Abstract
We report a case of reversible nonischemic dilated cardiomyopathy in a male in his 60s who presented with an acute heart failure syndrome. Both conventional two-dimensional echocardiography and cardiac magnetic resonance imaging (cMRI) demonstrated severe left ventricular systolic dysfunction; however, both modalities were devoid of significant valvular heart disease as well as the presence of fibrosis, infiltration, inflammation, and scar. After six months of aggressive neurohumoral modulation, there was complete reverse remodeling and normalization of left ventricular function, which highlights the role of cMRI as an adjunct to two-dimensional echocardiography in the detection of a potentially reversible nonischemic cardiomyopathy.
Keywords: cardiology, nonischemic cardiomyopathy, congestive heart failure, cardiac magnetic resonance imaging, two-dimensional echocardiography
Introduction
Two-dimensional echocardiography is the diagnostic modality of choice for the initial detection and evaluation of structural heart disease in patients with symptomatic heart failure (HF). Recently, cardiac magnetic resonance imaging (cMRI) has emerged as an indispensible tool for cardiologists investigating the causes of HF due to its capability of detecting myocardial fibrosis, necrosis, and inflammation, hence predicting potential reversibility.
The following case study emphasizes the complementary role of cMRI along with two-dimensional echocardiography in the diagnosis of a reversible nonischemic cardiomyopathy.
Case Report
A male in his 60s with a past medical history significant for well-controlled hypertension and moderate alcohol consumption was presented to the hospital with a two-month duration of dyspnea and fatigue on minimal effort (New York Heart Association [NYHA] functional class III [Stage C]). Associated symptoms included cough productive of clear phlegm, orthopnea and paroxysmal nocturnal dyspnea. Furthermore, he reported anorexia and a weight loss of 20 lbs. He had no significant past medical or surgical history and no family history of any cardiac disease. Approximately one day prior to admission, he developed sinus tachycardia and was placed on oral furosemide and digoxin in an outpatient setting.
A complete physical examination was performed. The vital signs were notable for a temperature of 97.3 °F, blood pressure of 100/71 mmHg, heart rate of 118 bpm, and respiratory rate of 20/minute. The oxygenation was 100% with an oxygen flow rate of 2 L/minute via nasal cannula. He appeared well with no acute distress. There were diminished breath sounds at the right lung base with dullness to percussion. The jugular venous pulsations were elevated and estimated at 14 cm H2O with a V-wave predominance. There was a rapid regular rhythm with some ectopic beats, soft third heart sound, and grades I and II/VI holosystolic murmurs heard at the lower left parasternal border and apex, respectively. There were no ventricular heaves or lifts, but percussion of the cardiac border demonstrated cardiomegaly. The abdominal examination was significant for a palpable, mildly enlarged liver, which was nonpulsatile. The extremities were warm and free of edema. The distal dorsalis pedis arterial pulses were present, but pulsus alternans was evident. There were no gross neurological deficits.
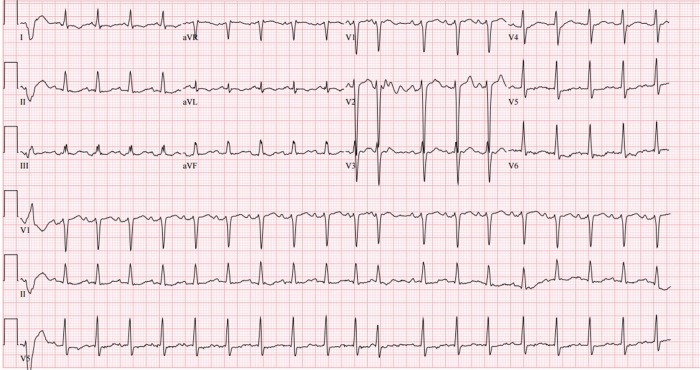
Laboratory data were suggestive of systemic congestion with an elevated brain natriuretic peptide (BNP) level of 2,755 pg/mL and total bilirubin of 2.2 mg/dL. His low-density lipoprotein and high-density lipoprotein were 106 and 24 mg/dL, respectively. Furthermore, there was an elevated galactin-3 level of 29.4 ng/mL. The remaining blood work was normal, including serum sodium, ferritin level, renal, and thyroid functions. A 12-lead electrocardiography showed sinus tachycardia at a rate of 118 bpm with nonspecific ST-T wave changes and occasional premature ventricular complexes. The QRS complex was narrow (98 ms; Fig. 1). Chest roentgenography demonstrated cardiomegaly, pulmonary vascular congestion, and a small right pleural effusion.
Figure 1.
Admission electrocardiogram (ECG) demonstrating sinus tachycardia, a narrow QRS complex and non-specific ST and T wave changes.
The patient was admitted to the telemetry unit with a diagnosis of acute HF. He was started on an initial anticongestive medical regimen of intravenous furosemide 40 mg three times daily, ramipril 2.5 mg at night, carvedilol IR 6.25 mg twice daily, spironolactone 25 mg daily, and continuation of outpatient digoxin 0.25 mg daily.
Conventional two-dimensional transthoracic echocardiography obtained during the acute phase of HF revealed a severely dilated left ventricular cavity with a left ventricular end-diastolic dimension (LVEDD) of 5.91 cm. There was severe global left ventricular hypokinesis with severely reduced left ventricular systolic function. The calculated left ventricular ejection fraction (LVEF) was 20% using Simpson’s method. There was no concentric left ventricular hypertrophy. The right ventricular cavity was severely dilated with moderate-to-severely reduced right ventricular systolic function. There was mild-to-moderate mitral regurgitation and moderate tricuspid regurgitation. The right ventricular systolic pressure was estimated to be 53 mmHg, suggestive of moderate pulmonary hypertension. There was a moderate left atrial (LA) enlargement with LA diameter of 4.5 cm and LA area of 33 cm2 with severe right atrial enlargement. The inferior vena cava appeared normal.
Subsequently, left and right heart catheterizations were performed. Selective coronary arteriography revealed a total occlusion of the mid right coronary artery, which received collateral flow from the left anterior descending coronary artery. Contrast left ventriculography exhibited severe diffuse global left ventricular hypokinesis with an LVEF of 15%. This clearly indicated the presence of nonischemic disease due to the absence of localized asymmetric wall motion abnormalities. The aortic and left ventricular end-diastolic pressures were 120/81 mmHg and 33 mmHg, respectively. There was no significant gradient across the aortic valve. The right heart catheterization hemodynamics demonstrated a mean right atrial pressure of 16 mmHg, right ventricular pressure of 50/12 mmHg, pulmonary artery pressure of 49/23 mmHg with a mean of 35 mmHg and a mean pulmonary capillary wedge pressure of 30 mmHg (V wave 35 mmHg). Utilizing the Fick principle, the cardiac index and output were calculated to be 1.5 L/minute/m2 and 3.5 L/minute, respectively. The transpulmonary gradient was 5 mmHg, and the pulmonary vascular resistance was 1.4 Woods units. On the next day, an electrophysiologic study demonstrated inducible sustained monomorphic ventricular tachycardia with syncope.
Based on the above findings, cMRI was performed to characterize the cardiomyopathy. The study was performed on 1.5 T Siemens scanner. The techniques used in performing the cMRI were steady-state free precession (SSFP) cine for cardiac function, phase velocity, and late gadolinium imaging in the phase-sensitive inversion recovery (PSIR) technique for delayed enhancement with gadolinium 0.15 mmol/kg to obtain standard two-chamber, three-chamber, four-chamber, long-, and short-axis left ventricular views.
On cMRI, there was severe biventricular dilatation with a left ventricular end-diastolic volume of 137 mL/m2 and a right ventricular end-diastolic volume of 124 mL/m2 cal culated using Simpson’s method (Fig. 2). There was no evidence of biventricular hypertrophy. Biventricular global systolic function was severely reduced. The biventricular regional systolic function revealed severe diffuse global hypokinesis. The LVEF and right ventricular ejection fraction were measured to be 17% and 18%, respectively. There was mild mitral and tricuspid regurgitation. The pulmonary artery appeared normal. The left atrium was severely dilated, and the right atrium was moderately dilated. The LA area in the four-chambered view was measured to be 38 cm2, while the volume was noted to be 203 mL (Fig. 2). Additionally, the right atrial area was cal culated to be 29 cm2. On delayed gadolinium-enhanced imaging PSIR sequences, no gadolinium uptake was observed in two-chamber (Fig. 3A), four-chamber (Fig. 3B), or sagittal two-chamber (Fig. 3C) views. Thus, there was no evidence of fibrosis or infarction (scar). Inflammation was also excluded by utilization of the relative enhancement technique.
Figure 2.
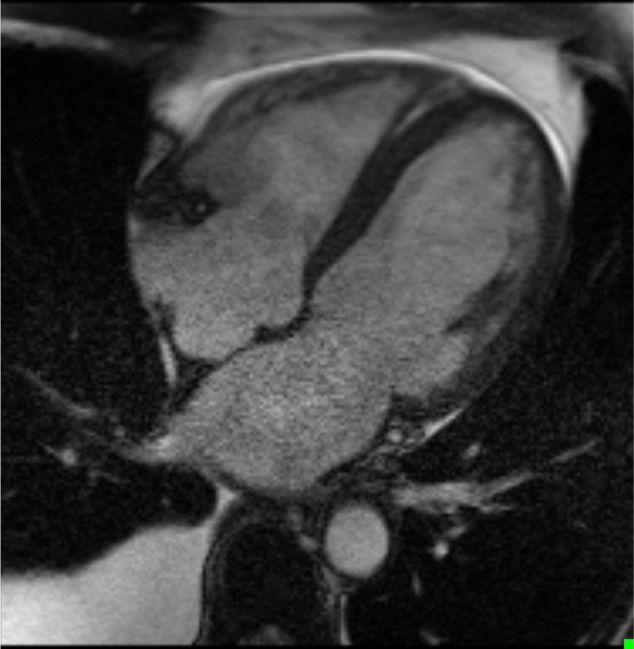
End diastolic cine cMRI image, 4 chambers view.
Figure 3.
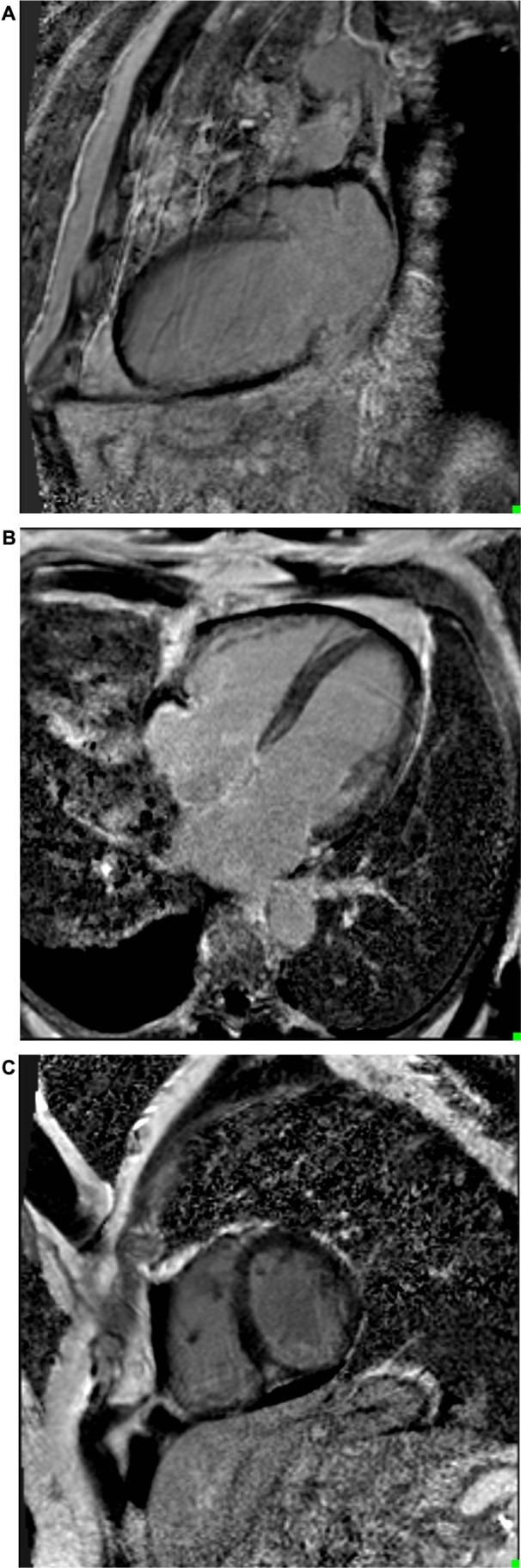
(A) 2-chamber LGE imaging devoid of any enhancement. (B) 4-chamber LGE imaging devoid of any enhancement. (C) Sagittal 2-chamber LGE imaging devoid of any enhancement.
After achieving an euvolemic state, the patient was discharged. Due to the positive electrophysiology study, he was fitted with a LifeVest for the primary prevention of sudden cardiac death. Additionally, he was maintained on a medical regimen of furosemide 40 mg orally twice daily, ramipril 2.5 mg at night, carvedilol IR 6.25 mg twice daily, spironolactone 25 mg daily, and digoxin 0.25 mg daily. Both aspirin 81 mg daily and simvastatin 20 mg at bedtime were added due to the presence of coronary artery disease (CAD). Digoxin was utilized due to the fact that it has been shown to improve ventricular systolic function and decrease hospitalizations in CHF with reduced ejection fraction. Over the next six months, furosemide was slowly discontinued, while ramipril and carvedilol IR were titrated to maximally recommended doses of 10 mg daily and 25 mg twice daily, respectively. The basic metabolic panel remained unremarkable and the BNP level normalized to 36 pg/mL. Galactin-3 level, however, remained elevated at 20.1 ng/mL. The patient’s clinical status improved drastically, and he became asymptomatic, regressing to NYHA functional class I (Stage A). A cardiopulmonary exercise tolerance test was done to objectively assess his cardiopulmonary exercise capacity. He was able to achieve a peak workload of 7.0 Metabolic Equivalents of Task (METS) with a peak oxygen consumption of 21.3 mL/kg/minute (74% predicted), which was equivalent to normal functional fitness.
Conventional two-dimensional echocardiography was again obtained with three-dimensional echocardiographic reconstruction. The left ventricular chamber size was now normal with a LVEDD of 4.83 cm (Fig. 4A and B). The left ventricular systolic function was normal with a calculated LVEF of 55%. The right ventricular cavity size and global systolic function were normal. Mild mitral regurgitation was present. There was no evidence of tricuspid regurgitation. There was no evidence of pulmonary hypertension. The LA area was now 25 cm2, and LA diameter was 3.9 cm. The infer ior vena cava appeared normal. At this point, the spironolactone and digoxin were discontinued. Furthermore, he no longer required the LifeVest support. At last follow-up, he remained asymptomatic with NYHA functional class I (Stage A) only with modulation of the rennin–angiotensin and sympathetic nervous systems, namely, ramipril 10 mg daily and carvedilol IR 25 mg twice daily.
Figure 4.
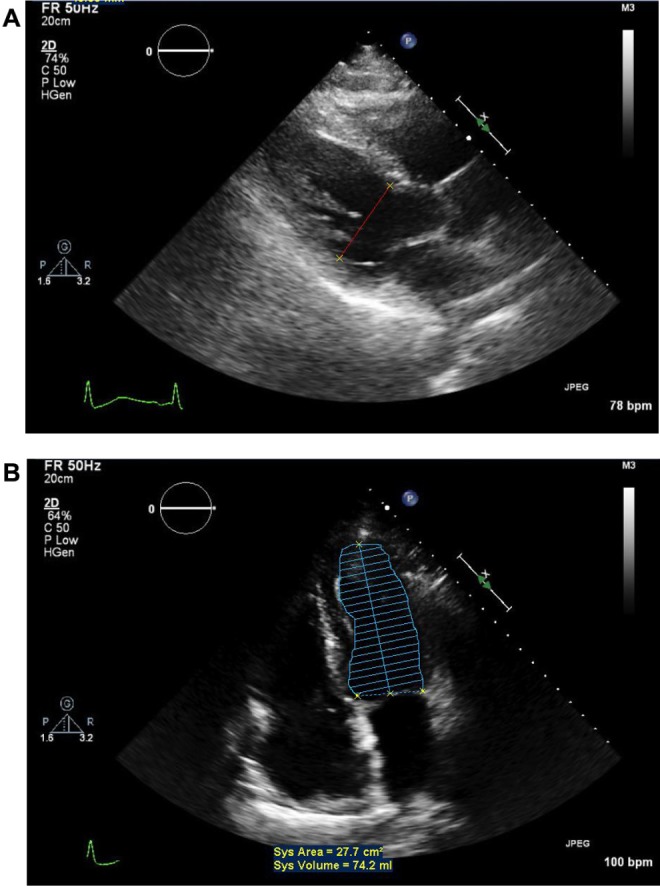
Post-treatment two-dimensional echocardiography parasternal long-axis and apical two-chamber views. Note the normalization of left ventricular end-diastolic dimension, systolic area and volume.
Discussion
Left ventricular systolic dysfunction associated with advanced HF symptomatology is thought to be a condition characterized by progressive deterioration. Although the goal of the treatment is to prevent further damage and to alleviate symptoms, morbidity and mortality rates remain quite high. However, there are certain nonischemic cardiomyopathies that are reversible with neurohumoral modulation.1,2 cMRI has been a useful complementary tool to two-dimensional echocardiography in the identification of these particular cases.
cMRI assesses left ventricular function by using SSFP cine imaging of the left ventricle.3 SSFP provides the best differentiation between blood in the chambers, which is visualized as white and myocardial tissue that is visualized as dark.4
In contrast to two-dimensional echocardiography, cMRI has the capability to obtain images in any desired anatomical plane. Data obtained from SSFP cMRI images include global left ventricular and right ventricular masses and volumes. Additionally, the three-dimensionality of cMRI allows the clinician to examine the right ventricle in greater detail, a task that is quite difficult when utilizing conventional two-dimensional transthoracic echocardiography.4
cMRI has unique advantages in tissue characterization. On T1-weighted sequence, fat has bright signal, whereas myocardium has dark signal. On T2-weighted images, water has a bright signal.
Gadolinium-enhanced imaging is used to ascertain the presence of prior infarcts, necrosis, or fibrosis. Damaged myocardium is characterized by increased extracellular volume caused by reduced functioning of the intracellular capillaries. This in turn causes hyperenhancement of damaged tissue on T1 imaging.4 cMRI along with gadolinium contrast can differentiate between ischemic and nonischemic variants of HF. Patients diagnosed with dilated cardiomyopathy who have a history of prior infarction primarily display subendocardial or transmural gadolinium uptake. Dilated cardiomyopathy patients without a prior history of infarction have blotchy or vertical striae along with mid-wall enhancement that cannot be isolated to a specific coronary artery perfusion area. This unique enhancement pattern is indicative of focal fibrosis or myocarditis.4
The edema discernable on T2 imaging is precipitated by inflammatory processes or acute ischemia. T2 imaging also enables differentiation between acute and chronic injury. The presence of edema indicates acute ischemic injury that can be salvaged before the onset of irreversible tissue damage.
A study by Bello et al evaluated the role of gadolinium-enhanced cMRI images in assessing the improvement of cardiomyopathy in patients treated with β-blockers and has shown an inverse relationship between transmural extent of scarring and the likelihood of improvement. In fact, in the study population, those with >75% regional scarring showed no improvement in contractile function in those regions after β-blocker treatment.5
Another prospective trial evaluated the capability of Late Gadolinium Enhancement (LGE) cMR imaging in distinguishing LV dysfunction related (presence of subendocardial ± transmural LGE) and not related to CAD. The study found that LGE had 91% specificity and 87% sensitivity in determining the presence of obstructive CAD when angiography was used as a diagnostic standard.6
Galactin-3 has recently emerged as an independent prognostic biomarker in the evaluation of HF and is associated with cardiac fibrosis.7,8 Galactin-3 is known to induce fibroblast production and collagen synthesis which are processes that play a pivotal role in promoting fibrosis. Gala ctin-3 has also been well documented as being a criterion for the diagnosis of HF. Its efficacy in establishing a diagnosis was examined in a study that compared 35 patients who were diagnosed as having HF with preserved ejection fraction (HFpEF) versus 43 healthy volunteers.9 Plasma levels of galactin-3 and BNP were measured in both groups to assess each marker’s respective efficacy in diagnosing HF. Both galactin-3 and BNP levels were found to be elevated in the HFpEF group in contrast to the control group. When a cutoff of 17.8 ng/mL was utilized, 33 of 35 patients were found to have HF. When a BNP level of 100 pg/mL was used as a benchmark, 27 of 35 patients were assessed as having HF. Fifteen of 43 patients in the control group were found to have HF when galactin-3 was used as a criterion for diagnosis, whereas only four subjects were labeled as having HF when BNP was used as the primary criterion. Galactin-3 was able to diagnose HF with a sensitivity of 94.3% (P = 0.04) and a specificity of 65.1% (P = 0.004), while BNP was able to establish a diagnosis with a sensitivity of 77.1% and a specificity of 90.7%.9 These data seem to suggest that galactin-3 along with BNP levels can be helpful to screen for the presence of HF, but both markers should not be used as definitive evidence of decompensated HF. Our case illustrates this principle since the galactin-3 levels remained elevated even after sufficient treatment.
Although the galectin-3 level in this case remained elevated after treatment, there was a relative decrease from 29.4 ng/mL pretreatment to 20.1 ng/mL posttreatment. Despite a markedly elevated posttherapy galectin-3 level in our patient, cMRI was devoid of hyperenhancement suggestive of fibrosis. Although fibrosis was not detected using cMRI with gadolinium, it is quite possible that diffuse fibrosis might have been present. Diffuse fibrosis is visible on T1 mapping, which was not performed due to the hospital protocol.
Through aggressive neurohumoral modulation with renin–angiotensin and sympathetic nervous system antagonists, the cornerstone of HF therapy,8,10–12 complete left ventricular recovery was achieved.
Automatic implantable cardioverter defibrillators and cardiac resynchronization devices are available in the management of HF.13,14 Our patient demonstrated inducible ventricular tachycardia with syncope. One might hypothesize that an elevated Left Ventricular End-Diastolic Pressure (LVEDP) rendered enough diastolic wall stress in accordance with the Law of Laplace. Despite the elevated galectin-3 level and positive electrophysiologic study, the absence of hyperenhancement on cMRI was reassuring that the arrhythmia burden was indeed low. Although our patient received a LifeVest support during uptitration and optimization of the medical therapy, this was discontinued once left ventricular recovery was noted.
Finally, the American College of Cardiology (ACC)/American Heart Association (AHA) now classifies HF based on risk.15,16 The Stages A–D are thought to be irreversible and progressive. It is entirely possible for a patient to regress from an advanced NYHA functional class to an asymptomatic state. However, our case indicates that regression from Stage C to Stage A (symptomatic left ventricular systolic dysfunction to an asymptomatic state with normalization of left ventricular systolic function) is also possible.
Conclusion
cMRI is a useful adjunct in the evaluation of cardiomyopathy due to its unique advantages of accurate quantification of left and right ventricles and tissue characterization capabilities. Valuable information such as scar/inflammation detection and quantification in such cases can be proven useful as powerful prognostic tools to predict potentially reversible cardiomyopathies.
In our case, due to the absence of hyperenhancement on cMRI, fibrosis, inflammation, and infarction were readily rejected as causes for the patient’s cardiomyopathy, and along with 2D-echocardiography findings, cMRI was instrumental in determining a potentially favorable outcome for this patient.
Footnotes
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1901 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Acquisition of data, drafting the article, critical revision of the article, and final approval of the version to be published: SM, PS, SKV. Conception and design, analysis and interpretation of data, critical revision of the article, and final approval of the version to be published: TJV, CEK, RM, AM. Conception and design, critical revision, drafting the article, and final approval of the version to be published: TJV.
REFERENCES
- 1.Hainstein ME, Berliner JI, Shah SJ, Taegtmeyer H, Gheorghiade M. Normalization of ejection fraction and resolution of symptoms in chronic severe heart failure is possible with modern medical therapy: clinical observations in 11 patients. Am J Ther. 2008;15:206–13. doi: 10.1097/MJT.0b013e3181728a1d. [DOI] [PubMed] [Google Scholar]
- 2.Huffman C, Wagman G, Fudim M, Zolty R, Vittorio T. Reversible cardiomyopathies – a review. Transplant Proc. 2010;42(9):3673–8. doi: 10.1016/j.transproceed.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 3.West AM, Kramer CM. Cardiovascular magnetic resonance imaging of myocardial infarction, viabilities and cardiomyopathies. Curr Probl Cardiol. 2010;35:176–220. doi: 10.1016/j.cpcardiol.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol. 2009;54:1407–24. doi: 10.1016/j.jacc.2009.04.094. [DOI] [PubMed] [Google Scholar]
- 5.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy. Circulation. 2003;108:1945–53. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 6.Soriano CJ, Ridocci F, Estornell J, Jimenez J, Martinez V, De Velasco JA. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol. 2005;45:743–8. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 7.de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–8. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–56. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin QS, Shi B, Dong L, Bi L. Comparative study of galectin-3 and B-type natriuretic peptide as biomarkers for the diagnosis of heart failure. J Geriatr Cardiol. 2014;11:79–82. doi: 10.3969/j.issn.1671-5411.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure: US Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Zannad F, Remme WJ, et al. The effect of spironlactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 13.Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. N Engl J Med. 2005;352:225–37. [Google Scholar]
- 14.Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. J Heart Lung Transplant. 2002;21:189–203. doi: 10.1016/s1053-2498(01)00776-8. [DOI] [PubMed] [Google Scholar]
- 16.Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation and assessment of myocardial viability. Circulation. 2008;117:103–14. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]