobes that are inversely correlated with the Enterobacteriaceae, Gram-negative aerobes. In turn, the Enterobacteriaceae are positively correlated with the Bifidobacteriaceae, which decrease in abundances after cessation of breastfeeding. Although there are many exceptions to general trends, we observed a decrease in Gram-negative bacteria over time, and found that early colonizers are aerobic whereas later colonizers tend to be anaerobic. Similar trends have been observed previously (Dominguez-Bello et al., 2010; Koenig et al., 2011; Palmer et al., 2007); however, the significantly higher density of sampling, size, and longitudinal nature of our cohort provide a high resolution map of these dynamics and demonstrate how universal they are across infants.
Third, we demonstrated a surprising stability in the maintenance of specific strains through time. Although the strain composition is quite distinct between individuals as expected, the strain composition within an individual remains essentially constant throughout infancy for almost all individuals and almost all species that have sufficiently high abundance for stability analysis.
In addition to shared trends, we identified aspects of infant gut microbiome development that are unique to the T1D state. We observed a significant alteration in the structure of the T1D-associated gut microbiome: a relative 25% reduction in alpha-diversity compared to non-converters and seroconverters, associated with shifts in both microbial phylogenetic and metabolic pathways. Importantly, this shift is seen in children who are diagnosed with T1D within the study time frame, but not in seroconverters without disease. Although the probability of progression to T1D after positivity for two islet autoantibodies is greater than 80% by the age of 15 (Ziegler et al., 2013), there is significant variability in when progression occurs, ranging from weeks to more than two decades (Knip et al., 2010), and the factors contributing to this variability are not well understood. The logistics of densely sampling a large cohort of individuals through T1D diagnosis limits the time frame of such a study, and we therefore are reporting on a special subset of T1D cases with early onset diabetes (EOD) (Harjutsalo et al., 2013). We provide evidence that pronounced alterations occur in the gut microbiome that precede overt disease.
Although previous studies of human cohorts have been constrained by the availability of sufficient longitudinal samples and subject groupings that distinguished seroconverting non-progressors from T1D progressors, they have shown a decreased microbial diversity in children with long-lasting beta-cell autoimmunity and in progressors to clinical T1D compared to non-seroconverted controls (Brown et al., 2011; Giongo et al., 2011; de Goffau et al., 2013). Here, we demonstrated that this shift occurs prior to onset of disease but after seroconversion, and identified that it is specific to T1D progressors and not seen in seroconverters without disease. Decreased microbial diversity is a hallmark of dysbiosis and has been observed in obesity (Turnbaugh et al., 2008), inflammatory bowel disease (Manichanh et al., 2012), and Clostridium difficile-associated diarrhea (Chang et al., 2008). A recent study showed that a failure to establish a critical level of diversity in the gut microbiota of developing mice resulted in long-term IgE levels, thus predisposing mice to immune-mediated disorders (Cahenzli et al., 2013). Decreased diversity results from the blooming of a small subset of the community that crowds out other community members.
Additionally, we find higher levels of human β-defensin 2 (hBD2) in early samples of children who develop T1D (Figure S5). hBD2 is an antimicrobial product induced by colonic epithelial cells during inflammation (O’Neil et al., 1999; Wehkamp et al., 2005); therefore, this result is supportive of increased intestinal inflammation in the cohort of children who go on to develop T1D. It has been proposed that an aberrant gut microbiota, a permeable intestinal mucosal barrier, and an altered mucosal immune response collectively contribute to the development of T1D (Vaarala et al., 2008). Our results prompt further functional studies to determine whether the pro-inflammatory microbiome we observe to bloom prior to clinical disease onset may take advantage of or drive increased intestinal permeability and intestinal inflammation to contribute to T1D pathogenesis.
EXPERIMENTAL PROCEDURES
Study Cohort
Please see Supplemental Experimental Procedures for cohort recruitment and sample and information collection details.
Stool Sample Collection and DNA Extraction
Stool samples were collected by participants’ parents and stored in the household freezer (−20°C) until the next visit to the local study center; samples were then shipped on dry ice to the DIABIMMUNE Core Laboratory. The samples were then stored at −80°C until shipping to the Broad Institute for DNA extraction. DNA extractions from stool were carried out using the QIAamp DNA Stool Mini Kit (Qiagen, Inc., Valencia, CA, USA).
Sequencing and Analysis of the 16S Gene and Shotgun Metagenomics
16S sequencing and metagenomics was performed essentially as previously described (Gevers et al., 2014). Additional details are available in the Supplemental Experimental Procedures.
Phylogenetic Abundance Trajectory Network Analysis
Analysis was limited to the 29 individuals without T1D. Samples from the 16S OTU abundance table were binned into 20 time-windows from 50 to 1,100 days, selecting the nearest sample in time for each bin. Read counts from the 16S OTU abundance table were collapsed at each phylogenetic level, from phylum to genus, and compositionally normalized such that the abundance in each sample sums to one. At each phylogenetic level, on a per-individual basis, the correlation between every clade-clade pair was performed in CCREPE (http://huttenhower.sph.harvard.edu/ccrepe; previously known as ‘ReBoot’ (Faust et al., 2012)). The default similarity metric, Spearman, was used. Only correlations with a Q-value < 0.1 were included in analysis. For each filtered clade-clade pair, the Z-statistic was summed across all 29 individuals, and only pairs with a cumulative absolute Z-statistic value of >20 were carried forward, as this was a conservative cut-off for consistent correlations across many subjects. The cumulative Z-statistic was scaled without centering using the R ‘scale’ function, and then visualized as a network diagram on Cytoscape.
Human β-defensin 2 Measurement from Stool Samples
Frozen stool samples were thawed at room temperature immediately prior to analysis. Fecal human β-defensin 2 (hBD2) levels were determined by the use of the enzyme linked immunoabsorbent assay (ELISA) using the [beta]-defensin 2 ELISA Kit (Immundiagnostik, Bensheim, Germany) adapted for fecal samples as described previously (Kapel et al., 1999).
Metabolomics and Lipidomics Profiling from Serum and Stool
Please see Supplemental Experimental Procedures for detailed metabolomics and lipidomics protocols and sample handling information.
Community Stability Analysis
The Jaccard index for a given sample pair is defined as: (sample A ∩ sample B)/(sample A ∪ sample B), and calculated using the compositionally normalized 16S OTU table. On a per-individual basis, the Jaccard index was calculated for all samples that fell into time windows of 1.5 months in length, beginning at 0 to 1.5 months up to 31.5 to 33 months.
MaAsLin (Multivariate Association with Linear Models) Analysis
MaAsLin analysis was performed using default parameters (http://huttenhower.sph.harvard.edu/maaslin). Subject ID was used as a random effect. The following variables were used as fixed effects for every analysis, in addition to the variable of interest: T1D status, age, gender, country, delivery mode, time and name of antibiotic exposure, total reads per sample, sequencing batch ID, breastfeeding (on/off), solid food (on/off), eggs (on/off), fish (on/off), soy products (on/off), rye (on/off), barley (on/off), and buckwheat and millet (on/off).
Alpha-diversity
Alpha-diversity analysis of the 16S OTU table was performed in QIIME 1.5.0 (Caporaso et al., 2010) with the alpha_diversity.py script using the ‘chao1’ metric and default parameters. Permutation-based analysis of significance was performed on a per-subject basis by shuffling the T1D subject label through all individuals and recalculating the difference in Chao1 mean between T1D subjects versus control and seroconverted subjects. 10,000 permutations were performed.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tiffany Poon (Broad Institute) for sample, sequencing, and data coordination, Timothy L. Tickle, Emma Schwager, and Xochitl C. Morgan (Harvard School of Public Health) for assistance with statistical analysis and helpful discussions, Natalia Nedelsky (MGH) for editorial assistance, Niina Lietzen, Leena Öhrnberg, Anna-Liisa Ruskeepää and Heli Nygren (VTT Technical Research Centre of Finland) for assistance in metabolomics analysis, and Katriina Koski and Matti Koski (Institute of Clinical Medicine, University of Helsinki) for the coordination and data base work of the DIABIMMUNE Study. Supported by the European Union Seventh Framework Programme FP7/2007-2013 under grant agreement No. 202063, Juvenile Diabetes Research Foundation grant 17-2011-529, and The Academy of Finland Centre of Excellence in Molecular Systems Immunology and Physiology Research grant Decision No. 250114, 2012–2017.
CONSORTIA
The members of the DIABIMMUNE Study Group are Mikael Knip, Katriina Koski, Matti Koski, Taina Härkönen, Samppa Ryhänen, Heli Siljander, AnuMaaria Hämäläinen, Anne Ormisson, Aleksandr Peet, Vallo Tillmann, Valentina Ulich, Elena Kuzmicheva, Sergei Mokurov, Svetlana Markova, Svetlana Pylova, Marina Isakova, Elena Shakurova, Vladimir Petrov, Natalya V. Dorshakova, Tatyana Karapetyan, Tatyana Varlamova, Jorma Ilonen, Minna Kiviniemi, Kristi Alnek, Helis Janson, Raivo Uibo, Tiit Salum, Erika von Mutius, Juliane Weber, Helena Ahlfors, Henna Kallionpää, Essi Laajala, Riitta Lahesmaa, Harri Lähdesmäki, Robert Moulder, Viveka Öling, Janne Nieminen, Terhi Ruohtula, Outi Vaarala, Hanna Honkanen, Heikki Hyöty, Anita Kondrashova, Sami Oikarinen, Hermie J.M. Harmsen, Marcus C. De Goffau, Gjal Welling, Kirsi Alahuhta, Tuuli Korhonen, Suvi M. Virtanen, and Taina Öhman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014;6:237ra65–ra237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubucker S, Segata N, Goll J, Schubert AM, Izard J, Cantarel BL, Rodriguez-Mueller B, Zucker J, Thiagarajan M, Henrissat B, et al. Metabolic Reconstruction for Metagenomic Data and Its Application to the Human Microbiome. PLoS Comput. Biol. 2012;8:e1002358. doi: 10.1371/journal.pcbi.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach P, Bonifacio E, Koczwara K, Ziegler A-G. Natural History of Type 1 Diabetes. Diabetes. 2005;54:S25–S31. doi: 10.2337/diabetes.54.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- Alcantara LM, Silveira NE, Dantas JR, Araujo PB, de Oliveira MM, Milech A, Zajdenverg L, Rodacki M, de Oliveira JE. Low triglyceride levels are associated with a better metabolic control in patients with type 1 diabetes. Diabetol. Metab. Syndr. 2011;3:22. doi: 10.1186/1758-5996-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Ertürk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL. Sphingolipids from a Symbiotic Microbe Regulate Homeostasis of Host Intestinal Natural Killer T Cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Intestinal Microbial Diversity during Early-Life Colonization Shapes Long-Term IgE Levels. Cell Host Microbe. 2013;14:559–570. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME. J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 2008;197:435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J, Mazmanian SK. Getting the Bugs out of the Immune System: Do Bacterial Microbiota “Fix” Intestinal T Cell Responses? Cell Host Microbe. 2009;5:8–12. doi: 10.1016/j.chom.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Consortium THMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. The Long-Term Stability of the Human Gut Microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, Huttenhower C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012;8:e1002606. doi: 10.1371/journal.pcbi.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D, Kugathasan S, Denson La, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME. J. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Goffau MC, Luopajärvi K, Knip M, Ilonen J, Ruohtula T, Härkönen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- Harjutsalo V, Sund R, Knip M, Groop P-H. Incidence of type 1 diabetes in Finland. JAMA. 2013;310:427–428. doi: 10.1001/jama.2013.8399. [DOI] [PubMed] [Google Scholar]
- Kapel N, Matarazzo P, Haouchine D, Abiola N, Guérin S, Magne D, Gobert JG, Dupont C. Fecal tumor necrosis factor alpha, eosinophil cationic protein and IgE levels in infants with cow’s milk allergy and gastrointestinal manifestations. Clin. Chem. Lab. Med. 1999;37:29–32. doi: 10.1515/CCLM.1999.004. [DOI] [PubMed] [Google Scholar]
- Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK. Environmental Triggers and Determinants of Type 1 Diabetes. Diabetes. 2005;54:S125–S136. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- Knip M, Korhonen S, Kulmala P, Veijola R, Reunanen A, Raitakari OT, Viikari J, Akerblom HK. Prediction of type 1 diabetes in the general population. Diabetes Care. 2010;33:1206–1212. doi: 10.2337/dc09-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013 doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Park SY, Baik SK, Kwon SO, Chung JM, Oh E-S, Kim HS. [Deoxycholic acid-induced signal transduction in HT-29 cells: role of NF-kappa B and interleukin-8] Korean J. Gastroenterol. 2004;43:176–185. [PubMed] [Google Scholar]
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- Mochida T, Tanaka T, Shiraki Y, Tajiri H, Matsumoto S, Shimbo K, Ando T, Nakamura K, Okamoto M, Endo F. Time-dependent changes in the plasma amino acid concentration in diabetes mellitus. Mol. Genet. Metab. 2011;103:406–409. doi: 10.1016/j.ymgme.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Morgan X, Tickle T, Sokol H, Gevers D, Devaney K, Ward D, Reyes J, Shah S, Leleiko N, Snapper S, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbauer M, Allard B, Bosserhoff AK, Kiessling S, Herfarth H, Rogler G, Schölmerich J, Jobin C, Hellerbrand C. Differential effects of deoxycholic acid and taurodeoxycholic acid on NF-kappa B signal transduction and IL-8 gene expression in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G1000–G1008. doi: 10.1152/ajpgi.00338.2003. [DOI] [PubMed] [Google Scholar]
- O’Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- Orešič M, Simell S, Sysi-Aho M, Näntö-Salonen K, Seppänen-Laakso T, Parikka V, Katajamaa M, Hekkala A, Mattila I, Keskinen P, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J. Exp. Med. 2008;205:2975–2984. doi: 10.1084/jem.20081800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the Human Infant Intestinal Microbiota. PLOS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CM, Weber C, Crowley-Skillicorn C, Dvorak K, Bernstein H, Bernstein C, Holubec H, Dvorakova B, Garewal H. Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28:215–222. doi: 10.1093/carcin/bgl139. [DOI] [PubMed] [Google Scholar]
- Sailer M, Dahlhoff C, Giesbertz P, Eidens MK, de Wit N, Rubio-Aliaga I, Boekschoten MV, Müller M, Daniel H. Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome. PLoS One. 2013;8:e63950. doi: 10.1371/journal.pone.0063950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears CL, Garrett WS. Microbes, Microbiota, and Colon Cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods. 2012a doi: 10.1038/nmeth.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods. 2012b;9:811–814. doi: 10.1038/nmeth.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva M, Jaggers GK, Verstraeten SV, Erlejman AG, Fraga CG, Oteiza PI. Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, p38, and Akt activation in Caco-2 cells. Free Radic. Biol. Med. 2012;52:151–159. doi: 10.1016/j.freeradbiomed.2011.10.436. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science. 2013 doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014 doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2008;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarala O, Atkinson MA, New J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Marchesini G, Forlani G, Angiolini A, Ciavarella A, Zoli M, Pisi E. Branched-chain amino acids and alanine as indices of the metabolic control in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1982;22:217–219. doi: 10.1007/BF00283757. [DOI] [PubMed] [Google Scholar]
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Borén J, Oresic M, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 2010;51:1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergès B. Lipid disorders in type 1 diabetes. Diabetes Metab. 2009;35:353–360. doi: 10.1016/j.diabet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Schmid M, Fellermann K, Stange EF. Defensin deficiency, intestinal microbes, and the clinical phenotypes of Crohn’s disease. J. Leukoc. Biol. 2005;77:460–465. doi: 10.1189/jlb.0904543. [DOI] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012 doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.