Abstract
Background
Recent neuroimaging studies have demonstrated resting-state functional connectivity (rsFC) abnormalities among intrinsic brain networks in Major Depressive Disorder (MDD); however, their role as predictors of treatment response has not yet been explored. Here, we investigate whether network-based rsFC predicts antidepressant and placebo effects in MDD.
Methods
We performed a randomized controlled trial of two weeklong, identical placebos (described as having either “active” fast-acting, antidepressant effects or as “inactive”) followed by a ten-week open-label antidepressant medication treatment. Twenty-nine participants underwent a rsFC fMRI scan at the completion of each placebo condition. Networks were isolated from resting-state blood-oxygen-level-dependent signal fluctuations using independent component analysis. Baseline and placebo-induced changes in rsFC within the default-mode, salience, and executive networks were examined for associations with placebo and antidepressant treatment response.
Results
Increased baseline rsFC in the rostral anterior cingulate (rACC) within the salience network, a region classically implicated in the formation of placebo analgesia and the prediction of treatment response in MDD, was associated with greater response to one week of active placebo and ten weeks of antidepressant treatment. Machine learning further demonstrated that increased salience network rsFC, mainly within the rACC, significantly predicts individual responses to placebo administration.
Conclusions
These data demonstrate that baseline rsFC within the salience network is linked to clinical placebo responses. This information could be employed to identify patients who would benefit from lower doses of antidepressant medication or non-pharmacological approaches, or to develop biomarkers of placebo effects in clinical trials.
Keywords: resting-state functional connectivity, large-scale connectivity networks, biomarkers of treatment response, placebo effects, Major Depression
INTRODUCTION
Major depressive disorder (MDD) has recently been conceptualized as a disorder with a network-based pathophysiology (1). Particularly, MDD manifests in cortico-limbic network dysregulation reflected by deficits in cognitive control and increased sensitivity of limbic networks (2-5), which is thought to result in excessive and negatively-skewed focus on introspective processes, difficulty regulating emotions, and persistent difficulties in sustaining attention (6, 7). Regions involved in these networks have emerged as biological markers of response to antidepressant treatments (8-13). Yet, the ability of these biomarkers to selectively distinguish drug-specific effects from other non-specific elements of the treatment response, such as the placebo effect, is still limited, with very few studies specifically addressing biomarkers of non-specific elements of antidepressant treatment response (14, 15). This is not a small concern, as placebo response rates in antidepressant clinical trials average 31-45% compared with response rates to antidepressants of ~50% and have increased at a rate of 7% per decade over the last 30 years (16, 17). Hence, further investigation is warranted in order to dissect the neural predictors of drug-specific and non-specific effects in MDD treatment.
Of the major functionally connected networks identified within the brain’s inherent organization (18-20), three have received special attention in MDD and the prediction of treatment response: the default-mode (DMN), salience (SN) and executive (EN) network (21, 22). The DMN, with key regions in the posterior cingulate, medial prefrontal, and bilateral parietal and temporal cortices (23), is associated with introspective cognition (24) and demonstrates heightened connectivity and abnormal down-regulation in MDD, which may contribute to the disorder’s hallmark attributes of excessive self-focus, inattention and rumination (25-28). Elevated pretreatment activity of the rostral anterior cingulate (rACC), a region encompassed within the main anterior DMN node (24) has consistently been identified as a predictive marker of treatment response across imaging and treatment modalities (9, 10, 29, 30). It has been hypothesized that heightened baseline rACC activity fosters better treatment outcome in patients with MDD by implementing adaptive self-reflection through its connectivity within the DMN (31). Moreover, antidepressant medication has been shown to decrease functional connectivity of the DMN (32). The SN, anchored by the anterior insula (aINS) and dorsal anterior cingulate cortex (dACC), is enlisted during the integration of salient stimuli and interoceptive information to guide motivated behavior (33). In particular, the aINS is a hub of meta-awareness and affective processing (34, 35) and has long been associated with MDD pathophysiology (25, 36-39). A recent meta-analysis illustrates its activity as a neural predictive marker of MDD treatment response, where increased baseline insular activity is associated with poor clinical response (40). Finally, MDD is characterized by reduced functional connectivity of the EN (4) and hypo-activation of the network’s key node: the dorsolateral prefrontal cortex (dlPFC) (41). The EN, which includes cohesive functional communication between the dlPFC and parietal cortex, is responsible for orienting to and engaging in attentive, goal-directed behavior (33); its dysfunction may contribute to a lack of control over heightened affective responses in MDD (21, 42). The dlPFC is involved in current MDD treatments and successful recovery from the disorder (43-45); while reduced grey matter volume in the region is an indicator of non-response to standard MDD treatments (11).
Nodes within these three networks have also been implicated in the neurobiological mechanisms of non-specific treatment effects in the field of placebo analgesia, where substantial headway has been made to identify the cognitive, neural, and molecular bases of the neurobiology of placebo effects (46-51). These studies have demonstrated a key role of the rACC in the formation of placebo analgesia (48, 52, 53), potentially through its interactions with additional subcortical brain areas involved in endogenous opioid-mediated analgesic effects, such as the amygdala (54) and the periaqueductal gray (48), but also the anterior and posterior INS (50, 51, 55). A number of neuroimaging studies have also reported placebo-associated changes in dlPFC function, thought to be related to expectancies and anticipatory mechanisms (56-58), consistent with the role of this region in cognitive executive function (59). In this regard, activity in EN-associated regions during pain anticipation was found to be predictive of the magnitude of placebo analgesia (60). Conversely, minimal information has been acquired as to the mechanisms implicated in antidepressant placebo effects, with notable exception of one investigation demonstrating an overlap in regions involved in placebo and medication effects (15) and our recent work describing the role of the opioid system in the formation of placebo responses in MDD (61).
Here, we take a network-based univariate and multivariate approach to the prediction of the response to placebo and antidepressant treatment using resting-state functional connectivity (rsFC) with independent component analysis (ICA) (62), a data-driven approach that produces results within a framework of the brain’s intrinsic connectivity networks and allows for identification of reliable, exploratory-based treatment response predictors. We investigated the relationship between baseline rsFC of three networks (DMN, SN, left and right EN) and: 1) depression severity; 2) clinical response to one-week of placebo treatment; and 3) clinical response to ten-weeks of open-label antidepressant treatment. First, among the three networks, only the DMN has been previously related to MDD duration (63) and maladaptive rumination (25); therefore, we hypothesized that increased baseline DMN rsFC would be associated with greater depression severity. Second, with respect to the prediction of treatment response, none of the connectivity networks have been specifically related to placebo or antidepressant medication responses in patients with MDD. However, central regions of the DMN, SN, and EN — specifically the rACC, INS, and dlPFC, respectively — have a key role in mechanisms implicated in antidepressant and placebo responses, as described above, as well as the role of these networks in processes necessary for treatment responses: internal monitoring, saliency and higher-level cognition, respectively. Therefore, we hypothesized that increased baseline functional connectivity within central regions of the DMN, SN and EN would predict response to both one-week of placebo and ten-weeks of open-label antidepressant treatment. Finally, we applied multivariate relevance vector regression (RVR) to evaluate the hypothesis that baseline rsFC maps of the three networks would allow for prediction of placebo and antidepressant responses at an individual level.
METHODS and MATERIALS
Subjects
Twenty-nine right-handed, un-medicated subjects with a DSM-V diagnosis of MDD were recruited via local advertisements [21 females; age: 18-59 (mean ± S.D.: 32 ± 13)]. See Supplemental Methods and Material for additional subject information and description of informed consent and authorized deception. All of the procedures used were approved by the University of Michigan Investigational Review Board for Human Subject Use Research Committee.
Placebo Randomized Controlled Trial (RCT) and Antidepressant Open-Label Trial
As previously described (61), subjects were randomized into either 1) one-week “active” placebo treatment (two pills/day), with expectations that the pill represented a potentially fast-acting antidepressant agent or 2) one-week “inactive” placebo treatment described as an control condition, without pharmacological effects (two pills/day). After a three-day “washout” period of no pills, subjects crossed over into the alternative condition to which they had not been assigned. After each week of placebo, subjects underwent a resting-state scanning session (Fig. 1).
Figure 1. Experimental Design.
A) After pre-randomization (screening), subjects are randomized into one of two conditions each lasting seven days: 1) Active: placebo administration with disclosure that it may provide antidepressant-like treatment effects; 2) Inactive: placebo administration with disclosure that it is an inactive agent. B) A three day washout occurs during which the patient receives no medication. C) Subjects cross-over to the alternative condition. D) Resting-state fMRI scans are obtained immediately after each condition. E) After full completion of the placebo trial, subjects undergo ten weeks of open-label antidepressant treatment. Depression measures are administered (* marks QIDS-16SR administration) at pre-randomization, pre- and post-active, pre- and post-inactive, and week 0, 2, 4, 8, 10 of the antidepressant trial.
Depression symptoms were assessed using the 16-item self-rated version of the Quick Inventory of Depressive Symptomatology (QIDS) (64) at the following time points: pre-randomization, baseline (pre)-, and post- active and inactive conditions. This measure was selected as a primary outcome measure for its sensitivity to fluctuations in depression symptoms and its availability as a self-reported measure. Additionally, patients completed the Montgomery Asberg Depression Rating Scale (MADRS) and the Hamilton Rating Scale for Depression (HDRS) at pre-randomization and during each visit within the antidepressant trial.
The change in QIDS (ΔQIDS = QIDSBASELINE - QIDSPOST) was calculated for active and inactive treatment conditions, and the difference between conditions was taken as an index of placebo response (ΔQIDSACTIVE – ΔQIDSINACTIVE). Positive values reflected greater reductions in depressive symptoms as a result of “active” placebo administration.
Following the placebo RCT and the two resting-state fMRI scan sessions, subjects received a ten-week open-label antidepressant trial with citalopram as an initial agent (starting at 20 mg/day and up to 40 mg/day in 45% of cases). When citalopram was not clinically indicated (e.g. prior non-response or side-effects), another antidepressant was given [sertraline (n=1), mirtazapine (n=1), fluoxetine (n=2), duloxetine (n=1), and bupropion (n=1)]. Participants’ symptom changes were evaluated at weeks 0, 2, 4, 8, and 10. Antidepressant response was measured by the difference in QIDS between week 0 and 10 (ΔQIDS = Week 0 – Week 10). In four cases, participants began the antidepressant treatment less than three days after the placebo experiment; because of this, a baseline QIDS Week 0 measure was created to measure antidepressant treatment response.
Resting State Functional Connectivity Networks
Image data acquisition, preprocessing, movement analysis, and ICA are detailed in Supplemental Methods. Among available rsFC analytical methods, in our opinion, ICA, a data-driven approach, is an optimal choice for isolating connectivity networks, to provide an inherent framework for any resultant predictors, versus manual selection of a seed region. Briefly, 20 components were output through ICA utilizing the Infomax algorithm within the Group ICA methodology in GIFT software (Medical Image Analysis Lab, University of New Mexico, Albuquerque, New Mexico; http://icatb.sourceforge.net/). Of the resultant components, the networks of interest were selected using templates from the BrainMap (http://www.brainmap.org/icns) database: a comprehensive resting-state fMRI data source (65). To determine the component with the “best-fit” for each particular network, a linear-template matching procedure was performed on all 20 components, as described elsewhere (63). Briefly, for each network template: all 20 components were scored based on their best-fit with the template by computing the average z-score of voxels falling outside the template in the component subtracted from the average z-score of voxels falling within the template. The component with the greatest value of this measure was identified as the network-of-interest: DMN, SN, right or left EN. For all networks, the component-of-interest had a best-fit score of at least two SD greater than the mean [network: best-fit score (mean ± SD); DMN: 15.7 (1.63 ± 3.58); SN: 5.2 (0.7±1.45); LEN: 12.0 (1.1 ± 2.7); REN: 4.6 (0.7 ±1.4)].
Data Analysis
All data analysis was performed using SPM8 (Welcome Department of Cognitive Neurology, University College, London, England) and Matlab (MathWorks, Natick, Massachusetts) software. One-sample t-tests and paired t-tests were used to analyze within-network functional connectivity within- and between-conditions, respectively (see Supplemental Methods for paired t-tests). For each network, one-sample t-tests were performed: network-of-interest components from all 29 subjects during the “post-inactive” scan (baseline) were entered into the analysis and ICA-assigned z-scores at each voxel were averaged across all subjects and compared to zero (Fig. 2). Significance threshold was set at p<0.05 family-wise corrected (FWE).
Figure 2. Functional Connectivity of Networks.
One-sample t-tests including baseline (inactive condition) resting-state fMRI scans for all 29 subjects for each ICA-component corresponding to the network: A) Default-mode, B) Salience, C) Left (left-side) and right (right-side) executive networks. The t-score bars are shown at the right; all images are displayed at a threshold of p<0.05 family-wise corrected. Images are shown in standard space of the Montreal Neurological Institute (MNI) template. N=29.
For each network, a whole brain voxel-by-voxel regression analysis was performed between network functional connectivity (z-score of the weight on the ICA component of interest) at baseline (post-inactive) with the following variables: 1) depression severity (as measured by QIDS score at pre-randomization); 2) reductions in depression symptoms in response to placebo administration with expectations of antidepressant effects [(ΔQIDSACTIVE – ΔQIDSINACTIVE), see above]; and 3) reductions in depression symptoms in response to ten weeks of antidepressant treatment (as measured by ΔQIDS scores = Week 0 - Week 10). All analyses were restricted to voxels within those defined by the network-of-interest’s one-sample t-test composed of corresponding network component maps from all 29 subjects’ inactive scan (masked at p<0.05-FWE). Depression severity score and scan order were input as covariates in all analyses.
All results were constrained within network component maps [one-sample t-test (above) masked at p<0.05-FWE]. For all a priori regions (rACC, INS, dlPFC), significance was set at a threshold height of p<0.005 uncorrected and 3dClustSim [AFNI (http://afni.nimh.nih.gov)] was used with 1000 Monte Carlo simulations to determine cluster-based, whole-brain correction at p<0.005 to account for testing of three bilateral regions-of-interest and four networks (total of ten). See Supplemental Material for extent cluster information, which is network-size dependent. Significance for all other peaks was set at p<0.05-FWE. These significant data clusters were extracted using MarsBar (66), for quantification of regional functional connectivity at baseline and placebo-induced changes, graphing, determination of correlation coefficients (Pearson/Spearman correlations at p<0.05). Data are expressed as the mean ± one S.D., unless otherwise indicated.
Multivariate Relevance Vector Regression (RVR) Analysis
We also investigated single-subject predictability of baseline (‘inactive’ scan) rsFC onto measured placebo responses using multivariate RVR as implemented in PRoNTo (http://www.mlnl.cs.ucl.ac.uk/pronto/) running under Matlab (Mathworks, 2012a release). RVR is detailed elsewhere (67). Briefly, it is a sparse kernel learning multivariate regression method formulated in Bayesian framework. The model weights are assigned a zero-mean Gaussian prior in which each weight is governed by its own hyperparameter. Iterative estimation from the fMRI data identifies the most probable values for these hyperparameters with sparseness achieved due to the posterior distributions of many of the weights peaking around zero. The voxels associated with non-zero weights are marked as relevance vectors, which can then be used to predict the target value (placebo-induced ΔQIDS) for a novel input vector (e.g. baseline SN rsFC). RVR’s major strength relative to other multivariate machine learning techniques [i.e. support vector machine (SVM), reviewed (68)] is that it computes quantitative prediction of a variable of interest without a need for discrete categorical estimation (e.g. patients vs. controls) (69). Here, the subjects’ SN components were mean centered and input into the RVR analysis. An estimate for the model’s predictability was calculated via leave-one-out cross validation, indexed using the Pearson correlation coefficient and mean squared error (MSE) between actual and the predicted placebo response measure. The significance of these metrics was determined through non-parametric permutation tests. In a single iteration, we randomly paired subjects’ SN functional connectivity with subjects’ placebo response values. Subsequently, we calculated the MSE and correlation for the random pairing. This was completed for 5000 iterations and built a distribution of MSE and correlation values from which p-values were calculated for accuracy of placebo response prediction.
RESULTS
Patient Characteristics
Twenty-nine participants with MDD [QIDS-16SR (mean ± SD): 16.1 ± 4.1; Hamilton Rating Scale for Depression (HRSD-17): 21.6 ± 5.1] were enrolled in the study and completed the two-week randomized placebo trial and entered the ten-week treatment with an open-label antidepressant (Fig. 1). 79% of them (23/29) completed the entire open-label antidepressant treatment portion; those who dropped out before full completion were not significantly different in their depression severity and placebo responsiveness from those who completed the study (see Supplemental Results, also for additional participant data).
Placebo- and Medication-induced Changes in Depression Symptoms
Reductions in depression symptoms after one week of the “active” placebo were significantly greater than after the “inactive” placebo treatment [ΔQIDS: 2.0 ± 3.4 for active; 0.17 ± 2.4 for inactive; F = 7.2, df = 1, p = 0.012]. The total placebo response measure (ΔQIDSACTIVE – ΔQIDSINACTIVE) was highly variable, ranging from −8 to 11 (mean ± S.D.: 1.8 ± 4.2). Ten weeks of open-label antidepressant treatment was also associated with a significant reduction in depression symptoms [QIDS at Week 0: 11.6 ± 4.3; at Week 10: 6.9 ± 3.9; t = 4.9, df = 22, p < 0.001].
Association of Baseline Resting Functional Connectivity, Pre-Randomization Depression Severity, Response to Placebo, and Response to Antidepressant Medication
Statistical component maps for each network were examined against symptoms of depression severity (QIDS-16SR) at time-point of pre-randomization. We did not find a significant relationship with any of the networks (a priori: p>0.005, mm3 > network-specific mm3; other: p>0.05-FWE).
Statistical component maps for each network were then examined against reductions in depression symptoms in response to one-week of placebo administration with expectations of antidepressant effects (Fig. 3) and in response to ten weeks of open-label antidepressant treatment. Significance was established at p<0.005 and extent > 928 mm3 for the SN (Supplemental Material). Increased baseline rsFC of the rACC within the SN was significantly associated with greater placebo response: rACC: 0, 38, 4, 1784 mm3; Z= 4.35. No other significant effects were observed.
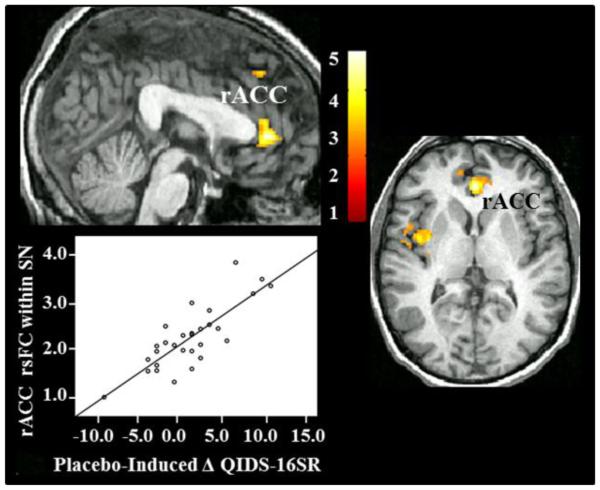
Figure 3. Baseline Functional Connectivity of the Salience Network Predicts Response to Placebo Administration.
N=29. (Top left and right): Voxel-by-voxel correlational analysis between baseline functional connectivity of the SN and decreases in depression symptoms in response to placebo administration. Clusters passing significance threshold are labeled. Image display is at p<0.01; t-score bar is shown in the bottom right. (Bottom Left): Association between reductions in depression symptoms in response to placebo administration and functional connectivity of rACC within the SN. Functional connectivity of this region predicted 65% of the variance in placebo responses (p<0.001).
A linear regression analysis that included baseline rsFC of the rACC within the SN predicted 65% of the variance in placebo responsiveness (adjusted R2=0.65).
Based on this initial finding and the rACC’s essential involvement in placebo effects (50-52) and the prediction of antidepressant treatment response (70, 71), we allowed subsequent activation of rACC rsFC to be significant after small volume correction (p<0.05-FWE) using a rACC region-of-interest sphere centered around the MNI coordinates 4, 42, 6 (72) with a 6mm radius [created using MarsBar (66)].
Increase baseline rsFC of the rACC within the SN was significantly associated with reductions in depression symptoms in response to ten weeks of open-label antidepressant treatment (rACC: 0, 40, 8; 64 mm3; Z = 3.09; p<0.05-SVC:FWE-corr). No other effects were observed.
Placebo-induced Changes in Network Functional Connectivity Predicts Response to Placebo and Antidepressant Medication
As expected, one-week of placebo administration with expectations of antidepressant effects did not result in significant changes within rsFC of the networks—as measured by the changes in rsFC from “active” to “inactive”—given high inter-individual variation in response to placebo (a priori: p>0.005; other: p>0.05-FWE-corr, see Supplemental for network-specific cluster size).
In order to account for the variability inherent in the placebo response measure, statistical component maps of rsFC changes in response to one-week of placebo administration were then examined against associated reductions in depression symptoms in response to one-week of placebo administration and ten weeks of open-label antidepressant treatment. Placebo-induced rsFC reduction in the rACC within the SN was significantly associated with greater placebo response (rACC: 0, 38, 4; 192 mm3; Z = 3.97; p<0.05-SVC:FWE-corr). No other significant effects were observed.
Multivariate Relevance Vector Regression (RVR): Single-Subject prediction of placebo and treatment response
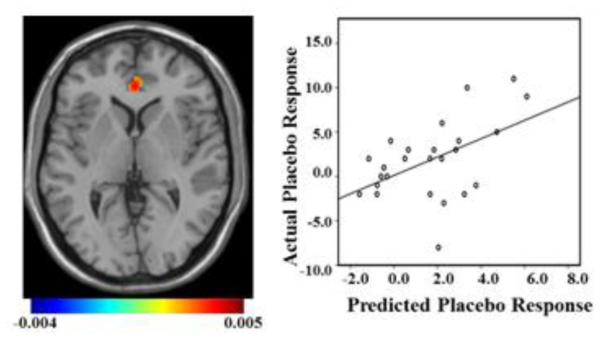
Based on the results described above, we first applied RVR to rsFC of the entire SN, where significant results were observed, in order to enable quantitative prediction of depression symptom reductions in response to placebo administration and in response to ten weeks of antidepressant treatment at the individual level. RsFC of the SN was significantly predictive of placebo responses [correlation = 0.41; p-value = 0.018; mean sum of squares = 14.36; p-value = 0.019; (Fig. 4)], with the greatest weights contributing to placebo response prediction within the rACC. SN rsFC was not a significant predictor of response to antidepressant treatment (correlation = 0.03; p-value = 0.34; mean sum of squares = 21.31; p-value = 0.36). No effects were found in the other networks.
Figure 4. Multivariate Relevance Vector Regression.
Left: Mean predictor map with an arbitrary threshold of >50% of minimum and maximum voxel weight values. The map shows the relative contribution of each voxel to the regression function in relation to all other voxels. Color bar signifies weight value for all voxels. Right: Scatter plot showing the predicted placebo response value derived from each subject’s baseline SN functional connectivity using RVR leave-one-out cross validation versus their actual placebo response value (r = 0.41; p-value = 0.018).
DISCUSSION
In this study, we illustrated the viability of network-based rsFC to identify potential imaging-based predictive markers of treatment response in MDD. We found that more cohesive rsFC of the SN, through connectivity of the rACC, predicted greater reductions in depressive symptoms following one-week of placebo administration with expectations of antidepressant effects and ten weeks of open-label antidepressant treatment. In addition, the clinical response to one-week of placebo treatment was also associated with placebo-induced decreases in rsFC of the rACC within the SN. Finally, multivariate RVR demonstrated that SN rsFC predicted placebo responses with significant accuracy at an individual level.
Elevated baseline rsFC of the rACC within the SN predicted greater reductions in depression symptoms in response to placebo administration with expectations of antidepressant effects. Specifically, the rACC alone predicted 65% of the response variance. This finding potentially advances a more general understanding of the neurobiology of placebo effects across diseases: Both opioidergic and BOLD activity within the rACC have been consistently implicated in placebo analgesia (48, 52, 53) which evinces a hypothesis that corresponding mechanisms of action are involved in the formation of placebo effects across disorders (47), with recent evidence in further support of this notion (61). Our data, to our knowledge, are also the first demonstration of SN involvement in MDD placebo effects and thus, supplement the small existing body of work in the neurobiology of placebo in patients with MDD (15, 61). This is consistent with the role of the SN in saliency attribution: particularly, selecting and integrating biologically relevant stimuli with interoceptive states to guide input of attentional resources and behavior (73). In this case, the network was involved in attending to and incorporating a set of expectations within a therapeutic environment with internal states resulting in a reduction of depression symptoms. If the former hypothesis remains valid, SN rsFC may be the functional context of prior placebo analgesia-associated rACC findings.
Mounting evidence from studies employing a variety of neuroimaging modalities and treatments indicate that increased pretreatment activity in the rACC is associated with better response to antidepressant treatment, as recently reviewed (31). More specifically, metabolism in the region has been observed to differentiate responders from non-responders to antidepressant medication (70). However, the open-label nature of these and prior studies (10, 70, 74, 75) makes it difficult to dissect drug-specific from non-specific treatment effects. Our study is an initial attempt in isolating predictors for different aspects of treatment effects, such as placebo. Here, we demonstrated that greater baseline rsFC of the rACC within the SN to be a significant predictor of placebo response and separately, open-label antidepressant treatment response. Consistent with these data, a recent RCT found that elevated pretreatment theta current density in the rACC predicted treatment outcome in both the medicated and placebo group (14).
In all, these findings point to the predictive role of rACC within the SN in the response to placebo and the overall response to antidepressant treatment (antidepressant + placebo + other non-specific effects). Furthermore, we observed the normalization of heightened baseline rACC rsFC after placebo administration to be associated with greater placebo responses; this illustrates that rACC modulation may be an important element of placebo neurobiology. A relevant hypothesis may state that enhanced rACC rsFC within the SN may represent a greater capacity for integrating salient stimuli with adaptive cognitive-affective functioning, which is conducive for the manifestation of a placebo effect. Normalization of heightened rACC activity has also been demonstrated in MDD responders to sleep deprivation, but absent in non-responders (a placebo-arm was not incorporated) (76). Thus, malleability within the rACC may extend to necessitate a successful antidepressant treatment response or to placebo mechanisms inherent in the antidepressant response.
Finally, we demonstrated that multivariate RVR application to baseline SN rsFC, with the greatest weight within the rACC, was significantly predictive of placebo responses at an individual-subject level, although this was not the case for antidepressant responses. The utilization of a similar method, SVM, has gained substantial attention in the last years as a predictive classifier in MDD with diagnostic and prognostic qualities (77-79). For example, in a sample of 37 depressed patients and 37 healthy controls, whole-brain structural MRI significantly classified nearly 90% of patients exhibiting clinical remission and nearly 70% of those with an MDD diagnosis (80). Instead, we employed RVR to enable quantitative prediction without need for group classification, as it has been recently described (69). The potential applicability of SN rsFC as an individual-subject predictor of placebo response will need to be further confirmed in larger samples; yet, this information could ultimately augment clinical treatment through identification of patients who possess a greater likelihood in benefiting from lower dosages of antidepressant medication or cognitive interventions. This may also be relevant in the context of clinical trials to better distinguish patients with greater susceptibility to placebo effects, allowing for patient stratification and possibly, more detailed clarification of the effects of treatment components (specific versus non-specific).
A limitation of the current study was the small sample size (N = 29 for the placebo phase; N = 23 for the antidepressant phase). This is especially relevant to the RVR analysis, where independent confirmation is needed to establish whether SN rsFC is a predictor of placebo response at an individual level. Finally, the subsequent nature of the open-label treatment after the placebo phase was not optimal for dissecting drug and placebo-specific effects. Future RCTs with parallel placebo and drug arms will need to be conducted to better identify and separate predictors of treatment response. However, our data have the potential to inform future designs of antidepressant clinical trials and personalized medicine for MDD patients by identifying individuals with greater likelihood in responding to non-pharmacologically specific interventions and who may ultimately be selected for less intensive treatments, lower dosages of medication, or for enhanced patient-clinician interaction.
Supplementary Material
ACKNOWLEDGEMENTS
Work was supported by R01 MH086858, (JKZ) and the Phil F. Jenkins Foundation, the Michigan Institute for Clinical & Health Research grant support (CTSA: UL1RR024986). We would also like to acknowledge the contribution of the technologists of the Department of Radiology at the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial name: Predictors of antidepressant response.
URL: http://clinicaltrials.gov/ct2/show/NCT02178696?term=zubieta&rank=3.
Trial number: NCT02178696.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA, et al. Distracted and down: neural mechanisms of affective interference in subclinical depression. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 6.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 7.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Clin Neurosci. 2011;65:175–182. doi: 10.1111/j.1440-1819.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 9.Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin Neurophysiol. 2009;120:1313–1319. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langguth B, Wiegand R, Kharraz A, Landgrebe M, Marienhagen J, Frick U, et al. Pre-treatment anterior cingulate activity as a predictor of antidepressant response to repetitive transcranial magnetic stimulation (rTMS) Neuro Endocrinol Lett. 2007;28:633–638. [PubMed] [Google Scholar]
- 11.Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50:347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 12.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizvi SJ, Salomons TV, Konarski JZ, Downar J, Giacobbe P, McIntyre RS, et al. Neural response to emotional stimuli associated with successful antidepressant treatment and behavioral activation. J Affect Disord. 2013;151:573–581. doi: 10.1016/j.jad.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 14.Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex activity and early symptom improvement during treatment for major depressive disorder. Psychiatry Res. 2011;192:188–194. doi: 10.1016/j.pscychresns.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 16.Stolk P, Ten Berg MJ, Hemels ME, Einarson TR. Meta-analysis of placebo rates in major depressive disorder trials. Ann Pharmacother. 2003;37:1891–1899. doi: 10.1345/aph.1D172. [DOI] [PubMed] [Google Scholar]
- 17.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 18.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophrenia bulletin. 2014;40:428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- 27.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 30.Holthoff VA, Beuthien-Baumann B, Pietrzyk U, Pinkert J, Oehme L, Franke WG, et al. [Changes in regional cerebral perfusion in depression.SPECT monitoring of response to treatment] Nervenarzt. 1999;70:620–626. doi: 10.1007/s001150050487. [DOI] [PubMed] [Google Scholar]
- 31.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry. 2013;70:373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 35.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- 40.Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 46.de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 47.Pecina M, Zubieta JK. Molecular mechanisms of placebo responses in humans. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia--imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 49.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 50.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 54.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic bulletin & review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 55.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 56.Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Pecina M, Stohler CS, Zubieta JK. Neurobiology of placebo effects: expectations or learning? Soc Cogn Affect Neurosci. 2014;9:1013–1021. doi: 10.1093/scan/nst079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 60.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pecina M, Bohnert A, Sikora M, Avery E, Langenecker S, Mickey BJ, Zubieta JK. Placebo-Activated Neural Systems are Linked to Antidepressant Responses. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.1335. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 65.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox; 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- 67.Tipping ME. Sparse Bayesian learning and the Relevance Vector Machine. Journal of Machine Learning Research. 2001;1:211–244. [Google Scholar]
- 68.Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Gong Q, Li L, Du M, Pettersson-Yeo W, Crossley N, Yang X, et al. Quantitative prediction of individual psychopathology in trauma survivors using resting-state FMRI. Neuropsychopharmacology. 2014;39:681–687. doi: 10.1038/npp.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 71.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. The American journal of psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 72.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 73.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 74.Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, et al. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, et al. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 76.Clark CP, Brown GG, Archibald SL, Fennema-Notestine C, Braun DR, Thomas LS, et al. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res. 2006;146:43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu CH, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SC, et al. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol Psychiatry. 2008;63:656–662. doi: 10.1016/j.biopsych.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 78.Gong Q, Wu Q, Scarpazza C, Lui S, Jia Z, Marquand A, et al. Prognostic prediction of therapeutic response in depression using high-field MR imaging. Neuroimage. 2011;55:1497–1503. doi: 10.1016/j.neuroimage.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 79.Marquand AF, Mourao-Miranda J, Brammer MJ, Cleare AJ, Fu CH. Neuroanatomy of verbal working memory as a diagnostic biomarker for depression. Neuroreport. 2008;19:1507–1511. doi: 10.1097/WNR.0b013e328310425e. [DOI] [PubMed] [Google Scholar]
- 80.Costafreda SG, Chu C, Ashburner J, Fu CH. Prognostic and diagnostic potential of the structural neuroanatomy of depression. PLoS One. 2009;4:e6353. doi: 10.1371/journal.pone.0006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.