Abstract
Background and aims
An accurate color reproduction represents the final validation level of an esthetic anterior or posterior restoration. The aim of this study was to evaluate the color of permanent maxillary incisors, canines and molars, using a clinical spectrophotometer.
Methods
The Vita Easyshade Advance 4.0® intraoral spectrophotometer was used by one clinician to determine the color of 369 permanent maxillary incisors, canines and molars. The best matches to Vitapan Classical® and 3D-Master® shade guides were recorded. A one-way analysis of variance and Kruskal-Wallis test were used to compare L*, a*, b*, c* and h* color coordinates among the 3 types of teeth. Differences between the mean values of all color coordinates were evaluated by use of Bonferroni corrections. Color difference (ΔE*) between incisors, canines and molars was calculated from ΔL*, Δa* and Δb* data and the results were compared to ΔE*=3.3 acceptability threshold.
Results
Except for Δa* and Δh* between canines and molars, statistically significant differences among the mean differences of all color coordinates were found when the 3 types of teeth were compared by pairs. The most frequently measured shades were A1 (48.4%), respectively 1M1 (31.5%) for incisors, B3 (36.6%), respectively 2M3 (39.8%) for canines and B3 (44.7%), respectively 2M3 (52%) for molars. Incisors had the highest lightness values, followed by canines and molars. Molars were the most chromatic with the highest a* and b* values.
Conclusions
Despite the limitations of this study, color differences among incisors, canines and molars were found to be statistically significant, above the clinical acceptability threshold established. In conclusion, successful esthetic restorations of permanent teeth of the same patient need an individual color assessment and reproduction of every type of tooth.
Keywords: color, spectrophotometer, permanent natural teeth
Background and aims
The complex scheme of shade selection represents the first step in attempting to achieve biomimetic anterior or posterior dental restorations. An accurate choice and a proper communication of tooth color offer the possibility to clinicians and dental technicians to mimic natural teeth and satisfy the increased esthetic expectations of patients. Visual color assessment, the oldest and most frequently used method of shade selection [1–3], accessible to any dentist, was regarded by several authors as subjective [4,5], prone to error [6], varying from one day to another and among individuals [2,4,7–9]. Moreover, the available dental color guides were not always adequately representing natural tooth color, being deficient in hue, value and chroma ranges [10–13]. It has been stated that there are no two identical commercially available shade guides [14,15] and that they vary between batches [16]. All these factors promoted the development of a new shade guide, Vita 3D-Master®, found to be arranged according to L*, C* and h* coordinates in groups of lightness, chroma and hue [17]. This shade guide has 5 groups of lightness, from 1 (the lightest) to 5 (the darkest). In each group of lightness there are 3 chroma levels, from 1 (the least chromatic) to 3 (the most chromatic) and in lightness group 2, 3 and 4 there are 3 possible hues L (more yellow), M (more orange) and R (more red). This new 3D Master system proved to be more accurate and reproducible, with a higher number of shade tabs extended to a wider color range and more uniformly spaced compared to Vitapan Classical® shade guide [18–20]. In 2007, in order to improve and simplify visual color assessment, a shade selection option was developed to an intraoral camera. This concept was found to be a reliable assistance to visual shade matching, by eliminating the colors of the background or of the surrounding colored objects and by creating standardized lighting conditions [21].
The high esthetic demands of nowadays patients corroborated with the variability and subjectivity of human eye has led to the development of color measurement devices that allow an objective choice of shade values. These devices are represented by spectrophotometers, tristimulus colorimeters, spectroradiometers and digital cameras. Spectrophotometers measure the full spectrum of reflected or transmitted light, converted afterwards into tristimulus data [22]. In several studies, spectrophotometers have been used as a reference [21,23,24] due to their sensitivity, accuracy and reproducibility [22,25]. Other investigations suggested that spectrophotometric shade selection and analysis was more accurate and reproducible compared to visual color assessment [14,26]. Colorimeters, as well as spectrophotometers, can provide readings from Commission Internationale de l’Eclairage (CIE) L*, a*, b* color space, where L* represents lightness (the amount of white and black within a color), a* is a measure of redness (positive a*) or greenness (negative a*) and b* represents the position on the blue (negative b*)-yellow (positive b*) axis [27,28]. This color notation system is widely used in dental research for both in vivo and in vitro color measurements [29]. However, color is described by CIE in terms of hue (h*), which is physically associated with the dominant wavelength of a color, value (L*), which indicates the lightness of a color measured on a scale from pure black (L*=0) to pure white (L*=100) and chroma (C*) which represents the amount or the intensity of hue of a given color [30]. Spectrophotometers have spectral data for the shade tabs of several shade guides incorporated in their database. Therefore, for each measurement they also display the best match of the shade guide chosen.
In color research, the Euclidean distance between two color points (ΔE) remains one of the most important parameter needed in the determination of color differences [31]. Delta E* was used in dentistry to establish clinical perceptibility [32–34] thresholds and clinically acceptability [34–36] thresholds after visual determination or instrumental measurement of tooth color coordinates. Tooth color and the optical properties of enamel and dentin were described in previous in vitro studies [37–38] as well as in vivo studies centered around the maxillary central incisors [39,40]. Mayoral et al. analyzed the relationship between the color coordinates of pure enamel and enamel-dentine complex of upper incisors and different age groups [40]. Even though the natural tooth color range has already been described in literature [8,13,41], to our knowledge, there have been no complete comparative reports regarding the color of permanent incisors, canines and molars.
The objectives of this study were to determine: (1) L*, a*, b*, C* and h* color parameters of permanent incisors, canines and molars; (2) the most frequently chosen color relying on Vitapan Classical and Vita 3D-Master shade guides; (3) the mean differences of all color parameters among the three groups of teeth; and (4) color differences (ΔE*) among incisors, canines and molars. The null hypothesis against which we performed our study assumed that differences in spectrophotometric color coordinates between maxillary incisors, canines and molars are not statistically significant.
Methods
1. Participants and color recordings
One hundred and twenty-three volunteers (45 men and 78 women) with ages between 21 and 29 years, all of them students at the local Dental Faculty were recruited in this study. All participating subjects received written information and signed an informed consent form which was approved by the Ethical Board of the Iuliu Hatieganu University of Cluj-Napoca (no. 249/06.05.2015). The teeth selected for color measurements were the maxillary right central incisor, canine and first molar. Only natural, unrestored teeth, without pathological discolorations, were included. Teeth with white spots in the middle third of the facial surface or with bleaching treatments in their history were excluded. Before measurements, the facial surface of each tooth was cleaned using polishing brushes and paste. Afterwards, every participant thoroughly rinsed with water.
Color recordings were performed by one experienced clinician using a Vita Easyshade spectrophotometer (Vita Easyshade Advance 4.0®; Vita Zahnfabrik) according to the manufacturer’s instructions. This digital shade matching device which uses D-65 illumination for color selection had previously been subjected to a validation test, in order to evaluate its reproducibility and inter-examiner reliability. Each tooth was measured once, so that a total of 369 teeth were measured in the end. Before measurements in every volunteer, an infection control shield was placed on the probe tip. To prevent the probe tip from slipping on teeth surfaces and to ensure that measurements were obtained from the same position, respectively from the middle third of the labial tooth surface, a set of special custom built acrylic jigs was used, one for each type of tooth. The 3 jigs were manufactured from transparent acrylic resin (Premacryl Plus®; Spofa Dental), on a class IV gypsum maxillary cast in a way that suited any type of incisor, canine or molar. The jigs were designed with a notch of 5 mm on their facial surface. The notch allowed the probe tip of the spectrophotometer to be fixed and in contact with the middle third of the facial enamel surface of the evaluated tooth. In order to mimic a standardized clinical situation, all measurements were performed under artificial light conditions, in a dental office from the Department of Odontology and Endodontics, Iuliu Hatieganu University of Cluj-Napoca, Romania. The intensity of the environmental light was measured before experimentation and was between 1178 and 1400 lux. After calibration on its standard white reflection port, the “Averaged measurement” operation mode was used to determine the basic color of the 3 types of teeth. Using the averaged measurement operation mode, up to 3 measurements were performed on every tooth. Between measurements, the probe tip had to be completely removed from the tooth surface and repositioned. The following measurements were recorded:
L*, a*, b*, C* and h* values for all teeth as well as the best matches to Vitapan Classical® and Vita 3D-Master® (Vita Zahnfabrik) shade guides.
-
ΔL*, Δa*,Δ b*, ΔC* and Δh* values between the groups of teeth were calculated. Color differences, measured as ΔE* between incisors, canines and molars were also calculated using the following formula:
2. Statistical methods
Color codes of incisors, canines and molars based on the two shade guides (Vitapan Classical and Vita 3D Master), as assessed using an intra-oral spectrophotometer, have been described by computing their frequencies and 95% confidence intervals (95% CI).
To describe the variability of measured color parameters L*, a*, b*, C* and h*, central tendency descriptors (mean, median) along with the 95% confidence intervals for means and standard deviations (SD) of these variables have been computed. Confidence intervals for mean L*, a*, b*, C* and h* for incisors, canines and molars have also been bootstrapped based on 1000 sample replications.
Normality of the measured color parameters has been investigated using Kolmogorov-Smirnov tests and Quantile-Quantile Plots.
One-way analyses of variance along with a Kruskal-Wallis test have been performed to compare L*, a*, b*, C* and h* among the different tooth types (incisors, canines and molars). Post-hoc multiple comparisons using Bonferroni corrections have been performed for pairwise comparisons of these measured color parameters between the three tooth types. Plots of means and their 95% CI have been used to graphically compare the three tooth types.
The level of statistical significance has been set at α=0.05 for uncorrected comparisons and at α=0.017 for Bonferroni corrected multiple comparisons.
Data has been collected using Microsoft Excel 2010 and analyzed using R 3.0.2 - a language and environment for statistical computing.
Results
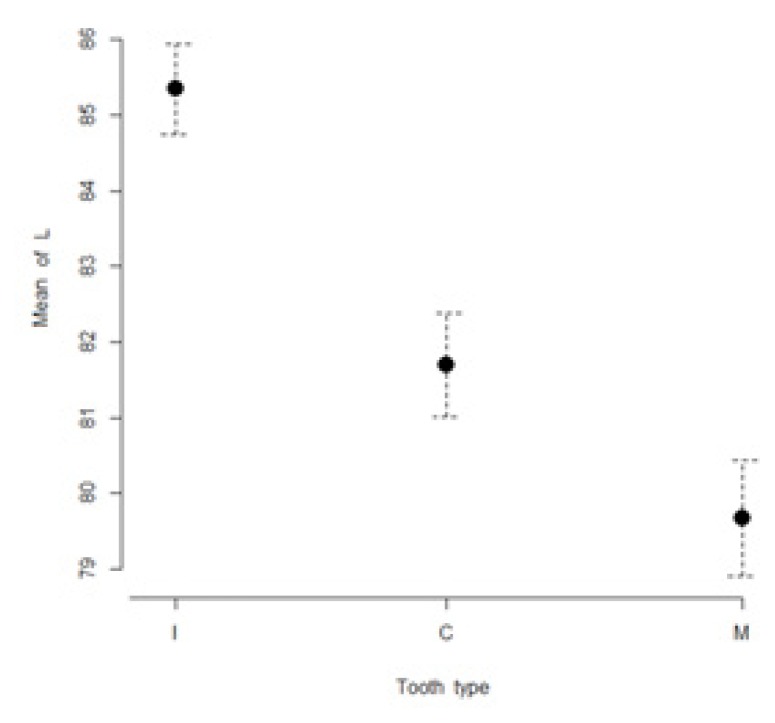
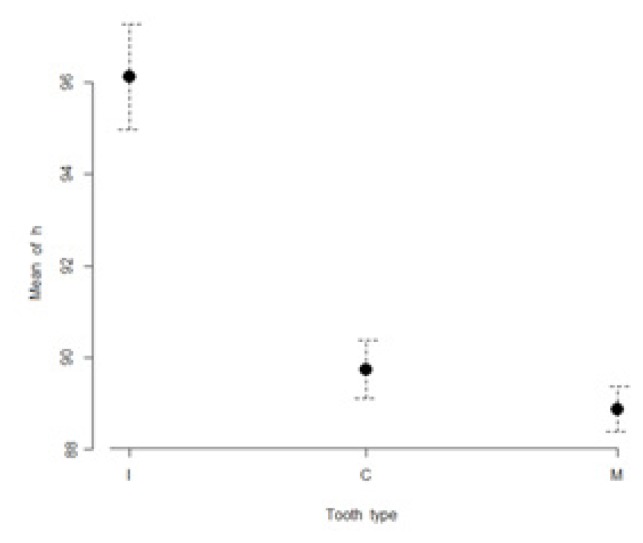
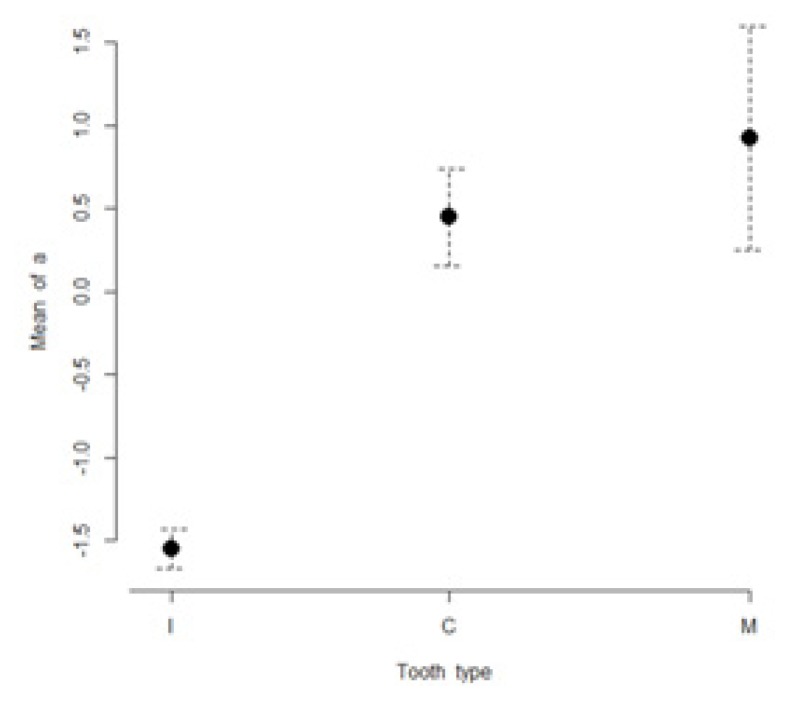
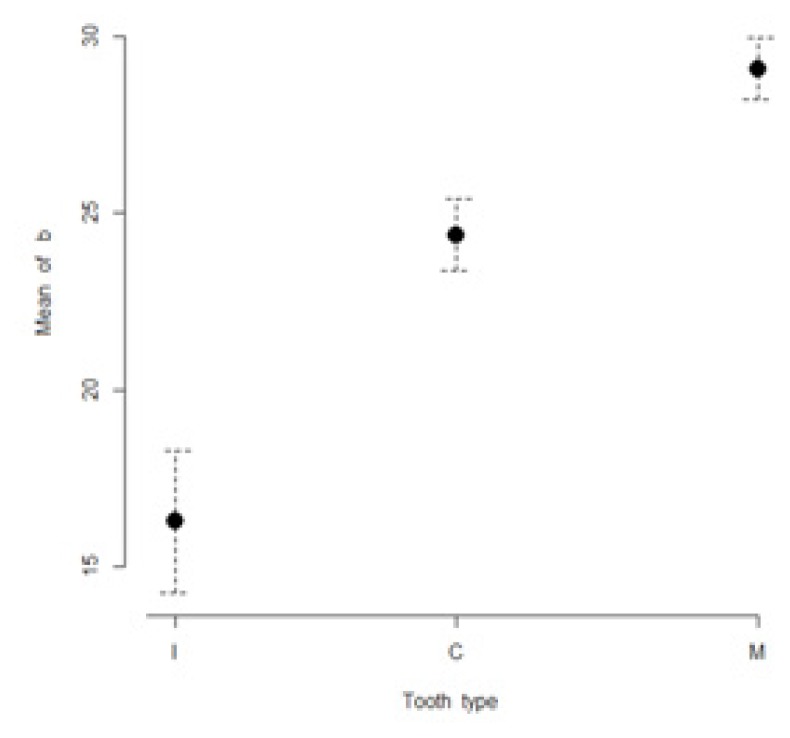
The mean values of L*, a*, b*, C* and h* color coordinates of the evaluated teeth are presented in Table I. The highest lightness values were found for incisors (85.3 ± 3.3), while the lowest lightness values were found for molars (79.7 ± 4.3). The most chromatic values (highest a* and b* values) were found in molars (a* = 0.9 ± 3.8; b*= 29.1 ± 4.9). The maxillary incisors were found to have the lowest a* and b* values (a* = −1.5 ± 0.7; b*= 16.3 ± 11.4). Figures 1 to 5 present visual comparisons between incisors, canines, and molars regarding the means and their 95% confidence intervals of L*, a*, b*, C* and h* color coordinates measured for the three tooth types in the studied sample of volunteers.
Table I.
Mean values, standard deviations and confidence intervals for means of color coordinates measured in the evaluated teeth.
| TOOTH TYPE | COLOR COORDINATES | ||||
|---|---|---|---|---|---|
| L* ± SD | a* ± SD | b* ± SD | C* ± SD | h* ± SD | |
| INCISORS 95% CI | 85.3±3.3 (84.7–85.9) | −1.5±0.7 (−1.6 – −1.4) | 16.3±11.4 (14.9–18.6) | 15.4±3.4 (14.8–16.0) | 96.1±6.4 (94.9–97.1) |
| CANINES 95% CI | 81.7±3.8 (81.0–82.3) | 0.4±1.6 (0.2–0.7) | 24.4±5.6 (23.4–25.4) | 24.4±5.6 (23.4–25.4) | 89.7±3.5 (89.2–90.4) |
| MOLARS 95% CI | 79.7±4.3 (78.8–80.4) | 0.9±3.8 (0.4–1.7) | 29.1±4.9 (28.2–30.0) | 29.0±4.9 (28.1–29.9) | 88.9±2.7 (88.4–89.4) |
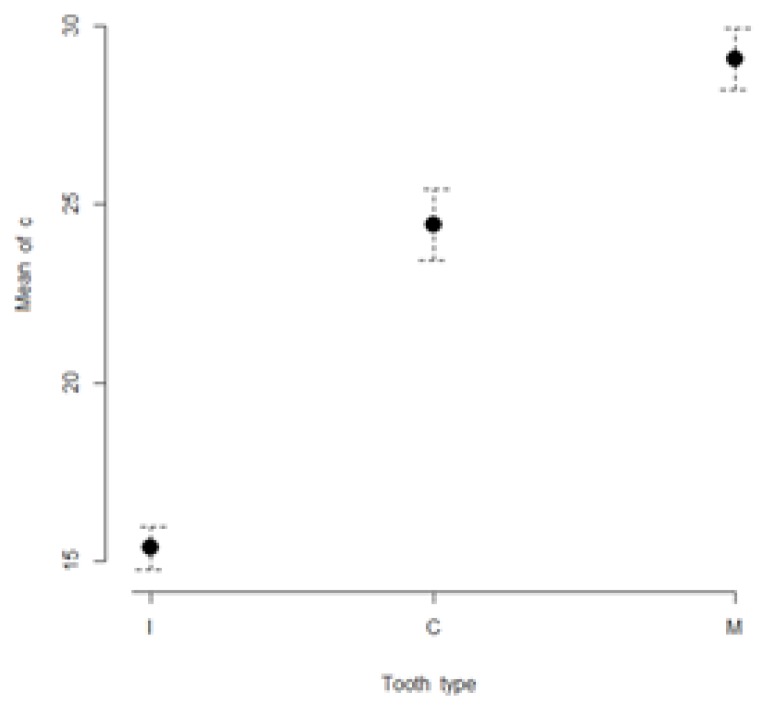
Figure 1.
Visual comparison of means and their 95% confidence intervals for L* color coordinates of incisors (I), canines (C), and molars (M) measured in the studied sample.
Figure 5.
Visual comparison of means and their 95% confidence intervals for h* color coordinates of incisors (I), canines (C), and molars (M) measured in the studied sample.
For the Vitapan Classical® shade guide, the most frequently chosen shades were in order: (1) A1 (48.4%); (2) B2 (25.8%) and (3) B1 (21.0%) for incisors; (1) B3 (36.6%); (2) A2 (16.3%) and (3) A3.5 (14.6%) for canines; (1) B3 (44.7%) ; (2) A3.5 (24.4%) and (3) A3 (7.3%) for molars (Table II).
Table II.
Vitapan Classical® shade tabs recordings (%) that best matched the examined teeth.
| VITAPAN CLASSICAL® | |||
|---|---|---|---|
| Shade tab | Incisors (%) | Canines (%) | Molars (%) |
| A1 | 48.4 | 12.2 | - |
| A2 | 4.0 | 16.3 | 4.9 |
| A3 | 0.8 | 6.5 | 7.3 |
| A3.5 | - | 14.6 | 24.4 |
| A4 | - | - | 5.7 |
| B1 | 21.0 | 2.4 | - |
| B2 | 25.8 | 11.4 | 4.9 |
| B3 | - | 36.6 | 44.7 |
| B4 | - | - | 6.5 |
| C3 | - | - | 0.8 |
| D3 | - | - | 0.8 |
The most frequently chosen shades relying on the Vita 3D-Master® shade guide were in order: (1) 1M1 (31.5%); (2) 1M2 (24.2%) and (3) 2M1 (15.3%) for incisors; (1) 2M3 (39.8%); (2) 2M2 (17.9%) and (3) 1M2 (9.8%) for canines; (1) 2M3 (52.0%); (2) 3M3 (15.4%) and (3) 2M2 (13.8%) for molars (Table III).
Table III.
Percentage of Vita3D-Master shade tabs that best matched measured incisors, canines and molars.
| VITA 3D-MASTER® | |||
|---|---|---|---|
| Shade tab | Incisors (%) | Canines (%) | Molars (%) |
| 0M2 | 0.8 | - | - |
| 0M3 | 4.0 | - | - |
| 1M1 | 31.5 | 4.9 | - |
| 1M2 | 24.2 | 9.8 | - |
| 2L1.5 | 8.1 | 0.8 | 0.8 |
| 2L2.5 | 1.6 | 0.8 | 0.8 |
| 2L3 | - | 1.6 | - |
| 2M1 | 15.3 | 4.1 | - |
| 2M1.5 | 0.8 | - | - |
| 2M2 | 13.7 | 17.9 | 13.8 |
| 2M3 | - | 39.8 | 52.0 |
| 2R1.5 | - | 0.8 | - |
| 2R2 | - | 0.8 | - |
| 2R2.5 | - | 7.3 | - |
| 3M1 | - | - | 1.6 |
| 3M2 | - | 0.8 | 3.3 |
| 3M3 | - | 8.1 | 15.4 |
| 3R2.5 | - | 1.6 | 1.6 |
| 4M2 | - | 0.8 | 1.6 |
| 4M3 | - | - | 6.5 |
| 4R1.5 | - | - | 1.6 |
| 5M3 | - | - | 0.8 |
In this study only 5 participants belonged to lightness group 0 and only 1 participant to lightness group 5. For incisors, lightness group 1 was the most frequently found (55.7%), followed by lightness group 2 (39.5%). Regarding chroma, the most frequent groups found in incisors were group 1 (46.8%) and group 2 (37.9%). Ninety point three percent of incisors belonged to the M hue group. In the case of canines, lightness group 2 was the most frequently found (73.9%), while the most frequent chroma groups were found to be the second (30.1%) and the third (49.5%) ones. Eighty point two percent of the measured canines belonged to the M hue group. Concerning molars, lightness group 2 was the most frequently recorded (67.4%), followed by lightness group 3 (21.9%). Chroma groups 2 (18.7%) and 3 (74.7%) were found to be the most frequently measured ones. Similar to incisors and canines, the most frequently measured hue group for molars has been the M group, with a frequency of 95%.
The mean values of ΔL*, Δa*, Δ b*, ΔC* and Δh* among the groups of teeth are presented in Table IV. The highest differences for all color coordinates were found among incisors and molars. Differences decreased slightly between the incisors-canines groups and were the lowest between canines and molars. Highly significant mean differences were found for all L*, a*, b*, C* and h* color coordinates between the 3 groups of teeth (p<0.001), except for the canines-molars groups for which the differences in a* and h* coordinates were not statistically significant (p>0.05).
Table IV.
Mean differences between the 3 groups of teeth for all color coordinates.
| ΔL* | Δa* | Δb* | ΔC* | Δh° | |
|---|---|---|---|---|---|
| I - C 95% CI | 3.6 (2.7–4.6) | −2.0 (−2.3 – −1.7) | −8.1 (−9.9 – −5.5) | −9.0 (−10.1 – −7.9) | 6.4 (5.0–7.5) |
| I - M 95% CI | 5.7 (4.6–6.6) | −2.5 (−3.2 – −1.9) | −12.8 (−14.5 – −10.4) | −13.7 (−14.7 – −12.6) | 7.2 (5.8–8.4) |
| C - M 95% CI | 2.0 (1.0–3.1) | −0.5† (−1.3 – −0.1) | −4.7 (−6.0 – −3.4) | −4.6 (−6.0 – −3.3) | 0.9† (0.1–1.7) |
p >0.05; I: incisors; C: canines; M: molars; CI: confidence interval.
Regarding color variations, the greatest color difference was found again between incisors and molars (ΔE=14.24). The color differences between the other two groups of teeth were: incisors-canines ΔE=9.13; canines-molars ΔE=5.15.
Discussion
A clinically validated esthetic restoration requires an individual approach in color matching and reproduction of the permanent teeth to be restored. Several studies presented data regarding the color range of permanent natural teeth [13] as well as of primary teeth [41]. Nevertheless, there are few published data regarding all five color coordinates of anterior and posterior permanent maxillary teeth.
The null hypothesis that no significant color difference can be detected between spectrophotometric color coordinates of maxillary incisors, canines and molars has been rejected in our study. The higher L* values found for incisors compared to canines and molars concurred with the results found in other studies [13]. Molars were darker than canines, which, in turn, were darker than incisors. Although the Vita Easyshade® spectrophotometer has its own light source, the reduced lightness of the molars and canines, in comparison with the incisors may be correlated with the reduced incidental light in the posterior area of the dental arch. Concerning a* and b* color coordinates, molars seem to be more chromatic than canines, with incisors having the lowest a* and b* values. Vitapan Classical® and Vita3D-Master® shade guides confirmed these results. A total of 48.4% of the incisors corresponded to A1 shade tab and more than 50% to lightness group 1 from the 3D Master shade guide, while B3 was the best match for 44.7% of molars and 67.4% belonged to the second group of lightness from the Vita 3D Master® shade guide. Among Vitapan Classical® shade tabs the widest range of color distribution was recorded for molars, where 9 different shade tabs constituted the best match. In the case of incisors only 5 shade tabs were found to be the best match (Table II). On the other hand, for the Vita3D-Master® shade guide the widest ranges of shade tabs found as best match were recorded in order: for canines (15 shade tabs), molars (12 shade tabs) and incisors (9 shade tabs) (Table II). This finding confirmed the results of other clinical investigations [41,42] and demonstrated once again the fact that Vitapan Classical® shade guide does not properly match the color of permanent teeth because of the small number of shade tabs, not adequately distributed in the color range of natural teeth [10,11].
The color differences detected by the human eye are limited to a certain degree. Acceptability and perceptibility thresholds of color differences have been a subject of debate in dental literature. Ruyter et al. established the clinical acceptability threshold to be 3.3 [34,35]. This means that color differences with ΔE* value less than approximately 3.3 are clinically acceptable. In other words, 50% of the observers will accept color differences between a restoration and the adjacent tooth when ΔE* value is lower than 3.3 and reject the restoration when ΔE*>3.4 [36]. Several authors determined that the clinical perceptibility thresholds ranged from ΔE*=1.0 to 3.7 [34,43]. In the present study, all ΔE* values between the three groups of teeth were higher than ΔE*=3.7. Based on the above thresholds, this qualifies as clinically perceptible and therefore inacceptable color differences, among incisors, canines and molars. This fact was also confirmed by post-hoc multiple comparisons using Bonferroni corrections between the color parameters of these 3 types of teeth. Only for a* (Δa* = −0.5) and h* (Δh* = 0.9) color coordinates between canines and molars, no significant differences could be demonstrated (Table IV). Perez et al. examined acceptability thresholds for the L*, C* and h* color coordinates and reported them to be ΔL* = 2.92, ΔC* = 2.52 and Δh* = 1.90 [44]. With respect to these thresholds and according to our results only the canine/molar pair exhibited ΔL* and Δh* differences below these thresholds (Table IV). Taking into account all these results it seems that the smallest differences for all color coordinates can be found between canines and molars.
The Vita Easyshade® spectrophotometer was specially designed for clinical shade selection of natural teeth and prosthetic restorations. The clinical accuracy of the Vita Easyshade® spectrophotometer has already been validated in several investigations. The CIE L*,C* and h* color coordinates and the Vita 3D-Master® corresponding shades provided by this objective device were considered to be very similar to those chosen by experienced clinicians [45]. However, this device encounters problems when measuring curved surfaces and translucent materials, such as dental structures. Due to the curved facial surfaces of canines and molars the probe tip of the instrument cannot be in direct contact with these surfaces, which means that edge-loss errors are present, leading to reduced L* values recorded for these two types of teeth. Therefore, despite being a standard for clinical color selection the spectrophotometer used in our study may have induced such a measurement bias, constituting hence an acknowledged limitation of our study. In addition, positioning errors of the probe tip cannot be excluded despite the use of a measuring jig, even though a higher reproducibility of measurements has been confirmed when an adjustment aid was used [46].
Conclusions
Within the limitations of this study, significant color variations among L*, a*, b*, C* and h* color coordinates of permanent incisors, canines and molars were found by spectrophotometric measurements. The most frequently selected shades were A1, respectively 1M1 for incisors and B3, respectively 2M3 for canines and molars. Further investigations are needed to transpose these reported instrumentally measured color differences into clinical observable color variations, detectable only by the human eye.
Figure 2.
Visual comparison of means and their 95% confidence intervals for a* color coordinates of incisors (I), canines (C), and molars (M) measured in the studied sample.
Figure 3.
Visual comparison of means and their 95% confidence intervals for b* color coordinates of incisors (I), canines (C), and molars (M) measured in the studied sample.
Figure 4.
Visual comparison of means and their 95% confidence intervals for C* color coordinates of incisors (I), canines (C), and molars (M) measured in the studied sample.
Acknowledgement
This paper was published under the frame of European Social Found, Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/S/138776.
References
- 1.van der Burgt TP, ten Bosch JJ, Borsboom PC, Kortsmit WJ. A comparison of new and conventional methods for quantification of tooth color. J Prosthet Dent. 1990;63:155–162. doi: 10.1016/0022-3913(90)90099-x. [DOI] [PubMed] [Google Scholar]
- 2.Hammad IA. Intrarater repeatability of shade selection with two shade guides. J Prosthet Dent. 2003;89:50–53. doi: 10.1067/mpr.2003.60. [DOI] [PubMed] [Google Scholar]
- 3.Hassel AJ, Grossmann AC, Schmitter M, Balke Z, Buzello AM. Interexaminer reliability in clinical measurement of L*C*h* values of anterior teeth using a spectrophotometer. Int J Prosthodont. 2007;20:79–84. [PubMed] [Google Scholar]
- 4.Culpepper WD. A comparative study of shade-matching procedures. J Prosthet Dent. 1970;24:166–173. doi: 10.1016/0022-3913(70)90140-x. [DOI] [PubMed] [Google Scholar]
- 5.Sproull RC. Color matching in dentistry. Part I. The three-dimensional nature of color. J Prosthet Dent. 1973;29:416–424. doi: 10.1016/s0022-3913(73)80019-8. [DOI] [PubMed] [Google Scholar]
- 6.Preston JD. Current status of shade selection and color matching. Quintessence Int. 1985;16:47–58. [PubMed] [Google Scholar]
- 7.van der Burgt TP, ten Bosch JJ, Borsboom PC, Plasschaert AJ. A new method for matching tooth colors with color standards. J Dent Res. 1985;64:837–841. doi: 10.1177/00220345850640051101. [DOI] [PubMed] [Google Scholar]
- 8.Russell MD, Gulfraz M, Moss BW. In vivo measurements of colour changes in natural teeth. J Oral Rehabil. 2000;27:786–792. doi: 10.1046/j.1365-2842.2000.00610.x. [DOI] [PubMed] [Google Scholar]
- 9.Yap AU, Sim CP, Loh WL, Teo JH. Human-eye versus computerized color matching. Oper Dent. 1999;24:358–363. [PubMed] [Google Scholar]
- 10.Paravina RD. Evaluation of a newly developed visual shade-matching apparatus. Int J Prosthodont. 2002;15:528–534. [PubMed] [Google Scholar]
- 11.Li Q, Yu H, Wang YN. In vivo spectroradiometric evaluation of colour matching errors among five shade guides. J Oral Rehabil. 2009;36:65–70. doi: 10.1111/j.1365-2842.2008.01894.x. [DOI] [PubMed] [Google Scholar]
- 12.Goodkind RJ, Keenan KM, Schwabacher WB. A comparison of Chromascan and spectrophotometric color measurements of 100 natural teeth. J Prosthet Dent. 1985;53:105–109. doi: 10.1016/0022-3913(85)90077-0. [DOI] [PubMed] [Google Scholar]
- 13.Goodkind RJ, Schwabacher WB. Use of a fiber-optic colorimeter for in vivo color measurements of 2830 anterior teeth. J Prosthet Dent. 1987;58:535–542. doi: 10.1016/0022-3913(87)90380-5. [DOI] [PubMed] [Google Scholar]
- 14.Paul S, Peter A, Pietrobon N, Hämmerle CH. Visual and spectrophotometric shade analysis of human teeth. J Dent Res. 2002;81:578–582. doi: 10.1177/154405910208100815. [DOI] [PubMed] [Google Scholar]
- 15.Miller L. Organizing color in dentistry. J Am Dent Assoc. 1987:26E–40E. doi: 10.14219/jada.archive.1987.0315. [DOI] [PubMed] [Google Scholar]
- 16.Cho BH, Lee YK. A shade guide model based on the color distribution of natural teeth. Color Res Appl. 2007;32:278–283. [Google Scholar]
- 17.Gómez-Polo C, Gómez-Polo M, Celemin Viñuela A, Martínez Vásquez De Parga JA. A clinical study relating CIELCH coordinates to the color dimensions of the 3D-Master System in a Spanish population. J Prosthet Dent. 2015;113:185–190. doi: 10.1016/j.prosdent.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Paravina RD. Performance assessment of dental shade guides. J Dent. 2009;37( Suppl 1):e15–e20. doi: 10.1016/j.jdent.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Paravina RD, Powers JM, Fay RM. Color comparison of two shade guides. Int J Prosthodont. 2002;15:73–78. [PubMed] [Google Scholar]
- 20.Yuan JC, Brewer JD, Monaco EA, Jr, Davis EL. Defining a natural tooth color space based on a 3-dimensional shade system. J Prosthet Dent. 2007;98:110–119. doi: 10.1016/S0022-3913(07)60044-4. [DOI] [PubMed] [Google Scholar]
- 21.Lasserre JF, Pop-Ciutrila IS, Colosi HA. A comparison between a new visual method of colour matching by intraoral camera and conventional visual and spectrometric methods. J Dent. 2011;39( Suppl 3):e29–e36. doi: 10.1016/j.jdent.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Chu SJ, Trushkowsky RD, Paravina RD. Dental color matching instruments and systems. Review of clinical and research aspects. J Dent. 2010;38( Suppl 2):e2–e16. doi: 10.1016/j.jdent.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Seghi RR. Effects of instrument-measuring geometry on colorimetric assessments of dental porcelains. J Dent Res. 1990;69:1180–1183. doi: 10.1177/00220345900690051101. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann KM, Igiel C, Schmidtmann I, Scheller H. Four color-measuring devices compared with a spectrophotometric reference system. J Dent. 2010;38( Suppl 2):e65–e70. doi: 10.1016/j.jdent.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Sarafianou A, Kamposiora P, Papavasiliou G, Goula H. Matching repeatability and interdevice agreement of 2 intraoral spectrophotometers. J Prosthet Dent. 2012;107:178–185. doi: 10.1016/S0022-3913(12)60053-5. [DOI] [PubMed] [Google Scholar]
- 26.Horn DJ, Bulan-Brady J, Hicks ML. Sphere spectrophotometer versus human evaluation of tooth shade. J Endod. 1998;24:786–790. doi: 10.1016/S0099-2399(98)80002-2. [DOI] [PubMed] [Google Scholar]
- 27.Commission Internationale de l’Eclairage. CIE Technical Report: Colorimetry. 3rd ed. Vienna: CIE Central Bureau; 2004. CIE Pub. No. 15. [Google Scholar]
- 28.Johnston WM. Color measurement in dentistry. J Dent. 2009;37( Suppl 1):e2–e6. doi: 10.1016/j.jdent.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Schanda J. Colorimetry: Understanding the CIE System. New York: John Wiley & Sons Inc; 2007. [Google Scholar]
- 30.CIE (Commission Internationale de l’Eclairage) CIE Technical Report 142. Vienna: CIE Central Bureau; 2001. Improvement to industrial color-difference evaluation. [Google Scholar]
- 31.Vichi A, Fazi G, Carrabba M, Corciolani G, Louca C, Ferrari M. Spectrophotometric evaluation of color match of three different porcelain systems for all-ceramic zirconia-based restorations. Am J Dent. 2012;25:191–194. [PubMed] [Google Scholar]
- 32.Kuehni RG, Marcus RT. An experiment in visual scaling of small color differences. Color Res Appl. 1979;4:83–91. [Google Scholar]
- 33.Seghi RR, Hewlett ER, Kim J. Visual and instrumental colorimetric assessments of small color differences on translucent dental porcelain. J Dent Res. 1989;68:1760–1764. doi: 10.1177/00220345890680120801. [DOI] [PubMed] [Google Scholar]
- 34.Johnston WM, Kao EC. Assessment of appearance match by visual observation and clinical colorimetry. J Dent Res. 1989;68:819–822. doi: 10.1177/00220345890680051301. [DOI] [PubMed] [Google Scholar]
- 35.Ruyter IE, Nilner K, Möller B. Color stability of dental composite resin materials for crown and bridge veneers. Dent Mater. 1987;3:246–251. doi: 10.1016/S0109-5641(87)80081-7. [DOI] [PubMed] [Google Scholar]
- 36.Ragain JC, Jr, Johnston WM. Color acceptance of direct dental restorative materials by human observers. Color Res Appl. 2000;25:278–285. [Google Scholar]
- 37.Pecho OE, Ghinea R, Ionescu AM, de Cardona JL, Pravaina RD, del Perez MM. Color and translucency of zirconia ceramics, human dentine and bovine dentine. J Dent. 2012;40( Suppl 2):e34–e40. doi: 10.1016/j.jdent.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Yu B, Ahn JS, Lee YK. Measurement of translucency of tooth enamel and dentin. Acta Odontol Scand. 2009;67:57–64. doi: 10.1080/00016350802577818. [DOI] [PubMed] [Google Scholar]
- 39.Ardu S, Feilzer AJ, Devigus A, Krejci I. Quantitative clinical evaluation of esthetic properties of incisors. Dent Mater. 2008;24:333–340. doi: 10.1016/j.dental.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Mayoral JR, Arocha MA, Dominguez S, Roig M, Ardu S. In vivo spectrophotometric evaluation of pure enamel and enamel-dentine complex in relationship with different age groups. J Dent. 2013;41:1245–1250. doi: 10.1016/j.jdent.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Paravina R, Chen JW. In vivo evaluation of color of primary teeth. Pediatr Dent. 2007;29:383–386. [PubMed] [Google Scholar]
- 42.Schwabacher WB, Goodkind RJ. Three-dimensional color coordinates of natural teeth compared with three shade guides. J Prosthet Dent. 1990;64:425–431. doi: 10.1016/0022-3913(90)90038-e. [DOI] [PubMed] [Google Scholar]
- 43.Seghi RR, Johnston WM, O’Brien WJ. Performance assessment of colorimetric devices on dental porcelains. J Dent Res. 1989;68:1755–1759. doi: 10.1177/00220345890680120701. [DOI] [PubMed] [Google Scholar]
- 44.del Pérez MM, Ghinea R, Herrera LJ, Ionescu AM, Pomares H, Pulgar R, et al. Dental ceramics: a CIEDE2000 acceptability thresholds for lightness, chroma and hue differences. J Dent. 2011;39( Suppl 3):e37–e44. doi: 10.1016/j.jdent.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Browning WD, Chan DC, Blalock JS, Brackett MG. A comparison of human raters and an intra-oral spectrophotometer. Oper Dent. 2009;34:337–343. doi: 10.2341/08-106. [DOI] [PubMed] [Google Scholar]
- 46.Shimada K, Kakehashi Y, Matsumura H, Tanoue N. In vivo quantitative evaluation of tooth color with hand-held colorimeter and custom template. J Prosthet Dent. 2004;91:389–391. doi: 10.1016/j.prosdent.2004.02.006. [DOI] [PubMed] [Google Scholar]