Abstract
Background
Fatigue is a common complication of adjuvant chemotherapy and compromises the quality of life of breast cancer survivors. We sought to correlate serial hemoglobin (Hb) levels with fatigue in a population of women on adjuvant chemotherapy, none of whom received erythropoietin-stimulating agents or red blood cell transfusions.
Patients and Methods
Seventy-five women participated in a study using quality-of-life questionnaires to assess changes in need for psychosocial support over time. Questionnaires were administered within 30 days of initiating adjuvant therapy and at 2, 6, and 12 months. Fatigue was assessed by the 36-Item Short-Form Health Survey (SF-36). Hemoglobin levels at each time point were captured retrospectively. Complete data are included for 40 of the 46 women who received adjuvant chemotherapy. Paired-samples t tests were conducted to compare mean SF-36 Energy/Fatigue scores between time points, and independent-samples t tests were conducted for comparisons against norms. Simple correlations (Pearson R) were conducted between SF-36 variables and Hb levels at each time point.
Results
At 2 months, 23.4% of women had Hb < 11 g/dL compared with 12.9% at 12 months. Compared with norms for women in the general population and breast cancer survivors, these women reported worse fatigue at baseline and at 2 and 6 months. A strong linear relationship was observed between Hb at 2 months and SF-36 Energy/Fatigue scores at 12 months (r = 0.71; P = .002).
Conclusion
Participants with high fatigue at 12 months had Hb levels at 2 months 13% lower than those with low fatigue. This finding suggests that chemotherapy-induced decline in Hb might be a marker of physiologic reserve.
Keywords: Physiologic reserve, Quality of life, Role limitations
Introduction
Fatigue is commonly reported by women undergoing treatment of early-stage breast cancer and significantly compromises quality of life (QOL).1-3 The relationship between fatigue and anemia has been difficult to establish.4 Cancer treatment, depression, menopause, and sleep disruption also contribute to fatigue in this population.
We undertook a trial to serially assess changes in psychosocial needs over time in a population of women with early-stage breast cancer who were receiving adjuvant chemotherapy. A battery of questionnaires was completed at baseline and serially over 1 year and included the 36-Item Short-Form Health Survey (SF-36) Energy/Fatigue subscale. None of these women received erythropoietin-stimulating agents (ESAs) or red blood cell transfusions. Thus, we sought to determine the effect of hemoglobin (Hb) levels on fatigue, unconfounded by treatment of anemia.
Patients and Methods
Participants
Patients from the medical oncology clinics at Washington University in St. Louis, MO, were approached for participation in a trial on clinical and psychosocial factors of QOL in breast cancer. Women were considered eligible if they were within 30 days of initiation of adjuvant chemotherapy for stage I or II breast cancer, had an Eastern Cooperative Oncology Group performance status of 0 or 1, and signed a consent form approved by the Institution Review Board of Washington University. Patients were ineligible if they had metastatic disease, were pregnant, or were unwilling to complete serial questionnaires for the study duration.
Seventy-five women with early-stage breast cancer were enrolled in a study addressing psychosocial support for women undergoing adjuvant therapy, and 46 of these women were treated with chemotherapy. The women completed a battery of questionnaires that addressed fatigue, general health-related QOL, and overall psychosocial adjustment to breast cancer at 4 time points during the study: at baseline and at 2, 6, and 12 months after initiation of chemotherapy. Hemoglobin levels were captured retrospectively at 3 time points for 40 of the 46 women treated with chemotherapy. Hemoglobin levels were drawn at the same clinic visit when the questionnaires were completed. No patient received ESAs or red blood cell transfusions during the course of adjuvant chemotherapy.
Measurements
Baseline information was obtained within 1 month of initiating the first cycle of adjuvant chemotherapy. Treatment and demographic information were collected, and measures of psychosocial functioning and QOL were administered. These measures were repeated 2, 6, and 12 months after the start of adjuvant chemotherapy. Hemoglobin levels were assessed at the same time as fatigue levels.
The SF-36 is a measure of health-related QOL that is not disease specific but has been used widely in patients with breast cancer and other chronically ill patients.5-8 This scale generates scores on the following 8 subscales: Energy/Fatigue, Physical Functioning, Role Limitations due to Physical Problems, General Health, Bodily Pain, Social Functioning, Role Limitations due to Emotional Problems, and Mental Health Index. Scores range from 0 to 100, with higher scores indicating better health. Energy/Fatigue, General Health, and Mental Health Index are “bipolar” subscales. If no limitations are reported on these bipolar subscales, scores are around the midpoint of 50. Positive QOL states are reflected in scores above 50, and negative QOL states are shown by scores below 50. When comparing scores, 5-point differences are considered clinically meaningful. The SF-36 subscales have been shown to be reliable (Cronbach’s α = 0.75-0.91) and to have adequate to substantial construct validity in a variety of medically ill populations.8
We were interested in the SF-36 Energy/Fatigue subscale as our primary measure of fatigue. There is no generally accepted definition of fatigue, although it is commonly thought to involve feeling tired and weak, with a lack of energy. Like pain, fatigue is nearly always assessed by self-report, and there is no gold standard. Most studies define fatigue by the instrument used for its measurement.5 The SF-36 Energy/Fatigue subscale assesses both energy (“pep, energy”) and fatigue (“worn out, tired”) over the past 4 weeks, using a 5-choice verbal rating scale format. This subscale has been shown to be highly correlated with other commonly used measures of fatigue and has distinguished patients with breast cancer from women with benign breast problems.9,10 It has shown excellent empirical validity and sensitivity to the effect of disease and treatment and has been used as the primary measure of fatigue in various recent studies of breast cancer patients and survivors.6,10,11 Although there is growing interest in the multidimensional nature of fatigue, the SF-36 Energy/Fatigue subscale is a venerable and still often-used instrument for assessing fatigue in various normal and patient populations, including patients with breast cancer. For normative comparisons, we included norms for women from the general US population and from a large study (N = 2582) of breast cancer survivors (averaging 2 years after completion of initial treatment).8,11
Statistical Analysis
Paired-samples t tests were conducted to compare participants’ mean SF-36 Energy/Fatigue scores between time points and independent-samples t tests for comparisons against norms. Simple correlations (Pearson R) were conducted between SF-36 variables and Hb levels at each time point. A more stringent criterion for significance (α = .004) was used to control for type I errors. This was determined by dividing the usual α level of .05 by 12 (3 Hb time points × 4 Energy/Fatigue time points, for a total of 12 simple correlations). Because it would be a major missed opportunity to not examine associations between Hb levels and the other SF-36 subscales at each time point, we conducted exploratory analyses of these relationships using simple correlations (α = .05). All analyses were conducted using SPSS version 12.0.2.
Results
Participant characteristics are shown in Table 1. The mean age was 47.7 years (range, 28-70 years), most women (72.5%) were married, and slightly > two thirds were non-Hispanic white. Approximately one third were employed, nearly one quarter were on medical leave, and 17.5% were retired. Participants had > 2 years of post–high school education on average and a reported mean household income of nearly $55,000. Lumpectomy was performed in 25 patients (63%), and mastectomy was performed in 15 (37%). Thus, the 25 patients who underwent lumpectomy also had adjuvant radiation during the study. The surgical stage was I in 15 patients and II in 25 patients. An average of 5.1 cycles of chemotherapy were administered; 23 patients received 4 cycles, 12 received 6 cycles, and 5 received 8 cycles. At 2 months, 23.4% of patients had Hb < 11 g/dL compared with 12.9% at 12 months. There were no significant associations for Hb levels versus cancer variables (stage, type of surgery, number of cycles of chemotherapy, tamoxifen use) or demographic variables (age, marital status, education, income, hours worked per week); therefore, no covariates were controlled for in subsequent analyses.
Table 1.
Participant Characteristics
| Characteristic | Value |
|---|---|
| Number of Patients | 40 |
| Age (Years) | 47.7 ± 10.1 |
| Race/Ethnicity, n (%) | |
| Non-Hispanic White | 27 (67.5) |
| African American | 11 (27.5) |
| Asian-American | 1 (2.5) |
| Other | 1 (2.5) |
| Marital Status, (%) | |
| Single | 2 (5) |
| Married | 29 (72.5) |
| Divorced | 5 (12.5) |
| Widowed | 1 (2.5) |
| Separated | 3 (7.5) |
| Years of Education | 14.3 ± 2.6 |
| Employment Status, n (%) | |
| Employed | 14 (35) |
| Medical leave | 9 (22.5) |
| Retired | 7 (17.5) |
| Semiretired | 1 (2.5) |
| Unemployed | 9 (22.5) |
| Household Income | $54,850 ± 35,102 |
| Stage of Disease, n (%) | |
| I | 15 (37.5) |
| II | 25 (62.5) |
| Surgery, n (%) | |
| Mastectomy | 15 (37.5) |
| Lumpectomy | 25 (62.5) |
| No. of Chemotherapy Cycles, n (%) | |
| 4 | 23 (57.5) |
| 6 | 12 (30) |
| 8 | 5 (12.5) |
| Tamoxifen, n (%) | |
| Yes | 12 (30) |
| No | 28 (70) |
| Hb Levels, g/dL | |
| 2 Months | 12.3 ± 1.4 |
| Percent with Hb < 11 | 23.4% |
| 6 Months | 12.1 ± 1.3 |
| Percent with Hb < 11 | 21.2% |
| 12 Months | 12.6 ± 1.3 |
| Percent with Hb < 11 | 12.9% |
Data are presented as mean ± SD or number and percentage.
Abbreviation: Hb = hemoglobin
Table 2 shows normative comparisons of participant SF-36 Energy/Fatigue scores. The patients’ scores at baseline (within 1 month of initiating chemotherapy) and at 2 months were significantly into the “negative QOL” range (< 50). Compared with norms for women in the general population and breast cancer survivors, these participants reported poorer SF-36 Energy/Fatigue scores at baseline, 2 months, and 6 months (all P < .05). All of these differences (8.6-15.3 points) exceeded the 5-point criteria for clinical meaningfulness. However, at 12 months, participants’ fatigue scores did not differ from either normative group. In addition, changes in Energy/Fatigue scores for these participants at 12 months, compared with scores at baseline and 2 and 6 months, were statistically significant and exceeded the 5-point criteria for clinical meaningfulness (P < .05).
Table 2.
Comparison of Participants’ SF-36 Energy/Fatigue Scores* with Norms for Women from the General Population and Breast Cancer Survivors
| Time Point | Participants (n = 40) | General Population Norms† | Breast Cancer Survivors (n = 2582)‡ |
|---|---|---|---|
| Baseline | 45.3 ± 19.5§∥ | 58.4 ± 21.5§ | 58.8 ± 20.9§ |
| 2 Months | 43.5 ± 18.8§∥ | 58.4 ± 21.5§ | 58.8 ± 20.9§ |
| 6 Months | 49.8 ± 22.2§∥ | 58.4 ± 21.5§ | 58.8 ± 20.9§ |
| 12 Months | 56.4 ± 25.7∥ | 58.4 ± 21.5 | 58.8 ± 20.9 |
Data are presented as mean ± SD.
Higher scores = better health.
Norms for women from the general US population.11
Study by Bardwell et al of health-related quality of life in breast cancer survivors.8
Differences for means for participants versus general population and breast cancer survivor norms exceeded the 5-point criteria for clinical meaningfulness and were statistically significant (independent-samples t tests, P < .05).
Compared with scores at 12 months, differences for means for participants at baseline and 2 and 6 months exceeded the 5-point criteria for clinical meaningfulness and were statistically significant (1-sample t tests, P < .05).
Abbreviation: SF-36 = 36-Item Short-Form Health Survey
Table 3 shows simple correlations between Hb levels and fatigue scores. Hemoglobin levels at 2 months were strongly correlated with SF-36 Energy/Fatigue scores at 12 months (r = 0.71; P = .002). This linear, dose-response relationship is shown graphically in Figure 1. None of the other relationships were significant. To determine whether Hb levels at 6 or 12 months might explain the relationship between Hb at 2 months and SF-36 Energy/Fatigue at 12 months, a hierarchical regression analysis was conducted. Hemoglobin levels at 6 and 12 months were forced into the model first but did not explain a significant degree of variance in Energy/Fatigue scores (adjusted R2 = 0.125; F = 1.999; P = .178). Next, when Hb at 2 months was forced into the model, explained variance increased by 30.4% (adjusted R2 = 0.429; F = 3.916; P = .027). We also examined whether Hb at 2 months would explain the change in fatigue from 2 to 12 months by controlling for Energy/Fatigue scores at 2 months. Energy/Fatigue at 2 months was not a significant predictor of Energy/Fatigue at 12 months (adjusted R2 = 0.163; F = 3.916; P = .068). However, when scores for Hb at 2 months were entered, the model became significant (adjusted R2 = 0.487; F = 8.129; P = .005). In addition, we compared Hb levels at each time point for women with high (SF-36 Energy/Fatigue > 50) versus low (SF-36 Energy/Fatigue ≤ 50) fatigue at 12 months. No differences were observed for Hb at 6 or 12 months. However, the high-fatigue group at 12 months had lower Hb at 2 months than the low-fatigue group (13.3 g/dL vs. 11.8 g/dL; P = .032).
Table 3.
Pearson Correlations (r; P): Hemoglobin Levels Versus SF-36 Energy/Fatigue
| SF-36 Energy/Fatigue | Hemoglobin | ||
|---|---|---|---|
| 2 Months | 6 Months | 12 Months | |
| 2 Months | 0.208; .298 (n = 27) | 0.253; .213 (n = 26) | 0.190; .364 (n = 25) |
| 6 Months | −0.119; .598 (n = 23) | 0.329; .135 (n = 23) | 0.117; .614 (n = 22) |
| 12 Months | 0.712; .002 (n = 16) | 0.462; .072 (n = 16) | 0.356; .193 (n = 15) |
Abbreviation: SF-36 = 36-Item Short-Form Health Survey
Figure 1.
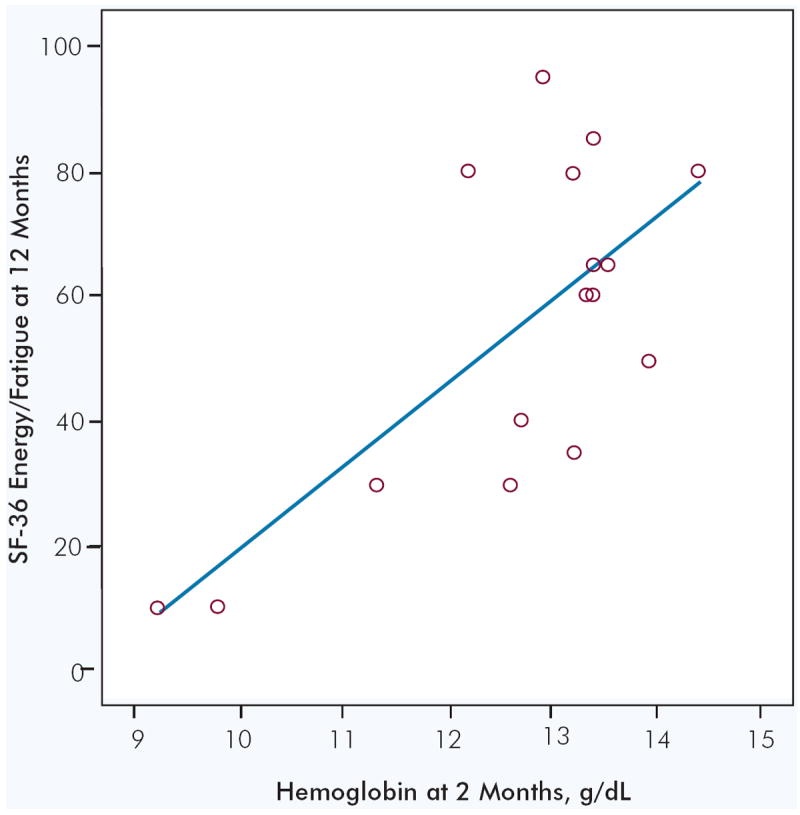
Scatter Plot of Relationship Between Hemoglobin at 2 Months and SF-36 Energy/Fatigue at 12 Months
r = 0.71; P = .002; n = 16. Each circle represents 1 patient.
Abbreviation: SF-36 = 36-Item Short-Form Health Survey
In exploratory analyses, we examined associations between Hb levels and the remaining SF-36 subscales. Significant relationships were observed for Hb levels at 6 months versus Role Limitations due to Physical Problems at 6 months (r = 0.44; P = .035) and Hb levels at 12 months versus Role Limitations due to Emotional Problems at 12 months (r = 0.54; P = .04).
Discussion
Our study includes a homogeneous population of women with early-stage breast cancer undergoing adjuvant chemotherapy, none of whom received ESAs or red blood cell transfusions. When compared with normative data from the general population and breast cancer survivors, fatigue in our patients was significantly poorer at baseline, 2 months, and 6 months. Indeed, mean fatigue scores for these women at baseline (within 1 month of initiating chemotherapy) and at 2 months were significantly into the “negative QOL” range. Patients were actively undergoing systemic therapy at these times and were expected to be more fatigued. That average fatigue scores at 12 months were identical to norms supports our contention about the role of active therapy contributing to fatigue.
We identified a significant relationship between Hb levels at 2 months and fatigue at 12 months. Despite the relatively small number of women included in this data set, the relationship was strong and consistent across the range of Hb levels, as shown in Figure 1. In addition, this association remained robust after controlling for subsequent Hb levels or fatigue at 2 months. After dividing the women into high- versus low-fatigue groups at 12 months, the groups differed on Hb levels only at 2 months. In fact, the group that would experience high fatigue at 12 months met current American Society of Clinical Oncology criteria for erythropoietin therapy at 2 months (mean Hb levels < 12 g/dL). Hemoglobin levels seemed to presage fatigue by 10 months in this study.12
We are uncertain about why we found a lack of contemporaneous links between Hb and fatigue across all time points. One possible explanation involves restriction of range for SF-36 fatigue at the earlier time points. Fatigue scores are lower and somewhat more tightly distributed at baseline through 6 months. It is only at 12 months that mean fatigue scores normalize and the distribution increases. That is, fatigue across the patients in this study was greater before the 12-month time point. Thus, early anemia might be indicative of fatigue levels only after fatigue scores have normalized at 12 months for those who did not have low Hb levels at 2 months.
Erythropoietin alfa was approved for patients with cancer by the US Food and Drug Administration on the basis of a demonstrated reduction in need for red blood cell transfusions during chemotherapy. However, the ESAs have not been shown to decrease fatigue or improve QOL in double-blind, placebo-controlled trials.13 Also, the use of ESAs in women with breast cancer might have adverse consequences. A randomized, double-blind, placebo-controlled trial testing the role of erythropoietin alfa was conducted in 939 women with metastatic breast cancer receiving chemotherapy. Although the primary endpoint of study was 1-year survival, the trial was stopped early because of an adverse outcome for patients receiving the ESA. Women assigned to the treatment arm had significantly shortened 1-year survival compared with the control group (70% vs. 76%; P = .0117). Shortened survival was attributed to both an increase in disease progression and thromboembolic complications in those receiving the ESA.14 Such data contributed to restriction on the use of ESAs in chemotherapy-induced anemia.15
Earlier clinical trials that have addressed fatigue and anemia generally followed up patients for < 6 months,13 yet breast cancer survivors consistently report fatigue years after completion of therapy and without evidence of recurrent cancer. We raise the possibility that the “stress” of adjuvant chemotherapy identifies a group of women who have a compromised “physiologic reserve.” By that, we mean they are physiologically older than their chronological age. In the geriatric population, anemia is an independent prognostic factor that correlates with shortened survival and predicts for hospitalizations.16-18 In the geriatric setting, Hb might be a marker for distinguishing physiologic age from chronologic age.19,20 Alternatively, one might consider that periods of sustained anemia produce hypoxia on normal tissues, and that can produce long-term effects on the body’s functional reserve, manifesting as fatigue.
Our study has a number of limitations. Because this study relied on a convenience sample, our findings might not be applicable to the general population of patients with breast cancer. Participants were accrued to the study after their surgical treatment and before the start of chemotherapy; we do not have Hb samples before surgical treatment. Although radiation therapy produces less fatigue that chemotherapy, it does cause fatigue, and 63% of women received radiation treatment during the 6-month time points. However, the 12-month fatigue levels should not be affected by radiation therapy. Another important limitation deals with sample size and statistical power. It is quite possible that the lack of significant relationships between Hb and fatigue at other time points is due to insufficient power. Therefore, this statistical limitation must be given consideration in the interpretation of these findings until a study with greater power can confirm or disconfirm these findings.
Conclusion
In summary, despite these limitations, the results raise the possibility that a decline in Hb after chemotherapy is a marker of physiologic reserve, independent of age. Further studies to test this hypothesis are warranted.
Acknowledgments
Supported by grant CA72554-02 from the National Institutes of Health.
References
- 1.Thompson P. The relationship of fatigue and meaning in life in breast cancer survivors. Oncol Nurs Forum. 2007;34:653–60. doi: 10.1188/07.ONF.653-660. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA, Bower JE. Cancer related fatigue: a focus on breast cancer and Hodgkin’s disease survivors. Acta Oncol. 2007;46:474–9. doi: 10.1080/02841860701367845. [DOI] [PubMed] [Google Scholar]
- 3.Schultz PN, Klein MJ, Beck ML, et al. Breast cancer: relationship between menopausal symptoms, physiologic health effects of cancer treatment and physical constraints on quality of life in long-term survivors. J Clin Nurs. 2005;14:204–11. doi: 10.1111/j.1365-2702.2004.01030.x. [DOI] [PubMed] [Google Scholar]
- 4.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616–34. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 5.Bardwell WA, Moore P, Ancoli-Israel S, et al. Fatigue in obstructive sleep apnea: driven by depressive symptoms instead of apnea severity? Am J Psychiatry. 2003;160:350–5. doi: 10.1176/appi.ajp.160.2.350. [DOI] [PubMed] [Google Scholar]
- 6.Bower JE, Ganz PA, Aziz N, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–11. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Bower JE, Ganz PA, Aziz N, et al. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277–80. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- 8.Ware JE., Jr SF-36 Health Survey update. Spine. 2000;25:3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 10.Hanson Frost M, Suman VJ, Rummans TA, et al. Physical, psychological and social well-being of women with breast cancer: the influence of disease phase. Psychooncology. 2000;9:221–31. doi: 10.1002/1099-1611(200005/06)9:3<221::aid-pon456>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Bardwell WA, Major JM, Rock CL, et al. Health-related quality of life in women previously treated for early-stage breast cancer. Psychooncology. 2004;13:595–604. doi: 10.1002/pon.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. J Clin Oncol. 2002;20:4083–107. doi: 10.1200/JCO.2002.07.177. [DOI] [PubMed] [Google Scholar]
- 13.Seidenfeld J, Piper M, Flamm C, et al. Epoetin treatment of anemia associated with cancer therapy: a systematic review and meta-analysis of controlled clinical trials. J Natl Cancer Inst. 2001;93:1204–14. doi: 10.1093/jnci/93.16.1204. [DOI] [PubMed] [Google Scholar]
- 14.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–72. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 15.The Cancer Letter. 2007 May 18;33 [Google Scholar]
- 16.Denny SG, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–34. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Chaves PH, Xue QL, Guralnik JM, et al. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52:1811–6. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 18.Penninx BW, Guralnik JM, Onder G, et al. Anemia and decline in physical performance among older persons. Am J Med. 2003;115:104–10. doi: 10.1016/s0002-9343(03)00263-8. [DOI] [PubMed] [Google Scholar]
- 19.Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systemactic review of the literature. Am J Med. 2004;116(suppl 1):3–10. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–20. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]


