Abstract
The incidence of diabetes mellitus is continuously growing worldwide, while the specific chronic complications that it induces have a negative impact on life expectancy andquality, entailing extremely high costs of healthcare services. Diabetic peripheral neuropathy is one of the most common chronic complications of diabetes, affecting almost half of diabetic people during life.
This review aims at summarizing the evidence on the advantages and the usefulness of current perception threshold measurement for peripheral diabetic neuropathy assessment. Among the different methods used for the screening and diagnosis of diabetic neuropathy, measurement of current perception threshold using the Neurometer® has the ability to assess three sub-types of nerve fiber by producing transcutaneous electrical stimuli at frequencies of 2000, 250 and 5 Hz.
Current evidence shows that this method provides a useful, noninvasive evaluation technique of patients with peripheral nervous system disorders, being able to detect neuropathy in the earliest and asymptomatic stages.
Keywords: diabetic neuropathies, peripheral nervous system, current perception threshold, Neurometer®
General aspects about diabetic neuropathy
We are witnessing a continuing growth in rates of diabetes incidence and prevalence worldwide, and the overwhelming burden of the disease is carried by underdeveloped countries, where four out of five people live with diabetes. The International Diabetes Federation (IDF) estimates that 382 million people have diabetes, and the number of people with the disease will dramatically escalate to 592 million in less than 25 years [1]. The chronic and progressive nature of the disease leads to specific microvascular (neuropathy, retinopathy, nephropathy) and macrovascular chronic complications (coronary heart disease, stroke, peripheral artery disease) having a negative impact on life expectancy, its quality and entails extremely high costs. Diabetes is the fifth cause of death worldwide [2], being responsible for more than 4.9 million deaths worldwide in 2014, according to IDF data [1]. Diabetes mellitus is considered a cardiovascular disease equivalent with a major impact on morbidity and mortality, people with diabetes having a two-fold increase in the risk of stroke [3]. Also, diabetes is the major cause of renal failure and the leading cause of visual impairment and blindness in developed countries [2].
Diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes, affecting more that 50% of the patients [4]. DPN is a major risk factor for development of foot ulcers in people with diabetes [5]; up to 25% of diabetic patients will develop a foot ulcer [6]. The majority of lower limb amputations are preceded, in most of the cases, by a foot ulcer. Approximately 70% of all leg amputations are being performed in people with diabetes, and annually more than 1 million diabetic people are suffering from an amputation, meaning that every 20 seconds, somewhere in the world, a person loses one foot because of diabetes. After a major amputation, up to 50% of the diabetic people will suffer an amputation to the other leg, within the next 5 years, and 5 years mortality rate after a leg amputation is 68% [7]. There is evidence that instituting a structured diabetic foot program can yield a 75% reduction in amputation rates and a near fourfold reduction in inpatient mortality. Given the high cost (both economic and personal) of treating foot ulcers, it is important that we understand the modifiable risk factors associated with the development of neuropathy, in order to delay or prevent its development, and also it is important to detect peripheral neuropathy early in the natural history of the disease so that specific education be provided, and preventive measures can be applied to avoid devastating complications associated with the diabetic foot [8].
Diabetic neuropathy encompasses a number of neuropathic syndromes, but, by far, the commonest form, encountered in more than 90% of diabetic patients, is the chronic diabetic peripheral neuropathy, also known as distal symmetrical polyneuropathy [4].
In daily practice, diabetic peripheral neuropathy is a clinical diagnosis, based on patient history and clinical examination, after the exclusion of other causes [9]. But, the diagnosis cannot rely on history alone, as up to 50% of patients may be asymptomatic.
For clinical research, the diagnosis of DPN requires at least two tests in addition to the clinical history and examination: these normally comprise quantitative sensory testing (QST) and electrophysiology (EP). Lately, promising new techniques have arised, such as intra-epidermal nerve fiber density, nerve biopsy and corneal confocal microscopy [10].
Current perception threshold using the Neurometer® - Description of the method
Among quantitative sensory testing, measurement of current perception threshold (CPT) using the Neurometer® (Neurotron Inc., Baltimore, Maryland, USA) has been proved to be a reliable method to asses DPN [10,11]. The technology has been available since 1989, and its employment has progressively increased in the past years, with applicability in both clinical use and human research.
The typical sensory nerve is composed of three distinct sub-populations of nerve fibers that are characterized by their diameter. These various sub-populations of nerve fibers transmit different sensations. The smallest (unmyelinated) fibers transmit dull pain and temperature, the middle diameter (small myelinated) fibers transmit fast pain, temperature and pressure sensation, and the largest (myelinated) fibers transmit touch and pressure sensation. Unmyelinated C fibers also play an important role in the peripheral autonomic nervous system providing the innervation to the smooth musculature. One third of the C fibers are autonomic efferents. Diabetic peripheral neuropathy was defined in literature as a small-fiber neuropathy [12,13]. The smallest diameter unmyelinated fibers represent more than eighty percent of the total fibers, have the longest refractory periods and the slowest average conduction velocities of 1 m/s. By contrast, large diameter myelinated fibers comprise less than ten percent of the total fibers, have the shortest refractory periods and fastest average conduction velocities of 60 m/s.
Neurometer® is able to evaluate the functional status of these three distinct nerve fiber types by measuring the CPT at frequencies of 2000, 250 and 5 Hz, respectively [11]. The 2000 Hz stimulus selectively induces large myelinated fiber responses and the 5 Hz stimulus selectively triggers unmyelinated fiber responses [14]. The device quantifies in a quickly and painless manner the conduction and functional integrity of the large and small myelinated and small unmyelinated sensory nerve fibers [15]. The device produces transcutaneous electrical stimuli through a pair of gold plated electrodes to quantify neuroselective CPT values. The patient can be tested at one or more body sites with three different alternating frequencies of electrical stimulus (2000, 250, 5 Hz) and the tested skin sites have to be intact. With the patient sitting in a comfortable position two electrodes coated with conductive gel are attached to the skin site to be evaluated. The current is increased (to a maximum of 9.99 mA) until the patient begins to feel the current at the skin where the electrodes are attached. The current is then terminated, decreased by 0.08 mA and reapplied. When a sufficient number of tests have been performed the CPT device determines and displays the CPT value for the test series. At each frequency (2000, 250 and 5 Hz), an R-CPT (rapid current perception threshold) value is generated and could range from 1 to 25. A value ranging from 6 to 13 is classified as normal, while a value ranging from 1 to 5 show hyperesthesia (increased sensation). A value between 14 and 25 shows hypoesthesia (decreased sensation). Both hyperesthesia and hypoesthesia indicate the presence of sensory neuropathy [16].
Using the Neurometer® for the screening and diagnosis of diabetic neuropathy - existing evidence
This test can be used to detect neuropathy in the earliest and asymptomatic stages. It is clearly proven and unanimously accepted that glycated hemoglobin (HbA1c) value is positively associated with specific diabetic microvascular complications, including neuropathy [17]. However, it has been suggested that impaired glucose tolerance (IGT) may also cause neuropathy [18]. One study, conducted on 45 subjects with IGT and 46 healthy volunteers, assessed the presence of neuropathy in people with IGF, by measuring the CPT using the Neurometer® as evaluating method for the sensory nerve function [19]. The results showed that subjects with IGT had a higher frequency of both hyperesthesia and hypoesthesia as detected by current perception threshold testing at 5 Hz as well as increased heat detection thresholds. Thus, this study has demonstrated that subclinical small-fiber neuropathy and autonomic neuropathy are commonly present in IGT subjects, and measurement of CPT using the Neurometer® demonstrated to be able of detecting neuropathy in the early stage.
Kempler and co, conducted a study in 1995 in which CPT and tests of autonomic function were applied on 22 patients with non-insulin dependent diabetes mellitus (NIDDM), 6 patients with newly diagnosed-NIDDM, and 12 healthy subjects. The authors concluded that CPT permits the diagnosis of sensory dysfunction early in the course of diabetes and in NIDDM [20].
The results of a study conducted in 116 patients with type 2 diabetes mellitus and 38 healthy controls [11], who were assessed for peripheral neuropathy by measurement of CPT using the Neurometer®, showed that the CPT at 2000 Hz was significantly higher in diabetic patients than in controls, and also showed a significant negative correlation with motor and sensory nerve conduction velocities. Significantly higher CPT values were obtained in patients with proliferative diabetic retinopathy and macroalbuminuria. So, the authors concluded that CPT is useful in detecting abnormalities of myelinated as opposed to unmyelinated nerve fibers in patients with type 2 diabetes.
We also conducted a study on 60 patients with type 2 diabetes, who were divided in three groups based on their diabetes durations (<5 years, 5–10 years, >10 years), and the patients were assessed for the presence of DPN using two different methods: Semmes - Weinstein Monofilament testing (SWMT) and rapid-current perception threshold measurement using the Neurometer® [15].
Our results also showed a higher incidence of sensory neuropathy in patients with more than 10 years duration of diabetes when compared with those with less than 5 years of diabetes duration. Neurometer® detected sensory neuropathy in a higher percent of subjects (250Hz: 30%, 5Hz: 46.6%) compared with monofilament testing method (21.7%), statistically significant only for 5Hz frequency (p=0.01). Also, frequency of 5Hz proved to be the most sensitive in detecting patients with hyper and hypoesthesia. Giving the fact that a significant incidence of sensory neuropathy was found in diabetics with less than 5 years duration of diabetes it is important to recommend screening for sensory neuropathy in patients as soon as diabetes is diagnosed.
The findings of a similar study including 60 type 2 diabetic patients, conducted by Nather and co [21], suggested that sensory testing using both Neurometer® and Pin-Prick tests detected a higher incidence of sensory neuropathy compared with Semmes-Weinstein monofilament testing.
The results of our study are also consistent to those found by Cheng et al. [22] in a study involving 558 type 2 diabetic people, from which 59 had sensory neuropathy after SWMT assessment, 45 were positive on Vibration Perception Threshold and 189 were diagnosed with sensory neuropathy according to R-CPT measurements by the Neurometer®. The conclusion was that the Neurometer® is capable of detecting peripheral sensory neuropathy in significantly more patients as compared to SWMT and Vibration Perception Threshold.
A very recent paper published in 2014 [23] involving 241 Korean subjects with type 2 diabetes, evaluated the usefulness of CPT for diagnosing distal polyneuropathy and cardiovascular autonomic neuropathy in diabetic patients. The conclusions were that even though the subjects with DPN had significantly higher CPT at all of the frequencies than the subjects without DPN, and the CPT was significantly associated with neuropathic symptoms or signs corresponding to the nerve fiber stimulated, it provides little additional information compared with conventional evaluations.
Conclusions
Assessment of current perception threshold is a useful evaluation method of patients with peripheral nervous system diseases resulting in altered cutaneous sensation. By applying this method it is possible to detect both clinical and subclinical large and small fiber neuropathy. The Neurometer® CPT can independently evaluate those three sub-types of nerve fibers (Aβ, Aδ and C fibers) by using different frequencies of an alternating current, providing improved detection sensitivity over other methods testing. However, conflicting information and methodological problems exist regarding the usefulness of the Neurometer® CPT for the evaluation of specific conditions such as diabetic neuropathy, although an important body of literature describes many of the benefit features attributed to this instrument [20]. Therefore, in the light of these data, future research is needed in order to establish the real diagnostic value of Neurometer® CPT data.
Figure 1.
The Neurometer® device (Neurotron Inc., Baltimore, Maryland, USA).
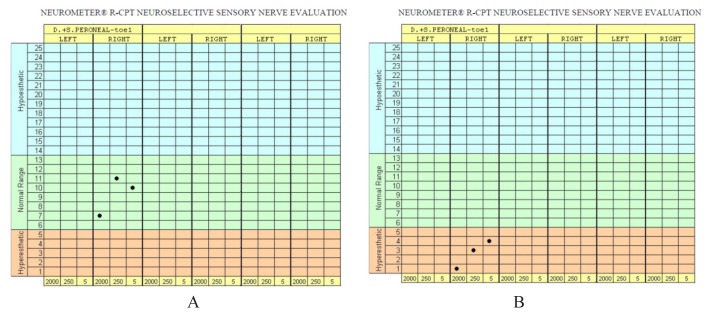
Figure 2.
R-CPT nerve evaluation in a patient without DPN (A) and a patient with sensory DPN (B).
Acknowledgement
We wish to thank Unirea Medical Center from Cluj-Napoca (part of “Regina Maria” The Private Healthcare Network) for lending us free of charge the Neurometer® device.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 6th edn. Brussels Belgium: International Diabetes Federation; 2013. Available from: http://www.idf.org/diabetesatlas. [Google Scholar]
- 2.World Health Organization. GLOBAL STATUS REPORT on noncommunicable diseases. 2014. Available from: http://www.who.int/nmh/publications/ncd-status-report-2014/en/
- 3.Boden-Albala B, Cammack S, Chong J, Wang C, Wright C, Rundek T, et al. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS) Diabetes Care. 2008;31(6):1132–1137. doi: 10.2337/dc07-0797. [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJM. Management of diabetic peripheral neuropathy. Clin Diabetes. 2005;23(1):9–15. [Google Scholar]
- 5.Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ, 3rd, O’Brien PC. Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care. 1999;22(9):1479–1486. doi: 10.2337/diacare.22.9.1479. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 7.Britton JP, Barrie WW. Amputation in the diabetic: ten years experience in a district general hospital. Ann R Coll Surg Engl. 1987;69(3):127–129. [PMC free article] [PubMed] [Google Scholar]
- 8.Weck M, Slesaczeck T, Paetzold H, Muench D, Nanning T, von Gagern G, et al. Structured health care for subjects with diabetic foot ulcers results in a reduction of major amputation rates. Cardiovasc Diabetol. 2013;12:45. doi: 10.1186/1475-2840-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott LV, Tesfaye S. Measurement of somatic neuropathy for clinical practice and clinical trials. Curr Diab Rep. 2001;1(3):208–215. doi: 10.1007/s11892-001-0036-4. [DOI] [PubMed] [Google Scholar]
- 10.Inceu G, Demea H, Veresiu IA. Corneal Confocal Microscopy – A Novel, Noninvasive Method to Assess Diabetic Peripheral Neuropathy. Rom J Diabetes Nutr Metab Dis. 2014;21(4):319–26. [Google Scholar]
- 11.Matsutomo R, Takebayashi K, Aso Y. Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. J Int Med Res. 2005;33(4):442–453. doi: 10.1177/147323000503300410. [DOI] [PubMed] [Google Scholar]
- 12.Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–2154. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 13.Vinik A. Diabetic Neuropathy: A Small-Fiber Disease. 2015. Available from: http://www.medscape.org/viewarticle/418568.
- 14.Donaghue VM, Giurini JM, Rosenblum BI, Weissman PN, Veves A. Variability in function measurement of three sensory foot nerves in neuropathic diabetic patients. Diabetes Res Clin Pract. 1995;29:37–42. doi: 10.1016/0168-8227(95)01107-o. [DOI] [PubMed] [Google Scholar]
- 15.Inceu G, Veresiu IA. Assessment of diabetic peripheral neuropathy: new versus conventional methods. IFMBE Proceedings; International Conference on Advancements of Medicine and Health Care through Technology; 5th – 7th June 2014; Cluj-Napoca, Romania. Springer International Publishing; 2014. pp. 177–180. [Google Scholar]
- 16.Nather A, Keng Lin W, Aziz Z, Hi Ong C, Mc Feng B, Lin C. Assessment of sensory neuropathy in patients with diabetic foot problems. Diabet Foot Ankle. 2011;2:6367. doi: 10.3402/dfa.v2i0.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross R. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(18):1925–1927. [PubMed] [Google Scholar]
- 18.Singleton JR, Smith AG. Neuropathy associated with prediabetes: what is new in 2007? Curr Diab Rep. 2007;7(6):420–424. doi: 10.1007/s11892-007-0070-y. [DOI] [PubMed] [Google Scholar]
- 19.Putz Z, Tabak AG, Toth N, Istenes I, Nemeth N, Gandhi R, et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care. 2009;32(1):181–183. doi: 10.2337/dc08-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Technology review: the Neurometer Current Perception Threshold (CPT) AAEM Equipment and Computer Committee. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1999;22(4):523–531. [PubMed] [Google Scholar]
- 21.Nather A, Neo SH, Chionh SB, Liew SCF, Sim EY, Chew JL. Assessment of sensory neuropathy in diabetic patients without diabetic foot problems. J Diabetes Complications. 2008;22(2):126–131. doi: 10.1016/j.jdiacomp.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Cheng WY, Jiang YD, Chuang LM, Huang CN, Heng LT, Wu HP, et al. Quantitative sensory testing and risk factors of diabetic sensory neuropathy. J Neurol. 1999;246(5):394–398. doi: 10.1007/s004150050370. [DOI] [PubMed] [Google Scholar]
- 23.Koo BK, Ohn JH, Kwak SH, Moon MK. Assessment of diabetic polyneuropathy and autonomic neuropathy using current perception threshold in korean patients with diabetes mellitus. Diabetes Metab J. 2014:285–293. doi: 10.4093/dmj.2014.38.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]