Abstract
Meaningful social interactions modify behavioral responses to sensory stimuli. The neural mechanisms underlying the entrainment of neutral sensory stimuli to salient social cues to produce social learning remains unknown. We used odor-driven behavioral paradigms to ask if oxytocin, a neuropeptide implicated in various social behaviors, plays a crucial role in the formation of learned associations between odor and socially significant cues. Through genetic, optogenetic and pharmacological manipulations, we show that oxytocin receptor signaling is crucial for entrainment of odor to social cues, but is dispensable for entrainment to non-social cues. Furthermore, we demonstrate that oxytocin directly impacts the piriform, the olfactory sensory cortex, to mediate social learning. Lastly, we provide evidence that oxytocin plays a role in both appetitive and aversive social learning. These results suggest that oxytocin conveys saliency of social stimuli to sensory representations in the piriform cortex during odor-driven social learning.
INTRODUCTION
Animals continuously interact with conspecifics throughout their lifetime. Some of these interactions, like mating and aggression, are crucial for the survival of an individual and the propagation of species, while other interactions are behaviorally insignificant. However, how animals classify these social events as salient has not been extensively explored.
A set of sensory stimuli elicits stereotyped innate behaviors (Tinbergen, 1951). These stimuli are thought to be processed through developmentally determined, highly specific neural pathways connecting sensory inputs to behavioral outputs (Choi et al., 2005; Dulac and Wagner, 2006; Haga-Yamanaka et al., 2014; Holy et al., 2000; Hong et al., 2014; Li and Liberles, 2015; Stowers et al., 2002). Behavioral significance can also be imposed on sensory stimuli through experience. Learned behaviors, especially in social contexts, may be as important as innate behaviors as they allow animals to maximize their chance of survival and reproduction (Beny and Kimchi, 2014; Pfaus et al., 2001). For instance, it would be crucial to remember cues predictive of encountering potential mates or aggressive individuals.
Oxytocin (Oxt) is a neuropeptide produced by neurons in the paraventricular nucleus (PVH), the medial preoptic nucleus (MPO), and the supraoptic nucleus (SON) of the hypothalamus (Landgraf and Neumann, 2004; Sofroniew, 1983; Swanson and Sawchenko, 1983). There is a single known oxytocin receptor (Oxtr), a seven transmembrane protein, that is functionally coupled to Gq/11α (Gimpl and Fahrenholz, 2001). Oxt is released into the circulatory system and promotes contractions of the uterus during parturition, and milk production. Oxt is also directly released within the brain, where it has been implicated in various social behaviors (Insel, 2010; Lee et al., 2009; Ross and Young, 2009; Stoop, 2012). Genetically removing or pharmacologically inactivating the Oxt-Oxtr pathway produces deficits in maternal behavior (Bosch and Neumann, 2012; Pedersen and Prange, 1979; Takayanagi et al., 2005), social recognition (Ferguson et al., 2000; Ferguson et al., 2001; Lee et al., 2008; Takayanagi et al., 2005) and social reward (Dölen et al., 2013). Despite its expansive involvement in social behaviors, whether Oxt plays a crucial role in associative learning in social contexts, has not been addressed.
The ubiquity of olfactory-guided social behaviors across vertebrate (Isogai et al., 2011; Kaur et al., 2014; Leypold et al., 2002; Liberles, 2014; Lin et al., 2005; Pfaus et al., 2001; Stowers et al., 2002) and invertebrate species (Li and Liberles, 2015; Michener, 1974; Sokolowski, 2010; Suh et al., 2004) strongly suggest that olfactory systems are important for recording and processing social information. Thus, in this study, we used odor-driven behavioral paradigms that capture the essence of social learning – the pairing of an olfactory conditioned stimulus (CS) with a social unconditioned stimulus (US) – to investigate the role of Oxt in social learning. Our experiments reveal that Oxt is selectively required for social learning but is dispensable for learning tasks that do not involve social cues. Also, optogenetic activation of Oxt+ neurons promotes social learning with a non-salient social stimulus. Furthermore, we show that Oxtr signaling in the piriform, the olfactory sensory cortex, is necessary to entrain initially neutral sensory representations to social cues. Finally, we demonstrate that Oxt is required for aversive as well as appetitive social learning. These results suggest that Oxt conveys saliency of social stimuli (US) to sensory representations (CS) in the piriform cortex during social learning.
RESULTS
Oxytocin receptor signaling is required for social learning
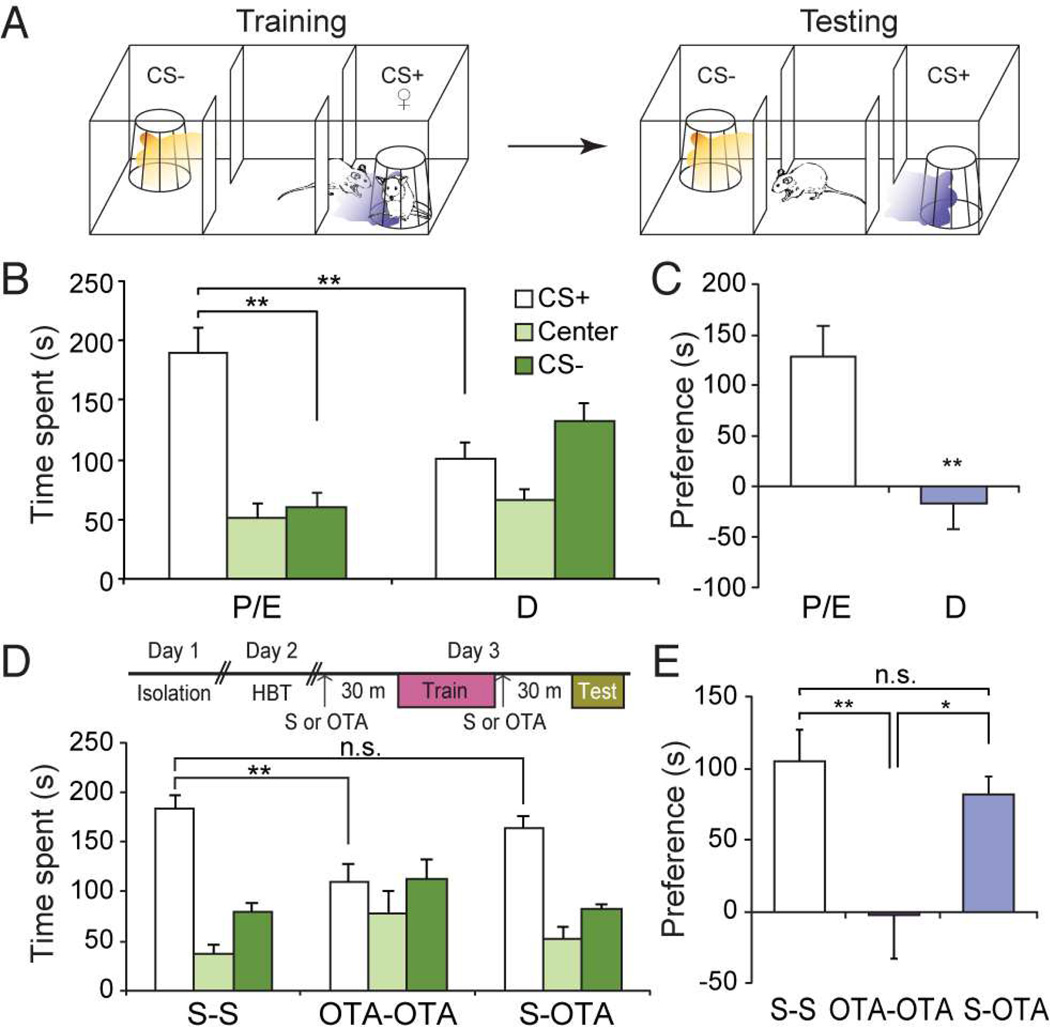
In order to assess the role of oxytocin (Oxt) in social learning, we used an odor-driven appetitive social learning paradigm (Choi et al., 2011), in which an initially neutral odor served as a conditioned stimulus (CS) and was paired with a socially rewarding unconditioned stimulus (US). During training, male mice were allowed to explore a three-chambered arena, housing a female in a wire cage on one side, and an empty wire cage on the other side (Figure 1A). CS+ odor or CS− odor was delivered when the subjects explored the female-containing wire cage or the empty cage, respectively. The subject’s preference for the CS+ or CS− odor was subsequently tested in the same arena in the absence of a female.
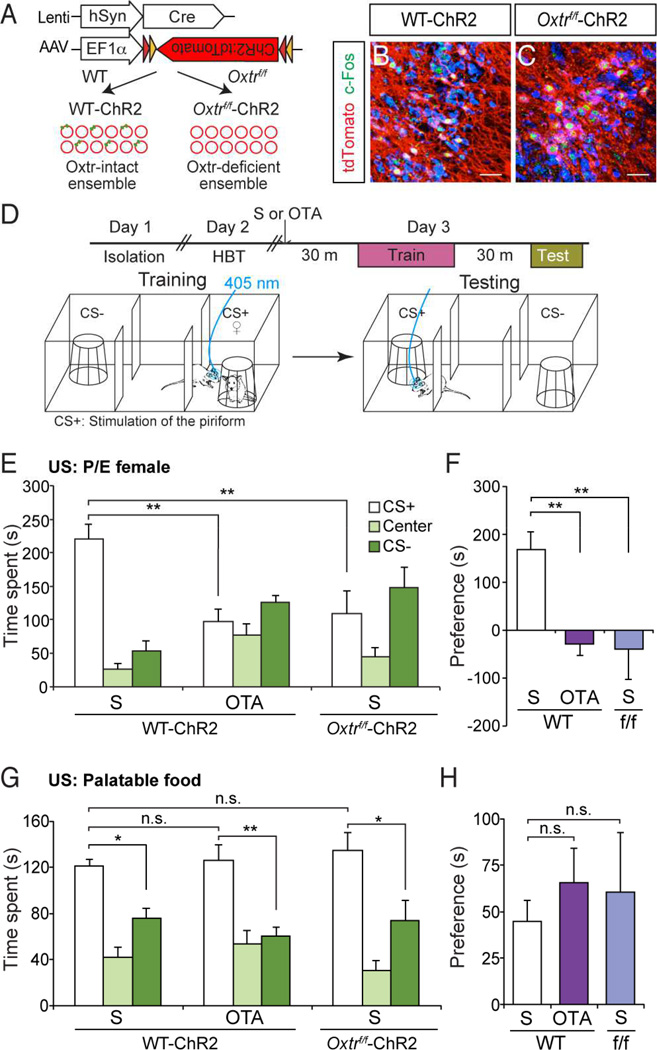
Figure 1.
Oxytocin receptor signaling is required for social learning. (A) Schematic of the odor-driven appetitive social learning paradigm. During training, the CS+ odor (purple shade) was presented when the male subject approached the wire cage containing a female (♀), while the CS− odor (orange shade) was presented when the subject approached the empty wire cage. During testing, the CS+ or the CS− odor was presented without females when the subject entered the randomly pre-determined CS+/CS− chamber. (B) Time spent in each chamber during the 5-min testing period after training with proestrus and estrus (P/E) or diestrus females (D) as unconditioned stimuli. **: P < 0.01 by Tukey HSD post hoc test. (C) Preference score (difference in time spent in the CS+ and CS− compartments) for data presented in (B). **: P < 0.01 by Student’s t-test. (D) Time spent in each chamber for subjects injected with saline before training and testing (S–S), with Oxtr antagonist (OTA) before training and testing (OTA-OTA), or saline before training and OTA before testing (S-OTA). HBT: habituation. **: P < 0.01 by Tukey HSD post hoc test. (E) Preference score for data presented in (D). *: P < 0.05 and **: P < 0.01 by Tukey HSD post hoc test. Data presented as mean ± SEM.
We initially asked if females in estrus phase serve as a more potent (attractive) social cue. Subject males were entrained to either females in reproductively receptive (proestrus or estrus: P/E) or non-receptive phases (diestrus phase: D). During testing, males trained with proestrus/estrus females (P/E) preferred the chamber with the CS+ odor threefold more than the CS− chamber (Figure 1B and C) (CS+ chamber = 189.34 ± 20.74 s; Center = 50.56 ± 13.22 s; CS− chamber = 60.13 ± 11.68 s, n = 6). On the other hand, males trained with diestrus females (D) spent an approximately equal amount of time in the CS+ and CS− chambers (Figure 1B and C) (CS+ chamber = 101.46 ± 13.62 s; Center = 65.57 ± 9.58 s; CS− chamber = 132.88 ± 15.21 s, n = 7). This could not be attributed to any differences in the time spent investigating estrus versus diestrus females during training, since males spent a similar amount of time investigating females regardless of their estrus phase (Figure S1) (For P/E, Female = 16.23 ± 1.25 s, Empty = 5.26 ± 0.53 s, n = 4; For D, Female = 18.10 ± 2.12 s, Empty = 2.60 ± 0.78 s, n = 4). Thus, estrus phase does not influence innately driven approach behavior toward females, but impacts socially learned approach behavior toward the conditioned stimulus.
We next asked if oxytocin receptor (Oxtr) signaling is required for social learning using estrus females as a social cue. We blocked Oxtr signaling by administering L-368,899, a non-peptidergic Oxtr antagonist that penetrates the blood-brain barrier (OTA, 5 mg/kg, intraperitoneally) (Boccia et al., 2007; 4), before training and/or testing. Males injected with saline (Figure 1D and E) (S-S: CS+ chamber = 184.07 ± 13.79 s; Center = 36.73 ± 8.79 s; CS− chamber = 79.10 ± 9.11 s, n = 7) or the antagonist before testing only (S-OTA: CS+ chamber = 163.61 ± 11.96 s; Center = 52.58 ± 12.48 s; CS− chamber = 82.37 ± 4.99 s, n = 6) maintained a preference for the CS+ chamber. On the contrary, subject animals injected with Oxtr antagonist both before training and testing failed to exhibit a preference and spent a similar amount of time in the CS+ and CS− chambers (Figure 1D and E) (OTA-OTA, CS+ chamber = 109.26 ± 17.96 s; Center = 78.69 ± 22.57 s; CS− chamber = 112.08 ± 20.19 s, n = 6). This deficit does not reflect potential effects of oxytocin receptor antagonist on innately driven approach behavior toward social cues. Animals treated with saline (S–S) or antagonist (OTA-OTA) spent an approximately equal amount of time investigating females (Figure S2A) (Average investigation time per trial: For S-S, Female = 14.15 ± 1.85 s/trial, Empty = 4.67 ± 0.71 s/trial, For OTA-OTA, Female = 16.58 ± 1.08 s/trial, Empty = 4.38 ± 0.54 s/trial, n = 6 each). Animals in both groups also traveled a similar distance during testing (Figure S2B) (For S-S, 2.01 ± 0.23 m, For OTA-OTA, 2.12 ± 0.37 m, n = 6 each). These data demonstrate that Oxtr signaling is necessary for association of an initially neutral odor with a rewarding social cue.
Oxytocin receptor signaling is dispensable for non-social learning
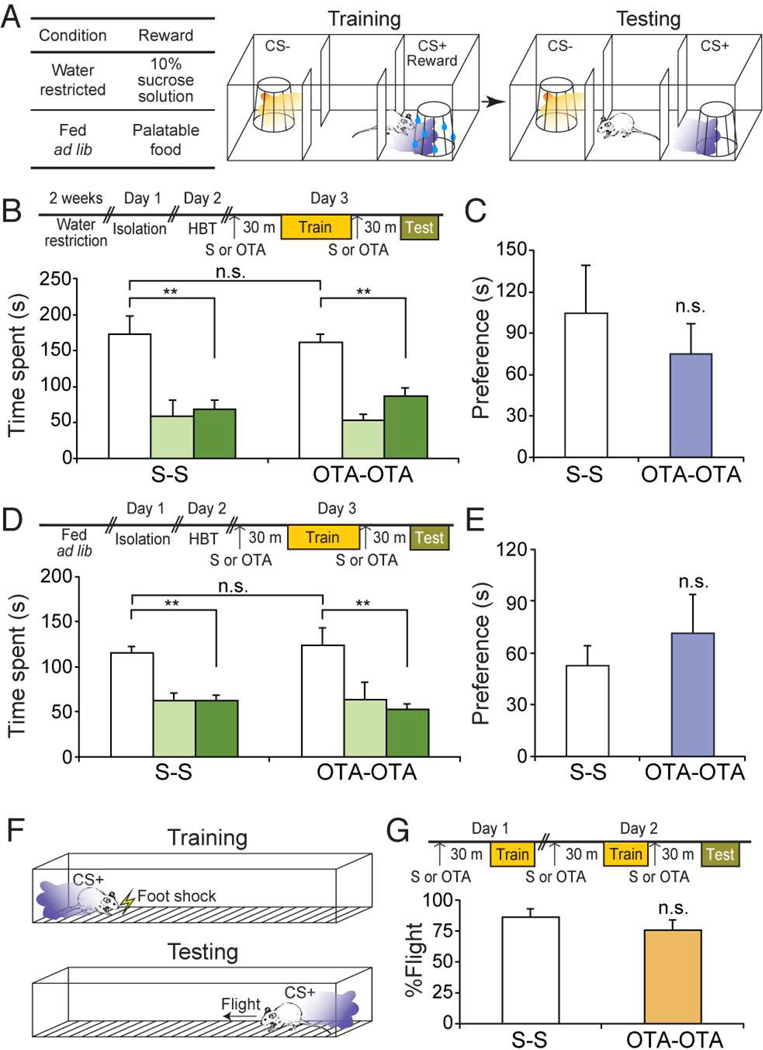
We next asked if Oxtr signaling is also required for association of odor with a rewarding non-social cue. The social learning paradigm was modified such that males were exposed to the CS+ odor in the presence of a non-social reward: sucrose solution or palatable food (Figure 2A). In the scheme with sucrose solution as the reward, saline-injected animals spent 2.5 fold more time in the CS+ chamber than the CS− chamber (Figure 2B and C) (S-S: CS+ chamber = 172.22 ± 26.15 s; Center = 58.41 ± 22.29 s; CS− chamber = 68.17 ± 13.23 s, n = 7). This preference was maintained in animals treated with Oxtr antagonist (OTA-OTA: CS+ chamber = 160.78 ± 11.43 s; Center = 53.06 ± 8.61 s; CS− chamber = 86.17 ± 12.30 s, n = 7). Similarly, in the scheme with palatable food as the reward, the treatment with Oxtr antagonist did not impair the learned preference toward the CS+ chamber (Figure 2D and E) (S-S: CS+ chamber = 115.25 ± 8.00 s; Center = 62.22 ± 8.57 s; CS− chamber = 62.53 ± 6.50 s, n = 7; OTA-OTA: CS+ chamber = 123.92 ± 19.59 s; Center = 64.10 ± 18.50; CS− chamber = 52.18 ± 6.25 s, n = 5).
Figure 2.
Oxytocin receptor signaling is dispensable for non-social learning. (A) Schematic of the odor-driven non-social appetitive learning paradigm. For water-restricted animals, 10% sucrose solution was used as a reward. For animals fed ad lib, palatable food was used as a reward. During training, presentation of the CS+ odor (purple shade) was paired with reward presented on a wire cage. The CS− odor (orange shade) was paired with an empty wire cage. For testing, the CS+ or the CS− odor was presented without reward when the subject entered the randomly pre-determined CS+/CS− chamber. (B) Time spent in each chamber of saline (S–S) or OTA (OTA-OTA)-injected mice in the sucrose-reward scheme. **: P < 0.01 by Tukey HSD post hoc test. (C) Preference score for data presented in (B). P = 0.84 by Student’s t-test. (D) Time spent in each chamber of S-S or OTA-OTA mice in the food-reward scheme. **: P < 0.01 by Tukey HSD post hoc test. (E) Preference score for data presented in (D). P = 0.39 by Student’s t-test. (F) Schematic of the aversive non-social learning paradigm. During training, the CS+ odor was presented on the side where the subject was located, followed by application of a mild foot shock. During testing, the CS+ odor was presented in the absence of shock. (G) Percentage of flight behavior displayed by S-S or OTA-OTA mice in response to the CS+ presentation. P = 0.33 by Student’s t-test. Data presented as mean ± SEM.
We also asked if Oxtr signaling is required for association of odor with an aversive nonsocial cue. During training, delivery of the CS+ odor was followed by a mild foot shock presented only on the side where the subject was located, allowing the subject to escape toward the opposite side of the chamber (Figure 2F). During testing, flight behavior in response to the CS+ odor was measured in the absence of shock. Both saline- and OTA-injected animals robustly avoided the CS+ odor (Figure 2G) (S-S: 86.0 ± 7.1%, n = 10; OTA-OTA: 75.6 ± 8.7%, n = 9). These results collectively suggest that Oxtr signaling is required for social learning, but is dispensable for other associative learning tasks that do not involve social cues.
Optical activation of Oxt+ neurons in the PVH promotes social learning
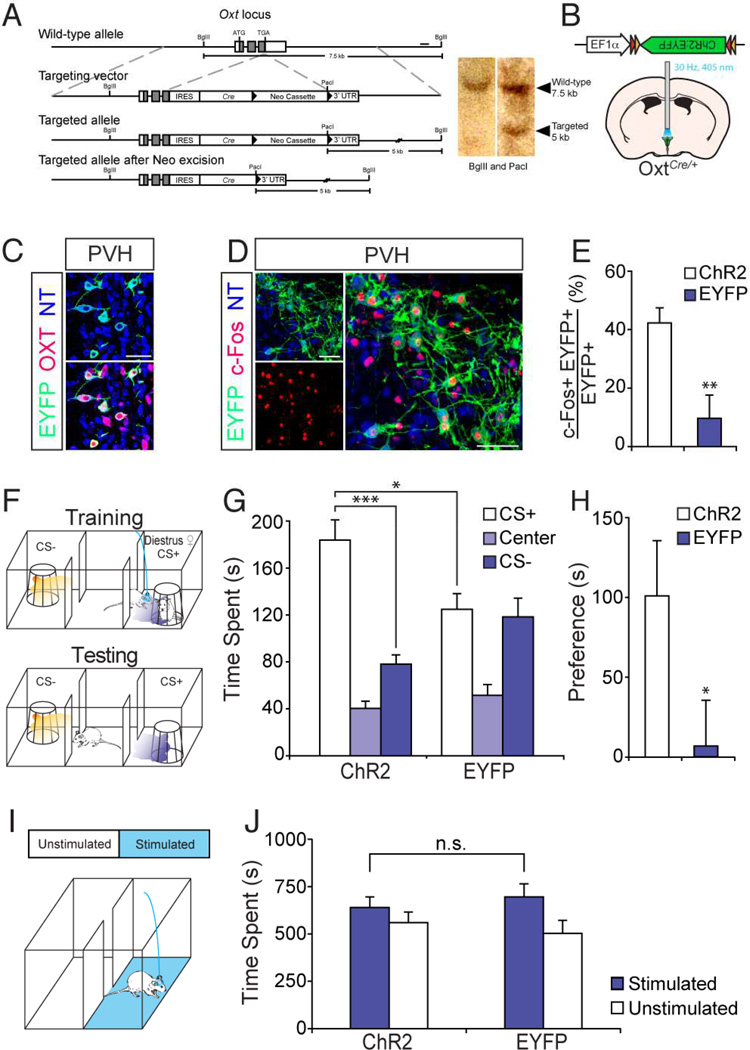
We next asked if stimulation of Oxt+ neurons is capable of promoting social learning. We generated a knock-in mouse line to gain genetic access to Oxt+ neurons, by targeting Cre recombinase to the 3’ end of the Oxt coding sequence (Figure 3A). To exogenously activate Oxt+ neurons in these OxtCre/+ mice, we then delivered adeno-associated virus (AAV) driving Cre-dependent ChR2 fused to EYFP (ChR2:EYFP) under control of the EF1α promoter into the PVH (Figure 3B), a major source of centrally released Oxt (Landgraf and Neumann, 2004). Double labeling experiments confirmed that Cre expression recapitulates endogenous expression of Oxt; the majority of ChR2:EYFP-expressing neurons co-expressed OXT (Figure 3C) (91.5% of 255 cells from 2 mice). Furthermore, analysis of c-Fos expression, a marker of neuronal activation, confirmed photostimulation-dependent activation of ChR2-expressing neurons. We observed that ~42% of cells expressing ChR2:EYFP also expressed c-Fos (Figure 3D and E) (ChR2: 42.46 ± 5.23%, n = 6), whereas ~9% of cells expressing EYFP were positive for c-Fos in control animals (EYFP: 9.72 ± 7.98%, n = 4).
Figure 3.
Stimulation of oxytocin producing neurons facilitates odor-driven social learning. (A) Strategy for targeting Oxt locus. Gray boxes indicate coding regions. Solid bar represents probe for southern blot. 3’ UTR: 3’ untranslated region. (B–E) Validation of OxtCre knock-in mouse line. (B) Strategy for optogenetic activation of Oxt+ neurons in the paraventricular nucleus of the hypothalamus (PVH). OxtCre/+ mice injected with AAV encoding Cre-dependent ChR2:EYFP into the PVH were stimulated with 405-nm laser at 30-Hz pulse frequency. (C) Immunostaining for EYFP (green) and OXT (red) in the PVH. (D) Immunostaining for EYFP (green) and c-Fos (red) in the PVH after photostimulation. Scale bars in (C and D) represent 50 µm. (E) Percentage of EYFP+ neurons expressing c-Fos upon photostimulation in the PVH. **: P < 0.01 by Student’s t-test. (F) Schematic of odor-driven social learning with diestrus females supplemented with optogenetic stimulation of Oxt+ neurons. During training, photostimulation was applied to the animal upon entrance into the randomly pre-determined CS+ chamber. In conjunction with the photostimulation, the CS+ odor (purple shade) was presented when male subject approached the wire cage containing a diestrus female (♀). The CS− odor (orange shade) was presented, in the absence of photostimulation, when the subject approached the empty wire cage. During testing, the CS+ or CS− odors were presented without females or photostimulation, when the subject entered the randomly pre-determined CS+/CS− chamber. (G) Time spent in each chamber for OxtCre/+ mice expressing ChR2 (ChR2) and EYFP (EYFP). ***: P < 0.001, *: P < 0.05 by Tukey HSD post hoc test. (H) Preference score for data presented in (G). *: P < 0.05 by Student’s t-test. (I) Schematic demonstrating real-time place preference test. (J) Time spent in each chamber during 20-min testing period for ChR2 and EYFP animals. P = 0.53 by Student’s t-test. Data presented as mean ± SEM.
We asked if diestrus females, which are normally incapable of driving learning in our behavioral scheme (Figure 1B and C), can serve as an effective US when supplemented with optogenetic stimulation of Oxt+ neurons in subject animals. In this experiment, photostimulation of Oxt+ neurons was applied during training when animals were exposed to a diestrus female in the CS+ chamber. Testing was performed in the absence of both the female and photostimulation (Figure 3F). ChR2-expressing animals spent substantially more time in the CS+ chamber than the CS− chamber (Figure 3G and H) (ChR2: CS+ chamber = 181.53 ± 18.57 s; Center = 39.21 ± 7.03 s; CS− chamber = 78.73 ± 12.69 s, n = 8). In contrast, EYFP-expressing animals failed to exhibit a significant preference for the CS+ chamber (Figure 3G and H) (EYFP: CS+ chamber = 126.95 ± 10.84 s, Center = 54.77 ± 9.97 s; CS− chamber = 112.30 ± 14.99 s, n = 8). Stimulation of Oxt+ neurons did not enhance investigation time toward diestrus females during training (Figure S3) (ChR2: Female = 14.35 ± 1.97 s/trial, Empty = 5.40 ± 1.44 s/trial, n = 7; EYFP: Female = 18.53 ± 0.90 s/trial, Empty = 5.47 ± 1.17 s/trial, n = 6). Furthermore, photostimulation of Oxt+ neurons did not generate reward-related effects in a real-time place preference test (RTPP) (Stamatakis and Stuber, 2012). In the RTPP arena, Oxt+ neuron stimulation occurred when animals were in one, but not in the neighboring compartment (Figure 3I). Both ChR2-and EYFP-expressing animals spent a similar amount of time in the stimulated and unstimulated compartments (Figure 3J) (ChR2: Stimulated = 640.00 ± 55.75 s, Unstimulated = 560.04 ± 55.75 s, n = 10; EYFP: Stimulated = 696.27 ± 68.73 s, Unstimulated = 503.12 ± 68.62 s, n = 8), suggesting that stimulation of Oxt+ neurons in the PVH does not elicit approach or avoidance behavior. The foregoing experiments demonstrate that stimulation of Oxt+ neurons in the PVH facilitates entrainment of odor to a non-salient social cue.
Oxt neurons project to the piriform cortex
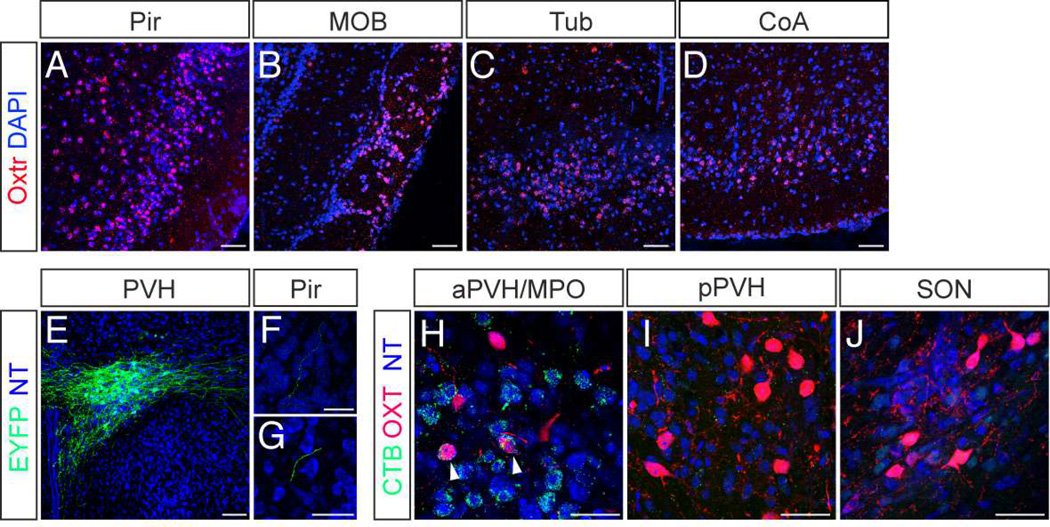
Anatomical, physiological, and behavioral studies have suggested that the piriform cortex serves as a likely neural substrate for odor-driven associative learning (Choi et al., 2011; Ghosh et al., 2011; Illig and Haberly, 2003; Miyamichi et al., 2011; Poo and Isaacson, 2009; Sosulski et al., 2011; Stettler and Axel, 2009). We carried out fluorescent in situ hybridization for Oxtr mRNA to determine if Oxt acts on the piriform cortex. Dense Oxtr expression was observed in all three layers of the piriform cortex (Figure 4A) (layer 1 = 24 ± 6% of total DAPI+ nuclei; layer 2 = 64 ± 4%; layer 3 = 48 ± 2%, n = 3), in accordance with a previous autoradiography study (Yoshida et al., 2009). Oxtr expression was also evident in other olfactory centers, such as the glomerular layer of the main olfactory bulb (MOB) (Figure 4B), the olfactory tubercle (Tub) (Figure 4C), and the cortical amygdala (CoA) (Figure 4D).
Figure 4.
The piriform cortex expresses Oxtr and receives projections from hypothalamic Oxt+ neurons. (A–D) In situ hybridization for Oxtr mRNA (red) in (A) the piriform cortex (Pir), (B) the main olfactory bulb (MOB), (C) the olfactory tubercle (Tub), and (D) the cortical amygdala (CoA). Counterstained with 4',6-diamidino-2-phenylindole (DAPI, blue). Scale bars in (A–D) represent 50 µm. (E–G) Anterograde tracing of Oxt+ neurons to the piriform cortex. (E) AAV encoding Cre-dependent ChR2:EYFP was injected into the PVH of an OxtCre/+ mouse. (F and G) EYFP+ fibers in sections of the piriform cortex counterstained with NT (blue). Scale bar in (E) represents 200 µm and in (F and G) represent 50 µm. (H–J) Hypothalamic regions enriched in Oxt+ neurons, in mice injected with retrograde tracer cholera toxin B subunit (CTB) in the piriform. (H) The anterior PVH (aPVH) and the medial preoptic area (MPO), (I) the posterior PVH (pPVH), and (J) the supraoptic nucleus (SON) were stained for CTB (green) and OXT (red) and counterstained with NT (blue). Arrowheads indicate co-labeled neurons. Scale bars in (H–J) represent 50 µm.
We next asked if Oxt+ neurons in the hypothalamus directly project to the piriform cortex. We labeled Oxt+ neurons by injecting AAV encoding Cre-dependent ChR2:EYFP into the PVH of OxtCre/+ mice (Figure 4E) and examined the piriform cortex for the presence of labeled fibers. In these animals, sparse EYFP+ fibers originating from Oxt+ neurons were found in the piriform cortex (Figure 4F and G). We also injected cholera toxin B subunit (CTB), a retrograde tracer, into the piriform (Figure S4). CTB was detected in Oxt+ neurons in the anterior PVH (aPVH) and the medial preoptic area (MPO) (Figure 4H), but not in Oxt+ neurons in the posterior PVH (pPVH) (Figure 4I) or the supraoptic nucleus (SON) (Figure 4J). These results demonstrate that the piriform cortex contains a dense population of Oxtr-expressing neurons and receives direct input from Oxt+ neurons in the aPVH/MPO.
Oxtr expression in piriform ensembles is necessary for appetitive social learning
Based on the foregoing observations in conjunction with the suggested role of the piriform in odor-driven learning, we hypothesized that Oxtr signaling in the piriform is involved in entrainment of odor to social cues. However, the large size of the brain structure and lack of piriform-specific Cre driver lines pose a technical challenge to interrogating its function in social learning. Thus, we used exogenously activated piriform neural ensembles (Choi et al., 2011) to ask if Oxtr expression is required in the piriform cortex for odor-driven social learning. Activation of these ensembles expressing ChR2, like odorants, can be entrained to elicit appetitive or aversive behavior when associated with reward or punishment.
We virally introduced ChR2 in layer 2 and 3 of the piriform cortex. We co-injected AAV driving Cre-dependent ChR2:tdTomato with lentivirus encoding Cre from the human Synapsin1 promoter. This dual virus strategy was applied to either wild-type (WT) or Oxtrf/f mice, in which the Oxtr coding region is conditionally deleted in a Cre-dependent manner (Lee et al., 2008). This generated Oxtr-intact ChR2-expressing ensembles in WT (WT-ChR2) and Oxtr-deficient ChR2-ensembles in Oxtrf/f mice (Oxtrf/f-ChR2), respectively (Figure 5A). In Oxtrf/f-ChR2 mice, the level of Oxtr mRNA was significantly reduced in ChR2-expressing neurons (ChR2+) compared to neighboring non-infected neurons (ChR2−) (Figure S5) (Normalized intensity of Oxtr mRNA: ChR2− = 100.00 ± 9.24; ChR2+ = 37.08 ± 3.84, n = 167 cells from 3 mice for ChR2− and 72 cells from 3 mice for ChR2+). ChR2 expression was detected in approximately 5–10% of the neurons in a localized region (Figure 5B and C) (Percentage of ChR2-expressing neurons: WT = 6.32 ± 1.35%; Oxtrf/f = 4.53 ± 1.18%, n = 11 for WT and 6 for Oxtrf/f). Analysis of c-Fos expression revealed that ChR2-ensembles both in WT and Oxtrf/f mice were activated upon photostimulation (Figure 5B and C) ([c-Fos+ ChR2+]/[ChR2+]: WT-ChR2 = 60.73 ± 4.52%; Oxtrf/f-ChR2 = 66.26 ± 5.06%, n = 11 for WT-ChR2 and 6 for Oxtrf/f-ChR2).
Figure 5.
Oxtr expression in ChR2-expressing piriform ensembles is necessary for entrainment to social cues. (A) Strategy to generate Oxtr (green dot)-intact or Oxtr-deficient ensembles. Red circles indicate individual piriform neurons. Dual virus strategy employs lentivirus encoding Cre from the human Synapsin1 promoter (hSyn) and AAV encoding Cre-dependent ChR2:tdTomato to generate Oxtr-intact ensembles in wild-type mice (WT-ChR2) but Oxtr-deficient ensembles in Oxtrf/f mice (Oxtrf/f-ChR2). (B and C) Expression of c-Fos in piriform ChR2-ensembles after photostimulation. Coronal sections of WT-ChR2 (B) or Oxtrf/f-ChR2 (C) were stained for c-Fos (green) and tdTomato (red) and counterstained with NT (blue). Scale bars in (B and C) represent 50 µm. (D) Schematic of social learning with activation of piriform ChR2-ensembles as the CS. The subjects were injected with saline (S) or Oxtr antagonist (OTA) before training. During training, photostimulation, instead of odor, was applied as the CS+ when the subject was in the vicinity of the wire cage containing a female (♀). For testing, photostimulation was applied when the subject was in the randomly pre-determined CS+ chamber. (E) Female in proestrus or estrus phase was used as US. Time spent in each chamber for WT-ChR2 mice injected with saline (S) or Oxtr antagonist (OTA), and Oxtrf/f-ChR2 mice injected with saline. **: P < 0.01 by Tukey HSD post hoc test. (F) Preference score for data presented in (E). **: P < 0.01 by Tukey HSD post hoc test. (G) Palatable food was used as US. Time spent in each chamber for WT-ChR2 mice injected with S or OTA, and Oxtrf/f-ChR2 mice injected with S. *: P < 0.05 and **: P < 0.01 by Tukey HSD post hoc test. (H) Preference score for data presented in (G). Data presented as mean ± SEM.
We first confirmed that ChR2-expressing piriform ensembles, just like odorants, require Oxtr signaling for social learning by entraining these ensembles in the presence or absence of Oxtr antagonist. During training, we replaced the CS+ odor with photostimulation such that ChR2-ensembles were activated when males investigated the females (Figure 5D). Males injected with saline prior to training spent significantly more time in the CS+ chamber (Figure 5E and F) (CS+ chamber = 220.53 ± 22.16 s; Center = 25.94 ± 9.31 s; CS− chamber = 53.08 ± 15.24 s, n = 5). The observed preference for CS+ chamber was independent of the anterior-posterior position of the ChR2-ensemble within the piriform (Figure S6). Males injected with Oxtr antagonist, however, failed to exhibit preference for the CS+ chamber and spent a similar amount of time in the CS+ and CS− chambers (Figure 5E and F) (CS+ chamber = 97.60 ± 18.62 s; Center = 76.29 ± 17.73 s; CS− chamber = 126.15 ± 10.71 s, n = 6).
We next asked if Oxtr expression is required in the piriform ChR2-ensembles for entrainment to social cues. Unlike WT animals, Oxtrf/f animals harboring Oxtr-deficient ensembles spent a similar amount of time in the CS+ and CS− chambers (Figure 5E and F) (CS+ chamber = 108.62 ± 35.03 s; Center = 44.17 ± 14.15 s; CS− chamber = 147.23 ± 30.52 s, n = 6). Our results are not explained by deficits intrinsic to Oxtrf/f mice since Oxtrf/f animals injected with control virus encoding EGFP were still capable of odor-driven social learning (Figure S7) (CS+ chamber = 170.15 ± 3.18 s; Center = 45.40 ± 11.19 s; CS− chamber = 82.87 ± 11.43 s, n = 4). In contrast to entrainment to social cues, entrainment of ChR2-expressing ensembles to non-social cues (i.e. palatable food) was affected neither by systemic Oxtr signaling blockade nor by ChR2-ensemble specific knock-out of Oxtr (Figure 5G and H) (WT-ChR2-S: CS+ chamber = 121.09 ± 5.78 s; Center = 42.62 ± 8.53 s; CS− chamber = 76.29 ± 8.21 s, n = 8; WT-ChR2-OTA: CS+ = 125.97 ± 13.84 s; Center = 53.66 ± 11.78 s; CS− chamber = 60.37 ± 7.40 s, n = 7; Oxtrf/f-ChR2-S: CS+ = 135.06 ± 15.74 s; Center = 30.56 ± 8.45 s; CS− chamber = 74.38 s, n = 5). Therefore, these results demonstrate that representations in the piriform cortex require Oxtr expression specifically for entrainment to social cues.
Oxytocin receptor signaling is required for aversive social learning
Our data suggest that oxytocin is crucial for appetitive social learning. We next asked if Oxt is also required for learning with aversive social cues. We designed an odor-driven aversive social learning paradigm, in which odor served as a CS and was paired with an aversive social US (Figure 6A). Prior to training, male mice were introduced into the home cage of a CD1 male, an aggressive strain of mouse, and allowed to interact in a resident-intruder scheme. This social encounter was characterized by frequent attacks directed towards the intruding subject mouse. During training, subjects were confined to one of the side compartments in the three-chambered arena, while they were exposed to odor in the presence or absence of the previously encountered CD1 male. After interleaved CS+ (CS+ odor with the CD1 male in a wire cage) and CS− trials (CS− odor with an empty wire cage), the subjects were tested in the same arena, which contained only the CS+ and CS− odors. Subjects that directly interacted with an aggressive CD1 prior to training avoided the CS+ chamber (Figure 6B and C) (CS+ chamber = 53.28 ± 4.42 s; Center = 101.58 ± 10.66 s; CS− chamber = 138.37 ± 12.65 s, n = 7), while the subjects that had not been exposed to the CD1 prior to training spent an approximately equal amount of time in the CS+ and CS− chambers (Figure 6B and C) (CS+ chamber = 93.52 ± 15.47 s; Center = 96.05 ± 17.31 s; CS− chamber= 100.74 ± 10.05 s, n = 6). Thus, odor can be entrained to an aversive social stimulus to produce avoidance behavior, and this learning requires an aggressive encounter prior to training in our behavioral scheme.
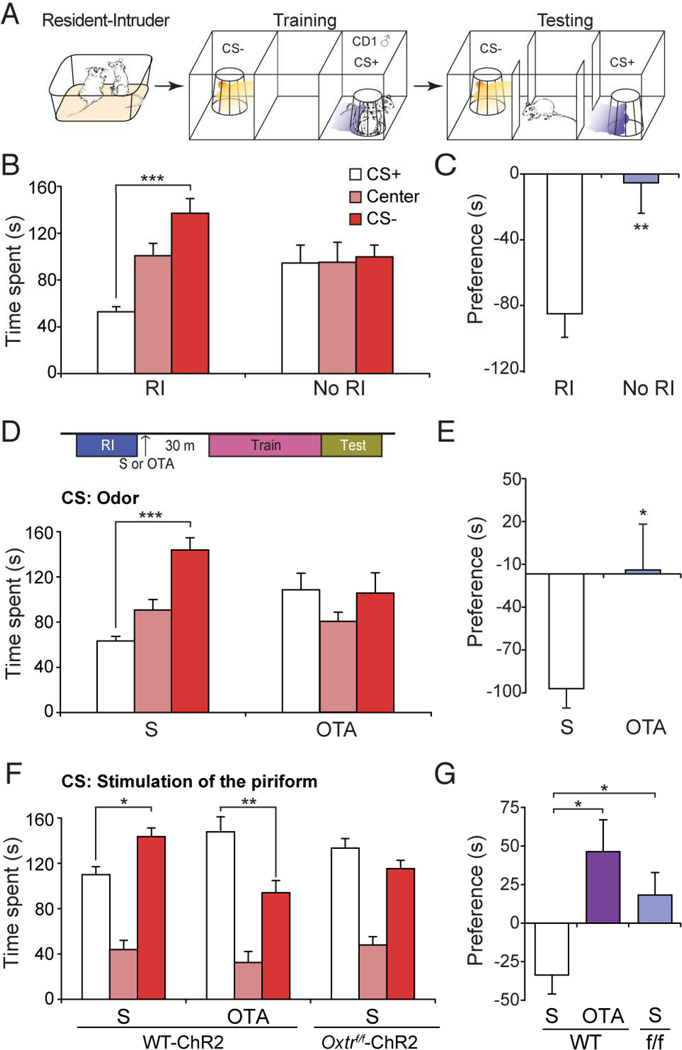
Figure 6.
Oxtr signaling is required for aversive social learning. (A) Schematic of odor-driven aversive social learning. After resident-intruder interaction, subjects were confined to one side chamber and presented with the CS+ odor and the wire cage containing the CD1 (♂) that served as resident. The CS− odor was presented with an empty wire cage. For testing, the CS+ or the CS− odor was presented, without the CD1, when the subject entered the randomly predetermined CS+/CS− chamber. (B) Time spent in each chamber during testing, with resident-intruder pretraining (RI), or without pretraining (No RI). ***: P < 0.001 by Tukey HSD post hoc test. (C) Preference score for data presented in (B). **: P < 0.01 by Student’s t-test. (D) Time spent in each chamber for subject mice injected with saline (S) or with OTA (OTA). (E) Preference score for data presented in (D). *: P < 0.05 by Student’s t-test. (F) Time spent in each chamber for WT-ChR2 mice injected with S or OTA, and Oxtrf/f-ChR2 mice injected with S. *: P < 0.05, **:P < 0.01 by Tukey HSD post hoc test. (G) Preference score for data presented in (F). *: P < 0.05 by Tukey HSD post hoc test.
We next asked if Oxtr signaling is required for aversive social learning. Subjects were injected with either saline or Oxtr antagonist after interaction with the CD1, but before training. While subjects injected with saline avoided the CS+ chamber (Figure 6D and E) (CS+ chamber = 63.57 ± 4.10 s; Center = 91.04 ± 9.34 s; CS− chamber = 144.39 ± 11.00 s, n = 7), animals injected with antagonist did not exhibit avoidance of the CS+ chamber (Figure 6D and E) (CS+ chamber = 108.96 ± 14.95 s; Center = 80.74 ± 8.43 s; CS− chamber = 106.18 ± 18.01 s, n = 6). We quantified the fraction of time the subject spent away from the pencil cup to assess avoidance of the CD1 during training. Avoidance of the CD1 was indistinguishable between animals injected with saline or Oxtr antagonist (Figure S8) (S: Avoidance score = 0.82 ± 0.03, n = 7; OTA: Avoidance score = 0.78 ± 0.06, n = 6). These results suggest that Oxtr signaling does not influence the avoidance of the CD1, but rather it is necessary for entrainment of odor to an aversive social cue.
To test whether Oxtr signaling in the piriform is involved in aversive social learning, we entrained Oxtr-intact (WT-ChR2) and Oxtr-deficient (Oxtrf/f-ChR2) ChR2-ensembles to a CD1. Males injected with saline avoided the CS+ chamber (Figure 6F and G) (CS+ chamber = 110.37 ± 6.73 s; Center = 44.12 ± 7.63 s; CS− chamber= 144.02 ± 7.37 s, n = 10), although to a lesser extent than what was observed with odor. However, subjects injected with Oxtr antagonist, rather than avoiding the CS+ chamber, spent more time in the CS+ chamber (CS+ chamber = 148.45 ± 12.12 s; Center = 32.70 ± 8.82 s; CS− chamber = 94.49 ± 9.78 s, n = 6). Furthermore, Oxtrf/f animals harboring Oxtr-deficient ensembles failed to avoid the CS+ chamber (Figure 6F and G) (CS+ chamber = 133.92 ± 7.91 s; Center = 48.18 ± 6.83 s; CS− chamber = 115.67 ± 6.93 s, n = 7). These results demonstrate that representations in the piriform cortex require Oxtr expression for entrainment to aversive social stimuli.
DISCUSSION
We asked if Oxt plays a crucial role in promoting odor-driven social learning by pairing an initially neutral olfactory stimulus with social cues. We show that oxytocin is required for entrainment of odor to salient social cues, and capable of promoting entrainment of odor to nonsalient social cues. Oxtr signaling carries out these functions by acting on the piriform cortex. Furthermore, we show that Oxtr signaling is required for aversive as well as for appetitive social learning. Taken together, these results suggest that Oxt conveys social saliency of unconditioned stimuli to sensory representations in the piriform cortex during odor-driven social learning (Figure 7).
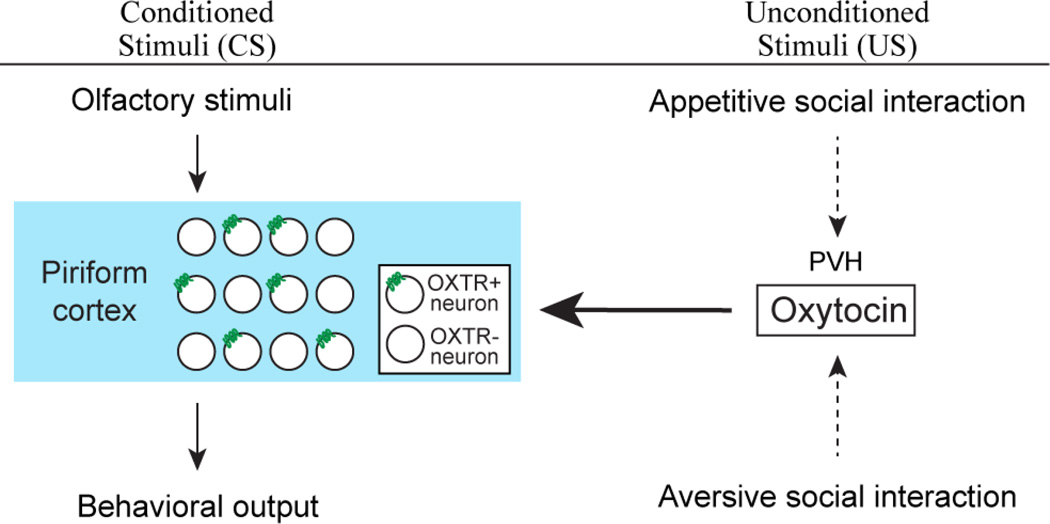
Figure 7.
Model for entrainment of odor to social cues of opposing valence. Associative learning transforms initially neutral olfactory representations (CS) in the piriform cortex to produce learned behavioral responses. Oxytocin, representing saliency of social cues of both appetitive and aversive nature (US), modulates this process by directly impacting the piriform cortex.
Oxt has been widely viewed as a molecule promoting prosocial behaviors or transmitting positive value of social interactions (Insel, 2010; Lee et al., 2009; Ross and Young, 2009). Consistent with these notions, we show that Oxt is required for entrainment of an odor to an appetitive social cue. The role of Oxt is not limited to appetitive social learning, but rather it is also involved in entrainment of an odor to an aversive social cue. Our results indicate that Oxt mediates social learning of opposing valence depending on the context. This is consistent with previous observations that Oxt is released by both mating and aggressive encounters in rodents (Ebner et al., 2000; Engelmann et al., 1999; Waldherr and Neumann, 2007).
Behavioral changes as a consequence of learning occur following conspicuous social interactions; females in estrus, but not in diestrus, served as an effective US in our learning paradigm. Furthermore, CD1 stimulus males, when presented without prior resident-intruder encounter, failed to drive learning in our aversive social scheme. However, females in diestrus, when supplemented with optogenetic activation of Oxt+ neurons in the subject animals, were capable of producing learned approach behavior. These data, taken together with the observation that Oxt mediates social learning of opposing valence, suggest that Oxt may convey saliency of social encounters. This model may explain the paradoxical observations made in humans that exogenous administration of Oxt improves social cognition and increases prosocial behavior in positive contexts (Guastella et al., 2008; Kosfeld et al., 2005; Rimmele et al., 2009), but promotes antisocial effects in negative contexts (Bartz et al., 2011; Bartz and Hollander, 2006; Heinrichs et al., 2009).
The notion that Oxt may facilitate opposing behavioral responses dependent upon the appetitive or aversive nature of the US, raises the question of how Oxt accomplishes this context-dependent function. In one model, context of social encounter may be conveyed through distinct neural pathways (Gunaydin et al., 2014), parallel to the oxytocin system. In an alternative, but not mutually exclusive model, Oxt itself may provide contextual information by acting on distinct brain regions. For instance, the resident-intruder encounter assay has been shown to induce Oxt release into the septum (Ebner et al., 2000), while Oxt in the ventral striatum has been shown to encode positive value of social interactions (Dölen et al., 2013). In the latter scenario, the Oxt+ neuronal population could be comprised of heterogeneous subpopulations that differentially process either positive or negative social stimuli.
If Oxt conveys saliency of social encounters, it may be dispensable for learning that involves non-social unconditioned stimuli. This stimulus-selectivity is observed upon inhibiting Oxtr signaling; Oxtr antagonist treatment impairs entrainment of odor to males and females, but not to sucrose solution, palatable food, or mild foot shock. These results are consistent with social-specific effects of Oxt previously reported in other rodent studies (Dölen et al., 2013; Ferguson et al., 2000). Moreover, in humans, intranasal administration of Oxt improved memory of faces, but not of non-social objects (Rimmele et al., 2009). These observations suggest that the social-specific effect of Oxt may be conserved across mammalian species.
Where does Oxt operate to permit odor-driven social learning? Anatomical and physiological studies have suggested that the piriform cortex, one of the recipients of olfactory information, is a neural substrate for odor-driven associative learning (Ghosh et al., 2011; Illig and Haberly, 2003; Miyamichi et al., 2011; Poo and Isaacson, 2009; Sosulski et al., 2011; Stettler and Axel, 2009; Giessel and Datta, 2014). Consistent with this notion, optogenetic activation of arbitrarily chosen ensembles of piriform neurons, just like odorants, could be entrained to various unconditioned stimuli to produce both aversive and appetitive learned behaviors (Choi et al., 2011). These results demonstrate that representations in the piriform cortex are sufficient to drive learned behaviors. On the other hand, another recipient of olfactory information, the cortical amygdala, recently has been identified as a neural substrate for odordriven innate behaviors (Root et al., 2014). We show here that ChR2-expressing piriform ensembles require Oxtr expression for entrainment to social cues. This observation, in conjunction with the tracing experiments (Figure 4E–J), suggests that Oxt directly impacts the piriform cortex to permit odor-driven social learning. Considering the neuromodulatory action of Oxt and the distribution of oxytocin receptor reported in other brain regions (Owen et al., 2013; Marlin et al., 2015), oxytocin’s role in social learning may be generalized across different sensory modalities.
Oxtr is expressed at multiple nodes along both the main and accessory olfactory pathways as well as in brain regions implicated in generation of motivated behaviors (Stoop, 2014). It is plausible that Oxtr operates at additional nodes of the olfactory pathways to support odor-driven social learning. For example, Oxt has been shown to act on the medial amygdala to mediate social recognition (Ferguson et al., 2001). In general, Oxt is poised to influence social learning at multiple loci from perception of both conditioned and unconditioned stimuli to behavioral output. How Oxt coherently orchestrates these distributed circuits to produce social learning remains to be determined.
EXPERIMENTAL PROCEDURES
Stereotaxic Injection and Fiber Optic Implantation into the Mouse Brain
Strains of mice used for experiments were wild-type C57BL/6J, CD-1, OxtCre/+, and Oxtrf/f. The surgeries were carried out using aseptic techniques. Stereotaxic injections of viruses were made at the rate of <0.1 µl/min at the layer 2 and 3 of the piriform cortex (AP = −0.6 mm, ML = +/− 3.8 mm from bregma, DV = −4.0 mm from brain surface; For the anterior piriform cortex: AP = +1.54 mm, ML = +/− 2.80 mm, DV = −3.50 mm; For the posterior piriform cortex: AP = −2.06 mm, ML = +/− 4.20 mm, DV = −4.10 mm) or the paraventricular nucleus of the hypothalamus (AP = −1.0 mm, ML = 0.0 mm , DV = −3.9 mm). The tip of the fiber optic implant was subsequently placed 300–400 µm above the virus injection site. All animal procedures were approved by the Committee on Animal Care of Massachusetts Institute of Technology.
Tissue Slice Preparation and Immunohistochemistry
The prepared slices were labeled with the following primary antibodies: chicken anti-GFP (Abcam, ab5450, 1:1000), goat anti-c-Fos (Santa Cruz, sc-52-G, 1:300), rabbit anti-c-Fos (Santa Cruz, sc-7270, 1:500), rabbit anti-DsRed (Clontech, 632496, 1:500), goat anti-CTB (List Biological laboratories, 703, 1:500), mouse anti-oxytocin (Millipore, MAB5296, 1:1000), and rabbit anti-oxytocin (Millipore, AB911, 1:1000). The following fluorophore-conjugated secondary antibodies were used: Alexa 488 goat anti-chicken (Invitrogen, A11039), Alexa 633 donkey anti-goat (Invitrogen, A21082), Alexa 488 donkey anti-goat (Invitrogen A11055), Alexa 647 donkey anti-mouse (Invitrogen, A31571), Alexa 633 goat anti-rabbit (Invitrogen, A21071), Alexa 568 goat anti-rabbit (Invitrogen, A11036). All secondary antibodies were diluted 1:250. The slices were counterstained with NeuroTrace (NT) fluorescent Nissl stain (Invitrogen, N-21479 or N-21483). All images were taken using a Zeiss LSM-710 confocal microscope system.
For quantitative analysis of photostimulation-dependent activation of ChR2− and EYFP-expressing neurons in OxtCre/+ mice, animals were photostimulated (30 sec per min for 10 min) one hour before they were sacrificed. Brain slices were double-labeled for c-Fos and EYFP. The percentage of neurons expressing c-Fos was obtained by dividing the number of EYFP+ c-Fos+ cells by the number of EYFP+ cells within the area beneath the optical fiber placement.
Quantitative analysis of piriform ensemble images was carried out as previously described (Choi et al., 2011). Briefly, WT-ChR2 or Oxtrf/f-ChR2 mice were photostimulated (30 sec per min for 10 min) one hour before they were sacrificed. Brain slices were double-labeled for c-Fos and DsRed. The percentage of neurons expressing ChR2 was obtained by dividing the number of ChR2+ NT+ cells by the number of NT+ cells within a 500 µm × 500 µm area around the center of injection site. The number of c-Fos+ ChR2+ neurons was counted within the same 500 µm × 500 µm area. The neurons were counted at least three different anterior-posterior levels and averaged for each animal.
Tracing Studies
For anterograde tracing, Cre-dependent ChR2 fused to EYFP was targeted to the PVH (AP: −1.0 mm, ML: 0.0 mm DV: −3.9 mm) of adult male OxtCre/+ mice. Following a two-week survival time, 100-µm sections were cut and stained with antibodies against GFP and counterstained with NT. For retrograde tracing, we injected 0.5% cholera toxin B subunit (CTB) at three points along the anterior-posterior extent of the piriform of male wild-type animals. Injections were targeted to the anterior (AP: +1.5 mm, ML: +2.5 mm, DV: −3.5 mm), middle (AP: −0.7 mm, ML: +3.5 mm, DV: −4.4 mm), and posterior (AP: −1.8 mm, ML: +3.85 mm, DV: −4.1 mm) aspects of the piriform. After four days of survival time, 50-µm sections were cut and co-stained for CTB and Oxt for 48 hrs. Sections were counterstained with secondary antibodies and with NT for 24 hrs.
Behavioral analysis
Social Appetitive Learning Paradigm
Behavioral training and testing were carried out in a custom-built three-chambered arena with modifications from a previously established paradigm (Choi et al., 2011). The middle chamber included two 10-cm-wide openings in each long wall, allowing free movement between all three chambers. One or two days before the experiment, subject males were singly caged and habituated for 15–20 min to the arena containing empty wire cages (11 cm height, 10.5 cm bottom diameter, bars spaced 1 cm apart), that were to be used later, in each side chamber. C57BL/6J females were transferred to a clean cage one day before the experiment and examined for their estrus phase by vaginal smear examination on the experiment day. During training, a wire cage containing a C57BL/6J female was placed in one side chamber while an empty wire cage was placed in the opposite side chamber. An odor set comprised of orange and anise extracts or another set comprised of octanol and ethyl acetate were randomly chosen for experiments. Individual odors were randomly designated as either CS+ or CS− odors. At the beginning of each trial, subject males were placed in the middle chamber. Odor was perfused into the wire cage only when the center point of the male subject entered approximately 3-cm proximity (training zone) to the wire cage. Each female was used for 2 consecutive trials, and placement of the female-containing cage was alternated every other trial. Ten trials were completed during training, with an intertrial interval of 2.5 min. Wire cages and side chambers were thoroughly cleaned with paper towel and 70% ethanol in between trials. During testing, in the absence of a female, odor was delivered when the male was in one of the arbitrarily chosen side chambers (CS+ chamber). Odor presentation was controlled using custom-built olfactometers and automated tracking software (EthoVision XT) during training and testing. Time spent in each chamber was quantified by the software and investigation time during training was manually counted. The preference score was obtained by subtracting the time spent in the CS− chamber from the time spent in the CS+ chamber. The subjects that spent more than half of testing session (150 sec) in the center chamber were excluded from analysis.
For optogenetic stimulation of Oxt+ neurons, OxtCre/+ male mice were injected with virus encoding either Cre-dependent ChR2 fused to EYFP, or Cre-dependent EYFP, into the PVH. After two weeks of recovery, animals were trained in our social-appetitive learning paradigm. During training, laser stimulation (405 nm, 30 Hz, 30% duty cycle) (Knobloch et al., 2012) was triggered upon the subject animal entering the CS+ compartment containing a female in diestrus. Photostimulation was controlled by a waveform generator and Ethovision XT. All other conditions were the same as described earlier for the appetitive social learning paradigm.
For ensemble-driven social appetitive learning, photostimulation (405 nm, 7.5–8 mW, 20 Hz, 50% duty cycle) was applied, instead of odor, when the center point of the male subject entered training zone. Oxytocin receptor antagonist (L-368,899) was purchased from Tocris and prepared in saline as 25 mg/mL stock solution. The stock solution was further diluted with saline to make 0.625 mg/mL working solution. The subjects were briefly anesthetized with isoflurane for intraperitoneal (i.p.) injection of 5 mg/kg of antagonist.
Non-social Learning Paradigm
For entrainment to sucrose solution, subject males were water restricted for 2 weeks before the experiment. Two days before the experiment, subjects were singly housed. One day before the experiment, subjects were habituated for 15–20 min to the arena and to wire cages carrying 6 drops of tap water (20 µL total) on their wires. During training, a wire cage carrying 8–12 drops of 10% sucrose solution, instead of female, was placed in one side chamber while an empty wire cage was placed in the opposite side chamber. Pairing and testing were performed in the same way as with the appetitive social learning paradigm. For entrainment to food, males fed ad lib were trained with palatable foods (white chocolate, peanut butter mixed with nutella, and NUTRI-CAL for ferrets) applied on the wire cage. During the first three trials of training, one type of food was used for each trial. For the first six trials, each food was presented twice in random order. For the 7th and 8th trials, the two foods that were highly investigated during previous trials were sequentially used as the US. If mice investigated both cues during the 7th and the 8th trials, the same foods were used during the 9th and the 10th trials in the same order. Otherwise, the food that was investigated the most during the 7th and the 8th trials was used for the final two trials. Testing was carried out for 4 min. Non-social aversive learning paradigm was performed as previously described (Choi et al., 2011).
Social Aversive Learning Paradigm
The three-chambered arena used for appetitive learning was modified for aversive learning. Side chambers were constrained (22.00 × 17.75 × 30.00 cm) and the center chamber was constrained (14.00 × 17.75 × 30.00 cm) using acrylic dividers. All subject mice were group housed prior to training. Prior to training, subject mice were exposed to a sexually experienced CD1 male for five minutes. If the CD1 did not initiate attack before 1.5 min, subject mice were placed in the home cage of a different CD1. Immediately following CD1 exposure, the subject mice were confined to the center of the three-chamber arena for five minutes before training began. Training consisted of 10 trials lasting 1 min each. During each trial, the subjects were confined to one side of the three-chamber arena with either the CS+ odor perfusing through the wire cage containing the previously encountered CD1, or the CS− odor perfusing through the empty wire cage. Each trial type alternated sides of the arena every trial. Immediately following training, animals were confined to the center chamber for five minutes. Animals were then assayed for odor preference in the same manner as in our appetitive social learning paradigm. Animals that spent greater than half of the test session (150 sec) in the center compartment were excluded from analysis. Avoidance scores during training were calculated by dividing the amount of time in the half of training compartment away from the wire cage by the total trial duration. Trials in which photostimulation was used as a CS utilized a narrowed center chamber (5.00 × 17.75 × 30.00 cm). Side chambers were constrained (13.00 × 17.75 × 30.00 cm) during training using acrylic dividers. Training consisted of 16 trials lasting 40 sec each. Baseline and testing trials were unchanged. Animals that spent greater than 100 sec in the center compartment were excluded from analysis. The ROUT method (with Q set to 5%) was used to detect outliers (n = 2).
Real-Time Place Preference (RTPP)
We used a custom built chamber (10 × 10 cm, red acrylic sheet) divided into two equal compartments. Presence in one compartment triggered onset of laser stimulation. Presence in the other compartment was unstimulated. Trials lasted for 20 minutes. We used 30 Hz, 30% duty cycle laser stimulation. Tracking and laser stimulation triggering were accomplished using Ethovision XT software.
Statistics
Group differences in either social appetitive or aversive learning were evaluated by two-way analysis of variance (ANOVA) followed by Tukey HSD post hoc test. Group differences in preference toward CS+ were evaluated by Student’s t-test or one-way ANOVA followed by Tukey HSD post hoc test. Investigation time during training along trial was evaluated by repeated-measures ANOVA (RM-ANOVA) followed by Bonferroni post hoc tests.
Supplementary Material
Highlights.
Oxytocin receptor signaling is specifically required for social learning
Oxytocin neuron stimulation facilitates social learning
Oxytocin directly impacts the piriform cortex to mediate social learning
Both appetitive and aversive social learning requires oxytocin receptor signaling
ACKNOWLEDGEMENTS
We thank Richard Axel, Barbara Noro, Jun Huh, and Charles Jennings for critical reading of the manuscript and helpful discussions; Ashley Park and Kristin Wiseman for technical assistance; Hyun-Ji Lee for preparation of schematic illustration. This work was supported by the Simons Center for the Social Brain Seed Grant (020362-056) and National Institute of Mental Health of the National Institute of Health under award number R01MH106497. H.K.C. was supported by postdoctoral fellowships from the Human Frontier Science Program (LT000692/2014-L) and Basic Science Research Program through National Research Foundation of Korea (NRF). M.D.R. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1122374.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
H.K.C. and M.D.R designed the experiments, conducted the experiments, analyzed the data, and wrote the manuscript. N.B., D.M., N.S., and Y.S.Y. conducted the experiments. G.B.C. conceived the project, designed the experiments, conducted the experiments and wrote the manuscript.
REFERENCES
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Beny Y, Kimchi T. Innate and learned aspects of pheromone-mediated social behaviours. Animal Behaviour. 2014;97:301–311. [Google Scholar]
- Boccia ML, Goursaud AP, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Horm Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Res. 2000;872:87–92. doi: 10.1016/s0006-8993(00)02464-1. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. [DOI] [PubMed] [Google Scholar]
- Giessel AJ, Datta SR. Olfactory maps, circuits and computations. Curr Opin Neurobiol. 2014;24:120–132. doi: 10.1016/j.conb.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, Yu CR. Integrated action of pheromone signals in promoting courtship behavior in male mice. Elife. 2014;3:e03025. doi: 10.7554/eLife.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Holy TE, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science. 2000;289:1569–1572. doi: 10.1126/science.289.5484.1569. [DOI] [PubMed] [Google Scholar]
- Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–1361. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AW, Ackels T, Kuo TH, Cichy A, Dey S, Hays C, Kateri M, Logan DW, Marton TF, Spehr M, Stowers L. Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell. 2014;157:676–688. doi: 10.1016/j.cell.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS. A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS. Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Liberles SD. Aversion and Attraction through Olfaction. Curr Biol. 2015;25:R120–R129. doi: 10.1016/j.cub.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD. Mammalian pheromones. Annu Rev Physiol. 2014;76:151–175. doi: 10.1146/annurev-physiol-021113-170334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D'Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener CD. The social behavior of the bees; a comparative study. Cambridge, Mass: Belknap Press of Harvard University Press; 1974. [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: a review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: "sparse" coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Denny CA, Hen R, Axel R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515:269–273. doi: 10.1038/nature13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB. Social interactions in "simple" model systems. Neuron. 2010;65:780–794. doi: 10.1016/j.neuron.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr Opin Neurobiol. 2014;29:187–193. doi: 10.1016/j.conb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N. The study of instinct. Oxford Eng: Clarendon Press; 1951. [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PD, Anderson PS, Ball RG, Bock MG, Carroll L, Chiu SH, Clineschmidt BV, Culberson JC, Erb JM, Evans BE, et al. 1-((7,7-Dimethyl-2(S)-(2(S)-amino-4-(methylsulfonyl)butyramido)bicyclo [2.2.1]-heptan-1(S)-yl)methyl)sulfonyl)-4-(2-methylphenyl)piperaz ine (L-368,899): an orally bioavailable, non-peptide oxytocin antagonist with potential utility for managing preterm labor. J Med Chem. 1994;37:565–571. doi: 10.1021/jm00031a004. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.