Abstract
Background
We investigated the functional roles of microRNA-125a-5p in regulating human prostate carcinoma.
Methods
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was conducted to evaluate the gene expression levels of miR-125a-5p in eight prostate cancer cell lines and nine biopsy specimens from patients with prostate cancer. miR-125a-5p was genetically knocked down in prostate cancer cell lines, DU145 and VCaP cells by lentiviral transduction. The effects of miR-125a-5p downregulation on prostate cancer cell proliferation and migration were evaluated by MTT assay and transwell assay, respectively. Direct regulation of miR-125a-5p on its downstream targets, NAIF1, and apoptotic gene caspase-3 were evaluated through dual-luciferase reporter assay, qRT-PCR, and Western blot, respectively. NAIF1 was then ectopically overexpressed in DU145 and VCaP cells to modulate prostate cancer cell proliferation and migration. Finally, the effects of miR-125a-5p downregulation or NAIF1 overexpression on the growth of in vivo prostate cancer xenograft were evaluated.
Results
miR-125a-5p was upregulated in prostate cancer cell lines and human prostate carcinomas. Lentivirus induced miR-125a-5p downregulation in DU145 and VCaP cells inhibited prostate cancer cell proliferation or migration. NAIF1 was the direct target of miR-125a-5p, as both gene and protein expression levels of NAIF1, as well as caspase-3 were upregulated by miR-125a-5p. Forced overexpression of NAIF1 had similar antitumor effects as miR-125a-5p downregulation on prostate cancer cell proliferation and migration. In vivo prostate xenograft assay confirmed the tumor-suppressive effect of miR-125a-5p downregulation or NAIF1 overexpression.
Conclusion
miR-125a-5p regulates prostate cancer cell proliferation and migration through NAIF1.
Keywords: miR-125a-5p, NAIF1, caspase-3, proliferation, migration
Introduction
Prostate cancer is one of the most common cancers in male patients.1 In the USA alone, the estimated yearly new cases of prostate cancer were over 220,000 and the estimated yearly death from prostate cancer was over 25,000 in 2015.1 While patients with prostate cancer may be treated with several efficient therapies, including immunotherapy,2 radiation therapy,3 or surgery,4 understanding the molecular mechanisms of human prostate cancer, developing early diagnostic methods or noninvasive focal therapies for patients with prostate cancer are still the greatest challenge ahead.
MicroRNAs (miRNAs) are groups of noncoding small RNAs that transcription-ally or posttranslationally degrade genes or proteins by binding the 3′-untranslated regions (3′-UTR) of targeted genes.5 In human prostate cancer, miRNAs are broadly involved in many aspects of cancer development, such as prostate cancer pathogenesis, proliferation, metastasis, or apoptosis.6–9 Among many of the regulatory miRNAs in human cancer, miRNA-125-5p is an active epigenetic regulator, acting as either an oncogene or a tumor-suppressive gene.10 In hepatocellular carcinoma, miR-125a-5p is a tumor suppressor, as it was downregulated in cancer tissues, and the upregulation of miR-125a-5p inhibited cancer cell proliferation and migration by targeting MMP11 and VEGF.11 On the other hand, miR-125a-5p acts as an oncogene to enhance lung cancer invasion by regulating Rock-1.12 However, it is not clear whether miR-125a-5p is acting as an oncogene or tumor suppressor in human prostate carcinoma.
NAIF1 is an apoptotic pathway gene that induces apoptosis in various human cancers. In gastric cancer, NAIF1 inhibits gastric cancer migration and invasion by targeting MAPK or caspase-3.13,14 In non-small-cell lung cancer, NAIF1 interacts with miR-24 to inhibit cancer growth.15 In human prostate cancer, NAIF1 was suggested to be a potential biomarker,16 yet its exact expression pattern or functional role remains elusive.
In our work, we evaluated the expression and molecular mechanism of miR-125a-5p in human prostate cancer. We found that miR-125a-5p was generally upregulated in both prostate cancer cell lines and biopsy specimens from patients with prostate cancer. Functional assays demonstrated that downregulating miR-125a-5p inhibited prostate cancer cell proliferation and migration. We also showed that NAIF1 was the target gene of miR-125a-5p in prostate cancer and that overexpressing NAIF1 had similar tumor-suppressive effects as miR-125a-5p.
Materials and methods
Cancer cell lines and prostate specimens
Prostate cancer cell lines, including androgen receptor (AR)-negative DU145, PC-3, NCI-H660 and AR-positive 22RV-1, LNCaP, VCaP cells, were all obtained from American Type Culture Collection (ATCC, Shanghai, People’s Republic of China). The nontumorigenic human prostate epithelial cell line RWPE-1 was obtained from the Cell Bank of the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, People’s Republic of China). All in vitro cell lines were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Invitrogen) in a humidified tissue culture chamber at 37°C. The biopsy prostate specimens, including carcinoma tissues (T) and adjacent noncarcinoma tissues (ANT), were obtained from prostate cancer patients through radical prostatectomy in the Department of Urology and Surgery at the Oilfield General Hospital of Daqing in Daqing, People’s Republic of China between June 2014 and May 2015. Signed consent forms were obtained from all patients. All protocols of the current study were approved by the Ethic Committee at the Oilfield General Hospital of Daqing, Daqing, People’s Republic of China, and was conducted in accordance with the Declaration of Helsinki.
RNA extraction and quantitative reverse transcription-polymerase chain reaction
RNA extraction and reverse transcription were performed using a Trizol Kit (Invitrogen) and a Superscript III kit (Invitrogen) according to the manufacturer’s protocol. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) for miR-125a-5p was performed using a TaqMan miRNA qRT-PCR assays (Applied Biosystem, Waltham, MA, USA) on an ABI-Prism 7300 System (Applied Biosystem) according to the manufacturer’s protocol. U6 transcript was used as internal control. qRT-PCR on NAIF1 and caspase-3 genes was performed using a SYBR Green PCR Master Mix kit (Applied Biosystem), also on the ABI-Prism 7300 System, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as internal control. The (2−ΔΔCT) method was used to quantitatively compare gene expression levels between samples.
miR-125a-5p knockdown
The knockdown of miR-125a-5p in prostate carcinoma cell lines, DU145 and VCaP, was performed by lentiviral transduction. The paired lentiviruses expressing hsa-miR-125a-5p inhibitor (125a-5p-inhibitor) or its negative control-miRNA (C-miRNA) were purchased from SunBio Medical Biotechnology (Shanghai, People’s Republic of China). In a 6-well plate, DU145 or VCaP cells were transduced with 3 mL of lentiviral supernatants with 8 µg/mL polybrene at multiplicity of infection of 50 for 4 hours. After replenishing culture medium, cells were continuously cultured and selected in 2 µg/mL of puromycin until stabilized.
Cancer cell proliferation assay
The in vitro proliferation/viability of DU145 and VCaP cells were measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Invitrogen) according to the manufacturer’s protocol. Briefly, DU145 and VCaP cells were incubated with MTT solution (1 mg/mL) for 4 hours at 37°C. After aspirating the medium, formazan crystals were dissolved in dimethyl sulfoxide (Sigma-Aldrich, St Louis, MO, USA). The absorbance at 570 nm was measured for each experiment and normalized to control.
Cancer migration assay
The in vitro migration of DU145 and VCaP cells was measured using a Transwell chamber (Becton Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer’s protocol. Briefly, 1×105 DU145 or VCaP cells were plated in transwell inserts precoated with Matrigel. The culture medium was without serum. The lower chamber was filled with culture medium and 10% fetal bovine serum as chemoattractant. Twenty-four hours after culture, nonmigrated cells were removed and the insert was fixed with 4% paraformaldehyde and stained with hematoxylin and eosin. In each insert, the averaged migrated cells were counted among three randomly assigned regions of interest. The relative migration was then measured by normalizing the averaged migrated cells of each experiment against control.
Dual-luciferase reporter assay
A human prostate cDNA library was used to amplify the 3′-UTR of NAIF1 gene. The cDNA was cloned into the SpeI and HindIII sites of pMir-Report (Ambion, Foster City, CA, USA), resulting in a wild-type (WT) NAIF1 firefly luciferase vector (Firefly-WT-NAIF1). Alternatively, the miR-125a-5p binding site on NAIF1 3′-UTR was modified by a Site-Directed Mutagenesis Kit (SBS Genetech, Beijing, People’s Republic of China). In HEK293T cells, the synthesized miR-125a-5p oligonucleotides (SunBio, Beijing, People’s Republic of China) were cotransfected with Firefly-WT-NAIF1, Firefly-MT-NAIF1, or a control Renilla luciferase vector for 48 hours. Forty-eight hours after transfection, luciferase activity of each experiment was measured by a dual-luciferase reporter assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
Western blot assay
DU145 or VCaP cells were lysed with a lysis buffer (Millipore, Billerica, MA, USA). Proteins (10 µg/sample) were dissolved on 10% of sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes. After blocking, the membranes were treated with primary antibodies against human NAIF1 and caspase-3 (1:200 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. After washing, the membranes were then treated with horseradish peroxidase-conjugated secondary antibodies (1:1,000 dilution) at room temperature for 2 hours. The blots were visualized with the enhanced chemiluminescence kit (Amersham Pharmacia Biotechnology, Piscataway, NJ, USA) according to the manufacturer’s protocol.
NAIF1 overexpression
DNA sequence of NAIF1 was amplified from a human prostate cDNA library and inserted into a recombinant plasmid eukaryotic expression vector pcDNA3.1 (Invitrogen), resulting in a NAIF1 overexpressing plasmid (pcDNA3.1-NAIF1). DU145 or VCaP cells were then transfected with pcDNA3.1-NAIF1, or a control empty plasmid (pcDNA3.1-C) using a Lipofectamine 2000 kit (Invitrogen, Carlsbad, CA, USA) for 24 hours.
Tumor xenograft assay
DU145 cells were transduced with 125a-5p-inhibitior or C-miRNA, or transfected with pcDNA3.1-NAIF1 or pcDNA3.1-C for 24 hours. After that, healthy DU145 cells (1×106) were subcutaneously injected into the right flank of 6-week-old female nude mice. The volume of the in vivo tumor xenograft was calculated with the equation, length × width2/2. Five weeks after initial injection, mice were sacrificed and prostate cancer xenograft was prepared for paraffin-embedded sectioning and Ki-67 immunostaining (BD Bioscience, Franklin Lakes, NJ, USA, Catalog #556027).
Statistical analysis
All data are presented as mean ± standard errors. Statistical difference was measured by Student’s t-test on a Windows-based SPSS software (Version 13.0; SPSS Inc., Chicago, IL, USA), with significance of P<0.05. All experiments were repeated at least three times.
Results
miRNA-125a-5p is generally upregulated in prostate cancer
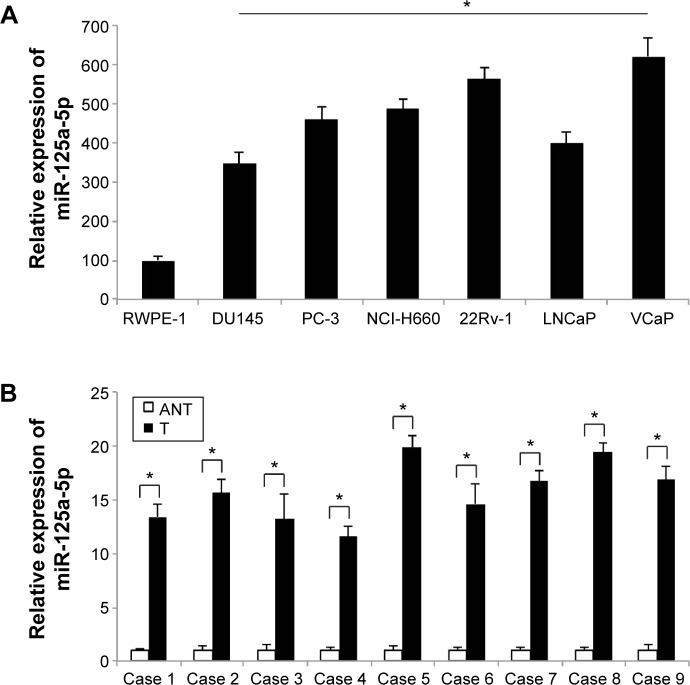
In this study, we first evaluated the expression level of miR-125a-5p in prostate cancer cells and human carcinoma by qRT-PCR. In prostate cancer cell lines, we found that the expression levels of miR-125a-5p were generally upregulated, in both AR-negative DU145, PC-3, NCI-H660 cells and AR-positive 22RV-1, LNCaP, VCaP cells, as compared to the expression level of miR-125a-5p in nontumorigenic human prostate epithelial cell line RWPE-1 (Figure 1A, *P<0.05). In addition, through qRT-PCR examination on the biopsy specimens from nine patients with prostate cancer, we found miR-125a-5p was highly expressed in cancer specimens (T) rather than in ANTs (Figure 1B, *P<0.05).
Figure 1.
Expression of miR-125a-5p in prostate cancer cell lines and clinical specimens.
Notes: (A) qRT-PCR was used to evaluate the expression levels of miR-125a-5p in AR-negative prostate cancer cell lines, DU145, PC-3, NCI-H660 cells and AR-positive prostate cancer cell lines, 22RV-1, LNCaP, VCaP cells. The control cell line is a nontumorigenic human prostate epithelial cell line RWPE-1. (*P<0.05 vs RWPE-1). (B) qRT-PCR was used to compare the expression levels of miR-125a-5p between paired T and ANT specimens in nine patients with prostate cancer (*P<0.05).
Abbreviations: qRT-PCR, quantitative reverse transcription-polymerase chain reaction; AR, androgen receptor; T, carcinoma tissues; ANT, adjacent noncarcinoma tissues.
Downregulating miR-125a-5p inhibits proliferation and migration in prostate cancer
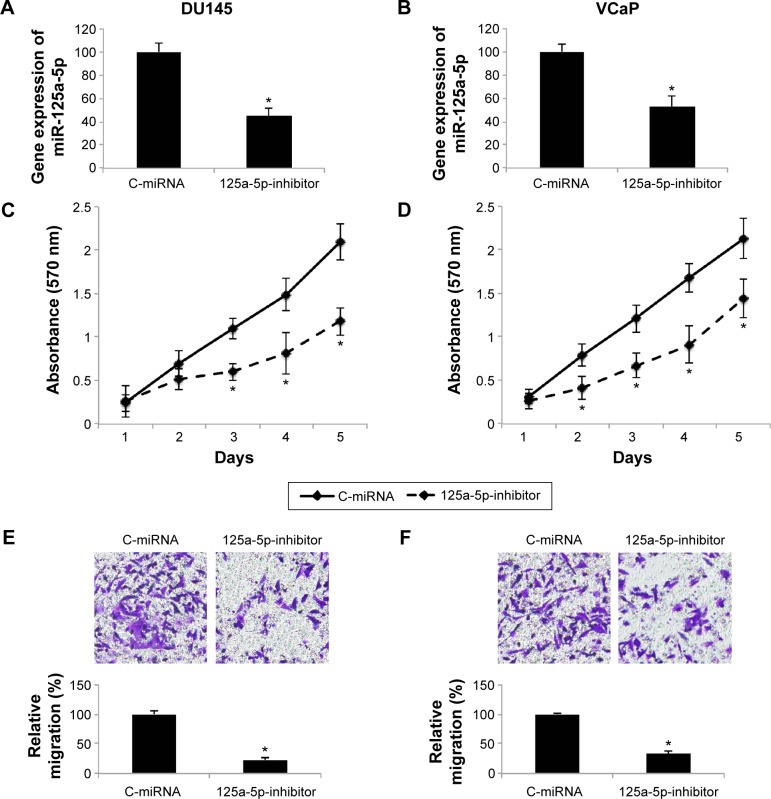
As we found that, overall, miR-125a-5p was upregulated in prostate cancer, we sought to understand its functional role in prostate cancer regulation. We transduced AR-negative DU145 cells, as well as AR-positive VCaP cells with miR-125a-5p inhibitor lentivirus (125a-5p-inhibitor) to downregulate miR-125a-5p. The control cells were transduced with a negative C-miRNA. The knockdown efficiency was verified by qRT-PCR in DU145 and VCaP cells (Figure 2A and B, *P<0.05). Prostate cancer cell proliferation was evaluated through an MTT assay for 5 days in vitro. It showed that in both DU145 and VCaP cells, prostate cancer cell proliferation was significantly inhibited by miR-125a-5p downregulation (Figure 2C and D, *P<0.05). We also evaluated the effect of miR-125a-5p downregulation on prostate cancer migration. The result of a transwell assay showed that the migrating capabilities of both DU145 and VCaP cells were significantly inhibited by miR-125a-5p downregulation (Figure 2E and F, *P<0.05).
Figure 2.
Effect of miR-125a-5p downregulation on prostate cancer cell proliferation and migration.
Notes: DU145 and VCaP cells were transduced with miR-125a-5p inhibitor lentivirus, 125a-5p-inhibitor, or C-miRNA. After lentiviral transduction, qRT-PCR was used to evaluate the knockdown efficiency of lentiviral transduction in DU145 (A) and VCaP (B) cells (*P<0.05). Cancer cell proliferation in DU145 (C) and VCaP (D) cells was evaluated by MTT assay (*P<0.05). Cancer migration in DU145 (E) and VCaP (F) cells was evaluated by a transwell assay and H&E staining (*P<0.05).
Abbreviations: C-miRNA, control-microRNA; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; H&E, hematoxylin and eosin.
miR-125a-5p regulates apoptotic pathways in prostate cancer
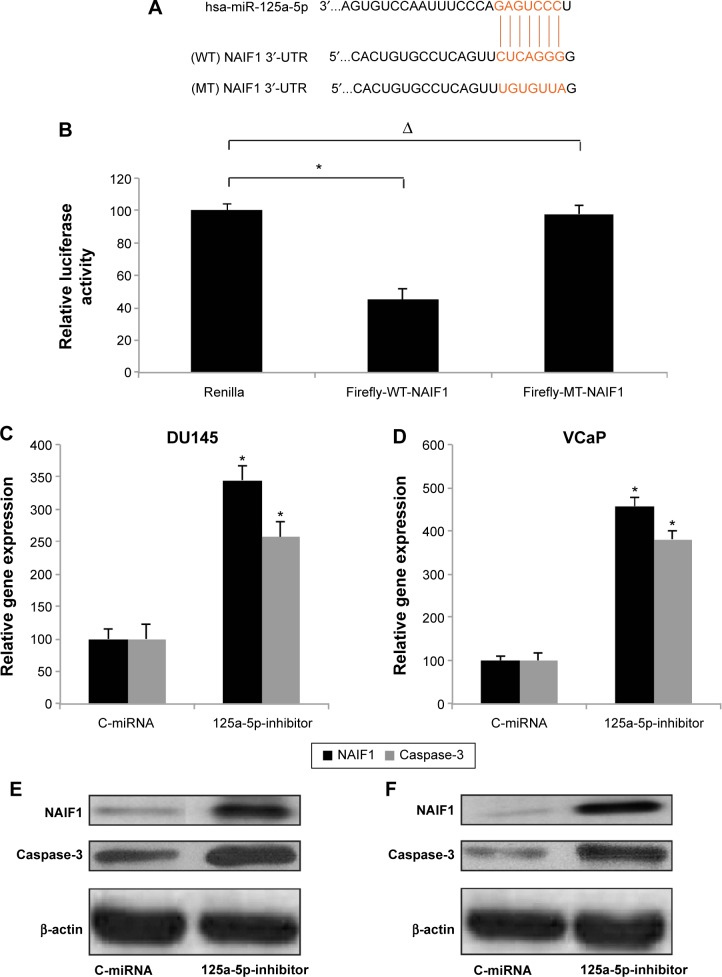
We then sought the molecular pathways involved in the regulation of miR-125a-5p in prostate cancer. Using some of the microRNA target searching tools, including TargetScan (www.targetscan.org) and miRANDA (www.microRNA.org), we found that NAIF1 was very likely the binding target of miR-125a-5p (Figure 3A). We thus constructed dual-luciferase firefly vectors expressing WT NAIF1 3″-UTR, or a mutant (MT) NAIF1 3″-UTR with modified miR-125a-5p binding site, and conducted a dual-luciferase reporter assay in HEK293T cells. It showed there was significant binding between miR-125a-5p and WT NAIF1 firefly luciferase vector, confirming that NAIF1 was the direct target of miR-125a-5p (Figure 3B, *P<0.05).
Figure 3.
Effect of miR-125a-5p on apoptotic pathway in prostate cancer.
Notes: (A) Diagram shows the binding of miR-125a-5p on WT NAIF1 3′-UTR. An MT NAIF1 3′-UTR with modified miR-125a-5p binding site was also demonstrated. (B) HEK293T cells were cotransfected with miR-125a-5p and firefly-WT-NAIF1, or firefly-MT-NAIF1, or Renilla luciferase vector (Renilla) for 48 hours. A dual-luciferase reporter assay was then used to evaluate the binding between miR-125a-5p and NAIF1 (*P<0.05, ∆P>0.05). DU145 and VCaP cells were transduced with 125a-5p-inhibitor or C-miRNA. qRT-PCR was used to evaluate the gene expression levels of NAIF1 and caspase-3 in DU145 (C) and VCaP (D) cells (*P<0.05 vs C-miRNA). Western blot was used to evaluate the protein expressions of NAIF1 and caspase-3 in DU145 (E) and VCaP (F) cells.
Abbreviations: WT, wild type; 3′-UTR, 3′-untranslated regions; MT, mutant; firefly-WT-NAIF1, WT NAIF1 firefly luciferase vector; firefly-MT-NAIF1, MT NAIF1 firefly luciferase vector; C-miRNA, control-microRNA; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
To elaborate the mechanisms of regulation of miR-125a-5p on apoptotic pathway in prostate cancer, we transduced DU145 and VCaP cells with 125a-5p-inhibitor or C-miRNA. Then, we used qRT-PCR to evaluate the gene expression levels of NAIF1 and caspase-3 in lentivirus transduced cells. It showed that, in both DU145 and VCaP cells, NAIF1 and caspase-3 genes were significantly upregulated by miR-125a-5p downregulation (Figure 3C and D, *P<0.05 vs C-miRNA). In addition, the result of Western blot showed that the protein expression levels of NAIF1 and caspase-3 were also significantly upregulated by miR-125a-5p down-regulation in DU145 and VCaP cells (Figure 3E and F).
NAIF1 overexpression inhibits proliferation and migration in prostate cancer
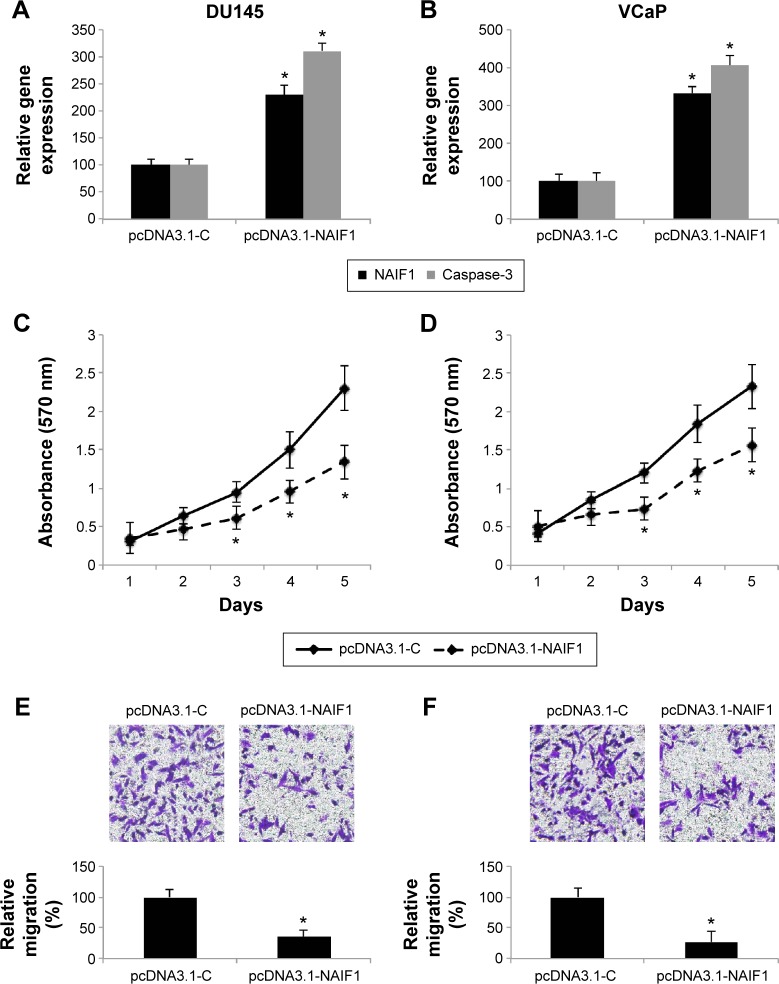
As we discovered that miR-125a-5p regulated apoptotic pathways, including NAIF1 and caspase-3 in prostate cancer, we then sought to find out whether NAIF1 was directly involved in prostate cancer regulation. We constructed a NAIF1 overexpression vector (pcDNA3.1-NAIF1) and transfected DU145 and VCaP cells with it. The control cells were transfected with an empty overexpression vector (pcDNA3.1-C). The overexpression efficiency was verified by qRT-PCR in DU145 and VCaP cells (Figure 4A and B, *P<0.05 vs pcDNA3.1-C). In addition, qRT-PCR demonstrated that in both DU145 and VCaP cells, caspase-3 genes were also significantly upregulated by NAIF1 overexpression (Figure 4A and B, *P<0.05 vs pcDNA3.1-C).
Figure 4.
Effect of NAIF1 upregulation on prostate cancer cell proliferation and migration.
Notes: (A, B) DU145 and VCaP cells were transfected with NAIF1 overexpression vector, pcDNA3.1-NAIF1 or an empty overexpression vector, pcDNA3.1-C. After transfection, qRT-PCR was used to evaluate the gene expression levels of NAIF1 and caspase-3 in DU145 (A) and VCaP (B) cells (*P<0.05). Cancer cell proliferation in DU145 (C) and VCaP (D) cells was evaluated by MTT assay (*P<0.05). Cancer migration in DU145 (E) and VCaP (F) cells was evaluated by a transwell assay and H&E staining (*P<0.05).
Abbreviations: qRT-PCR, quantitative reverse transcription-polymerase chain reaction; H&E, hematoxylin and eosin.
We then evaluated the functional role of NAIF1 overexpression in DU145 and VCaP cells. Using MTT assay, we found that NAIF1 overexpression significantly inhibited prostate cancer cell proliferation in DU145 and VCaP cells (Figure 4C and D, *P<0.05). Moreover, in a transwell assay, we found that NAIF1 overexpression inhibited prostate cancer migration in DU145 and VCaP cells (Figure 4E and F, *P<0.05). Therefore, our data showed that overexpressing NAIF1 had similar tumor-suppressive effects as miR-125-a-5p downregulation in prostate cancer.
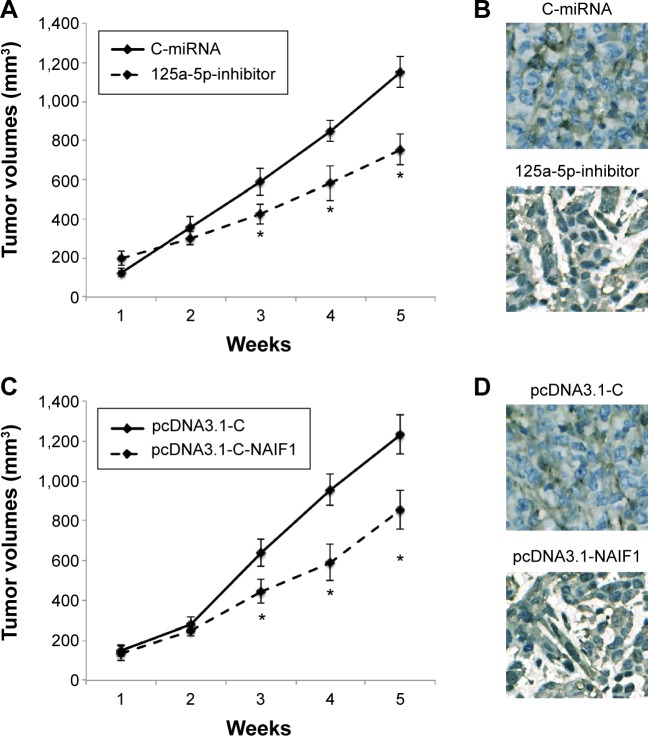
miR-125a-5p inhibition or NAIF1 overexpression inhibits in vivo tumor growth in prostate cancer xenograft
Finally, we evaluated the in vivo effects of miR-125a-5p downregulation or NAIF1 overexpression in prostate carcinoma. For miR-125a-5p inhibition, DU145 cells were transduced with 125a-5p-inhibitor or C-miRNA. For NAIF1 overexpression, DU145 cells were transfected with pcDNA3.1-NAIF1 or pcDNA3.1-C. After that, healthy DU145 cells were selected and injected into 6-week-old female null mice. The volume of prostate cancer xenograft was monitored weekly. The 5-week growth curves showed that both miR-125a-5p downregulation and NAIF1 overex-pression significantly reduced the in vivo growth of prostate cancer xenografts (Figure 5A and C, *P<0.05). At the end of in vivo growth assay, prostate tumors were extracted and processed with paraffin-embedded sectioning and Ki-67 staining. It showed that in both cases, miR-125a-5p down-regulation or NAIF1 overexpression markedly reduced Ki-67 expression in tumor xenografts. (Figure 5B and D).
Figure 5.
Effect of miR-125a-5p downregulation or NAIF1 overexpression on prostate cancer in vivo xenograft.
Notes: (A) DU145 cells were transduced with miR-125a-5p inhibitor lentivirus, 125a-5p-inhibitor or C-miRNA lentivirus. One million healthy DU145 cells were then inoculated into 6-week-old female null mice. The growth curves were plotted to monitor tumor volumes for 5 weeks (*P<0.05). (B) After 5-week in vivo growth, prostate cancer xenografts were extracted. Ki-67 immunostaining was then performed. (C, D) DU145 cells were transfected with NAIF1 overexpression vector, pcDNA3.1-NAIF1 or an empty overexpression vector, pcDNA3.1-C, followed by in vivo xenograft assay. The growth curves were also monitored for 5 weeks (C, *P<0.05); and Ki-67 staining was performed after that (D).
Abbreviation: C-miRNA, control-microRNA.
Discussion
In our work, we explored the molecular expression and epigenetic functions of miR-125a-5p in human prostate cancer. We showed that miR-125a-5p was significantly upregulated in both prostate cancer cell lines and clinical prostate carcinoma obtained from human patients. Interestingly, we found that miR-125a-5p was upregulated in both AR-negative and AR-positive prostate cancer cell lines. It was previously shown that, another member of the miR-125 family, miR-125b was differentially expressed in androgen-dependent or -independent prostate cells.17 The underlying mechanism contributing to the expression disparity between miR-125a-5p and miR-125b is unknown. However, our data showing predominant upregulation of miR-125a-5p in both AR-negative and AR-positive prostate cancer cells suggest that it may act through differential regulatory pathways than miR-125b in human prostate cancer.
Through functional assays, including MTT and transwell assays, we demonstrated that downregulating miR-125a-5p inhibited cancer cell proliferation and migration in DU145 and VCaP cells. It was shown in previous studies that miR-125a-5p may act as either an oncogene or a tumor-suppressive gene, depending on the cancer type. miR-125a-5p is oncogenic in lung cancer as it enhanced lung cancer invasion,12 whereas miR-125a-5p is tumor suppressive in hepatocellular carcinoma as it inhibits cancer proliferation and migration.11 Therefore, our data showing that miR-125a-5p was upregulated, and its subsequent downregulation inhibited cancer cell proliferation or migration in DU145 and VCaP cells, suggest that miR-125a-5p is acting as an oncogenic effector in human prostate cancer.
Also in our work, we demonstrated that NAIF1 was directly targeted by miR-125a-5p in prostate cancer. Along with caspase-3, NAIF1 was upregulated by miR-125a-5p in DU145 and VCaP cells, suggesting that the anticancer effects of miR-125a-5p downregulation may act through apoptotic pathway and include NAIF1 and caspase-3. It was then confirmed by our functional assays, showing that ectopically forced NAIF1 overexpression upregulated caspase-3, inhibited in vitro cancer cell proliferation/migration and in vivo prostate tumor xenografts. Therefore, along with previous studies showing proapoptotic effects of NAIF1 in gastric cancer and non-small-cell lung cancer,13–15 it is very likely that NAIF1 may predominantly act as a tumor-suppressive gene, possibly through the activation of apoptotic pathways, in various types of cancers, including prostate cancer.
In summary, we demonstrated for the first time that miR-125a-5p expression is elevated in prostate cancer cell lines and prostate tumors and that downregulating miR-125a-5p inhibited proliferation and migration of prostate cancer. We also discovered that miR-125a-5p regulated prostate cancer through NAIF1 and apoptotic pathway. The findings of our work may not only contribute to the understanding of molecular mechanism of epigenetic regulation of miR-125 in prostate cancer, but also provide a novel therapeutic target for treating patients with prostate cancer.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri I, Cattarino S, Agliano AM, Collalti G, Sciarra A. Beyond the immune suppression: the immunotherapy in prostate cancer. Biomed Res Int. 2015;2015:794968. doi: 10.1155/2015/794968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose JN, Crook JM. The role of radiation therapy in the treatment of metastatic castrate-resistant prostate cancer. Ther Adv Urol. 2015;7(3):135–145. doi: 10.1177/1756287215576647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nazim SM, Abbas F. Role of surgery in locally advanced prostate cancer. Pak J Med Sci. 2015;31(3):710–716. doi: 10.12669/pjms.313.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15(5):563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Casanova-Salas I, Rubio-Briones J, Fernandez-Serra A, Lopez-Guerrero JA. MiRNAs as biomarkers in prostate cancer. Clin Trans Oncol. 2012;14(11):803–811. doi: 10.1007/s12094-012-0877-0. [DOI] [PubMed] [Google Scholar]
- 7.Wang YL, Wu S, Jiang B, Yin FF, Zheng SS, Hou SC. Role of microRNAs in prostate cancer pathogenesis. Clin Genitourin Cancer. 2015;13(4):261–270. doi: 10.1016/j.clgc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Wen X, Deng FM, Wang J. MicroRNAs as predictive biomarkers and therapeutic targets in prostate cancer. Am J Clin Exp Urol. 2014;2(3):219–230. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Zang J, Jing X, et al. Identification of candidate miRNA biomarkers from miRNA regulatory network with application to prostate cancer. J Transl Med. 2014;12:66. doi: 10.1186/1479-5876-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun YM, Lin KY, Chen YQ. Diverse functions of miR-125 family in different cell contexts. J Hematol Oncol. 2013;6:6. doi: 10.1186/1756-8722-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi Q, Tang S, Xia L, et al. Ectopic expression of miR-125a inhibits the proliferation and metastasis of hepatocellular carcinoma by targeting MMP11 and VEGF. PLoS One. 2012;7(6):e40169. doi: 10.1371/journal.pone.0040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang L, Zhang Q, Chang H, Qiu X, Wang E. hsa-miR-125a-5p Enhances invasion in non-small cell lung carcinoma cell lines by upregulating rock-1. Zhongguo Fei Ai Za Zhi. 2009;12(10):1069–1073. doi: 10.3779/j.issn.1009-3419.2009.10.03. Chinese. [DOI] [PubMed] [Google Scholar]
- 13.Luo Q, Zhao M, Zhong J, et al. NAIF1 is downregulated in gastric cancer and promotes apoptosis through the caspase-3 pathway in human MKN45 cells. Oncol Rep. 2011;25(4):1117–1123. doi: 10.3892/or.2011.1171. [DOI] [PubMed] [Google Scholar]
- 14.Yang M, Gu YY, Peng H, et al. NAIF1 inhibits gastric cancer cells migration and invasion via the MAPK pathways. J Cancer Res Clin Oncol. 2015;141(6):1037–1047. doi: 10.1007/s00432-014-1865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao G, Liu L, Zhao T, et al. Upregulation of miR-24 promotes cell proliferation by targeting NAIF1 in non-small cell lung cancer. Tumour Biol. 2015;36(5):3693–3701. doi: 10.1007/s13277-014-3008-4. [DOI] [PubMed] [Google Scholar]
- 16.Cheng WS, Tao H, Hu EP, et al. Both genes and lncRNAs can be used as biomarkers of prostate cancer by using high throughput sequencing data. Eur Rev Med Pharmacol Sci. 2014;18(22):3504–3510. [PubMed] [Google Scholar]
- 17.Shi XB, Xue L, Yang J, et al. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci U S A. 2007;104(50):19983–19988. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]