Abstract
Data show that viral genotype 1 may increase the risk of cirrhosis and hepatocellular carcinoma (HCC) compared to genotype 2 in patients with chronic hepatitis C virus (HCV) infection. However, the effect of HCV genotype 3 on cirrhosis and HCC risk is uncertain. We identified patients with active HCV infection, confirmed by positive PCR and a known HCV genotype, from the VA HCV Clinical Case Registry between 2000 and 2009. We examined the effect of HCV genotype on the risk of cirrhosis and HCC in a Cox proportional hazards model adjusting for patients’ age, period of service (World War I/II, Vietnam era, post-Vietnam era), race, gender, HIV infection, alcohol use, diabetes, body mass index, and antiviral treatment receipt. Of the 110,484 patients with active HCV viremia, 88,348 (79.9%) had genotype 1, 13,077 (11.8%) genotype 2, 8337 (7.5%) genotype 3, and 1082 (0.9%) patients had genotype 4 infection. Despite being younger, patients with genotype 3 had a higher risk of developing cirrhosis (unadjusted hazard ratio, HR=1.40, 95% CI=1.32–1.50) and HCC (unadjusted HR=1.66, 95% CI=1.48–1.85) than HCV genotype 1 patients. After adjustment for pre-specified demographic, clinical, and antiviral treatment factors, the risk of cirrhosis and HCC was 31% (adjusted HR=1.31, 95% CI=1.22–1.39) and 80% (adjusted HR=1.80, 95%CI=1.61–2.03) higher in patients with genotype 3 compared to genotype 1 infected patients.
Conclusion
HCV genotype 3 is associated with a significantly increased risk of developing cirrhosis and HCC compared to HCV genotype 1. This association is independent of patients’ age, diabetes, body mass index, or antiviral treatment.
Keywords: Cohort, longitudinal, Veterans Administration, viral factors, association
BACKGROUND
Chronic infection with hepatitis C virus (HCV) is a common and progressive condition. Of the estimated ~4 million persons in the U.S. who are chronically infected with HCV, up to one-third will progress to advanced fibrosis and cirrhosis—a subset at high risk for subsequent complications, including hepatocellular cancer (HCC).(1–3) Several host factors are associated with increased risk of cirrhosis and HCC in HCV. These include older age at infection, longer duration of infection, male sex, alcohol consumption > 50 g/day, human immunodeficiency virus (HIV) co-infection, high body mass index (BMI), and diabetes.(4, 5)
In addition, viral factors— particularly HCV viral genotype—may influence the natural course of HCV. Several published studies show that HCV genotype 1 infection may increase the risk of cirrhosis and HCC compared to the other HCV genotypes,(6) although a birth cohort effect in which patients with genotype 1 infection were infected earlier than those with other genotypes cannot be excluded. The effect of HCV genotype 3 on cirrhosis and HCC risk in U.S cohorts has been less clear. Evolving data suggest that HCV genotype 3 may have a negative impact on histological and clinical outcomes in patients with HCV. Specifically, HCV genotype 3 was associated with faster progression of fibrosis in a recent meta-analysis of cross-sectional single-biopsy studies.(7) In addition, few recent cohort studies found that patients with HCV genotype 3 may be at a greater risk for HCC and all-cause mortality than patients with other HCV genotypes.(8–10)
These data, although suggestive of high risk of cirrhosis and HCC with genotype 3, are limited by relatively small sample of patients with HCV genotype 3 in previous studies and inability to adjust for the range of factors associated with risk of cirrhosis and HCC. Furthermore, available studies combined disparate clinical outcomes into one composite endpoint or aggregated data across multiple HCV genotypes into one comparison group. Consequently, the risk of cirrhosis and HCC attributable to the full range of viral genotypes in HCV remains unclear. Moreover, most cohorts were from outside the U.S. with limited applicability to diverse populations of HCV-infected patients in the U.S.(9, 10) There are no data on the effect of HCV viral genotype on the risk of cirrhosis and HCC (and progression from cirrhosis to HCC) in U.S. populations infected with HCV while accounting for racial case-mix, clinical, and birth cohort factors.
Examining the relationship between HCV genotype 3 and clinically meaningful outcomes in HCV has become particularly relevant as we await the new direct acting antiviral agents (DAAs). Recent data show that sustained viral response rates with the combination of sofosbuvir and ribavirin may be substantially lower in patients with genotype 3 (~56–60%) compared to those with genotype 2 infection (>90%).(11, 12) A significant proportion of patients with HCV genotype 3 may thus remain at risk for progression to cirrhosis and HCC. Therefore, if genotype 3 is associated with accelerated disease progression, then HCV genotype status may have a major role as a risk screener, with implications in regards to prevention and screening.
We conducted a retrospective cohort study of approximately 110,000 US veterans with chronic HCV infection and an average follow up of over 5 years to examine the differences between HCV genotypes in the risk of progression to cirrhosis and HCC, as well as the risk of progression from cirrhosis to HCC.
METHODS
Data Sources
This study was approved by Baylor College of Medicine’s Institutional Review Board and all procedures conform to the ethical guidelines of the 1975 Declaration of Helsinki. We used data from the VA HCV Clinical Case Registry (CCR), which contains health information for all known HCV-infected patients from 128 VA facilities nationwide. Data elements in the CCR include demographics; laboratory test results; outpatient and inpatient VA pharmacy data; and inpatient and outpatient diagnoses codes. Additional details of the CCR data are published elsewhere.(13) We examined data sets obtained from the VA HCV CCR database for patients diagnosed with HCV in the VA between October 1, 1999 (fiscal year 2000) and September 31, 2009 (fiscal year 2009).
Study Population
The study cohort included patients with chronic HCV infection, defined as a positive test for HCV RNA in plasma by qualitative or quantitative assays or detectable HCV genotype between VA fiscal year 2000 and 2009. Patients had to be 18 to 90 years old to be included in the study. We excluded patients with less than one year of follow up to minimize bias related to incomplete ascertainment of patients’ cirrhosis and HCC risk. We defined the date of first positive HCV RNA as the index date for this analysis.
Study Exposure
HCV genotype was categorized as 1, 2, 3, and 4. We excluded patients without a documented HCV genotype test (n=51,000) from the primary analysis. We also excluded 33 patients with genotype 5 or 6 because this represented a very small subgroup of patients with HCV.
Study Outcomes
The primary outcomes of the study were new cases (incident cases) of cirrhosis (ICD-9 codes 571.2, 571.5, 571.6) and HCC (ICD-9 code 155.1) that were first recorded after one year of the HCV index date. In secondary analyses, we also examined the association between viral genotypes and cirrhosis and HCC recorded during the 3 years before and one year after the HCV index date (prevalent cases). Our ICD-9 code based definitions for both cirrhosis and HCC was validated in our previous studies against detailed chart reviews and shown to have a high positive predicative value.(14)
To calculate the incidence of cirrhosis, we excluded patients who had prevalent diagnosis of cirrhosis from the denominator. For the HCC incidence calculation, we excluded patients who had a prevalent diagnosis of either cirrhosis or HCC from the denominator. The study follow up ended at the time of HCC, patient’s death, last visit in the VA, or September 30, 2009.
Potential confounders
We ascertained several risk factors that may be associated with HCV genotype and an accelerated progression to cirrhosis and HCC in patients with HCV: age at the time of HCV diagnosis, year of birth, period of service (World War I/II, Vietnam era, post-Vietnam era), race, gender, diabetes, alcohol use, obesity, HIV infection, and receipt and success of antiviral treatment. We identified HIV, diabetes, and alcohol use by the presence of outpatient or inpatient ICD-9 diagnosis codes recorded during one year before or after the HCV index date. We classified a patient as obese if the BMI closest to the HCV index date was 30 kg/m2 or greater. We defined antiviral treatment as at-least one filled prescription of interferon or pegylated interferon any time after HCV index date. We defined sustained virologic response (SVR) as all RNA tests being negative after treatment completion with one being recorded at least 12 weeks after treatment completion, as previously described.(15)
Data Analysis
For the primary analyses (incidence), we calculated the incidence rates per 1000 person-years of follow up for newly diagnosed cirrhosis and HCC for each HCV genotype. We generated Kaplan-Meier curves to illustrate and compare the cumulative incidence of cirrhosis and HCC in the four HCV genotype groups (genotype 1, 2, 3 and 4) starting 12 months after the HCV index date till the end of follow up period. We used the log rank test to evaluate the differences among these rates.
We constructed 2 separate Cox proportional hazards models to examine the association between HCV genotype and time to cirrhosis or HCC while adjusting for potential confounders. We also conducted several pre-specified subgroup analyses. Specifically, we limited the analysis to only those patients with documented cirrhosis and considered incident HCC cases that were recorded after one year of the cirrhosis index date (defined as the first instance of cirrhosis ICD-9 code). We also conducted stratified analyses by patients’ age (<50 years, >50 years), race (white, African American), and diabetes status to determine if the effect of HCV genotype 3 on cirrhosis and HCC risk was differential across demographic and clinical subgroups. We conducted three sensitivity analyses 1) using the using year of birth in lieu of patients’ age to adjust for birth cohort effect; 2) restricting our cohort to patients with at-least 2 years of follow up in the VA; and 3) excluding patients if they developed HCC within 3 years after the index date to ensure that we did not misclassify prevalent cirrhosis and HCC.
The results of these regressions are expressed as hazard ratios (HR) and corresponding 95% confidence intervals (CIs). The proportional hazard assumption was tested and fulfilled in all models.
For our secondary analyses (prevalence), we calculated the proportions (and their accompanying 95% confidence intervals) of patients with prevalent cirrhosis and HCC for each HCV genotype group. We used 2 separate logistic regression analyses to examine the association between HCV genotype and prevalent cirrhosis and HCC while adjusting for potential confounders described above. We reported these results as odd ratios (OR) and 95% CI.
We used SAS version 9.1 (SAS Institute, Cary, NC) to conduct all analyses.
RESULTS
Our study cohort included 110,484 patients with HCV (Table 1) who were followed for a mean of 5.4 years (standard deviation, SD, 2.5 years). The mean age was 51.9 years (SD 6.7 years), almost all were male, 52.6% were White, and 33.2% were African Americans. Most patients were Vietnam era Veterans (70.2%). Approximately 11% had diabetes, 38% had BMI ≥ 30, 51.2% had a diagnosis of alcohol abuse, and 4.3% had HIV co-infection. A total of 21.7% received antiviral treatment and 6.9% achieved SVR.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Variables | All patients (n=110 484) | HCV Genotype 1 (n=88 348) | HCV Genotype 2 (n=13 077) | HCV Genotype 3 (n=8 337) | HCV Genotype 4 (n=1 082) | P-value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age in years mean (SD) | 51.9 (6.7) | 51.9 (6.6) | 52.7 (7.5) | 50.2 (6.4) | 51.5 (6.3) | <0.0001 |
| Male gender, (%) | 97.0 | 97.0 | 96.9 | 97.1 | 97.4 | 0.74 |
| Race, (%) | <0.0001 | |||||
| White | 52.6 | 47.5 | 71.9 | 76.2 | 44.3 | |
| African American | 33.2 | 39.3 | 10.1 | 5.3 | 37.2 | |
| Hispanic | 5.6 | 5.1 | 7.7 | 7.4 | 9.4 | |
| Others | 1.5 | 1.5 | 1.8 | 1.7 | 1.3 | |
| Missing | 7.0 | 6.6 | 8.5 | 9.1 | 7.3 | |
| Period of Service, (%) | <0.0001 | |||||
| World War I/II | 4.3 | 4.1 | 7.1 | 2.1 | 2.8 | |
| Vietnam | 70.2 | 70.6 | 70.3 | 66.5 | 70.1 | |
| Post-Vietnam | 25.5 | 25.3 | 22.6 | 31.4 | 27.2 | |
| Clinical, (%) | ||||||
| Yes of HCV diagnosis | ||||||
| 2000–2002 | 36.0 | 36.2 | 35.4 | 35.5 | 34.9 | 0.04 |
| 2003–2005 | 38.5 | 38.3 | 39.5 | 38.1 | 37.9 | |
| 2005–2009 | 25.5 | 25.4 | 25.1 | 26.3 | 27.2 | |
| Diabetes | 11.1 | 11.7 | 9.3 | 7.3 | 13.5 | <0.0001 |
| Alcohol use | 51.2 | 51.7 | 46.4 | 53.1 | 52.4 | <0.0001 |
| HIV coinfection | 4.3 | 4.6 | 2.5 | 2.9 | 5.7 | <0.0001 |
| Body mass index | ||||||
| <18.5 | 1.3 | 1.3 | 0.9 | 1.3 | 0.9 | <0.0001 |
| 18.5–24.9 | 31.9 | 32.1 | 29.4 | 33.7 | 28.3 | |
| 25.0–29.9 | 39.4 | 39.6 | 38.4 | 38.7 | 43.5 | |
| >30 or higher | 28.3 | 27.9 | 31.7 | 27.2 | 27.9 | |
| Antiviral treatment | ||||||
| No treatment | 78.5 | 80.4 | 70.8 | 70.6 | 80.4 | <0.0001 |
| Sustained response | 6.9 | 4.9 | 16.4 | 14.0 | 4.7 | |
| No response | 7.9 | 8.3 | 5.4 | 7.8 | 8.3 | |
| Undeterminable | 6.6 | 6.4 | 7.4 | 7.6 | 6.6 |
A total of 88,348 patients (79.9%) had HCV genotype 1, 13,077 (11.8%) genotype 2, 8337 (7.5%) genotype 3, and 1082 (0.9%) patients had genotype 4 infection. There were significant demographic and clinical differences among the HCV genotype groups (Table 1). Patients with genotype 3 were younger (mean age, 50.2 year, SD 6.4 year) whereas those with genotype 2 were older (mean age 52.7 year, SD 7.5 year) than patients with genotype 1 infection (mean age, 51.9 year, SD 6.6 year) (p<0.0001). Genotype 3 patients were more likely to have served in the post Vietnam era (31.4%) compared to HCV genotype 1 (25.3%) and 2 patients (22.6%) (p<0.0001). Both genotype 2 and 3 patients were more likely to be white non-Hispanic compared to genotype 1 patients. HCV genotype 3 patients were less likely to have diabetes, HIV co-infection, and had lower BMI than genotype 1 patients. As expected, significantly more patients with HCV genotypes 2 and 3 received antiviral treatment and achieved SVR compared to genotype 1 patients. HCV genotype 4 patients were more likely to be Hispanics and diabetics than HCV genotype 1 patients.
Association between HCV genotype and risk of incident cirrhosis and HCC
After an overall follow up of 589,205 person-years, 11,306 (11.1%) patients developed cirrhosis for an incidence rate of 19.2 per 1000 person-years, and 2854 (2.6%) patients developed HCC for an incidence rate of 4.8 per 1000 person-years. The incidence rates of cirrhosis were 21.5 (95% CI=21.1–21.9), 16.6 (95% CI=15.6–17.7), 30.0 (95% CI=28.2–31.8), and 20.4 (95% CI=16.8–24.7) per 1000 person-years in patients with genotype 1, 2, 3 and 4, respectively. Similarly, the incidence rates for HCC were 4.8 (95% CI=4.6–5.1) per 1000 person-years for genotype 1, 2.9 (95% CI=2.5–3.3) per 1000 person-years for genotype 2, 7.9 (95% CI=7.1–8.8) per 1000 person-years for genotype 3, and 4.7 (95% CI=3.3–6.9) per 1000 person -years for genotype 4 patients.
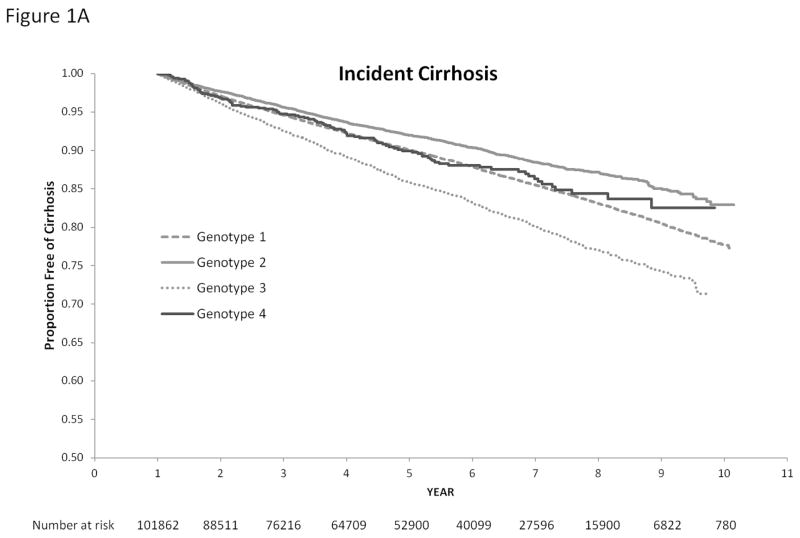
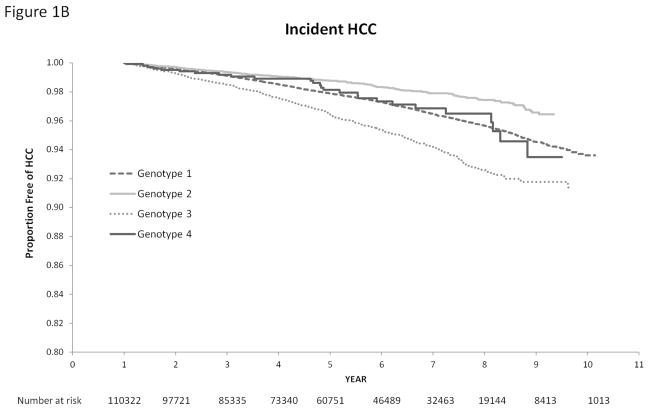
Figure 1 displays the relationship of HCV genotypes with the cumulative incidence of cirrhosis and HCC. HCV genotype was strongly associated with time until development of cirrhosis and HCC (log rank test p value <0.0001). In univariate Cox analyses, HCV genotype 3 was associated with a 40% increase in the risk of cirrhosis (unadjusted HR=1.40, 95% CI=1.32–1.50) and 66% increase in the risk of HCC unadjusted (HR=1.66, 95% CI=1.48–1.85) compared with HCV genotype 1 infection. Compared to patients with HCV genotype 1, those with genotype 2 had a lower risk of cirrhosis (unadjusted HR, HR=0.77, 95% CI=0.73–0.82) and HCC (unadjusted HR=0.60, 95% CI=0.52–0.69). There was no statistical difference in the risk of cirrhosis and HCC in HCV genotype 1 and genotype 4 patients.
Figure 1.
Cumulative Incidence of Cirrhosis (1A) and Hepatocellular Cancer (1B) in Patients with HCV Genotypes 1, 2, 3 and 4. We use the log rank test to test the differences among these rates. HCC-hepatocellular cancer
We examined the independent association between HCV genotypes and risk of incident cirrhosis and HCC after adjusting for pre-specified demographic, clinical, and treatment factors. The adjusted HRs are displayed in Table 2. The risk of cirrhosis and HCC was 31% (adjusted HR=1.31, 95% CI=1.22–1.39) and 80% (adjusted HR=1.80, 95%CI=1.61–2.03) greater in patients with HCV genotype 3 compared to genotype 1.
Table 2.
Association between HCV Genotypes and Risk of Incident Cirrhosis (column 2) and Hepatocellular Cancer (column 3) – Results of Multivariable Cox Regression Analyses
| Characteristics | Adjusted Hazard Ratio (95% confidence Interval) | |
|---|---|---|
| Incident Cirrhosis | Incident HCC | |
| HCV genotype | ||
| 1 | 1.0 | 1.0 |
| 2 | 0.68 (0.64, 0.73) | 0.55 (0.47, 0.63) |
| 3 | 1.30 (1.22, 1.39) | 1.80 (1.60, 2.03) |
| 4 | 0.94 (0.78, 1.14) | 0.99 (0.68, 1.45) |
| Demographics | ||
| Age | 1.03 (1.02, 1.03) | 1.07 (1.06, 1.08) |
| Gender | ||
| Female | 1.0 | 1.0 |
| Male | 1.16 (1.03, 1.32) | 2.78 (1.77, 4.38) |
| Race | ||
| White | 1.0 | 1.0 |
| African American | 0.58 (0.56, 0.61) | 0.73 (0.66, 0.79) |
| Hispanic | 1.28 (1.19, 1.37) | 1.53 (1.34, 1.74) |
| Others | 0.89 (0.79, 1.05) | 0.86 (0.63, 1.19) |
| Missing | 0.79 (0.73, 0.86) | 0.82 (0.69, 0.98) |
| Period of Service | ||
| World War I/II | 1.0 | 1.0 |
| Vietnam | 1.29 (1.16, 1.44) | 1.38 (1.15, 1.66) |
| Post-Vietnam | 1.12 (0.98, 1.29) | 0.95 (0.74, 1.22) |
| Clinical factors | ||
| Yes of HCV diagnosis | ||
| 2000–2002 | 1.0 | 1.0 |
| 2003–2005 | 1.09 (1.04, 1.14) | 1.24 (1.14, 1.36) |
| 2005–2009 | 1.36 (1.27, 1.46) | 1.94 (1.68, 2.24) |
| Diabetes | ||
| No | 1.0 | 1.0 |
| Yes | 1.34 (1.26, 1.42) | 1.34 (1.21, 1.49) |
| Alcohol use | ||
| No | 1.0 | 1.0 |
| Yes | 1.16 (1.12, 1.20) | 1.21 (1.12, 1.30) |
| HIV co-infection | ||
| No | 1.0 | 1.0 |
| Yes | 1.09 (1.00, 1.20) | 0.91 (0.75, 1.13) |
| Body mass index | ||
| <18.5 | 1.0 | 1.0 |
| 18.5 – 24 | 1.05 (0.86, 1.27) | 1.18 (0.82, 1.72) |
| 25 – 29 | 1.18 (0.97, 1.43) | 1.14 (0.78, 1.64) |
| ≥30 | 1.42 (1.18, 1.73) | 1.21 (0.83, 1.75) |
| Antiviral treatment | ||
| No treatment | 1.0 | 1.0 |
| Sustained response | 0.74 (0.68, 0.81) | 0.36 (0.29, 0.46) |
| No response | 1.99 (1.89, 2.10) | 1.30 (1.16, 1.46) |
The results of subgroup analyses are shown in Table 3. Genotype 3 was associated with a higher risk of HCC in all subgroups, although the magnitude was somewhat attenuated for African Americans and patients with diabetes. Limiting the analysis to only patients with cirrhosis did not change the direction of magnitude of the association between HCV genotypes and HCC. Similarly replacing patients’ age with the year of birth, restricting our analysis to patients with at-least 2 years of follow, or excluding patients who developed HCC within 3 years after the index date did not affect the results (adjusted HRs for incident HCC in HCV genotype 3 versus genotype 1 patients=1.83, 95% CI=1.60–1.95; 1.77, 95% CI=1.56–2.02; and 1.80, 95% CI=1.60–2.03, respectively).
Table 3.
Association between HCV Genotypes (1–4) and Risk of Incident Hepatocellular Cancer in Subgroup Analyses – Table presents adjusted hazard ratios from Cox regression models in various defined subgroups
| Defined subgroup | * Adjusted hazard ratio (95% confidence interval) |
|---|---|
| Patients with cirrhosis (n=21,716) | |
| 1 | 1.0 |
| 2 | 0.62 (0.50, 0.77) |
| 3 | 1.44 (1.23, 1.68) |
| 4 | 0.96 (0.96, 1.22) |
| Younger patients (50 year and younger) (n=55,424) | |
| 1 | 1.0 |
| 2 | 0.34 (0.24, 0.46) |
| 3 | 1.86 (1.56, 2.22) |
| 4 | 1.21 (0.71, 2.05) |
| Older patients (older than 50 year) (n=54,898) | |
| 1 | 1.0 |
| 2 | 0.65 (0.55, 0.76) |
| 3 | 1.79 (1.53, 2.11) |
| 4 | 0.81 (0.47, 1.40) |
| White (n=57,970) | |
| 1 | 1.0 |
| 2 | 0.59 (0.49, 0.70) |
| 3 | 1.93 (1.68, 2.21) |
| 4 | 1.66 (1.07, 2.56) |
| African American (n=36,693) | |
| 1 | 1.0 |
| 2 | 0.44 (0.26, 0.73) |
| 3 | 1.23 (0.67, 2.37) |
| 4 | 0.40 (0.05, 1.00) |
| Patients without diabetes (n=98,143) | |
| 1 | 1.0 |
| 2 | 0.55 (0.47, 0.64) |
| 3 | 1.87 (1.65, 2.12) |
| 4 | 1.13 (0.76, 1.67) |
| Patients with diabetes (n=12,179) | |
| 1 | 1.0 |
| 2 | 0.54 (0.36, 0.80) |
| 3 | 1.30 (1.88, 1.90) |
| 4 | 0.38 (0.09, 1.53) |
Adjusted for age at the time of HCV diagnosis, year of birth, period of service (World War I/II, Vietnam era, post-Vietnam era), race, gender, diabetes, alcohol use, obesity, HIV infection, and receipt and success of antiviral treatment
Association between HCV genotype and prevalent cirrhosis and HCC
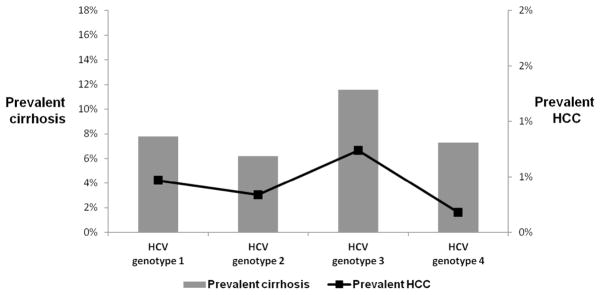
A total of 8769 (7.9%) and 522 (0.5%) patients had prevalent cirrhosis and HCC (diagnosed within one year of HCV index date). Figure 3 displays the distribution of prevalent cases of both cirrhosis and HCC stratified by HCV genotype. Patients with HCV genotype 3 were 53% (unadjusted OR=1.53, 95% CI=1.43–1.65) and 59% (unadjusted OR=1.59, 95% CI=1.22–2.08) more likely to have both prevalent cirrhosis and HCC compared to genotype 1 patients. Adjusting for potential confounders did not change the magnitude or direction of the associations between genotype 3 and cirrhosis or HCC (Appendix Table).
DISCUSSION
In this large U.S. cohort of patients with HCV, we found that HCV genotype was significantly associated with the risk of developing cirrhosis and HCC. Specifically, we found that patients with HCV genotype 3 were 31% and 80% more likely to develop cirrhosis and HCC, respectively compared to patients with the most common HCV genotype 1 infection. In contrast, infection with HCV genotype 2 was associated with a decreased risk of subsequently developing HCC and cirrhosis relative to genotype 1. We also found that the negative effect of HCV genotype 3 persisted after we adjusted for confounders in the multivariable regression analyses, in the pre-specified sensitivity analyses, and was consistent across several subgroups based on age, race, and diabetes. HCV genotype 3 also significantly increased the risk of HCC when we limited the analysis to patients with documented cirrhosis.
Other than a true causal association, we considered several possibilities as potential explanations underlying the association between HCV genotype 3 and adverse clinical outcomes. First, it is plausible that HCV genotype 3 entered the U.S. population earlier than other genotypes. If this were to be the case then patients with genotype 3 might be older and also have had the infection for a longer duration; this birth cohort effect could translate into a higher risk of cirrhosis and HCC. However, we found patients with genotype 3 HCV were indeed younger and likely to have acquired the infection later than individuals infected with other genotype, rendering this explanation untenable. Second, genotype 3 (and 2) has traditionally been considered easier to treat compared with genotype 1 and 4 infections. Indeed, we found that a significantly higher proportion of patients with genotype 3 received and subsequently responded to antiviral treatment than genotype 1. This therapeutic advantage, however, did not counterbalance the negative impact of genotype 3 on cirrhosis and HCC risk; therefore the observed negative association could have been even greater. Third, we considered the possibility of a bias introduced by our analytical technique where we excluded patients with prevalent cirrhosis or HCC from the main analyses (i.e. incidence prevalence bias). Specifically, differential exclusion of patients with prevalent cirrhosis or HCC from non-genotype 3 groups could have spuriously magnified the association between genotype 3 and study endpoints. In order to guard against this possible bias, we constructed separate models that examined the association between HCV genotypes and prevalent cirrhosis and HCC and found similar results. Collectively, these data demonstrate that HCV genotype 3 contributes to excess risk of cirrhosis and HCC in patients with HCV.
HCV genotype 3-associated hepatic steatosis results from a direct viral effect that is independent of other predisposing conditions such as overweight, diabetes, or alcohol use. (16–18) Indeed, patients with HCV genotype 3 in our cohort were less likely to be obese or to have diabetes—findings that were consistent with previous reports.(9, 18) Hepatic steatosis may underlie the accelerated fibrosis observed in genotype 3 infection. (18, 19) However, due to lack of information on hepatic steatosis in our database we could not adjust for it in our analysis. Age at the time of HCV infection is correlated with the risk of fibrosis progression, but the study database did not include information on the estimated date of HCV acquisition. Although patients with HCV genotype 3 were younger than those with other genotypes, age at first HCV diagnosis may not correspond with the age at infection. Therefore, it remains possible that patients with HCV genotype 3 may have been infected at an older age compared to those with genotype 1 resulting in more rapid progression of fibrosis. Differences in host genetic factors not captured by our study may also explain some of the observed associations between HCV genotypes and cirrhosis and HCC risk. For example, recent studies found that the C allele at IL28B related single nucleotide polymorphism is more common in Caucasian patients with HCV genotypes 2 and 3 than in patients with HCV genotype 1 infection.(20, 21) IL28B CC genotype may be associated with an increased risk of advanced fibrosis, although the available data show mixed results.(22) Nonetheless, differences in host genotype per se are unlikely to explain the opposing effects of HCV genotypes 2 and 3 on the risk of cirrhosis and HCC.
Our findings have implications that involve the entire spectrum of care from antiviral treatment to prevention and screening in patients with HCV genotype 3 infection. Given the accelerated progression to advanced liver disease, patients with HCV genotype 3 may serve as a high-risk group that will need to be prioritized in the era of new antiviral treatments. Unfortunately, SVR rates with the all-oral regimen combination of sofosbuvir and ribavirin may be lower in patients with genotype 3 compared to those with genotype 2 infection (11, 12). Newer and more efficacious treatments for HCV genotype 3 patients may eventually become available. However, our data show that a substantial proportion of patient with genotype 3 infection already have developed cirrhosis (24.6%); these patients will likely remain at risk for HCC regardless of whether they receive or respond to antiviral treatment. Thus, HCV genotype 3 infection may have a major role to screen for patients who are at an increased risk for HCC.
Our study is limited by the observational retrospective nature of its design. However, large prospective studies with sufficient long term follow up to document clinical outcomes in HCV are not likely to be forthcoming due to cost and feasibility issues. Furthermore, the absence of temporal ambiguity combined with the consistency of our results across several subgroups and in pre-specified sensitivity analyses, and biological plausible mechanism of effect collectively suggest that HCV genotype 3 is causally linked with a higher risk of cirrhosis and HCC. Our results were derived from diagnosed HCV infected patients who sought care in the VA healthcare system, and although the generalizability of the biologic process of cirrhosis progression probably extends to other HCV infected individuals in the VA as well as nonveterans, further research would be needed to confirm that. We were also limited by the validity of ICD-9 coding system, which may vary within the VA facilities as well as between VA and non-VA practitioners. Finally, while we accounted for dispensed prescriptions, we did not have information on adherence with HCV antiviral medication; therefore, we were unable to account for differences in treatment adherence across patients with different genotypes.
In summary, HCV genotype 3 was significantly associated with the risk of developing cirrhosis and HCC. This association is independent of patients’ age or year of birth and persisted after adjusting for a range of factors including diabetes and BMI. These data are relevant to the thousands of HCV patients with genotype 3 infection, and to their physicians who provide care and counseling to this population. Our results are also important from a healthcare system standpoint and may be useful in prioritizing the next generation/s of DAA so that the patients in greater need receive the treatment in an equitable and timely manner.
Figure 2.
Prevalence of Cirrhosis and Hepatocellular Cancer (HCC) at the Time of HCV Diagnosis Stratified by Hepatitis C Virus Genotypes (1–4)
Acknowledgments
GRANT SUPPORT: This material is based upon work supported in part by the Houston VA HSR&D Center of Excellence (HFP90-020).
LIST OF ABBREVIATIONS
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- HIV
human immunodeficiency virus infection
- BMI
body mass index
- DAA
direct acting antiviral agent
- CCR
Clinical Case Registry
- SVR
sustained virologic response
- OR
odds ratio
Footnotes
CONFLICT OF INTEREST: No conflict of interest to declare
DISCLAIMER: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veterans Affairs
Reference List
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006 May 16;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010 Feb;138(2):513–21. 521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F, Kramer J, Asch SM, El-Serag H, Spiegel BM, Edmundowicz S, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010 Aug;8(8):709–717. doi: 10.1016/j.cgh.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008 May;134(6):1699–1714. doi: 10.1053/j.gastro.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 5.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002 Nov;36(5 Suppl 1):S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 6.Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009 Jun;50(6):1142–1154. doi: 10.1016/j.jhep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Probst A, Dang T, Bochud M, Egger M, Negro F, Bochud PY. Role of hepatitis C virus genotype 3 in liver fibrosis progression--a systematic review and meta-analysis. J Viral Hepat. 2011 Nov;18(11):745–759. doi: 10.1111/j.1365-2893.2011.01481.x. [DOI] [PubMed] [Google Scholar]
- 8.McMahon BJ, Bruden D, Bruce MG, Livingston S, Christensen C, Homan C, et al. Adverse outcomes in Alaska natives who recovered from or have chronic hepatitis C infection. Gastroenterology. 2010 Mar;138(3):922–931. doi: 10.1053/j.gastro.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 9.Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011 Oct;18(10):e516–e522. doi: 10.1111/j.1365-2893.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012 Dec 26;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013 May 16;368(20):1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 12.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013 May 16;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 13.Backus LI, Gavrilov S, Loomis TP, Halloran JP, Phillips BR, Belperio PS, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009 Nov;16(6):775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008 Feb 1;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 15.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007 Jul;46(1):37–47. doi: 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 16.Castera L, Hezode C, Roudot-Thoraval F, Lonjon I, Zafrani ES, Pawlotsky JM, et al. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004 Mar;53(3):420–424. doi: 10.1136/gut.2002.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piodi A, Chouteau P, Lerat H, Hezode C, Pawlotsky JM. Morphological changes in intracellular lipid droplets induced by different hepatitis C virus genotype core sequences and relationship with steatosis. Hepatology. 2008 Jul;48(1):16–27. doi: 10.1002/hep.22288. [DOI] [PubMed] [Google Scholar]
- 18.Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E, et al. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004 Mar;53(3):406–412. doi: 10.1136/gut.2003.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstal R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002 Dec;37(6):837–842. doi: 10.1016/s0168-8278(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 20.Lagging M, Askarieh G, Negro F, Bibert S, Soderholm J, Westin J, et al. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6(2):e17232. doi: 10.1371/journal.pone.0017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ydreborg M, Westin J, Rembeck K, Lindh M, Norrgren H, Holmberg A, et al. Impact of IL28B-Related Single Nucleotide Polymorphisms on Liver Transient Elastography in Chronic Hepatitis C Infection. PLoS One. 2013;8(11):e80172. doi: 10.1371/journal.pone.0080172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noureddin M, Wright EC, Alter HJ, Clark S, Thomas E, Chen R, et al. Association of IL28B genotype with fibrosis progression and clinical outcomes in patients with chronic hepatitis C: A longitudinal analysis. Hepatology. 2013 Nov;58(5):1548–1557. doi: 10.1002/hep.26506. [DOI] [PMC free article] [PubMed] [Google Scholar]