Abstract
Mechanisms determining final organ size are poorly understood. Animals undergoing regeneration or ongoing adult growth are likely to require sustained and robust mechanisms to achieve and maintain appropriate sizes. Planarians, well known for their ability to undergo whole-body regeneration using pluripotent adult stem cells of the neoblast population, can reversibly scale body size over an order of magnitude by controlling cell number. Using quantitative analysis, we showed that after injury planarians perfectly restored brain:body proportion by increasing brain cell number through epimorphosis or decreasing brain cell number through tissue remodeling (morphallaxis), as appropriate. We identified a pathway controlling a brain size set-point that involves feedback inhibition between wnt11-6/wntA/wnt4a and notum, encoding conserved antagonistic signaling factors expressed at opposite brain poles. wnt11-6/wntA/wnt4a undergoes feedback inhibition through canonical Wnt signaling but is likely to regulate brain size in a non-canonical pathway independently of beta-catenin-1 and APC. Wnt/Notum signaling tunes numbers of differentiated brain cells in regenerative growth and tissue remodeling by influencing the abundance of brain progenitors descended from pluripotent stem cells, as opposed to regulating cell death. These results suggest that the attainment of final organ size might be accomplished by achieving a balance of positional signaling inputs that regulate the rates of tissue production.
KEY WORDS: Wnt signaling, Notum, Organ size, Planaria, Regeneration, Tissue remodeling
Highlighted article: Restoration of brain:body proportion after injury in planarians involves spatial Wnt/Notum feedback inhibition to control brain cell differentiation and target organ size.
INTRODUCTION
Most animal species possess stereotyped body forms, in which organs and appendages grow to defined proportions with respect to total body size. Mutations that alter organ proportion can underlie evolutionary changes (Abzhanov et al., 2004; Jones et al., 2012) and result in human developmental disorders, such as microcephaly (Mochida and Walsh, 2001). Many molecular pathways have been described as contributing to growth regulation (Conlon and Raff, 1999; Lander, 2011; Schwank and Basler, 2010; Tumaneng et al., 2012), primarily through genetic studies of species that cease or largely dampen growth at the end of embryogenesis, but despite considerable interest, the developmental mechanisms explaining size attainment largely remain a mystery. By contrast, species that undergo regeneration and ongoing growth throughout adulthood must possess robust mechanisms to control animal form and proportion actively (Elliott and Sánchez Alvarado, 2013; Rink, 2013; Wills et al., 2008a,b); hence, they represent model systems well suited for investigating the developmental mechanisms that underlie the extent of growth and attainment of size (Baguñà and Romero, 1981; Oviedo et al., 2003; Wada et al., 2013).
Planarians are flatworms that continue to undergo significant tissue turnover throughout adulthood and exhibit an extreme capacity for growth control that allows reversible alteration of body and organ size over an order of magnitude through the regulation of cell number. Nutrient uptake leads to an increase in body size, whereas prolonged starvation results in ‘degrowth’, a reduction of body size and cell number without an alteration in relative tissue proportions or function (Baguñà and Romero, 1981; Morgan, 1898; Oviedo et al., 2003; Romero and Baguñà, 1991; Takeda et al., 2009). Additionally, planarians exhibit robustness in growth control through the process of regeneration. Planarians do not appreciably increase in size during regeneration, so small amputated tissue fragments ultimately become small but well-formed individuals, in which overt body proportionality appears broadly restored (Morgan, 1898). Planarians accomplish regeneration both by the production of missing structures in a regeneration blastema (termed epimorphosis) and through remodeling of pre-existing tissues [originally termed morphallaxis (Morgan, 1898)]. The term morphallaxis has also been used to describe putative regeneration mechanisms that might occur independently of cell proliferation (Morgan, 1901); therefore, for clarity we will describe the process of injury-induced alterations to pre-existing tissues as ‘tissue remodeling’ (Forsthoefel et al., 2011; Oviedo et al., 2003). Planarians reproduce asexually by fission, so they are a suitable model to study the mechanisms of natural proportion regulation and organ sizing through control of cell number (Oviedo et al., 2003).
The relationships between epimorphosis and tissue remodeling are not fully understood, but several cellular events and molecular pathways have been identified as contributing to each of these processes. Growth from feeding or epimorphic regeneration depends on parenchymal cells termed neoblasts that are the only proliferating cells in adult planarians (Baguñà, 1976; Newmark and Sánchez Alvarado, 2000; Reddien et al., 2005b). Neoblasts are a heterogeneous population containing both adult pluripotent stem cells (Wagner et al., 2011) and more lineage-restricted dividing cells (Adler et al., 2014; Cowles et al., 2013; Currie and Pearson, 2013; Forsthoefel et al., 2012; Lapan and Reddien, 2012; Marz et al., 2013; Scimone et al., 2014a,b, 2011; van Wolfswinkel et al., 2014; Vásquez-Doorman and Petersen, 2014; Vogg et al., 2014; Wenemoser et al., 2012). Tissue removal results in proliferative activation of neoblasts thought to be necessary for production of missing tissue types within the regeneration blastema. By contrast, the cellular basis for regenerative tissue remodeling is less well understood but has been proposed to involve a wave of systemic injury-induced cell death proportional to the extent of tissue removed by injury (Pellettieri et al., 2010). Within the intestine, regeneration involves both substantial differentiation of new cells and incorporation of pre-existing cells, suggesting a complexity of tissue additions and alterations during regeneration (Forsthoefel et al., 2011). As planarians can perform tissue remodeling and blastema formation through periods of starvation, it is possible that regulated autophagy could contribute to these processes (González-Estévez, 2009; González-Estévez et al., 2007). Perturbation of several molecular processes can influence body and organ size in planarians, including bioelectric signaling (Beane et al., 2012), FGF signaling (Cebrià et al., 2002a), JNK signaling (Almuedo-Castillo et al., 2014), TORC1 signaling (González-Estévez et al., 2012b; Peiris et al., 2012; Tu et al., 2012) and insulin-like peptide signaling (Miller and Newmark, 2012). However, there is still a limited understanding of the developmental and molecular events that underlie the attainment and maintenance of appropriate organ size in planarians.
We investigated the planarian brain as a model for regenerative organ size control, because it undergoes prominent and easily identifiable changes in cell number (Oviedo et al., 2003). Here, we show that planarians completely restore appropriate brain:body proportions through either epimorphic growth or tissue remodeling. We find that the secreted Wnt inhibitor notum (Petersen and Reddien, 2011) is expressed in anterior brain neurons and promotes brain size in regeneration, remodeling and homeostasis. notum(RNAi) brain phenotypes are suppressed by inhibition of wnt11-6/wntA/wnt4a (Gurley et al., 2010; Kobayashi et al., 2007; Riddiford and Olson, 2011), hereafter referred to as wnt11-6, an inhibitor of brain size expressed in the posterior brain (Adell et al., 2009; Kobayashi et al., 2007). wnt11-6 signaling through beta-catenin-1 is required for notum expression in the brain, but is likely to inhibit brain size through β-catenin-independent signaling. This Wnt/notum negative feedback loop regulates brain:body proportion through control of neoblast differentiation, rather than cell death, in both epimorphosis and remodeling. The ability of the planarian to restore organ proportionality provides a unique system to study organ size determination and control of the extent of regeneration.
RESULTS
Planarians robustly restore brain:body proportionality through regeneration or remodeling
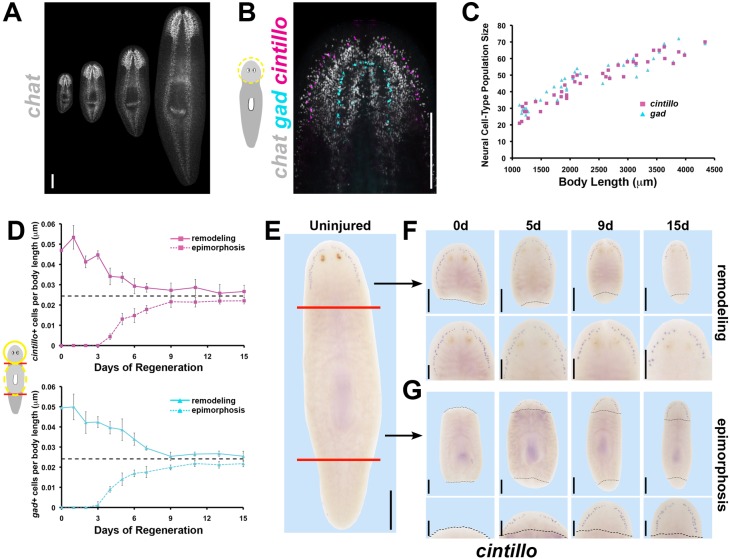
We first sought to clarify the precise relationships between brain cell number, brain size and body size. We examined uninjured adult planarians across a range of overall sizes using multiplex fluorescence in situ hybridizations (FISH) to measure numbers of abundant neurons [cholinergic neurons expressing choline acetyltransferase (chat) (Nishimura et al., 2010)] and more rare cell types of the brain [putative chemosensory neurons expressing a degenerin homolog cintillo (Oviedo et al., 2003) and GABAergic neurons expressing glutamine decarboxylase (gad) (Nishimura et al., 2008); Fig. 1A,B]. Populations of all three neuronal cell types were correlated strongly with brain length (Fig. S1B,C) and body size (Fig. 1C; Fig. S1D), consistent with previous analyses (Oviedo et al., 2003; Takeda et al., 2009). Neuronal cell size, as measured by in situ hybridization signal area for cintillo+ and gad+ neurons (labeling the cell body), did not vary with animal size, confirming previous analyses indicating that control of cell number is a principal method of body and organ size regulation in planarians (Fig. S1E) (Baguñà and Romero, 1981; Oviedo et al., 2003). Thus, adult planarians maintain a specific number of brain-related cells with respect to body size.
Fig. 1.
Planarian brain:body proportion is restored through regeneration by either increasing or decreasing brain cell number as necessary. (A) FISH detecting chat expression in intact animals 2-8 mm in length. (B) Triple FISH detects chemosensory neurons (expressing cintillo, magenta), GABAergic neurons (expressing gad, cyan) and cholinergic neurons (expressing chat, gray). (C) Numbers of neuronal subpopulations (cintillo+, magenta; gad+, cyan) from differently sized uninjured animals plotted against body length. (D) Average numbers of cintillo+ neurons (top, magenta) and gad+ neurons (bottom, cyan) normalized to animal length during epimorphic regeneration of a new brain (dotted lines) or remodeling of the pre-existing brain (solid lines; averages of n≥5 samples; bars, s.d.) measured by FISH. Black dashed lines in D indicate interpolated brain proportion based on analysis of intact brain proportions (see Materials and Methods). (E-G) WISH showing cintillo expression in intact animals (E), head fragments remodeling a pre-existing brain (F) and trunk fragments regenerating a new brain through epimorphosis (G). (F,G) Black dashed lines indicate amputation plane; bottom panels show higher magnification view of top panels. Scale bars: 300 µm in A,B,E and F,G top panels; 150 µm in F,G bottom panels. Anterior, top. d, day.
We next examined whether brain:body proportionality is precisely restored through regeneration by measuring brain cell numbers in decapitated animals forming a new brain through epimorphosis (Fig. 1D, dotted lines; Fig. 1E, lower) or amputated head fragments undergoing brain remodeling (Fig. 1D, solid lines; Fig. 1E, upper). In order to allow evaluation of regenerative progress with respect to the complete body plan and across replicates of varying sizes, neural cell numbers were normalized to the total fragment length to give a value for brain:body proportion at each time in regeneration (Fig. 1D). Relative brain size during regeneration was compared with values interpolated from a power law regression analysis of brain cell number versus body length in uninjured animals to determine when appropriate organ size was achieved (Fig. 1D, dashed black lines). In animals remodeling a pre-existing organ, brain:body proportion decreased rapidly during the 72 h following decapitation and continued to decrease until stabilizing around day 9. In epimorphic growth, cintillo+ and gad+ neurons emerged around day 3, coincident with appearance of the brain primordia (Cebrià, 2007), and brain:body proportion continued to increase until stabilizing by day 9. Throughout either regeneration scenario, brains had a constant ratio of neuronal cell numbers to brain length (Fig. S1F), indicating that proper scale within the brain is established early and maintained through periods of organ size change (Takeda et al., 2009), whereas the proportion of the organ with respect to the body is subject to regulation. Although remodeling and epimorphosis ultimately achieved similar brain:body proportions, the absolute brain sizes of these fragments were significantly different (Fig. S1G), indicating that whole-body regeneration in planarians is not the exact replacement of cell numbers but rather the restoration of appropriate body form. Therefore, regeneration can achieve correct brain proportions through either epimorphosis or remodeling programs, suggesting the existence of mechanisms that actively control organ proportionality.
notum and wnt11-6 are expressed in neurons at opposite poles of the brain
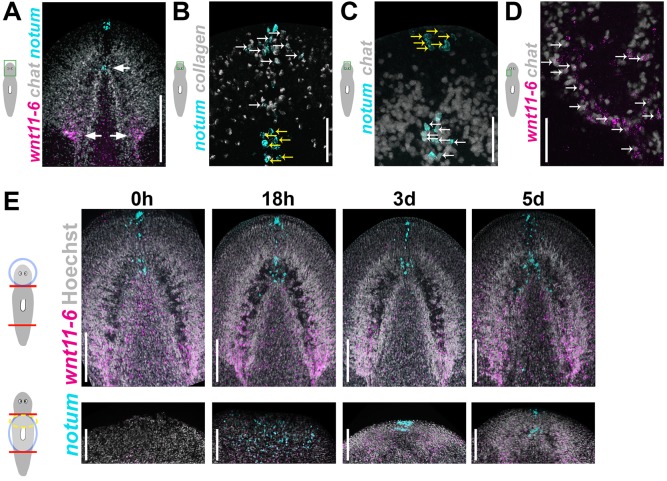
We took a candidate approach to identify molecules that control brain:body proportion in planarians, reasoning that mechanisms underlying robust size attainment would involve organ-specific secreted signals. Notum proteins are secreted lipases that deacylate Wnt ligands and prevent binding to Frizzled receptors (Kakugawa et al., 2015; Zhang et al., 2015), thus inhibiting Wnt signaling in many animals, including planarians, fruit flies, zebrafish and mammals (Flowers et al., 2012; Gerlitz and Basler, 2002; Giráldez et al., 2002; Petersen and Reddien, 2011; Traister et al., 2008). Planarians have a single notum homolog, and in uninjured animals, notum is expressed in the head at the anterior body pole and the anterior brain commissure (Fig. 2A, cyan) (Petersen and Reddien, 2011). Double FISH determined that notum expression at the anterior pole is within collagen+ body-wall muscle cells (Witchley et al., 2013) (Fig. 2B, white arrows; 85.5% of notum+ anterior pole cells were collagen+), whereas notum expression at the anterior brain commissure is within chat+ cholinergic neurons (90.1% of notum+ cells at the anterior commissure were chat+; Fig. 2C, white arrows). Notum proteins can inhibit Wnt signaling, so we sought to identify a planarian Wnt gene expressed near the brain. Among the nine planarian Wnt genes, wnt11-6 has prominent expression in the posterior brain (Fig. 2A, magenta) in addition to dispersed cells throughout the body (Adell et al., 2009; Gurley et al., 2010; Kobayashi et al., 2007). Double FISH determined that a majority of wnt11-6 cells associated with the brain (51.3%) are expressed in chat+ neurons (Fig. 2D, white arrows). notum and wnt11-6 are expressed at opposite ends of the brain in distinct expression domains that are maintained and restored during brain remodeling and epimorphic brain growth, respectively, and we were not able to identify cells that coexpress notum and wnt11-6 in any conditions (Fig. 2A,E).
Fig. 2.
notum and wnt11-6 are expressed in neurons at opposite poles of the brain. (A) Double FISH to detect notum (cyan), wnt11-6 (magenta) and chat (gray) expression in uninjured animals. notum is expressed at the anterior pole and in the brain commissure, whereas wnt11-6 is expressed at the posterior of each brain lobe (arrows). (B,C) Double FISH detecting expression of notum and either collagen (B) or chat (C). Of the notum+ cells at the anterior pole, 85.5±4.6% express collagen, marking musculature (out of 131 cells counted in five animals), whereas 90.1±4.4% of notum+ cells at the anterior commissure express chat, marking neurons (out of 81 notum+ cells counted in five animals; white arrows, double positive cells; yellow arrows, cells that express only notum). (D) Of the wnt11-6+ cells near the posterior brain, 51.3±0.7% express chat (out of 2362 wnt11-6+ cells counted in four animals). (E) Double FISH detecting expression of notum (cyan) and wnt11-6 (magenta) in head fragments undergoing brain remodeling (top panels) and trunk fragments forming a new brain through epimorphosis (bottom panels). Scale bars: 50 µm in A-D; 150 µm in E. Anterior, top. d, day.
notum and wnt11-6 are required for proper control of brain proportion
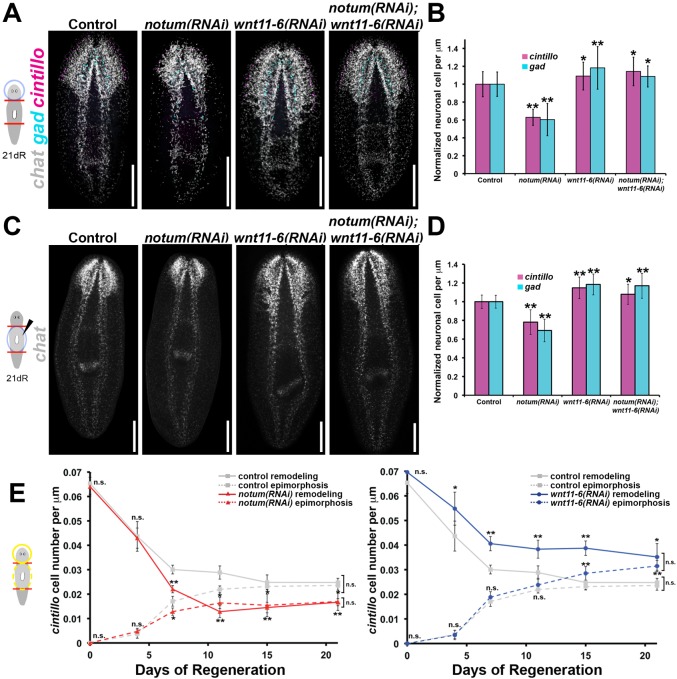
Given its regionalized expression within the brain, we next sought to identify putative functions of notum in the control of brain proportion. Inhibition of notum caused a reduction of cintillo+ and gad+ brain cell proportions in regenerating head fragments compared with control animals (Fig. 3A,B). Therefore, notum has a role in promoting brain cell numbers during conditions of brain cell loss through tissue remodeling. notum(RNAi) regenerating head fragments possessed normal regionalized expression of three homeotic transcription factors [orthopedia (otp) (Umesono et al., 1997), orthodenticle B (otxB) and orthodenticle A (otxA) (Umesono et al., 1999)] and a brain branch marker [G protein alpha subunit (gpas) (Cebrià et al., 2002b); Fig. S2], suggesting that notum primarily affects size but not pattern of the brain. The size reduction phenotype appeared to be specific to the brain, because notum RNAi did not change relative pharynx-to-body proportion (Fig. S3A), length of the pharynx neuropile at the distal end of that organ (Fig. S3B) or total body length (Fig. S3C). Finally, inhibition of notum did not significantly alter the density of neurons within the brain (Fig. S3D) or average neuronal cell size (Fig. S3E), indicating that notum is likely to regulate brain:body proportion principally through the regulation of brain cell number. Furthermore, although previous work identified notum as controlling anterior pole formation during head regeneration (Petersen and Reddien, 2011), inhibition of notum in head fragments did not eliminate expression of sFRP-1, a marker of the anterior pole (Fig. S4A). Therefore, the role of notum in brain sizing is likely to be independent of anterior pole formation. Collectively, these results demonstrate a specific function for notum in promoting appropriate brain size in regenerative degrowth.
Fig. 3.
notum inhibits wnt11-6 to control brain size in regenerative degrowth and growth. (A) Day 21 regenerating head fragments undergoing brain remodeling stained for expression of chat, gad and cintillo after indicated RNAi treatments. (B) Brain:body proportion [cintillo+ (magenta) or gad+ (cyan) cell numbers divided by body length and normalized to control treatments] from animals treated as in A, n≥18 worms per condition. (C,D) Animals were injected with indicated dsRNA 24 h after amputation of heads and tails, then fixed 21 days after amputation (21dR) and stained by multiplex FISH for chat (C) to show brain morphology and for cintillo (D; magenta) and gad (D; cyan) to quantify brain size normalized to body length; n≥18 animals per condition. (E) Animals undergoing epimorphosis (dashed lines) or remodeling (solid lines) were stained for expression of cintillo to measure brain proportion (cell number divided by body length) following administration of control (gray), notum dsRNA (left, red) or wnt11-6 dsRNA (right, blue); n≥4 animals per time point. All error bars indicate s.d. *P<0.05, **P<0.005, two-tailed t-test. Scale bars: 300 µm. Anterior, top.
notum(RNAi) head fragments undergoing brain remodeling additionally formed an ectopic set of photoreceptors within the head tip anterior to the original photoreceptors (19 of 21 worms; Fig. S4B). Such animals had anterior chat+ neural tissue associated with the ectopic photoreceptors and a more anterior placement of the posterior brain boundary (Fig. 3A). We suggest that brain size reduction through notum RNAi might change the position of an eye field that could be set up through underlying tissue of the brain. Alternatively, notum could have functions in eye placement that also affect brain size. We examined the relationship between the small brain and ectopic eye phenotypes by inhibiting ovo, a transcription factor required for production of photoreceptors (Lapan and Reddien, 2012). Dual inhibition of notum and ovo caused a reduction in brain size and absence of ectopic anterior photoreceptors (Fig. S4C,D). Additionally, prep(RNAi) homeostasis animals that form ectopic anterior photoreceptors (Fig. S4E) (Felix and Aboobaker, 2010), similar to notum(RNAi) animals, have normal numbers of cintillo+ cells (Fig. S4F), suggesting a potential separation in requirements for brain sizing and eye placement. We conclude that eye placement functions for notum are not required for its control of brain size and we did not investigate them further.
wnt11-6(RNAi) planarians undergo brain expansion (Adell et al., 2009; Kobayashi et al., 2007) and in the planarian Dugesia japonica form ectopic posterior photoreceptors (Kobayashi et al., 2007), suggestive of opposing functions to notum in brain size control. We therefore tested possible functional interactions between notum and wnt11-6 in Schmidtea mediterranea using double RNAi. wnt11-6(RNAi) head fragments had increased brain:body proportions compared with control animals (Fig. 3A,B) but no defect in photoreceptor number (Fig. S5A; 49 of 51 animals). wnt11-6(RNAi) animals also had no defects in mediolateral brain organization (Fig. S2) or significant changes in pharynx proportion, pharynx neuropile size, body size, neuron density or neuron cell size (Fig. S3), indicating that, like notum, wnt11-6 specifically regulates the relationship between brain cell number and body size. Simultaneous inhibition of notum and wnt11-6 in amputated head fragments undergoing brain remodeling completely suppressed the ectopic photoreceptor and small brain phenotype caused by notum inhibition (Fig. S5A; 65 of 67 animals) and instead resulted in an increased brain size similar to wnt11-6(RNAi) animals (Fig. 3A,B). qPCR confirmed that the suppressive effects of wnt11-6 dsRNA on the notum RNAi phenotype were not caused by alteration of notum RNAi efficiency (Fig. S5B). We conclude that wnt11-6 is required for the notum(RNAi) brain size phenotype during remodeling, consistent with a mechanism in which notum inhibits wnt11-6, which in turn normally suppresses brain size.
We next tested whether notum and wnt11-6 also control brain size during formation of a new head through epimorphic regeneration. After decapitation, notum is expressed by 18 h near the anterior-facing wound site, and subsequently by 48-72 h in the new anterior pole (Petersen and Reddien, 2011). Administration of notum dsRNA prior to injury results in a range of defects, including head/tail polarity transformations or defective head regeneration (Petersen and Reddien, 2011). To examine functions for notum specifically in brain growth, we delivered notum dsRNA to animals 24 h after head amputation, reasoning that establishment of pole identity is likely to precede head and brain formation (Fig. S6A). Such animals succeeded in regenerating a head and forming an anterior pole (Fig. S6B,C) but formed elongated or supernumerary photoreceptors (Fig. S6B; 8 of 12 worms) reminiscent of the photoreceptor phenotype in notum(RNAi) regenerating head fragments (Fig. S4B). notum(RNAi)-epimorphosis animals exhibited reduced brain proportions (Fig. 3C,D). These phenotypes were also dependent upon wnt11-6, as notum(RNAi);wnt11-6(RNAi)-epimorphosis animals lacked photoreceptor defects (Fig. S6D; 31 of 34 worms) and had increased brain proportions (Fig. 3C,D). The effect of Wnt signaling on brain proportion appears to be specific to wnt11-6, because inhibition of wnt1, another planarian Wnt gene whose activity is known to be affected by notum (Petersen and Reddien, 2009a, 2011), did not significantly affect brain size or suppress the notum(RNAi) small brain phenotype (Fig. S6E). notum and/or wnt11-6 inhibition in trunk fragments did not alter pharynx proportion or total body size, further indicating that the scaling functions of these genes are specific for the brain (Fig. S6F,G). These results support the hypothesis that notum inhibits wnt11-6 function to control the relationship between brain and body size and suggest that this regulation could occur through a process common to both epimorphosis and remodeling.
We then investigated the dynamic emergence of brain proportion phenotypes after notum and wnt11-6 RNAi in epimorphosis and remodeling (Fig. 3E). notum or wnt11-6 inhibition caused progressive defects in brain:body proportion (Fig. 3E) and in absolute neuronal cell number (Fig. S7A) that were stronger at later times during regeneration (after 4 days). notum/wnt11-6 signaling is therefore unlikely to affect general processes intrinsic to early wound-induced signaling or all instances of neurogenesis but rather has a function specific to brain size attainment. Notably, inhibition of notum and wnt11-6 each caused an identical change in ultimate brain:body proportion achieved through both brain epimorphosis and brain remodeling (Fig. 3E, day 21). Consistent with this interpretation, prolonged inhibition of notum or wnt11-6 in the absence of injury caused effects on brain size similar to those observed through regeneration (Fig. S7B,C). Together, these results suggest that notum/wnt11-6 signaling might specifically influence target brain size. We conclude that notum and wnt11-6 antagonism acts in an essentially constitutive process to control brain cell number, potentially through the determination and maintenance of a size set-point for the planarian brain.
wnt11-6 regulates notum expression at the anterior brain commissure to form an inhibitory spatial feedback loop
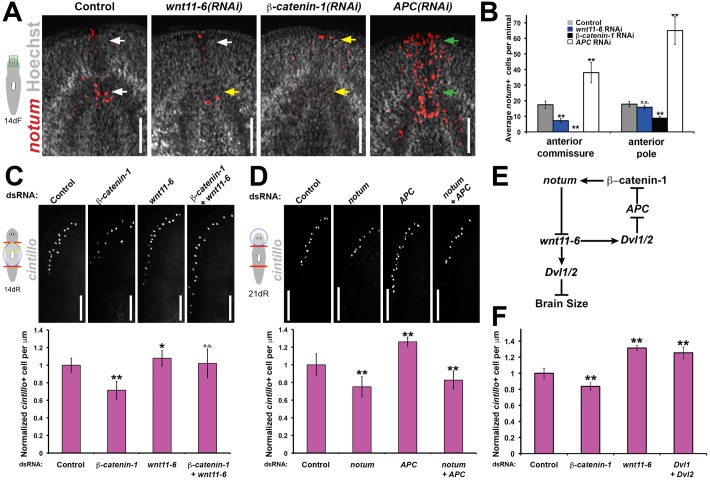
Homeostatic mechanisms, such as set-points, commonly rely on feedback inhibition to provide output stabilization (Stanger, 2011). Notum acts as a feedback inhibitor of Wnt signaling in multiple animal species (Flowers et al., 2012; Gerlitz and Basler, 2002; Giráldez et al., 2002; Petersen and Reddien, 2011); therefore, we examined the potential requirement of wnt11-6 for notum expression. Inhibition of wnt11-6 reduced numbers of notum-expressing cells in the anterior brain in conditions of homeostasis and remodeling (Fig. 4A,B; Fig. S8A). This function for wnt11-6 was specific for the brain, because wnt11-6 RNAi did not noticeably reduce numbers of notum+ cells at the anterior pole (Fig. 4A,B; Fig. S8A) or induced early after amputation (Fig. S8B), suggesting that other or redundantly acting Wnts might participate in these processes. Inhibition of beta-catenin-1 decreased notum+ cell numbers in the anterior commissure and anterior pole and, by contrast, inhibition of APC (encoding a component of the β-catenin destruction complex) increased the numbers of these notum+ cells (Fig. 4A,B). Therefore, transcriptional activation of notum in the anterior brain is likely to occur through canonical Wnt signaling. We additionally found that the notum+ cells of the brain commissure express four of the nine planarian frizzled genes [frizzled-5/8-2, frizzled-5/8-3, frizzled-5/8-4 and frizzled-1/2/7 (Liu et al., 2013)], suggesting that they should be capable of receiving Wnt signals (Fig. S8C). As notum and wnt11-6 were not coexpressed in any cells during regeneration (Fig. 2E), we conclude that wnt11-6 acts either at a distance or indirectly to activate notum expression. Together, these results indicate that wnt11-6 is required for the expression of its own secreted inhibitor, notum, within the brain to establish a negative feedback loop across the organ axis.
Fig. 4.
wnt11-6 activates anterior brain notum expression through canonical Wnt signaling to form a negative feedback loop. (A) notum expression (red) in uninjured control, wnt11-6(RNAi), beta-catenin-1(RNAi) and APC(RNAi) animals fixed after 14 days of RNAi feeding (14dF). Arrows indicate normal (white), reduced (yellow) or increased (green) notum+ cell numbers. (B) Quantification of notum+ cell numbers at the brain commissure and anterior body pole from A (n=5 animals per condition). (C,D) Day 14 regenerating trunk fragments (C) or day 21 regenerating head fragments (D) stained for cintillo expression after the indicated RNAi treatments [upper, animal images; lower, quantifications of cintillo+ cell number per animal length normalized to control animals. n≥9 (C) or n≥5 (D) animals per condition]. (E) Animals were fed dsRNA every 3 days for 14 (beta-catenin-1) or 22 days (control, wnt11-6, Dvl1;Dvl2) then fixed and analyzed for brain:body proportion as measured by cintillo+ cell numbers per animal length normalized to control animals (n≥5 animals per condition). All error bars indicate s.d. Scale bars: 100 µm in A; 150 µm in C,D. *P<0.05, **P<0.005, n.s. P>0.05, two-tailed t-test. Anterior, top.
wnt11-6 is likely to suppress brain growth through non-canonical Wnt signaling
Wnt family ligands can signal through either canonical, β-catenin-dependent pathways or non-canonical, β-catenin-independent pathways (Nusse, 2012). We examined intracellular components of canonical Wnt signaling and their relationships to notum and wnt11-6 to clarify the signaling pathways used in control of brain size. RNAi of beta-catenin-1 [at a time of regeneration prior to body-wide formation of ectopic anterior central nervous system structures (Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008)] caused a decrease in brain size in heads regenerated from anterior-facing wounds (Fig. 4C), opposite to the wnt11-6 RNAi phenotype and consistent with previous findings (Owen et al., 2015). wnt11-6(RNAi);beta-catenin-1(RNAi) animals had large brains similar to wnt11-6 inhibition alone (Fig. 4C), suggesting that wnt11-6 might act downstream or in parallel to beta-catenin-1 to control brain size. We inhibited APC to overactivate canonical Wnt signaling in order to test the hypothesis that beta-catenin-1 promotes brain size mainly through activating notum expression. In head fragments undergoing brain remodeling, APC RNAi phenocopied wnt11-6 RNAi to produce an enlarged brain (Fig. 4D). Furthermore, simultaneous inhibition of APC and notum resulted in small-brained animals (Fig. 4D), confirming that the large brain APC(RNAi) phenotype depends on notum. The simplest interpretation of these results is that wnt11-6 activates notum through canonical Wnt signaling but wnt11-6 suppresses brain growth through non-canonical, beta-catenin-1-independent signaling (Fig. 4E). Inhibition of Dishevelled (Dvl) homologs Dvl-1 and Dvl-2, genes that function in both canonical and non-canonical Wnt signaling pathways, caused brain enlargement similar to wnt11-6 RNAi and unlike beta-catenin-1 RNAi (Fig. 4F), consistent with this interpretation.
notum and wnt11-6 regulate neoblast differentiation to influence brain size
We then sought to identify the cellular mechanisms regulated by notum and wnt11-6 in control of brain size and hypothesized that they are likely to be common to epimorphosis and tissue remodeling. We first tested for candidate functions for notum and wnt11-6 in apoptosis because of their ability to control cell number and their activation during tissue remodeling (Pellettieri et al., 2010). We measured the role of notum and wnt11-6 in directing cell death using whole-mount terminal uridine nick-end labeling (TUNEL) in fixed tissue fragments undergoing brain remodeling (Fig. 5A,B) (Pellettieri et al., 2010). Both notum(RNAi) and wnt11-6(RNAi) fragments were able to activate cell death by 3 days post-amputation and return to basal levels around day 14 or 15 (Fig. 5A). Furthermore, these treatments did not significantly alter numbers of TUNEL+ cells throughout the animal at any regenerative time point (Fig. 5B). Therefore, we conclude that notum and wnt11-6 are unlikely to act through injury-induced cell death to direct a change in brain size.
Fig. 5.
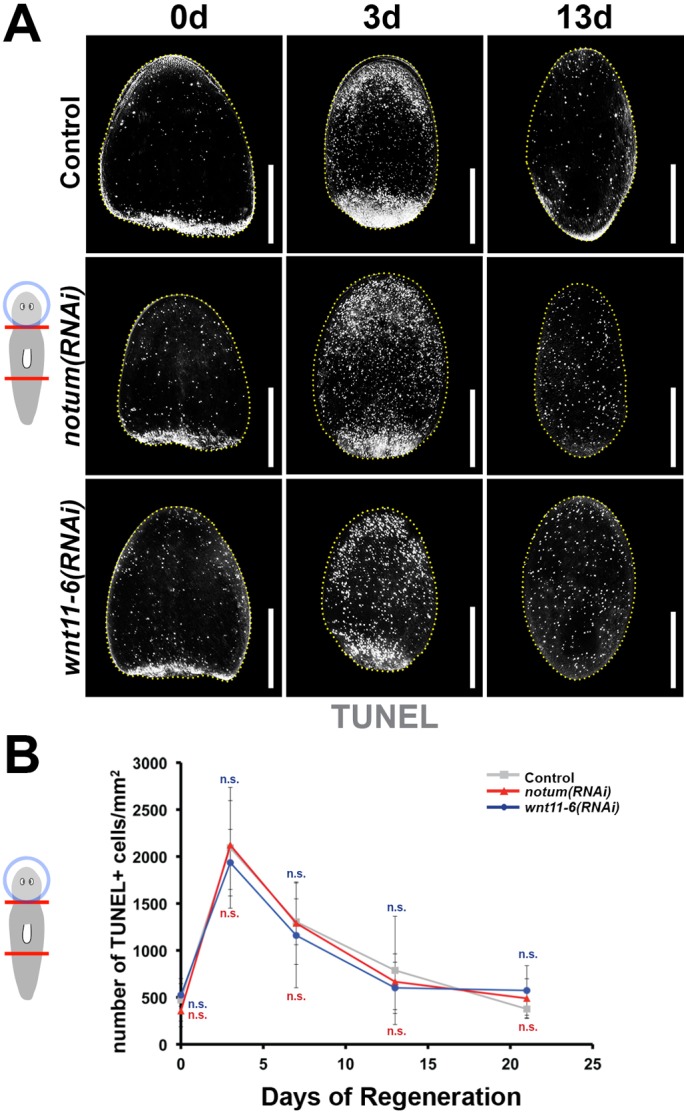
notum and wnt11-6 do not control injury-induced cell death. (A,B) Cell death during brain remodeling in regenerating head fragments was assayed by TUNEL staining over a time series of 21 days. (A) Animals were treated with control, notum or wnt11-6 dsRNA by injection (three injections over 3 days), amputated pre- and post-pharyngeally, then TUNEL stained. (B) TUNEL+ cell numbers per fragment area were quantified for all conditions and time points (n≥5 animals per time point; n.s. P>0.05, two-tailed t-test). All error bars indicate s.d. Scale bars: 300 µm. Anterior, top. d, day.
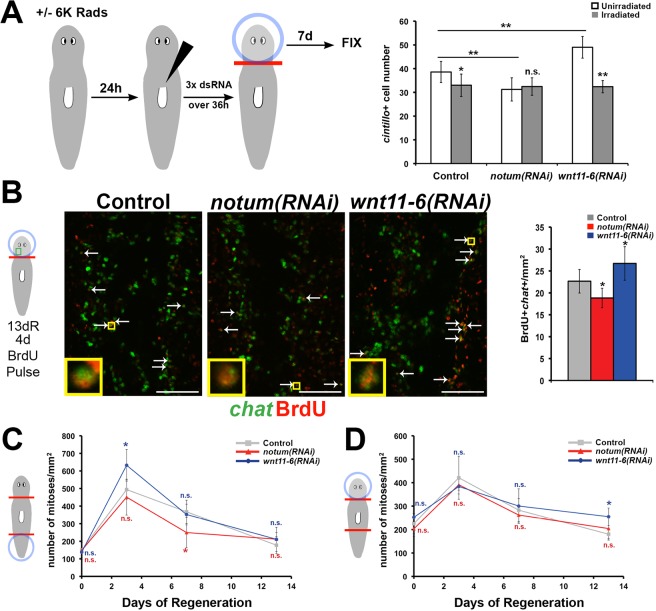
Ultimate organ size is likely to result from a balance between cell loss and cell production. Planarians use neoblasts, a population that includes pluripotent stem cells, for regeneration, growth and homeostatic cell replacement (Reddien et al., 2005b; Wagner et al., 2011). Therefore, we next investigated whether notum or wnt11-6 regulates neoblast-dependent tissue production to control brain size. To test this hypothesis, we measured cintillo+ cell number in lethally irradiated animals injected with control, notum or wnt11-6 dsRNA 2 days prior to head removal (Fig. 6A). As expected, decapitated irradiated trunk fragments did not produce a head blastema or cintillo+ cells (zero cells in three animals examined for each RNAi condition). Surprisingly, control RNAi irradiated head fragments undergoing brain remodeling lost an excess number of cintillo+ cells compared with non-irradiated control head fragments, indicating that normal tissue remodeling involves a neoblast-dependent activity that promotes brain cell number. notum(RNAi)non-irradiated or irradiated head fragments all attained a number of cintillo+ cells similar to irradiated control RNAi head fragments, indicating that notum requires neoblasts to promote brain size during remodeling (Fig. 6A). Additionally, irradiation completely suppressed the large brain phenotype observed with wnt11-6 RNAi, suggesting that wnt11-6 also requires neoblasts to affect brain size in all regenerative contexts (Fig. 6A). The simplest interpretation of these results is that remodeling involves the production of new brain cells in a manner regulated by notum and wnt11-6. To test this hypothesis, we measured the ability of notum(RNAi) or wnt11-6(RNAi) animals undergoing brain remodeling to produce new chat+ cells of the brain after a pulse of bromodeoxyuridine (BrdU) on day 9 and before fixation on day 13. notum RNAi caused a reduction in relative numbers of BrdU+chat+ cells, whereas wnt11-6 RNAi increased their numbers (Fig. 6B). Together, these results strongly suggest that new brain cell production contributes to attainment of appropriate proportion during regenerative tissue remodeling and that notum and wnt11-6 are likely to control brain size by influencing the formation of new brain cells.
Fig. 6.
notum and wnt11-6 regulate formation of new brain tissue in epimorphosis and in remodeling. (A) Non-irradiated or lethally irradiated (6000 rad) animals were injected with control, notum or wnt11-6 dsRNA three times over 60 h. Heads were removed, fixed 7 days later, and numbers of cintillo+ cells quantified by FISH. (B) Regenerating head fragments undergoing RNAi were injected with BrdU at day 9, fixed at day 13 and stained for chat by FISH (green) and BrdU (red) by immunofluorescence. Right, quantification of BrdU+chat+ cells from the posterior brain, normalized to animal size and control animal values. (C,D) Mitotic activity in tail fragments (C) and head fragments (D) was assessed by immunostaining for phospho-Ser10 of histone H3 (H3P) over a regeneration time series and quantified as average H3P+ cell number normalized to total fragment area (n≥5 animals per time point per condition; see Fig. S9 for representative images). Error bars, s.d. in A-D. *P<0.05, **P<0.005, n.s. P>0.05, two-tailed t-test. Scale bars: 50 µm.
We tested for possible functions of wnt11-6 on global proliferation, because neoblasts are the only proliferating cells in planarians (Baguñà, 1976; Newmark and Sánchez Alvarado, 2000; Reddien et al., 2005b). We measured global mitotic index in control, notum(RNAi) and wnt11-6(RNAi) head or tail fragments during brain remodeling or epimorphosis by staining with anti-phophoSer10-histone H3 (Wenemoser and Reddien, 2010). In both remodeling and epimorphosis, notum(RNAi) and wnt11-6(RNAi) animals had broadly similar global proliferative activities (Fig. 6C,D; Fig. S9), with differences at selected times (elevated after wnt11-6 RNAi in day 3 tail fragments and day 13 head fragments and reduced after notum RNAi in day 7 in tail fragments). Thus, wnt11-6 and notum do not appear to control cell division globally throughout regeneration as would be expected for regulation that constitutively maintains appropriate brain size, although we cannot rule out the possibility that they control proliferation of neoblast subpopulations at isolated windows in regeneration.
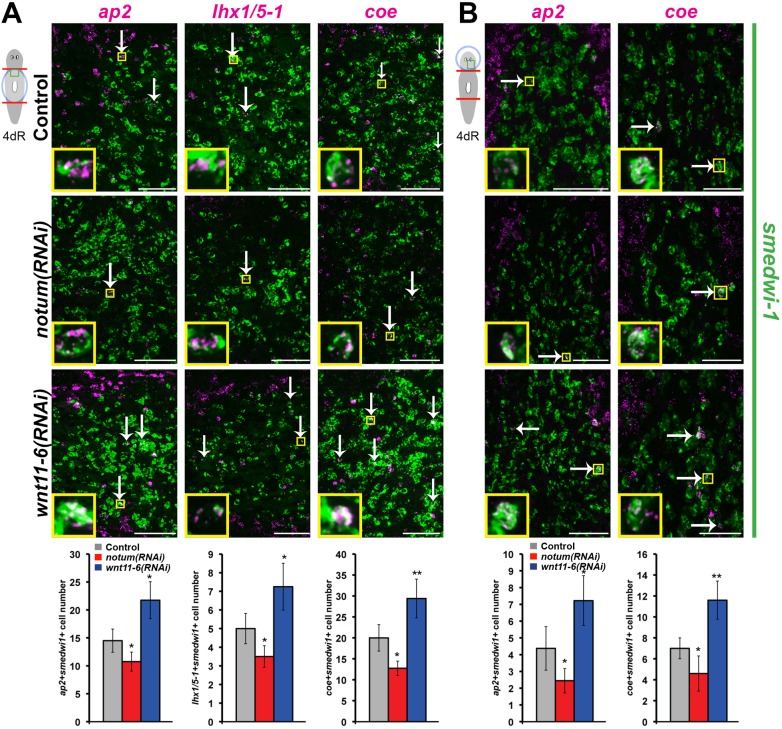
Neoblasts are a heterogeneous population that includes both pluripotent cells and distinct subpopulations specified for production of multiple differentiated tissues (Adler et al., 2014; Cowles et al., 2013; Currie and Pearson, 2013; Forsthoefel et al., 2012; Lapan and Reddien, 2012; Marz et al., 2013; Scimone et al., 2011, 2014a,b; van Wolfswinkel et al., 2014; Vásquez-Doorman and Petersen, 2014; Vogg et al., 2014; Wenemoser et al., 2012). We hypothesized that notum and wnt11-6 might regulate brain cell production by controlling numbers of brain cell progenitors marked by expression of lineage-specific transcription factors. We first examined ap2, which is required for production of trpA+ brain neurons and expressed in nearby smedwi-1+ neoblasts (Wenemoser et al., 2012). Like all other neuronal populations tested, trpA+ brain cell numbers increased after wnt11-6 RNAi and decreased after notum RNAi (Fig. S10A,B). As hypothesized, ap2+ smedwi-1+ cell numbers were elevated after wnt11-6 RNAi and reduced after notum RNAi both in formation of a new brain through epimorphosis (Fig. 7A) and in alteration of a pre-existing brain through remodeling (Fig. 7B). We examined two additional neoblast subpopulations that contribute to the brain, lhx1/5-1+smedwi-1+ cells that form serT+ serotonergic neurons of the brain (Currie and Pearson, 2013) (Fig. S10C,D) and coe+smedwi-1+ cells that form a variety of neuronal subtypes [including cpp-1+, chat+, gad+, tph+, th+ and tbh+ neurons (Cowles et al., 2013)]. In all cases, notum(RNAi) animals had fewer brain progenitors and wnt11-6(RNAi) animals had more brain progenitors (Fig. 7A,B). Taken together, we suggest that notum inhibits wnt11-6 activity, which in turn limits production of differentiated neurons through control of neoblast differentiation but not cell death. These results suggest that appropriate size attainment in regeneration involves regulation of the rates or extents of tissue production through organ-specific regionalized signaling.
Fig. 7.
notum and wnt11-6 regulate numbers of brain cell progenitors. (A,B) Double FISH to detect coexpression of smedwi-1 (green) and ap2, lhx1/5-1 or coe (magenta) in (A) trunk fragments regenerating a new brain or (B) head fragments undergoing brain remodeling 4 days after amputation and treated with indicated dsRNAs. Upper panels, images are representative planes from a confocal stack of the region indicated by the cartoon (white arrows, double positive cells; yellow insets show magnified representative double positive cells). Lower panels, graphs show quantifications of progenitor cell numbers (see Materials and Methods for details; n=4 animals per condition). Error bars indicate s.d. *P<0.05, **P<0.005, n.s. P>0.05, two-tailed t-test. Scale bars: 100 µm. Anterior, left in A; top in B.
DISCUSSION
The mechanisms underlying size attainment in development and regeneration are poorly understood, and planarians offer a powerful system for dissection of this process. Our studies support a model in which negative feedback signaling directs the size of the planarian brain through reversible regenerative growth (Fig. 8). We propose that wnt11-6 from the posterior brain inhibits the formation or division of brain progenitors to modify the rate of brain cell production. Through the canonical Wnt signaling pathway, wnt11-6 activates the expression of its own secreted inhibitor, notum, at the opposite end of the organ. In turn, notum promotes brain growth through its inhibition of non-canonical wnt11-6 activity. This process operates to maintain brain proportionality in uninjured animals and restore appropriate brain:body proportion in animals either growing a new brain through blastema formation or shrinking a pre-existing brain through remodeling. The notum/wnt11-6 feedback loop does not appear to control the ability to respond to injury or significantly affect the early rates of brain size increase in epimorphosis or decrease in brain remodeling, but rather controls a brain size set-point. The ability to perform large-scale unbiased RNAi screens in planarians, the unique ability to achieve organ proportions by either increases or decreases in cell number and to measure these processes quantitatively in whole animals will enable this system to be used to identify the genetic architecture underlying attainment of organ size.
Fig. 8.
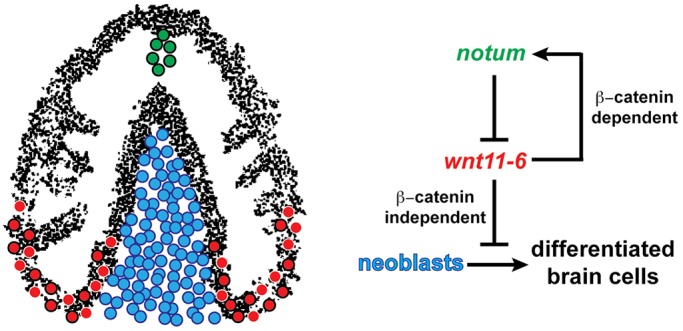
notum/wnt11-6 feedback regulation dampens brain cell differentiation to achieve proper brain:body scaling. (Left) Cartoon depicting planarian brain with neurons (black), wnt11-6-expressing neurons (red with black outline) and other brain-associated cell types (red with white outline) in the posterior brain region (red), notum-expressing neurons at the anterior commissure (green with black outline) and neoblasts that surround the brain (blue, medial neoblasts shown). (Right) Model for regulatory pathway influencing brain size. wnt11-6 inhibits neoblast production of differentiated brain cells (including cintillo+, gad+, trpA+ and tph+ neurons) by suppressing formation or division of neural progenitors (ap2+smedwi-1+, coe+smedwi-1+ and lhx1/5-1+smedwi-1+ cells) from pluripotent neoblasts. wnt11-6 signals directly or indirectly through β-catenin-dependent canonical Wnt signaling to activate expression of notum in neurons at the anterior brain commissure. wnt11-6 is likely to signal independently of beta-catenin-1 in control of neoblasts to suppress brain size. notum encodes a secreted protein that inhibits wnt11-6 function to promote ongoing synthesis of brain cells from neoblasts. Levels of wnt11-6 and notum signaling control numbers of neoblasts committing to brain cell fates to influence the size of the brain.
Our studies highlight that whole-body regeneration acts primarily to restore form rather than simply to replace missing tissues. For example, decapitation of animals with an average of 45 cintillo+ cells caused regeneration of only 30 new cintillo+ cells from regenerating trunk fragments (Fig. S1G), but this number perfectly restored the proportion of cintillo+ cells to body size (Fig. 1D). Therefore, planarian regeneration does not replace an absolute number of cells removed by injury. Likewise, amputated head fragments starting with 45 cintillo+ cells lost 20 of these cells through tissue remodeling (Fig. S1G), and this reduction also restored brain proportion (Fig. 1D). Given that uninjured planarians do not possess a fixed size (Reddien and Sánchez Alvarado, 2004), we argue that restoration of proportion is central to the completion of regeneration and might be a requirement to ensure fidelity in successive rounds of asexual reproduction or a property intrinsic to whole-body regeneration.
We find unexpected similarities between the mechanisms of growth control involved in epimorphosis, tissue remodeling and homeostatic maintenance. After or in parallel to early injury-induced proliferation (Wenemoser and Reddien, 2010), tissue polarization (Petersen and Reddien, 2009a, 2011) and injury-induced signaling (Gavino et al., 2013; Wenemoser et al., 2012), production of the regeneration blastema might involve an extreme form of tissue turnover shared among aspects of tissue remodeling and normal maintenance, similar to the concept of homeostatic regeneration (Wills et al., 2008a,b). The control of organ size is frequently described as the result of a balance between cell proliferation and cell death (Conlon and Raff, 1999). Tissue remodeling activated by injury coincides with waves of early wound-induced and later systemic cell death dependent on the extent of missing tissue (Pellettieri et al., 2010). We observed no role for notum/wnt11-6 signaling in programmed cell death during brain remodeling (Fig. 5) and instead found that notum and wnt11-6 controlled the size of the pool of brain progenitors descended from pluripotent neoblasts (Fig. 7). Additionally, depletion of stem cells by irradiation and notum RNAi caused identical excesses of cell loss to brains undergoing tissue remodeling, and perturbation of wnt11-6 and notum had opposite effects on the rate of neoblast differentiation to brain cells in remodeling (Fig. 6A,B). Together, these results suggest that an important component of size regulation through remodeling is the control of neoblast activity. Likewise, starvation-induced degrowth that reduces size globally involves body-wide reduction of differentiation (González-Estévez et al., 2012a), and intestinal remodeling coincides with a significant amount of new cell production in that organ (Forsthoefel et al., 2011). We suggest that the wnt11-6/notum signaling system can tune the size of the brain in contexts of regenerative growth and degrowth and in the absence of injury to allow alteration of the fraction of neoblasts specified to brain cell fates. Lineage-committed progenitor cell number during embryogenesis of several vertebrate model systems has been shown to be correlated with final organ size (Kicheva et al., 2014; Stanger et al., 2007), suggesting that the regulation of progenitor numbers could be an ancient and conserved mechanism for organ size control.
The use of Wnt signaling for organ size control and anterior patterning is also widespread (Petersen and Reddien, 2009b). Production of the Wnt inhibitor Dkk through cell differentiation regulates the size of zebrafish mechanosensory organs by counteracting Wnt signals required for progenitor proliferation (Wada et al., 2013). Overactivation of β-catenin increases the size of the mammalian brain through cell cycle regulation of neural progenitors (Chenn and Walsh, 2002, 2003), a phenotype broadly similar to planarian APC RNAi (Fig. 4D). Mammalian wnt1, wnt3a and wnt8 participate in brain patterning and proliferation (Erter et al., 2001; Lee et al., 2000; McMahon and Bradley, 1990; Thomas and Capecchi, 1990), but the precise mechanisms that relate Wnt ligands and secreted inhibitors for control of brain size remain unclear in vertebrates, as do their relationships with additional regulatory pathways. Perturbation of planarian insulin (Miller and Newmark, 2012) or hippo signaling (Lin and Pearson, 2014) causes body-wide defects in neoblast proliferation, suggesting that alternative pathways are used for control of brain size. Our results reveal a broadly conserved relationship between Wnt activity and brain size and suggest that the planarian brain is a tractable system for discovery of additional size regulatory pathways.
Growth inhibitors produced as a consequence of differentiation additionally have broad use in the control of organ size (Gamer et al., 2003; Gomer, 2001; Lander et al., 2009; Stanger, 2008). As a negative regulator of brain growth expressed in neurons of the brain and predicted to encode a secreted protein, wnt11-6 shares similarity with the action of molecules such as myostatin (McPherron and Lee, 1997; McPherron et al., 1997) and gdf11 (Wu et al., 2003), proposed chalones that limit cell numbers within the tissue from which they are expressed, the mammalian muscle and olfactory epithelium, respectively. Our studies demonstrate an additional layer of regulation in regenerative organ size control, in which the action of a putative chalone (wnt11-6) is tuned through the use of a feedback inhibitor (notum). In principle, restoration and maintenance of organ proportions could occur by achieving and preserving maximal activity of growth inhibitors in order to halt cell differentiation at an appropriate endpoint. However, it is unlikely that wnt11-6 activity levels are maximally high or low at the endpoint of regeneration, because perturbation of wnt11-6 or notum can reversibly affect brain size during homeostatic conditions in animals starting with optimal brain:body proportions (Fig. S7B,C). Instead, maintenance of ongoing growth and regeneration abilities might require constitutively expressed dampening mechanisms (Reddien, 2011), such as wnt11-6 feedback inhibition through notum, to prevent cessation of growth regulation. Spatial regulatory modules that achieve a sustained balance between growth inhibitors and activators could be essential for regenerative abilities, proportional growth and defining target organ size.
MATERIALS AND METHODS
Planarian culture and irradiation treatments
Asexual Schmidtea mediterranea (CIW4) were maintained in 1× Montjuic salts between 18 and 20°C. Gamma irradiation (6000 rad) was performed with a cesium-137 source irradiator at least 24 h prior to amputation to eliminate all dividing cells.
Whole-mount in situ hybridization
Animals were fixed and stained as described previously (Pearson et al., 2009). Antibodies were used in MABT containing 10% horse serum for FISH [anti-DIG-POD 1:2000 (Roche), anti-FL-POD 1:1000 (Roche), anti-DNP-POD 1:500 (PerkinElmer)] or NBT/BCIP in situ hybridization [(anti-DIG-AP 1:4000 (Roche)]. For multiplex FISH, peroxidase activity was quenched between tyramide reactions using 4% formaldehyde (Pearson et al., 2009) or 100 mM sodium azide (King and Newmark, 2013) for at least 1 h at room temperature. Nuclear counterstaining was performed using 1:1000 Hoechst 33342 (Invitrogen) in PBSTx (1× phosphate buffered saline with 0.1% Triton X-100).
RNA interference
RNA interference by feeding was performed using E. coli HT115 cultures expressing dsRNA from cDNA cloned into pPR244 (Gurley et al., 2008; Reddien et al., 2005a). For regeneration experiments, animals were fed liver-bacteria mixture four times over 9 days. For long-term homeostasis experiments, animals were fed RNAi bacterial food every 3 days. dsRNA targeting C. elegans unc-22 was used as a negative control. For RNAi by injection, dsRNA was synthesized by in vitro transcription and diluted to 2000 ng/μl, then administered to animal fragments by microinjection (Drummond Scientific). For double RNAi, liver-bacteria mixtures or in vitro transcribed dsRNA were mixed in equal volumes prior to administration, with single gene inhibition controls normalized with control dsRNA. notum, beta-catenin-1, APC, Dvl-1 and Dvl-2 plasmids were described previously (Gurley et al., 2008; Petersen and Reddien, 2011). wnt11-6 was cloned using primers 5′-TCGCATACAGCTTCAATCACA-3′ and 5′-AATGATTTTGTGCCATACGAA-3′.
BrdU labeling
Day 9 head fragments from RNAi-fed animals were injected with 5 mg/ml BrdU (Sigma-Aldrich) in water, then 4 days later fixed and stained as previously described (Vásquez-Doorman and Petersen, 2014).
Whole-mount immunostaining
Animals were sacrificed in 0.75 M HCl, then fixed with Carnoy's solution (60% ethanol, 30% chloroform and 10% acetic acid) and bleached overnight with 6% hydrogen peroxide in methanol. Animals were blocked for 6 h in 1× PBSTB (1x phosphate buffered saline, 0.3% Triton X-100, 0.25% bovine serum albumin) and primary and secondary antibody incubations performed overnight using rabbit anti-phospho-Histone H3 Ser10 (Cell Signaling; 1:3000 in 1× PBSTB) followed by anti-rabbit horseradish peroxidase conjugate (Invitrogen; 1:1000 in 1× PBSTB) and Alexa568-tyramide amplification (1:150; Invitrogen).
Terminal uridine nick-end labeling (TUNEL)
TUNEL was performed as described by Pellettieri et al. (2010), with modifications. Animals were sacrificed in 5% N-acetyl-cysteine in 1× PBS, fixed in 4% formaldehyde in 1× PBSTx, and bleached overnight in 6% hydrogen peroxide in 1× PBSTx. Samples were labeled with DIG-11-dUTP (Roche) by terminal deoxyuridine transferase (TdT) reaction (Fermentas) at 37°C for 2 h, then blocked and incubated overnight in anti-DIG-POD (Roche; 1:2000 in 10% horse serum in 1× PBSTx) prior to tyramide development (Invitrogen).
Image analysis
Imaging
Imaging was performed with a Leica M210F dissecting scope with a Leica DFC295 camera, a Leica DM5500B compound microscope with Optigrid, Leica SP5 or Leica TCS SPE confocal compound microscopes. Fluorescent images collected by compound microscopy are maximal projections of a z-stack and adjusted for brightness and contrast using Adobe Photoshop.
Cell counting
cintillo+ cells and gad+ or trpA+ cells in the medial brain region were counted manually. chat+ cells from one lobe of each animal were counted from a z-series of images using three-dimensional segmentation software in Imaris. Animal lengths were measured with ImageJ (National Institutes of Health) as visualized with Hoechst. Relative brain length was measured from the most posterior brain branch to the most anterior brain branch as visualized by chat FISH signal or Hoechst. Samples from similar fragments and time points were averaged and significant differences determined by two-tailed Student's t-tests. Cells coexpressing wnt11-6 and chat, notum and chat or notum and collagen were counted manually from z-stack confocal images (0.5-1 μm thick) using ImageJ. For notum coexpression, images were taken of the head region containing both the anterior body pole and anterior brain commissure. For wnt11-6, images were taken of the posterior of brain. Cells labeled with BrdU and chat or expressing both smedwi1 and either ap2, coe or lhx1/5 during regeneration were manually blind-counted from z-stack images near the posterior cephalic ganglia (BrdU), anterior-facing wound site (epimorphosis) or between the lobes of the cephalic ganglia (remodeling) by manual electronic labeling in using ImageJ and checking for consistency by comparing neighboring planes. H3P+ and TUNEL+ cell numbers were quantified using CellProfiler (Jones et al., 2008; Lamprecht et al., 2007) or ImageJ.
Regression analysis
Lines of best fit for cintillo+ and gad+ compared with either body (Table S1) or brain length (Table S2) in uninjured animals were determined by regression analysis in Microsoft Excel. Equations and r2 values are shown in Tables S1 and S2. Estimates of intact proportions, shown on graphs of regenerative time courses by a dashed black line, are averages of values interpolated from power regression (the analysis type with highest r2 values) using body or brain length measurements of animals on day 15 of remodeling or epimorphosis.
Acknowledgements
We thank the Pearson Laboratory (UToronto) for kindly sharing their TUNEL protocol and Dr Erik Andersen, Adam Hockenberry and all past and present members of the Petersen laboratory for helpful discussions of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceived and designed the experiments: E.M.H., C.P.P. Performed the experiments: E.M.H. Analyzed the data: E.M.H., C.P.P. Contributed reagents/materials/analysis tools: E.M.H., C.P.P. Wrote the paper: E.M.H., C.P.P.
Funding
The authors acknowledge support from a National Institutes of Health institutional predoctoral training program [Cellular and Molecular Basis of Disease Training Program 2T32GM008061-31 to E.M.H.] and an Ellison Medical Foundation New Scholar in Aging Research Award [AG-NS-0835-11 to C.P.P.], a National Institutes of Health Director's New Innovator Award [1DP2DE024365-01 to C.P.P.] and an American Cancer Society institutional research grant [ACS-IRG 93-037-15 to C.P.P.]. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.123612/-/DC1
References
- Abzhanov A., Protas M., Grant B. R., Grant P. R. and Tabin C. J. (2004). Bmp4 and morphological variation of beaks in Darwin's finches. Science 305, 1462-1465. 10.1126/science.1098095 [DOI] [PubMed] [Google Scholar]
- Adell T., Saló E., Boutros M. and Bartscherer K. (2009). Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development 136, 905-910. 10.1242/dev.033761 [DOI] [PubMed] [Google Scholar]
- Adler C. E., Seidel C. W., McKinney S. A. and Sánchez Alvarado A. (2014). Selective amputation of the pharynx identifies a FoxA-dependent regeneration program in planaria. eLife 3, e02238 10.7554/eLife.02238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuedo-Castillo M., Crespo X., Seebeck F., Bartscherer K., Salò E. and Adell T. (2014). JNK controls the onset of mitosis in planarian stem cells and triggers apoptotic cell death required for regeneration and remodeling. PLoS Genet. 10, e1004400 10.1371/journal.pgen.1004400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguñà J. (1976). Mitosis in the intact and regenerating planarian Dugesia mediterranea n.sp. I. Mitotic studies during growth, feeding and starvation. J. Exp. Zool. 195, 53-64. 10.1002/jez.1401950106 [DOI] [Google Scholar]
- Baguñà J. and Romero R. (1981). Quantitative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia 84, 181-194. 10.1007/BF00026179 [DOI] [Google Scholar]
- Beane W. S., Morokuma J., Lemire J. M. and Levin M. (2012). Bioelectric signaling regulates head and organ size during planarian regeneration. Development 140, 313-322. 10.1242/dev.086900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrià F. (2007). Regenerating the central nervous system: how easy for planarians! Dev. Genes Evol. 217, 733-748. 10.1007/s00427-007-0188-6 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Kobayashi C., Umesono Y., Nakazawa M., Mineta K., Ikeo K., Gojobori T., Itoh M., Taira M., Sánchez Alvarado A. et al. (2002a). FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature 419, 620-624. 10.1038/nature01042 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Kudome T., Nakazawa M., Mineta K., Ikeo K., Gojobori T. and Agata K. (2002b). The expression of neural-specific genes reveals the structural and molecular complexity of the planarian central nervous system. Mech. Dev. 116, 199-204. 10.1016/S0925-4773(02)00134-X [DOI] [PubMed] [Google Scholar]
- Chenn A. and Walsh C. A. (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365-369. 10.1126/science.1074192 [DOI] [PubMed] [Google Scholar]
- Chenn A. and Walsh C. A. (2003). Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb. Cortex 13, 599-606. 10.1093/cercor/13.6.599 [DOI] [PubMed] [Google Scholar]
- Conlon I. and Raff M. (1999). Size control in animal development. Cell 96, 235-244. 10.1016/S0092-8674(00)80563-2 [DOI] [PubMed] [Google Scholar]
- Cowles M. W., Brown D. D. R., Nisperos S. V., Stanley B. N., Pearson B. J. and Zayas R. M. (2013). Genome-wide analysis of the bHLH gene family in planarians identifies factors required for adult neurogenesis and neuronal regeneration. Development 140, 4691-4702. 10.1242/dev.098616 [DOI] [PubMed] [Google Scholar]
- Currie K. W. and Pearson B. J. (2013). Transcription factors lhx1/5-1 and pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development 140, 3577-3588. 10.1242/dev.098590 [DOI] [PubMed] [Google Scholar]
- Elliott S. A. and Sánchez Alvarado A. (2013). The history and enduring contributions of planarians to the study of animal regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2, 301-326. 10.1002/wdev.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erter C. E., Wilm T. P., Basler N., Wright C. V. E. and Solnica-Krezel L. (2001). Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development 128, 3571-3583. [DOI] [PubMed] [Google Scholar]
- Felix D. A. and Aboobaker A. A. (2010). The TALE class homeobox gene Smed-prep defines the anterior compartment for head regeneration. PLoS Genet. 6, e1000915 10.1371/journal.pgen.1000915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers G. P., Topczewska J. M. and Topczewski J. (2012). A zebrafish Notum homolog specifically blocks the Wnt/β-catenin signaling pathway. Development 139, 2416-2425. 10.1242/dev.063206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel D. J., Park A. E. and Newmark P. A. (2011). Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev. Biol. 356, 445-459. 10.1016/j.ydbio.2011.05.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel D. J., James N. P., Escobar D. J., Stary J. M., Vieira A. P., Waters F. A. and Newmark P. A. (2012). An RNAi screen reveals intestinal regulators of branching morphogenesis, differentiation, and stem cell proliferation in planarians. Dev. Cell 23, 691-704. 10.1016/j.devcel.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer L. W., Nove J. and Rosen V. (2003). Return of the chalones. Dev. Cell 4, 143-144. 10.1016/S1534-5807(03)00027-3 [DOI] [PubMed] [Google Scholar]
- Gaviño M. A., Wenemoser D., Wang I. E. and Reddien P. W. (2013). Tissue absence initiates regeneration through Follistatin-mediated inhibition of Activin signaling. eLife 2, e00247 10.7554/eLife.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz O. and Basler K. (2002). Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev. 16, 1055-1059. 10.1101/gad.991802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giráldez A. J., Copley R. R. and Cohen S. M. (2002). HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev. Cell 2, 667-676. 10.1016/S1534-5807(02)00180-6 [DOI] [PubMed] [Google Scholar]
- Gomer R. H. (2001). Not being the wrong size. Nat. Rev. Mol. Cell Biol. 2, 48-55. 10.1038/35048058 [DOI] [PubMed] [Google Scholar]
- González-Estévez C. (2009). Autophagy meets planarians. Autophagy 5, 290-297. 10.4161/auto.5.3.7665 [DOI] [PubMed] [Google Scholar]
- González-Estévez C., Felix D. A., Aboobaker A. A. and Saló E. (2007). Gtdap-1 promotes autophagy and is required for planarian remodeling during regeneration and starvation. Proc. Natl. Acad. Sci. USA 104, 13373-13378. 10.1073/pnas.0703588104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Estévez C., Felix D. A., Rodríguez-Esteban G. and Aboobaker A. A. (2012a). Decreased neoblast progeny and increased cell death during starvation-induced planarian degrowth. Int. J. Dev. Biol. 56, 83-91. 10.1387/ijdb.113452cg [DOI] [PubMed] [Google Scholar]
- González-Estévez C., Felix D. A., Smith M. D., Paps J., Morley S. J., James V., Sharp T. V. and Aboobaker A. A. (2012b). SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians. PLoS Genet. 8, e1002619 10.1371/journal.pgen.1002619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley K. A., Rink J. C. and Sánchez Alvarado A. (2008). Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319, 323-327. 10.1126/science.1150029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley K. A., Elliott S. A., Simakov O., Schmidt H. A., Holstein T. W. and Sánchez Alvarado A. (2010). Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev. Biol. 347, 24-39. 10.1016/j.ydbio.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias M., Gomez-Skarmeta J. L., Saló E. and Adell T. (2008). Silencing of Smed-βcatenin1 generates radial-like hypercephalized planarians. Development 135, 1215-1221. 10.1242/dev.020289 [DOI] [PubMed] [Google Scholar]
- Jones T. R., Kang I. H., Wheeler D. B., Lindquist R. A., Papallo A., Sabatini D. M., Golland P. and Carpenter A. E. (2008). CellProfiler Analyst: data exploration and analysis software for complex image-based screens. BMC Bioinformatics 9, 482 10.1186/1471-2105-9-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. C., Grabherr M. G., Chan Y. F., Russell P., Mauceli E., Johnson J., Swofford R., Pirun M., Zody M. C., White S. et al. (2012). The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484, 55-61. 10.1038/nature10944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakugawa S., Langton P. F., Zebisch M., Howell S. A., Chang T.-H., Liu Y., Feizi T., Bineva G., O'Reilly N., Snijders A. P. et al. (2015). Notum deacylates Wnt proteins to suppress signalling activity. Nature 519, 187-192. 10.1038/nature14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicheva A., Bollenbach T., Ribeiro A., Pérez Valle H., Lovell-Badge R., Episkopou V. and Briscoe J. (2014). Coordination of progenitor specification and growth in mouse and chick spinal cord. Science 345, 1254927 10.1126/science.1254927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. S. and Newmark P. A. (2013). In situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Dev. Biol. 13, 8 10.1186/1471-213X-13-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C., Saito Y., Ogawa K. and Agata K. (2007). Wnt signaling is required for antero-posterior patterning of the planarian brain. Dev. Biol. 306, 714-724. 10.1016/j.ydbio.2007.04.010 [DOI] [PubMed] [Google Scholar]
- Lamprecht M. R., Sabatini D. M. and Carpenter A. E. (2007). CellProfiler™: free, versatile software for automated biological image analysis. BioTechniques 42, 71-75. 10.2144/000112257 [DOI] [PubMed] [Google Scholar]
- Lander A. D. (2011). Pattern, Growth, and Control. Cell 144, 955-969. 10.1016/j.cell.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander A. D., Gokoffski K. K., Wan F. Y. M., Nie Q. and Calof A. L. (2009). Cell lineages and the logic of proliferative control. PLoS Biol. 7, e1000015 10.1371/journal.pbio.1000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapan S. W. and Reddien P. W. (2012). Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep. 2, 294-307. 10.1016/j.celrep.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Tole S., Grove E. and McMahon A. P. (2000). A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127, 457-467. [DOI] [PubMed] [Google Scholar]
- Lin A. Y. T. and Pearson B. J. (2014). Planarian yorkie/YAP functions to integrate adult stem cell proliferation, organ homeostasis and maintenance of axial patterning. Development 141, 1197-1208. 10.1242/dev.101915 [DOI] [PubMed] [Google Scholar]
- Liu S.-Y., Selck C., Friedrich B., Lutz R., Vila-Farré M., Dahl A., Brandl H., Lakshmanaperumal N., Henry I. and Rink J. C. (2013). Reactivating head regrowth in a regeneration-deficient planarian species. Nature 500, 81-84. 10.1038/nature12414 [DOI] [PubMed] [Google Scholar]
- Marz M., Seebeck F. and Bartscherer K. (2013). A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development 140, 4499-4509. 10.1242/dev.100081 [DOI] [PubMed] [Google Scholar]
- McMahon A. P. and Bradley A. (1990). The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62, 1073-1085. 10.1016/0092-8674(90)90385-R [DOI] [PubMed] [Google Scholar]
- McPherron A. C. and Lee S.-J. (1997). Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 94, 12457-12461. 10.1073/pnas.94.23.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron A. C., Lawler A. M. and Lee S.-J. (1997). Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83-90. 10.1038/387083a0 [DOI] [PubMed] [Google Scholar]
- Miller C. M. and Newmark P. A. (2012). An insulin-like peptide regulates size and adult stem cells in planarians. Int. J. Dev. Biol. 56, 75-82. 10.1387/ijdb.113443cm [DOI] [PubMed] [Google Scholar]
- Mochida G. H. and Walsh C. A. (2001). Molecular genetics of human microcephaly. Curr. Opin. Neurol. 14, 151-156. 10.1097/00019052-200104000-00003 [DOI] [PubMed] [Google Scholar]
- Morgan T. H. (1898). Experimental studies of the regeneration of Planaria Maculata. Arch. Entwickl. Mech. Org. 7, 364-397. 10.1007/bf02161491 [DOI] [Google Scholar]
- Morgan T. H. (1901). Regeneration. New York: Macmillan. [Google Scholar]
- Newmark P. A. and Sánchez Alvarado A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev. Biol. 220, 142-153. 10.1006/dbio.2000.9645 [DOI] [PubMed] [Google Scholar]
- Nishimura K., Kitamura Y., Umesono Y., Takeuchi K., Takata K., Taniguchi T. and Agata K. (2008). Identification of glutamic acid decarboxylase gene and distribution of GABAergic nervous system in the planarian Dugesia japonica. Neuroscience 153, 1103-1114. 10.1016/j.neuroscience.2008.03.026 [DOI] [PubMed] [Google Scholar]
- Nishimura K., Kitamura Y., Taniguchi T. and Agata K. (2010). Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience 168, 18-30. 10.1016/j.neuroscience.2010.03.038 [DOI] [PubMed] [Google Scholar]
- Nusse R. (2012). Wnt Signaling. Cold Spring Harb. Perspect. Biol. 4, a011163 10.1101/cshperspect.a011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo N. J., Newmark P. A. and Sánchez Alvarado A. (2003). Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea. Dev. Dyn. 226, 326-333. 10.1002/dvdy.10228 [DOI] [PubMed] [Google Scholar]
- Owen J. H., Wagner D. E., Chen C.-C., Petersen C. P. and Reddien P. W. (2015). teashirt is required for head-versus-tail regeneration polarity in planarians. Development 142, 1062-1072. 10.1242/dev.119685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson B. J., Eisenhoffer G. T., Gurley K. A., Rink J. C., Miller D. E. and Sánchez Alvarado A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev. Dyn. 238, 443-450. 10.1002/dvdy.21849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris T. H., Weckerle F., Ozamoto E., Ramirez D., Davidian D., García-Ojeda M. E. and Oviedo N. J. (2012). TOR signaling regulates planarian stem cells and controls localized and organismal growth. J. Cell Sci. 125, 1657-1665. 10.1242/jcs.104711 [DOI] [PubMed] [Google Scholar]
- Pellettieri J., Fitzgerald P., Watanabe S., Mancuso J., Green D. R. and Sánchez Alvarado A. (2010). Cell death and tissue remodeling in planarian regeneration. Dev. Biol. 338, 76-85. 10.1016/j.ydbio.2009.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. P. and Reddien P. W. (2008). Smed-βcatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319, 327-330. 10.1126/science.1149943 [DOI] [PubMed] [Google Scholar]
- Petersen C. P. and Reddien P. W. (2009a). A wound-induced Wnt expression program controls planarian regeneration polarity. Proc. Natl. Acad. Sci. USA 106, 17061-17066. 10.1073/pnas.0906823106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. P. and Reddien P. W. (2009b). Wnt signaling and the polarity of the primary body axis. Cell 139, 1056-1068. 10.1016/j.cell.2009.11.035 [DOI] [PubMed] [Google Scholar]
- Petersen C. P. and Reddien P. W. (2011). Polarized notum activation at wounds inhibits wnt function to promote planarian head regeneration. Science 332, 852-855. 10.1126/science.1202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P. W. (2011). Constitutive gene expression and the specification of tissue identity in adult planarian biology. Trends Genet. 27, 277-285. 10.1016/j.tig.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P. W. and Sánchez Alvarado A. (2004). Fundamentals of planarian regeneration. Annu. Rev. Cell Dev. Biol. 20, 725-757. 10.1146/annurev.cellbio.20.010403.095114 [DOI] [PubMed] [Google Scholar]
- Reddien P. W., Bermange A. L., Murfitt K. J., Jennings J. R. and Sánchez Alvarado A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635-649. 10.1016/j.devcel.2005.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P. W., Oviedo N. J., Jennings J. R., Jenkin J. C. and Sánchez Alvarado A. (2005b). SMEDWI-2 is a PIWI-Like protein that regulates planarian stem cells. Science 310, 1327-1330. 10.1126/science.1116110 [DOI] [PubMed] [Google Scholar]
- Riddiford N. and Olson P. D. (2011). Wnt gene loss in flatworms. Dev. Genes Evol. 221, 187-197. 10.1007/s00427-011-0370-8 [DOI] [PubMed] [Google Scholar]
- Rink J. C. (2013). Stem cell systems and regeneration in planaria. Dev. Genes Evol. 223, 67-84. 10.1007/s00427-012-0426-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R. and Baguñà J. (1991). Quantitative cellular analysis of growth and reproduction in freshwater planarians (Turbellaria; Tricladida). I. A cellular description of the intact organism. Invert. Reprod. Dev. 19, 157-165. 10.1080/07924259.1991.9672170 [DOI] [Google Scholar]
- Schwank G. and Basler K. (2010). Regulation of organ growth by morphogen gradients. Cold Spring Harb. Perspect. Biol. 2, a001669 10.1101/cshperspect.a001669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M. L., Srivastava M., Bell G. W. and Reddien P. W. (2011). A regulatory program for excretory system regeneration in planarians. Development 138, 4387-4398. 10.1242/dev.068098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M. L., Kravarik K. M., Lapan S. W. and Reddien P. W. (2014a). Neoblast specialization in regeneration of the planarian Schmidtea mediterranea. Stem Cell Rep. 3, 339-352. 10.1016/j.stemcr.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M. L., Lapan S. W. and Reddien P. W. (2014b). A forkhead transcription factor is wound-induced at the planarian midline and required for anterior pole regeneration. PLoS Genet. 10, e1003999 10.1371/journal.pgen.1003999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger B. Z. (2008). Organ size determination and the limits of regulation. Cell Cycle 7, 318-324. 10.4161/cc.7.3.5348 [DOI] [PubMed] [Google Scholar]
- Stanger B. Z. (2011). The Concept of the “Size Set Point” and Implications for Organ Size During Growth, pp. 3-12. New York, NY: Handbook of Growth and Growth Monitoring in Health and Disease. [Google Scholar]
- Stanger B. Z., Tanaka A. J. and Melton D. A. (2007). Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445, 886-891. 10.1038/nature05537 [DOI] [PubMed] [Google Scholar]
- Takeda H., Nishimura K. and Agata K. (2009). Planarians maintain a constant ratio of different cell types during changes in body size by using the stem cell system. Zoolog. Sci. 26, 805-813. 10.2108/zsj.26.805 [DOI] [PubMed] [Google Scholar]
- Thomas K. R. and Capecchi M. R. (1990). Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 346, 847-850. 10.1038/346847a0 [DOI] [PubMed] [Google Scholar]
- Traister A., Shi W. and Filmus J. (2008). Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 410, 503-511. 10.1042/BJ20070511 [DOI] [PubMed] [Google Scholar]
- Tu K. C., Pearson B. J. and Sánchez Alvarado A. (2012). TORC1 is required to balance cell proliferation and cell death in planarians. Dev. Biol. 365, 458-469. 10.1016/j.ydbio.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumaneng K., Russell R. C. and Guan K.-L. (2012). Organ size control by hippo and tor pathways. Curr. Biol. 22, R368-R379. 10.1016/j.cub.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono Y., Watanabe K. and Agata K. (1997). A planarian orthopedia homolog is specifically expressed in the branch region of both the mature and regenerating brain. Dev. Growth Diff. 39, 723-727. 10.1046/j.1440-169X.1997.t01-5-00008.x [DOI] [PubMed] [Google Scholar]
- Umesono Y., Watanabe K. and Agata K. (1999). Distinct structural domains in the planarian brain defined by the expression of evolutionarily conserved homeobox genes. Dev. Genes Evol. 209, 31-39. 10.1007/s004270050224 [DOI] [PubMed] [Google Scholar]
- van Wolfswinkel J. C., Wagner D. E. and Reddien P. W. (2014). Single-cell analysis reveals functionally distinct classes within the planarian stem cell compartment. Cell Stem Cell 15, 326-339. 10.1016/j.stem.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez-Doorman C. and Petersen C. P. (2014). zic-1 expression in planarian neoblasts after injury controls anterior pole regeneration. PLoS Genet. 10, e1004452 10.1371/journal.pgen.1004452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogg M. C., Owlarn S., Pérez Rico Y. A., Xie J., Suzuki Y., Gentile L., Wu W. and Bartscherer K. (2014). Stem cell-dependent formation of a functional anterior regeneration pole in planarians requires Zic and Forkhead transcription factors. Dev. Biol. 390, 136-148. 10.1016/j.ydbio.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Wada H., Ghysen A., Asakawa K., Abe G., Ishitani T. and Kawakami K. (2013). Wnt/Dkk negative feedback regulates sensory organ size in zebrafish. Curr. Biol. 23, 1559-1565. 10.1016/j.cub.2013.06.035 [DOI] [PubMed] [Google Scholar]
- Wagner D. E., Wang I. E. and Reddien P. W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811-816. 10.1126/science.1203983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenemoser D. and Reddien P. W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev. Biol. 344, 979-991. 10.1016/j.ydbio.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenemoser D., Lapan S. W., Wilkinson A. W., Bell G. W. and Reddien P. W. (2012). A molecular wound response program associated with regeneration initiation in planarians. Genes Dev. 26, 988-1002. 10.1101/gad.187377.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A. A., Holdway J. E., Major R. J. and Poss K. D. (2008a). Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development 135, 183-192. 10.1242/dev.010363 [DOI] [PubMed] [Google Scholar]
- Wills A. A., Kidd A. R., Lepilina A. and Poss K. D. (2008b). Fgfs control homeostatic regeneration in adult zebrafish fins. Development 135, 3063-3070. 10.1242/dev.024588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witchley J. N., Mayer M., Wagner D. E., Owen J. H. and Reddien P. W. (2013). Muscle cells provide instructions for planarian regeneration. Cell Rep. 4, 633-641. 10.1016/j.celrep.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-H., Ivkovic S., Murray R. C., Jaramillo S., Lyons K. M., Johnson J. E. and Calof A. L. (2003). Autoregulation of neurogenesis by GDF11. Neuron 37, 197-207. 10.1016/S0896-6273(02)01172-8 [DOI] [PubMed] [Google Scholar]
- Zhang X., Cheong S.-M., Amado N. G., Reis A. H., MacDonald B. T., Zebisch M., Jones E. Y., Abreu J. G. and He X. (2015). Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev. Cell 32, 719-730. 10.1016/j.devcel.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]