Abstract
Heparin is a highly sulfated polysaccharide and useful because of its diverse biological functions. However, because of batch-to-batch variability and other factors, there is significant interest in preparing biomimetics of heparin. To identify polymeric heparin mimetics, a cell-based screening assay was developed in cells that express fibroblast growth factor receptors (FGFRs) but not heparan sulfate proteoglycans. Various sulfated and sulfonated polymers were screened and poly(vinyl sulfonate) (pVS) was identified as the strongest heparin-mimicking polymer in its ability to enhance binding of basic fibroblast growth factor (bFGF) to FGFR. The results were confirmed by an ELISA-based receptor-binding assay. Different molecular weights of pVS polymer were synthesized by reversible addition fragmentation chain transfer (RAFT) polymerization. The polymers were able to facilitate dimerization of FGFRs leading to cell proliferation in FGFR-expressing cells, and no size dependence was observed. The data showed that pVS is comparable to heparin in these assays. In addition, pVS was not cytotoxic to fibroblast cells up to at least 1 mg/mL. Together this data indicates that pVS should be explored further as a replacement for heparin.
Keywords: poly(vinyl sulfonate), heparin-mimicking polymer, FGFR-binding assay, ELISA
INTRODUCTION
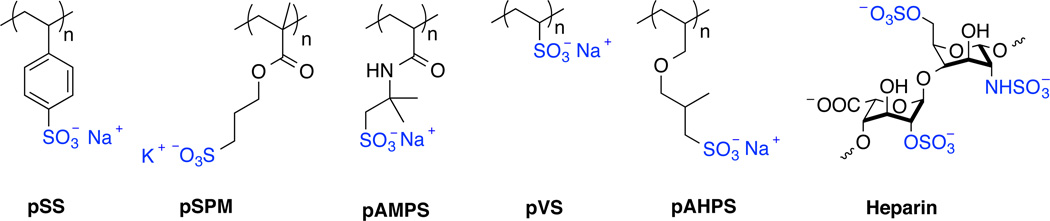
Polymers that mimic polysaccharides belong to an important area of polymer research.1 Polysaccharides exhibit a variety of biological functions ranging from energy storage or structural materials to inducing biological responses such as blood anticoagulation or anti-inflammation.2,3 Specifically, in biomimetic polymer research, the highly sulfated polysaccharide heparin has gained much interest. Originally, heparin-mimicking polymers were developed as antithrombotic alternatives to heparin.4,5 Over the years, heparin was recognized for its functions in diverse biological processes including in cell differentiation, angiogenesis, inflammation, and cell adhesion and interaction.6 Despite the heavy use of heparin in the clinic specifically as an anticoagulant, heparin is exclusively isolated from animal tissues, which could pose potential risk of virus contamination. Adverse effects from manufactured heparin contaminated with over sulfated chondroitin sulfate have been reported.7,8 Furthermore, variable patient-dependent dose-responses possibly due to heparin’s inherent heterogeneity and nonspecific interactions with plasma proteins9–11 and cytothrombopenia12 are of great concerns. These factors inspire researchers to look for heparin alternatives.
Synthetic heparin-mimicking polymers have been reported in literature, at first, as substitute anticoagulants such as carboxymethyl benzylamide sulfonate dextrans (CMDBS).5 This class of heparin-mimicking dextrans was further investigated for other biological functions including anti-inflammatory, antibacterial and antiviral, and regenerating activities, which depended on the degree of the chemical modification.13 The antiproliferative effect of heparin was later explored in heparin-mimicking polymers such as CMDBS,13 poly(4-hydroxyphenoxyacetic acid),14,15 and poly(2-acrylamido-2-methylpropane sulfonic acid).16 In the opposite function, the role of heparin mimics in potentiating binding of heparin-binding proteins to their receptors leading to cell proliferation was demonstrated for CMDBS17 and sulfated glycopolymers.18,19 In addition, sulfated glycopolymers20 reported by the Chaikof group and polysulfonated styrene polymers we reported were presented as stabilizers to basic fibroblast growth factor in vitro.20–22
A structure related to heparin is heparan sulfate (HS) proteoglycan, a low-affinity receptor to many heparin-binding proteins including basic fibroblast growth factor (bFGF).23 In the absence of HS proteoglycan, heparin can facilitate binding of bFGF to its high affinity receptor, fibroblast growth factor receptor 1 (FGFR1), which then leads to receptor dimerization and eventually phosphorylation, a key event in cell signaling.24 Although heparin-mimicking polymers that are involved in bFGF cell signaling in HS proteoglycan-deficient cells have been reported, their activities were approximately 60% that of heparin.18,25 A member of the CMDBS, a semi-synthetic functionalized dextran, has also been demonstrated to have similar bioactivity to heparin but its structure is generally heterogeneous and ununiformed.17 To our knowledge, there has been no report on a well-defined synthetic heparin-mimicking polymer that has equivalent activity to heparin.
Previously, we identified a polysulfonate polymer, poly(sodium 4-styrenesulfonate-copoly(ethylene glycol) methyl ether methacrylate) (p(SS-co-PEGMA)), that could bind to bFGF likely at the heparin-binding domain.20,21 However, further investigation showed that the polymer, unlike heparin, did not bind to and facilitate dimerization of FGFR.22 Herein, we report the development of a screening method to search for a heparin-mimicking polymer that is capable of potentiating FGFR dimerization. A small library of polysulfonated polymers were subjected to screening studies using Ornitz’s engineered BaF3-FR1C cell line that expresses FGFR1 but lacks HS proteoglycan.26 The results were confirmed by an ELISA-based receptorbinding assay. Upon screening, poly(vinyl sulfonate) (pVS) was identified as the strongest FGFR1 activator, comparable to heparin. Herein, we report the synthesis, screening and verification of the pVS heparin mimetic
EXPERIMENTAL SECTION
Materials
Poly(sodium sulfonate styrene) (pSS) polymers and poly(2-acrylamido-2-methylpropane sulfonic acid) (pAMPS) 70 kDa were purchased from Polymer Laboratories and Monomer- Polymer & Dajac Labs (Trevose, PA), respectively. Vinyl sulfonic acid, sodium salt (VS monomer) was purchased from Sigma-Aldrich as an aqueous solution and dried prior to use. The plasmid pET29c(+)hFGF-2 was obtained from Prof. Thomas Scheper (Leibniz University of Hannover, Germany), and the expression and purification procedures of bFGF were performed as described before.27 ToxinSensorTM Chromogenic LAL Endotoxin assay kit was purchased from Genscript (Piscataway, NJ). Recombinant human FGFR1α(IIIc) Fc chimera, and ELISA Development DuoSet® kits were purchased from R&D Systems. Heparin was purchased from PromoCell. Rabbit anti-fibroblast growth factor basic antibody and goat anti-rabbit IgG-HRP conjugate for Western blot were purchased from CALBIOCHEM and Bio-Rad, respectively. Normal HDF cells were purchased from Lonza. BaF3-FR1C cells expressing FGFR1 were provided by Prof. David Ornitz (Washington University, Saint Louis).26 Media and supplements for cell culture were either purchased from Lonza or Invitrogen. CellTiter-Blue® cell viability assay was obtained from Promega and was approximately 24 kDa. HiTrap™ Heparin HP 1 ml was purchased from GE Healthcare. Poly(methyl methacrylate) standards for GPC calibration were purchased from Polymer Laboratories. Merck 60 (230–400 mesh) silica gel was used for column chromatography. All other chemicals and reagents were purchased from Fisher or Sigma-Aldrich. THF and DCM were distilled over CaH2 prior to use. Prior to polymerizations, vinyl sulfonic acid, sodium salt was pre-treated with Na+-activated DOWEX 50WX8 200–400 mesh resin and then dried to produce the sodium salt. V501 initiator was dried prior to use.
Analytical techniques
1H NMR and 13C NMR spectroscopy were performed on Avance DRX 400 or 500 MHz spectroscopy instruments. UV-Vis spectrophotometry analyses were performed on a Biomate 5 Thermo Spectronic spectrometer. ELISA assay results were read on the ELX800 Universal Microplate Reader (Bio-Tek Instrument Inc., Winooski) with λ = 450 nm and 630 nm for signal and background, respectively. Fluorescent signals from CellTiter-Blue® assay were read using a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale). Fluorescent images of the cells from LIVE/DEAD® viability/cytotoxicity assays were acquired on an Axiovert 200 microscope equipped with an AxioCam MRm camera and FluoArc mercury lamp (Carl Zeiss, Thornwood). NIH ImageJ software was used to assist cell counting. GPC was conducted on a Shimadzu HPLC system equipped with a refractive index detector RID-10A, with a guard column and column from Tosoh. Acetonitrile (20%) in water with 0.3 M NaNO3 (flow rate: 0.70 mL/min). Calibration was performed using near-monodisperse poly(ethylene glycol) (PEG) standards from Polymer Laboratories.
Methods
Synthesis of poly(potassium 3-sulfopropyl methacrylate) (pSPM), poly(sodium 1-allyloxy-2 hydroxypropyl sulfonate) (pAHPS) and poly(sodium vinyl sulfonate) (pVS) polymers for screening studies
Free radical polymerization in water was employed to synthesize all these polymers for screening studies; V501 was used as the initiator. Prior to screening studies, all of the polymers were purified extensively by dialysis against MilliQ-water using MWCO 2,000 tubing and lyophilized. Two pSPM polymers were prepared by varying the ratio of [SPM]:[V501] = 4/200:1 at 80 °C and yielded polymers with number average molecular weights (Mn) and molecular weight dispersities by GPC of 83.0 kDa (Đ = 3.45, bimodal) and 403 kDa (Đ = 1.51). One pAHPS polymer was prepared with a ratio of [AHPS]:[V501] = 20:1 at 60 °C and yielded a polymer with a molecular weight of 2.4 kDa (Đ = 1.33). pVS was synthesized with a ratio of [VS]:[V501] = 40:1 at 60 °C and yielded a polymer with a molecular weight of 6.4 kDa (Đ = 1.21).
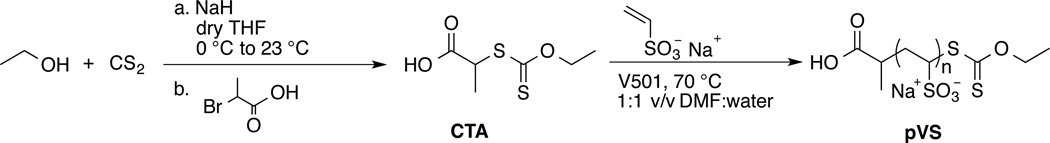
Synthesis of 2-((ethoxycarbonothioyl)thio)propanoic acid, CTA
The synthesis of the CTA was adapted from reported literature.28 In detail, in a three-neck round-bottom flask, 40 mL of dry THF under argon was pre-cooled to 0 °C in an ice-bath. Sodium hydride 60% in oil (1.76 g, 73.3 mmol) was added into the flask. Dry ethanol (2.6 mL, 44.4 mmol) was added drop-wise into the reaction flask over the course of 10 minutes followed by carbon disulfide (3 mL, 49.9 mmol). The reaction was allowed to stir for 20 minutes. Subsequently, 2-bromopropanoic acid (1.0 mL, 11.1 mmol) was added into the reaction flask. The reaction solution was stirred for 48 hours and allowed to warm to 23 °C. The reaction was quenched with methanol, then water. THF was removed via rotovap; the crude reaction was washed twice with DCM. The aqueous layer was acidified and extracted three times with DCM. The collected organic layer was dried over anhydrous magnesium sulfate and the solvent was removed by rotovap. The crude product was purified by silica column chromatography (9:1 v/v hexane: acetic acid), and lyophilized to give a slightly yellow solid (1.86 g, 86% yield). δ 1H NMR (400 MHz, CDCl3): 11.58 (1H, s, COOH), 4.67-4.61 (2H, qd, J = 7.18, 2.00 Hz, OCH2CH3), 4.44-4.38 (1H, q, J = 7.43 Hz, CHCH3), 1.61-1.59 (3H, d, J = 7.44 Hz, CHCH3), 1.43-1.39 (3H, t, J = 7.11 Hz, OCH2CH3). δ 13C NMR (400 MHz, CDCl3): 211.67, 178.10, 70.64, 46.91, 16.60, 13.72. FT- IR (cm−1): 2980, 2933, 2628, 2486, 1698, 1628, 1457, 1450, 1435, 1408, 1363, 1301, 1258, 1237, 1153, 1113, 1078, 1059, 1040, 1002, 984, 855, 805, 716, 696. ESI MS (expected, observed) (Da): [MH+] = (195.0071, 195.0135).
Synthesis of pVS polymers for size-dependent studies
RAFT polymerization was employed to polymerize the VS monomer. The polymerizations were performed with the initial feed ratios of [VS]:[CTA]:[V501] = 40/80/160/320/500:2:1 using standard Schlenk techniques. The concentration of the monomer was 1.4 – 2.0 M. For a typical polymerization of the system [VS]:[CTA]:[V501] = 40:2:1, the CTA (27.7 mg, 0.14 mmol), VS monomer (372 mg, 2.86 mmol), and V501 (20 mg, 0.07 mmol) were dissolved in 1 mL of Milli-Q water and 1 mL of DMF in a Schlenk tube. The system was sealed and subjected to four freeze-pump-thaw cycles before immersion in a 70 °C oil bath. Aliquots were removed at predetermined time points and diluted in D2O for 1H NMR analysis. The progress of the polymerization was monitored from the 1H NMR spectra using the integral value of the vinylic proton of VS monomer at 6.6 ppm and the integral value of the growing polymer protons (3.8-3.4 ppm, part of CHSO3Na polymer backbone). The polymerization was stopped after 3.5 hours. The polymer was purified by dialysis with Milli-Q water (MWCO 1000) and lyophilized to remove solvent. δ 1H NMR 500 MHz (D2O): 4.2-2.9 (CH2CHSO3Na polymer backbone), 2.9-1.4 (CH2CHSO3Na polymer backbone), 1.45 (2H, OCH2CH3), 1.45-1.20 (3H, CHCH3). IR (cm−1): 3435, 2942, 1642, 1544, 1525, 1499, 1453, 1156, 1031, 711, 613, 572, 540, 515. The NMR spectrum of each polymer was calibrated to the peak at 1.45-1.20 ppm, and the Mn of the polymers were calculated using the formula: Mn = Σ polymer backbone integrals 3 × MW monomer + MW CTA. The five pVS polymers had Mn (1H NMR) of 6.6, 14.4, 24.6, 35.9, and 80.3 kDa. The Mn and dispersity were also determined by GPC to be 2.3 kDa (Đ = 1.39), 4.1 kDa (Đ = 1.14), 6.9 kDa (Đ = 1.40), 46.5 kDa (Đ = 1.19), and 62.3 kDa (Đ = 1.22), respectively.
ELISA-based FGFR binding assay
A 96-well-plate was incubated with 100 µL of 0.5 µg/mL of rhFGFR1α(IIIc) prepared in D-PBS for 16 hours at 23 °C, then blocked with 1% BSA solution for 2 hours. A solution of 1 ng/mL of bFGF with or without 1 µg/mL of heparin or pVS polymer (35.9 kDa) was added to the wells and incubated for 2 hours. Subsequently, bFGF antibody-biotin conjugate solution was probed for 2 hours before streptavidin-horseradish peroxidase solution was added for 20 minutes. The colorimetric signals were developed by incubating the wells with 1-Step Ultra TMB solution (Pierce Biotechnology, Rockford) for 6 minutes. The assay was stopped by addition of 50 µL of 1 M H2SO4 in to each well. The absorbance signals were recorded at λ = 450 nm, and the background at λ = 630 nm was subtracted. Each group was done with three replicates, and the experiment was repeated two times.
ELISA-based pVS EC50 assay
A 96-well-plate was incubated with 100 µL of 0.5 µg/mL of rhFGFR1α(IIIc) prepared in D-PBS for 16 hours at 23 °C, then blocked with 1% BSA solution for 2 hours. A solution of 1 ng/mL of bFGF with increasing concentrations of 14.4 kDa pVS (0.038, 0.075, 0.125, 0.25, 0.5, 0.75, 1.0, and 6.0 ug/mL each) were added to the wells and incubated for 2 hours. Subsequently, bFGF antibody-biotin conjugate solution was probed for 2 hours before streptavidin-horseradish peroxidase solution was added for 20 minutes. The colorimetric signals were developed by incubating the wells with 1-Step Ultra TMB solution (Pierce Biotechnology, Rockford) for 5 minutes. The assay was stopped by addition of 50 µL of 1 M H2SO4 in to each well. The absorbance signals were recorded at λ = 450 nm and a standard curve was plotted. The half maximal effective concentration (EC50) was calculated by determining the concentration of pVS required to reach half of the maximal absorbance value. Each group was done with three replicates. The EC50 for pVS was determined to be 56 ug/mL with the concentration of bFGF is 1 ng/mL
Cell culture
HDF primary cells were cultured in Lonza FGM-2 medium containing 2% fetal calf serum (FCS), bFGF, insulin, 30 µg/mL Gentamicin and 15 ng/mL Amphotericin at 37 °C, 5% CO2. HDF cells were passaged every four days or after reaching 80% confluency. HDF cells were used up to passage 12. BaF3-FR1C cells were cultured as recommended. Specifically, the cells were grown in RPMI1640 medium containing 10% newborn bovine calf serum, 2 mM L-glutamine, 0.5 ng/mL of recombinant mouse IL-3, 600 µg/mL of G418, 50 nM of 2-mercaptoethanol, supplemented with 100 unit/mL penicillin and 100 µg/mL streptomycin at 37 °C, 5% CO2. The medium was changed every two days.
Cytotoxicity study on HDF cells
HDF cells were trypsinized and resuspended in fibroblast growth medium without bFGF. The cells were plated at a concentration of 5,000 cells/well in a 48-well plate and allowed to adhere over 16 hours at 37 °C, 5% CO2. Then, the medium in the wells was replaced with 200 µl of the working medium containing various concentrations of pVS 80.3 kDa ranging from 1 mg/mL to 1 ng/mL. After an incubation of 24 hours at 37 °C, 5% CO2, cell viability was assessed using LIVE/DEAD® viability/cytotoxicity assay. Briefly, the cells were washed twice with pre-warmed D-PBS, then incubated with 200 µl of 1 µM calcein AM and 4 µM ethidium homodimer-1 in D-PBS for 20 minutes at 37 °C, 5% CO2. The fluorescent images of each well under a green channel and red channel were captured on an Axiovert 200 microscope equipped with an AxioCam MRm camera and FluoArc mercury lamp. The numbers of live and dead cells were counted manually using the ‘cell counter’ function in NIH ImageJ software, and the percent live cells was calculated by dividing the number of live cells by the total number of live and dead cells. Each sample group had four repeats, and the whole experiment was repeated three times.
Proliferation assays
BaF3-FR1C cells were collected and washed two times with cultured medium without IL-3. The cells were plated at the density of 20,000 cells/well/50 µL in a 96-well plate using the working medium. The samples were prepared to contain the tested compounds at double the final desired concentrations. Subsequently, 50 µL of each sample were added into each corresponding well. After incubation for 48 hours at 37 °C, 5% CO2, CellTiter-Blue® assay was carried out to evaluate the extent of cell growth. All of the groups were normalized to the control group, which had blank medium. Each group was performed with four replicates. The proliferation assays with different sizes of pVS polymers were repeated three times.
Inhibition proliferation assays
BaF3-FR1C cells were collected and the cells were plated at a density of 20,000 cells/well/50 µL in a 96-well plate using the working medium. The samples were prepared to contain the test compounds at double the desired final concentrations. Each experimental group was prepared so that the concentration after plating contain 1 ng/mL bFGF, 1 ng/mL bFGF with 1 ug/mL pVS, or 1 ug/mL pVS; all samples contained 125 nM inhibitor PD173074. Control groups were replicates of experimental groups with the exception that no PD173074 was added. Subsequently, 50 µL of each sample were added into each corresponding well. After incubation for 48 hours at 37 °C, 5% CO2, CellTiter-Blue® assay was carried out to evaluate the extent of cell growth. All of the groups were normalized to the control group, which had blank medium. The assay was performed with six well replicates of both control and experimental groups. The entire proliferation assay was repeated 3 times.
Statistical analysis
The data was presented as the average and the standard deviation (STDEV) or standard error of the mean (SEM) where SEM = STDEV/(n−1)1/2 and n = the number of independent repeats. Individual comparisons were made with Student’s t-test. All tests were two-tailed, unpaired and p < 0.05 was considered significant.
RESULTS AND DISCUSSION
HS proteoglycan is known as a low affinity receptor to bFGF.23 Upon binding of two bFGF molecules to HS proteoglycan or heparin and to two FGFR molecules, dimerization and subsequent phosphorylation of the receptors occur.24,29 This event is essential to cascade the signals leading to cellular responses. In a previous report, we showed p(SS-co-PEGMA) as a protein-stabilizer for bFGF similar to heparin but unlike heparin the polymer was unable to facilitate the dimerization of FGFRs.22 This motivated us to search for another heparinmimicking polymer with this specific property. In order to accomplish this, a small library of polysulfonated polymers (Scheme 1) were either synthesized via free radical polymerization or purchased, and tested for their co-facilitating effect in a dose-dependent manner. BaF3-FR1C cells, which express FGFR1s but lack HS proteoglycans,26 were used for in vitro screening (Figure 1). Heparin was used in the positive control group, and all the samples were normalized to blank medium, which was set to 100%. In the presence of bFGF and heparin, BaF3-FR1C cell proliferation was very high compared to blank medium. This affect was screened for with various polysulfonated polymers. However, some polysulfonated polymers have been reported to inhibit cell proliferation at high concentrations,16,30 likely due to the competition with the high-affinity receptors for binding to bFGF. Such an inhibition effect was also carefully evaluated.
Scheme 1.
Chemical structures of the polysulfonated polymers used in the screening studies.
Figure 1.
Screening studies for a heparin-mimicking polymer. Proliferation studies of BaF3-FR1C cells in the presence of 1 ng/mL bFGF and various concentrations of pSS (a), pSPM or pAMPS (b), pVS or pAHPS polymers (c). Incubation of 20,000 cells/well in 96-well plate with each of the samples was carried out for 48 hours. CellTiter®-Blue assay was performed to quantify the extent of cell growth. Samples with heparin were used as positive control groups. Data was normalized to the blank medium group, which was set at 100%. Each sample had four replicates. Error bars represent STDEV. Statistical analysis was performed using Student’s t-test; p < 0.05 was consider significant. The percent cell growth from each polymer was compared to the control group, which had heparin at the same concentration. All polymers were significantly different than heparin except for pVS at 200 µg/mL, 0.5 µg/mL, and 0.02 µg/mL.
As shown in Figure 1 and summarized in Table 1, pSS polymers showed moderate inhibition effect only at high concentration. pSPM inhibited cell proliferation at high concentrations; for example, 100 µg/mL of pSPM decreased cell proliferation to approximately 50% compared to blank medium group (all sets were normalized to blank media, which was set to 100%). pAMPS polymer was also an inhibitor at high concentrations, and this finding is consistent with literature.16 pVS and pAHPS were strong activators at high concentrations tested. The activation effect of pAHPS decreased quickly as the concentration of the polymers dropped. Yet, pVS displayed a very similar profile to heparin at all of the concentrations tested. This made pVS a promising candidate for further testing. In addition, the results demonstrate that this cell based screening assay would be interesting to screen for both potentiators and inhibitors of bFGF activation.
Table 1.
Summary from the screening studies.
| Polymer screened | Inhibitor | Activator |
|---|---|---|
| pSS | Moderate at high concentrations | No |
| pSPM | Strong at high concentrations | No |
| pAMPS | Strong at high concentrations | No |
| pAHPS | No | Strong at high concentrations |
| pVS | No | Strong at all concentrations |
In the screening studies, only one pVS was prepared and tested; therefore, to understand how polymer size could influence cell proliferation, various sizes of pVS were prepared via RAFT polymerization. To our knowledge, RAFT polymerization of unprotected VS monomer has not been reported. Since VS monomer belongs to a class of “less activated” monomers similar to vinyl acetate monomer, a dithiocarbonate- or xanthate-functionalized CTA was synthesized to better facilitate RAFT polymerization of this monomer (Scheme 1).31
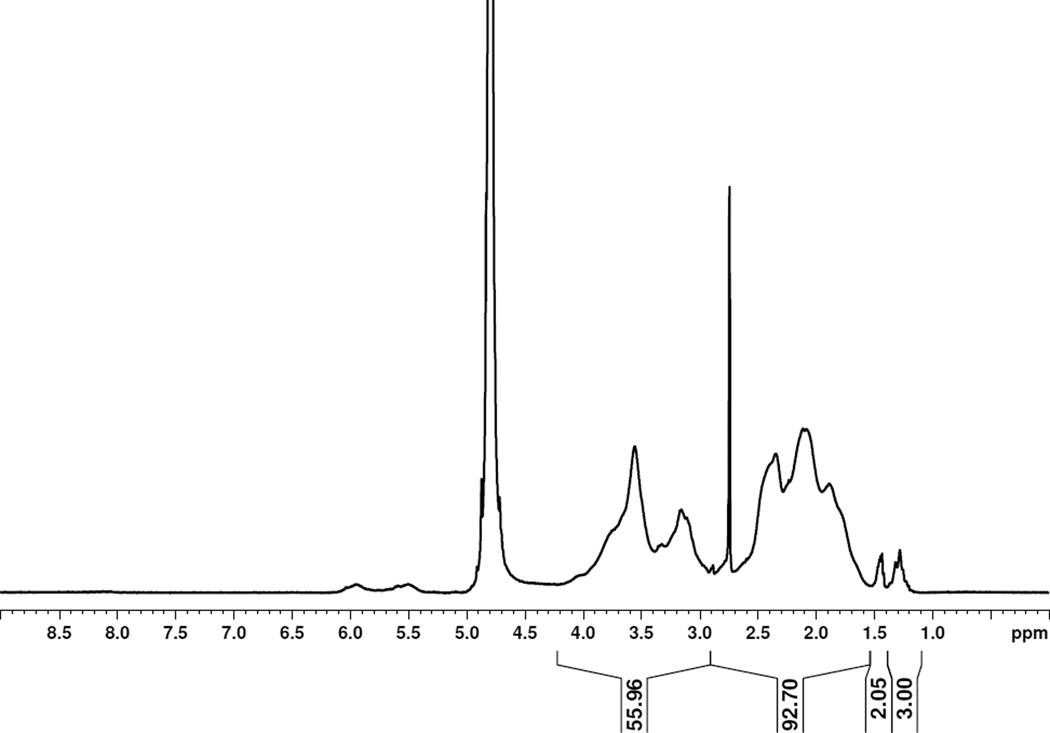
The xanthate-functionalized CTA was synthesized in one step from ethanol, carbon disulfide and 2-bromopropanoic acid in 86% yield (See supporting information Figure S1 for 1H NMR, 13C NMR, and IR spectrum of the CTA) using a slight modification of a reported procedure.28 Five pVS polymers with molecular weights ranging from 6.6 kDa to 80.3 kDa were synthesized using this CTA. The molecular weights of the polymers were calculated from their respective 1H NMR spectra using the integral value of the CTA peak at 1.25 ppm (CHCH3) and the integral value of the polymer backbone at 4.2-1.45 ppm. Figure 2 shows the 1H NMR spectrum of pVS 6.6 kDa, and the rest of the pVS polymer spectra are provided as Figure S2 in the ESI. The integration of the peak OCH2CH3 at 1.45 ppm was less than 3 for pVS 6.6 kDa, which indicates partial hydrolysis of the xanthate end group. Yet, the UV-Vis spectrum had a maximum absorbance at 287 nm, which is the known absorbance of the xanthate functional group.32 This absorbance peak was also observed in the UV-Vis spectrum of the CTA. A control pVS polymer prepared by free radical polymerization showed no such absorbance. The results together indicated that the xanthate was at least partially intact at the end of the polymer chain. The broad peaks at 6.0 and 5.5 ppm in the 1H NMR spectrum were assigned as internal olefins in the polymer backbone. Jiang and co-workers studied the thermal degradation of pVS and showed that the degradation involved the cleavage of the carbon sulfur bonds to give radicals along the polymer chain that coupled to form internal alkenes.33 We tested different polymerization conditions in which the water-soluble initiator and the polymerization temperature were varied; however, we were not able to prevent the side reaction. The molecular weight dispersities determined from GPC showed that the polymers had Đ values between 1.14 and 1.40 and thus relatively narrow molecular weight distributions.
Figure 2.
1H NMR spectrum of pVS 6.6 kDa in D2O.
The different sizes of pVS polymers were incubated with BaF3-FR1C cells in the presence of 1 ng/mL of bFGF to evaluate the extent of stimulated cell growth. Figure 3 shows the results from the studies. Heparin was used as the positive control group. The concentrations of heparin and the polymers were varied from 100 µg/mL to 0.001 µg/mL. The activation effect of heparin was concentration dependent, which has been observed by others.34 Different molecular weight pVS polymers were also concentration dependent; the maximum stimulated percent cell growth was observed at 1 µg/mL for both the polymers and heparin. There was no statistical difference between the pVS polymers of different molecular weights at all the concentrations tested, indicating that the polymer size in the tested range did not play an important role in influencing the biological activity of bFGF. More importantly, the activation effect from pVS polymers was not statistically different than that of heparin at all concentration tested. This result is significant because it demonstrates the first fully synthetic heparin mimic polymer that can induce mitogenic activity in HS-deficient cells comparable to heparin.
Figure 3.
Proliferation studies of different sizes of pVS polymers on BaF3-FR1C cells. The cells were incubated in the presence of 1 ng/mL bFGF and various concentrations of heparin, pVS 6.6 kDa, 14.4 kDa, 24.6 kDa, 35.9 kDa, and 80.3 kDa. Incubation of 20,000 cells/well in 96-well plate with each of the samples was carried out for 48 hours. CellTiter®-Blue assay was performed to quantify the extent of cell growth. Data was normalized to the blank medium group, which was set at 100%. Each sample had four replicates and the experiment was repeated three times. Error bars represent SEM. Statistical analysis was performed using Student’s t-test; p < 0.05 was consider significant. The percent cell growth from each polymer was compared to the control group, which had heparin at the same concentration. No statistical difference was found between the pVS polymers and heparin at all concentrations tested.
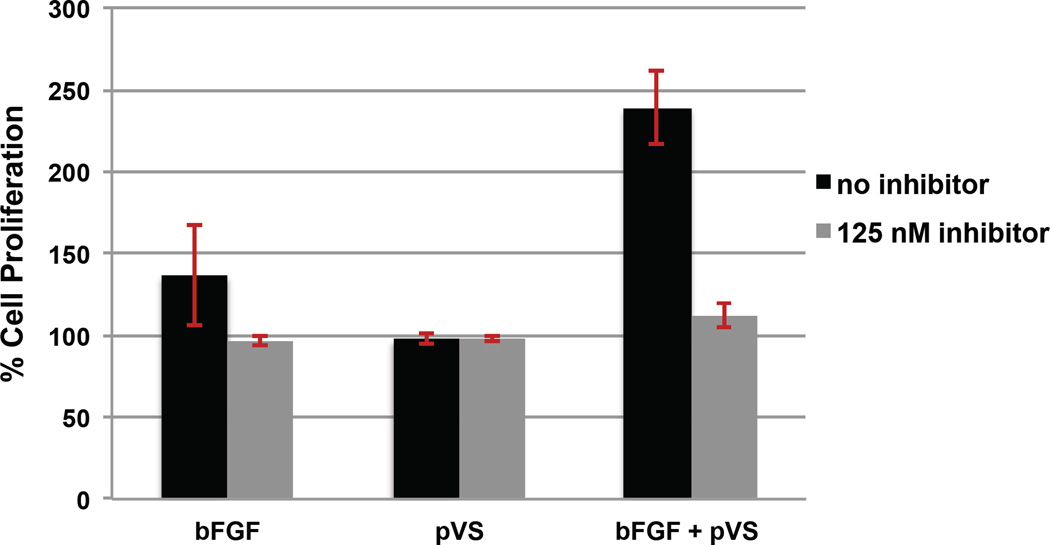
To verify that pVS was facilitating bFGF-induced FGFR1 receptor activation through the same signal transduction pathway as heparin, a cellular inhibition study was performed on BaF3 FR1C cells using pVS 14.4 kDa. An inhibitor of the FGF receptor 1 (FGFR1), PD173074, has been shown to deactivate FGFR1 phosphorylation,35 and we have shown that in the BaF3-FR1C cell assay that PD173074 inhibits heparin-induced bFGF proliferation.36 Samples containing 1ng/mL bFGF, 1 ug/mL pVS, or 1 ng/mL bFGF with 1 ug/mL pVS were incubated with and without 125 nM inhibitor PD173074 for 48 hours. Figure 4 shows that the inclusion of inhibitor into pVS samples decreases proliferation to that of the blank. This suggests that the increase in cell proliferation observed in Figure 3 with pVS polymers is due to facilitating binding of bFGF with FGFR1 receptor rather than through a different cellular route.
Figure 4.
FGFR1 inhibition studies of pVS polymer (14.4 kDa) in BaF3-FR1C cells. The cells were incubated in the presence of 1 ng/mL bFGF, 1 ug/mL pVS or 1 ug/mL pVS with bFGF, all samples with and without FGFR inhibitor. Incubation of 20,000 cells/well in 96-well plate with each of the samples was carried out for 48 hours. CellTiter®-Blue assay was performed to quantify the extent of cell growth. Data was normalized to the blank medium group, which was set at 100%. Each sample had six well replicates and the entire experiment was repeated three times. Error bars represent SEM. Statistical analysis was performed using Student’s t-test; p < 0.05 was considered significant compared to sample containing inhibitor.
pVS was compared side-by-side with native bFGF and bFGF in the presence of heparin for its ability to facilitate binding of bFGF to FGFR using an ELISA-based assay we developed for this purpose. In this assay, recombinant human FGFR1α(IIIc) was used to coat a 96-well-plate to capture bFGF. A stock solution of bFGF was prepared and divided in three sets. In one set, pVS was added; in another set, heparin was added to provide a positive control group; blank buffer was added to the last set to provide an untreated bFGF group. Then blank buffer was added accordingly to bring the final concentrations of bFGF and the polymer/heparin in the samples to 1 ng/mL and 1 µg/mL, respectively. Since ELISA is very sensitive to the concentration of the ligand, this preparation procedure ensured the same concentration of bFGF in all of the samples.
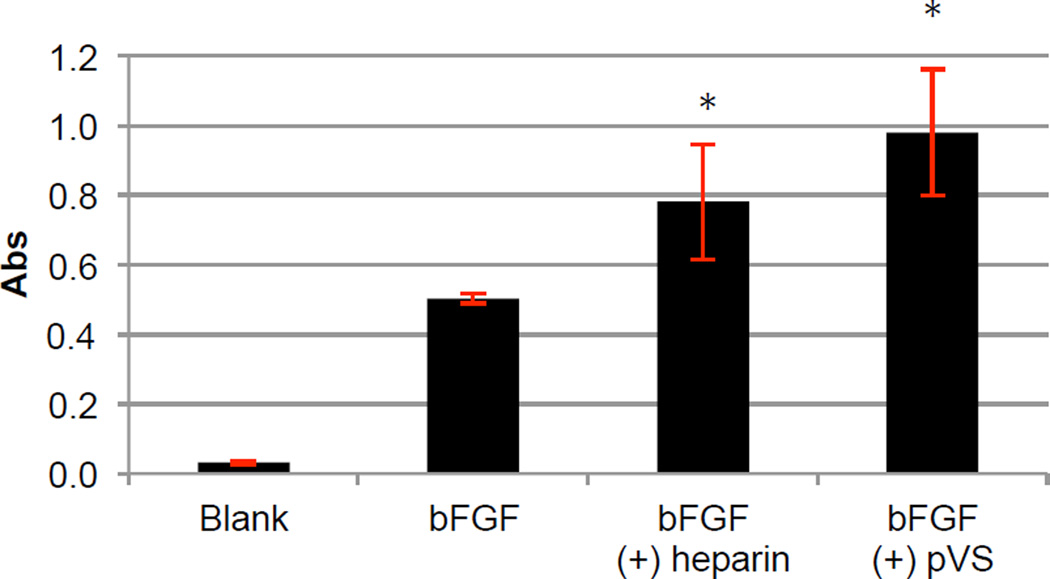
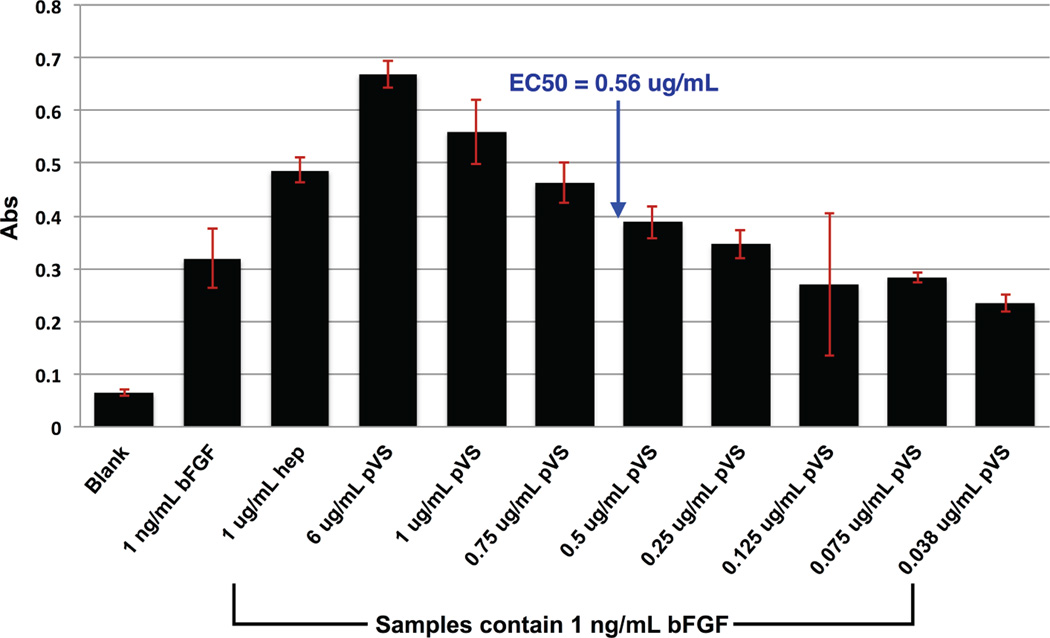
Figure 5 shows the results from the FGFR-binding assay. The absorbance signals from bFGF, bFGF (+) heparin, and bFGF (+) pVS (35.9 kDa) were 0.503 ± 0.014, 0.781 ± 0.167, and 0.979 ± 0.182 respectively. Heparin is known to enhance binding of bFGF to FGFR.23 Our data confirmed that in the presence of heparin and bFGF, the absorbance signal was higher than that of bFGF alone (p = 0.002) probably due to the high number of bFGF molecules able to remain binding to the coated receptors throughout the assay. The absorbance signal from the bFGF (+) pVS group was also higher than that of bFGF alone (p = 0.0001) showing that pVS was as effective as heparin at increasing binding affinity of bFGF to its receptors. By adding increasing concentrations of pVS from 0.03 to 1 µg/mL, it was determined that the half maximal effective concentration (EC50) of pVS in this assay was 0.56 ug/mL when the concentration of bFGF was 1 ng/mL (Figure 6). These results show that pVS facilitates binding of bFGF to its receptors in a dose dependent manner.
Figure 5.
ELISA-based FGFR-binding assay. FGFR1α(IIIc) was used to coat a 96-well-plate. The wells were treated with various formulations: blank buffer, 1 ng/mL of bFGF with or without 1 µg/mL of heparin or pVS 35.9 kDa. The procedure is detailed in the Methods section. The graphed absorbance was the difference between the signals at λ = 450 nm and the background signals at λ = 630 nm. The experiment was repeated two times, each had n = 3. Error bars represent STDEV. Statistical analysis was performed using Student’s t-test; * p < 0.05 for the ‘bFGF (+) heparin’ and ‘bFGF (+) pVS’ group compared to the control group ‘bFGF’.
Figure 6.
ELISA-based FGFR-binding assay to determine EC50 of pVS 14.4 kDa. FGFR1α(IIIc) was used to coat a 96-well-plate. The wells were treated 1 ng/mL bFGF with varying concentrations of pVS. The procedure is detailed in the Methods section, n = 3. Error bars represent STDEV.
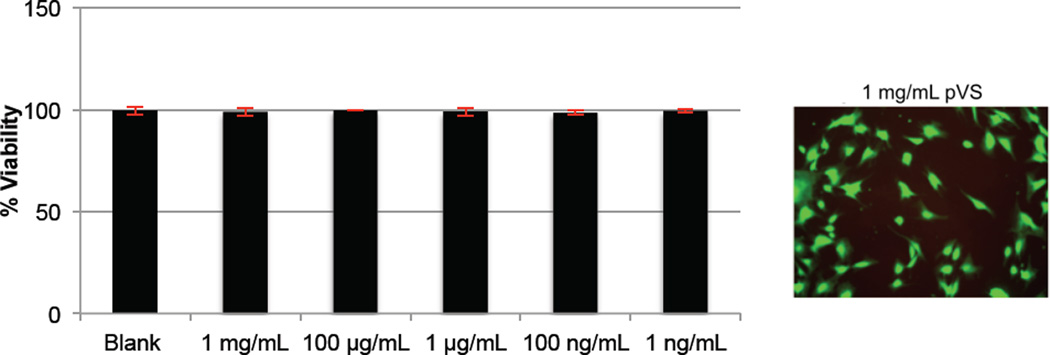
Lastly, the cytotoxicity of pVS polymer was evaluated. Figure 7 shows the results from the study. Various concentrations of pVS 80.3 kDa were incubated with HDF cells in the absence of bFGF for 24 hours. Then a LIVE/DEAD® viability/cytotoxicity assay was performed, which indicated that the polymer was not toxic to HDF cells up to at least 1 mg/mL.
Figure 7.
Cytotoxicity studies of pVS 80.3 kDa on HDF cells. Incubation of 5,000 HDF cells/well in 48-well plate with various concentrations of the polymer was carried out for 24 hours. Each well was washed twice with D-PBS and subsequently incubated with 1 mM calcein AM and 4 mM ethidium homodimer-1 for 20 minutes. The percentage of live cells was calculated by dividing the number of live cells by the total number of live and dead cells. Each sample had four replicates and the experiment was repeated three times. Error bars represent SEM.
PVS has been explored before as an antithrombogenic agent,37,38 HIV-inhibitor,39 coatings for antibacterial surfaces,40 and as polyelectrolytes for antibody and protein purification processes,41–43 but never before as co-activator for FGFRs. From screening studies, we identified pVS from other polysulfonated polymers as a new heparin-mimicking polymer. Heparin can enhance or inhibit cell proliferation.44 For example, Likens and co-workers studied polysulfonated polymers in the search for a cell proliferation inhibitor for FGFR-expressed cells.30 These inhibitors worked by competing with the cell surface HS for binding to bFGF, thus shutting down the FGFR dimerization and phosphorylation processes. The activities of the inhibitors were most pronounced at high concentrations, which could also be due their cytotoxicity. pVS was found not to have any inhibiting effect at all concentrations tested.30 The polymer was as efficient as heparin in facilitating binding of bFGF to FGFR1 and bFGF-induced cell proliferation in HS-deficient cells. It was also non-cytotoxic up to at least 1 mg/mL concentrations. This result suggests that pVS should be explored as a new heparin-mimicking polymer that could activate cells, while not exhibiting any inhibitory affect. This could be important in many applications including the use of pVS in biomaterials and for wound healing.
CONCLUSIONS
In this paper, we report screening studies of polysulfonated polymers to search for a polymer that was capable of stimulating cell proliferation in FGFR-expressed cells similar to heparin. pVS was identified because of its ability to stimulate cell proliferation as effectively as heparin in BaF3-FR1C cells by co-facilitating dimerization of FGFRs – an essential initial event responsible for cell signaling. pVS polymers ranging from 6.6 to 80.3 kDa were prepared via RAFT polymerization, and the activation effect was found independent of the polymer size. The results were confirmed by an FGFR binding ELISA assay. Furthermore, the polymer was found not cytotoxic to fibroblast cells up to at least 1 mg/mL. Altogether, we report a synthetic heparin-mimicking polymer with comparable activity to heparin in inducing proliferation in HSdeficient cells.
Supplementary Material
Scheme 2.
Synthesis of different sizes of pVS.
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB136774). The authors thank Prof. David Ornitz (Washington University) for providing the BaF3 cell line. The authors thank Thomas Scheper and the Helmholtz Centre for Infection Research, Braunschweig, Germany for providing the pET29c(+)hFGF-2 plasmid. S.J.P. thanks the NIH Chemistry Biology Interface Training Fellowship (T32 GM 008496) and UCLA Graduate Division for funding.
Footnotes
Experimental details and additional data. This information is available free of charge via the Internet at http://pubs.acs.org/.
REFERENCES
- 1.Ladmiral V, Melia E, Haddleton DM. Eur. Polym. J. 2004;40:431–449. [Google Scholar]
- 2.Kogan G, Soltes L, Stern R, Gemeiner P. Biotechnol. Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 3.Sasisekharan R, Venkataraman G. Curr. Opin. Chem. Biol. 2000;4:626–631. doi: 10.1016/s1367-5931(00)00145-9. [DOI] [PubMed] [Google Scholar]
- 4.Mauzac M, Aubert N, Jozefonvicz J. Biomaterials. 1982;3:221–224. doi: 10.1016/0142-9612(82)90023-0. [DOI] [PubMed] [Google Scholar]
- 5.Mauzac M, Jozefonvicz J. Biomaterials. 1984;5:301–304. doi: 10.1016/0142-9612(84)90078-4. [DOI] [PubMed] [Google Scholar]
- 6.Capila I, Linhardt RJ. Angew. Chem. Int. Ed. Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Blossom DB, Kallen AJ, Patel PR, Elward A, Robinson L, Gao GP, Langer R, Perkins KM, Jaeger JL, Kurkjian KM, Jones M, Schillie SF, Shehab N, Ketterer D, Venkataraman G, Kishimoto TK, Shriver Z, McMahon AW, Austen KF, Kozlowski S, Srinivasan A, Turabelidze G, Gould CV, Arduino MJ, Sasisekharan R. N. Engl. J. Med. 2008;359:2674–2684. doi: 10.1056/NEJMoa0806450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang ZQ, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Nat. Biotechnol. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsh J, van Aken WG, Gallus AS, Dollery CT, Cade JF, Yung WL. Circulation. 1976;53:691–695. doi: 10.1161/01.cir.53.4.691. [DOI] [PubMed] [Google Scholar]
- 10.Kroon C, ten Hove WR, de Boer A, Kroon JM, van der Pol JM, Harthoorn-Lasthuizen EJ, Schoemaker HC, van der Meer FJ, Cohen AF. Circulation. 1992;86:1370–1375. doi: 10.1161/01.cir.86.5.1370. [DOI] [PubMed] [Google Scholar]
- 11.Manson L, Weitz JI, Podor TJ, Hirsh J, Young E. J. Lab. Clin. Med. 1997;130:649–655. doi: 10.1016/s0022-2143(97)90115-3. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin TE, Levine MN, Hirsh J, Horsewood P, Roberts RS, Gent M, Kelton JG. N. Engl. J. Med. 1995;332:1330–1336. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 13.Logeart-Avramoglou D, Jozefonvicz J. J. Biomed. Mater. Res. 1999;48:578–590. doi: 10.1002/(sici)1097-4636(1999)48:4<578::aid-jbm26>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Benezra M, Ishai-Michaeli R, Ben-Sasson SA, Vlodavsky I. J. Cell. Physiol. 2002;192:276. doi: 10.1002/jcp.10136. [DOI] [PubMed] [Google Scholar]
- 15.Regan JR, Bruno JG, Chang MN, Sabatino R, Dalisa R, Bensasson SA, Eilat D. J. Bioact. Compatible Polym. 1993;8:317–337. [Google Scholar]
- 16.Liekens S, Neyts J, Degreve B, DeClercq E. Oncol. Res. 1997;9:173–181. [PubMed] [Google Scholar]
- 17.Ikeda Y, Charef S, Ouidja MO, Barbier-Chassefiere V, Sineriz F, Duchesnay A, Narasimprakash H, Martelly I, Kern P, Barritault D, Petit E, Papy-Garcia D. Biomaterials. 2011;32:769–776. doi: 10.1016/j.biomaterials.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Guan R, Sun XL, Hou SJ, Wu PY, Chaikof EL. Bioconjugate Chem. 2004;15:145–151. doi: 10.1021/bc034138t. [DOI] [PubMed] [Google Scholar]
- 19.Sun XL, Grande D, Baskaran S, Hanson SR, Chaikof EL. Biomacromolecules. 2002;3:1065–1070. doi: 10.1021/bm025561s. [DOI] [PubMed] [Google Scholar]
- 20.Christman KL, Vazquez-Dorbatt V, Schopf E, Kolodziej CM, Li RC, Broyer RM, Chen Y, Maynard HD. J. Am. Chem. Soc. 2008;130:16585–16591. doi: 10.1021/ja803676r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolodziej CM, Kim SH, Broyer RM, Saxer SS, Decker CG, Maynard HD. J. Am. Chem. Soc. 2012;134:247–255. doi: 10.1021/ja205524x. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TH, Kim SH, Decker CG, Wong DY, Loo JA, Maynard HD. Nature Chem. 2013;5:221–227. doi: 10.1038/nchem.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 24.Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Cell. 1999;98:641–650. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 25.Griffith BR, Allen BL, Rapraeger AC, Kiessling LL. J. Am. Chem. Soc. 2004;126:1608–1609. doi: 10.1021/ja037646m. [DOI] [PubMed] [Google Scholar]
- 26.Ornitz DM, Leder P. J. Biol. Chem. 1992;267:16305–16311. [PubMed] [Google Scholar]
- 27.Chen R, John J, Lavrentieva A, Muller S, Tomala M, Zhao YX, Zweigerdt R, Beutel S, Hitzmann B, Kasper C, Martin U, Rinas U, Stahl F, Scheper T. Eng. Life Sci. 2012;12:29–38. [Google Scholar]
- 28.Fleet R, McLeary JB, Grumel V, Weber WG, Matahwa H, Sanderson RD. Macromolecul. Symp. 2007;255:8–19. [Google Scholar]
- 29.Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Mol. Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 30.Liekens S, Leali D, Neyts J, Esnouf R, Rusnati M, Dell'Era P, Maudgal PC, De Clercq E, Presta M. Mol. Pharmacol. 1999;56:204–213. doi: 10.1124/mol.56.1.204. [DOI] [PubMed] [Google Scholar]
- 31.Destarac M, Blidi I, Coutelier O, Guinaudeau A, Mazieres S, Van Gramberen E, Wilson J. Acs Symp. Ser. 2012;1100:259–275. [Google Scholar]
- 32.Johnson RS, Finnegan PS, Wheeler DR, Dirk SM. Chem. Commun. 2011;47:3936–3938. doi: 10.1039/c1cc00090j. [DOI] [PubMed] [Google Scholar]
- 33.Jiang DD, Yao Q, McKinney MA, Wilkie CA. Polym. Degrad. Stab. 1999;63:423–434. [Google Scholar]
- 34.Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Mol. Cell. Bio. 1992;12:240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR. Embo J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen TH, Kim S-H, Decker CG, Wong DY, Loo JA, Maynard HD. Nature Chem. 2013;5:221–227. doi: 10.1038/nchem.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito Y, Iguchi Y, Kashiwagi T, Imanishi Y. J. Biomed. Mater. Res. 1991;25:1347–1361. doi: 10.1002/jbm.820251104. [DOI] [PubMed] [Google Scholar]
- 38.Ito Y, Liu L, Imanishi Y. J. Biomed. Mater. Res. 1991;25:99–115. doi: 10.1002/jbm.820250108. [DOI] [PubMed] [Google Scholar]
- 39.Mohan P, Schols D, Baba M, De Clercq E. Antiviral Res. 1992;18:139–150. doi: 10.1016/0166-3542(92)90034-3. [DOI] [PubMed] [Google Scholar]
- 40.Vasilev K, Sah VR, Goreham RV, Ndi C, Short RD, Griesser HJ. Nanotechnology. 2010;21:215102–215107. doi: 10.1088/0957-4484/21/21/215102. [DOI] [PubMed] [Google Scholar]
- 41.Braia M, Ferrero M, Rocha MV, Loureiro D, Tubio G, Romanini D. Protein Expession Purif. 2013;91:91–95. doi: 10.1016/j.pep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Braia M, Porfiri MC, Farruggia B, Pico G, Romanini D. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2008;873:139–143. doi: 10.1016/j.jchromb.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 43.McDonald P, Victa C, Carter-Franklin JN, Fahrner R. Biotechnol. Bioeng. 2009;102:1141–1151. doi: 10.1002/bit.22127. [DOI] [PubMed] [Google Scholar]
- 44.Ruoslahti E. J. Biol. Chem. 1989;264:13369–13372. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.