Abstract
Cytochrome P450 (CYP)-derived epoxyeicosatrienoic acids (EETs) exhibit potent cardiovascular protective effects in preclinical models, and promoting the effects of EETs has emerged as a potential therapeutic strategy for coronary artery disease (CAD). The relationship between circulating EET levels and CAD extent in humans, however, remains unknown. A panel of free (unesterified) plasma eicosanoid metabolites was quantified in 162 patients referred for coronary angiography, and associations with extent of CAD [no apparent CAD (N = 39), nonobstructive CAD (N = 51), and obstructive CAD (N = 72)] were evaluated. A significant relationship between free EET levels and CAD extent was observed (P = 0.003) such that the presence of obstructive CAD was associated with lower circulating EET levels. This relationship was confirmed in multiple regression analysis where CAD extent was inversely and significantly associated with EET levels (P = 0.013), and with a biomarker of EET biosynthesis (P < 0.001), independent of clinical and demographic factors. Furthermore, quantitative enrichment analysis revealed that these associations were the most pronounced compared with other eicosanoid metabolism pathways. Collectively, these findings suggest that the presence of obstructive CAD is associated with lower EET metabolite levels secondary to suppressed EET biosynthesis. Novel strategies that promote the effects of EETs may have therapeutic promise for patients with obstructive CAD.
Keywords: arachidonic acid, atherosclerosis, eicosanoids, heart, inflammation, pharmacometabolomics, precision medicine
Despite significant advances in its treatment, coronary artery disease (CAD) remains the leading cause of morbidity and mortality worldwide (1). Consequently, new therapeutic strategies are needed to further improve outcomes. The discovery of biomarkers involved in the pathogenesis of CAD offers considerable promise to facilitate the development of novel therapeutics and identify subsets of high-risk patients who may derive the greatest benefit from therapy (2).
In addition to their role as xenobiotic metabolizing enzymes, cytochromes P450 (CYPs) metabolize fatty acids to bioactive lipids that regulate numerous cellular and physiological processes relevant to the pathogenesis of CAD (3). Most notably, CYP epoxygenases from the CYP2C and CYP2J subfamilies metabolize arachidonic acid (AA) into four epoxyeicosatrienoic acid (EET) regioisomers (5,6-, 8,9-, 11,12-, and 14,15-EET) with varying regioselectivity (4). The EETs elicit potent vasodilatory, anti-inflammatory, and cellular protective effects in the cardiovascular system but are rapidly hydrolyzed by soluble epoxide hydrolase (sEH; EPHX2) into dihydroxyeicosatrienoic acids (DHETs) (5, 6).
Promoting the effects of EETs yields potent cardiovascular protective effects in preclinical models of vascular inflammation, atherosclerosis, and myocardial ischemia-reperfusion injury (5–7). In epidemiological studies, associations between functional polymorphisms in genes responsible for EET biosynthesis (CYP2C8, CYP2J2) or EET hydrolysis (EPHX2) and the development of CAD have been reported (8–10). Taken together, these data suggest that increasing CYP-derived EET levels may be a viable therapeutic strategy for CAD. However, because EETs are not measured on traditional metabolomic or eicosanoid analytical platforms (11), major gaps in knowledge surrounding the biological and therapeutic importance of EETs in human CAD exist. Quantifying EET metabolite levels offers the potential to identify key factors that impact EET biosynthesis and EET hydrolysis, beyond germ-line genetic polymorphisms, and facilitate the rational design of clinical trials with emerging therapies that target the EET metabolic pathway.
The primary objective of the current study was 2-fold: 1) to elucidate the relationship between CYP-derived EET levels and the angiographic extent of CAD and 2) to evaluate the strength of this association relative to eicosanoids derived from the CYP hydroxylase, cyclooxygenase (COX), and lipoxygenase (LOX) metabolic pathways. In order to accomplish these objectives, a panel of 28 CYP-, COX-, and LOX-derived lipid metabolites was quantified in a cohort of patients referred for coronary angiography with suspected or worsening obstructive CAD using a targeted metabolomics approach.
MATERIALS AND METHODS
Study population
A cohort of individuals 18–80 years of age referred for coronary angiography to detect new or worsening CAD were identified in the cardiac catheterization laboratories at the University of North Carolina at Chapel Hill (UNC) between September 2012 and February 2014. Exclusion criteria included severe concurrent illness (such as active pneumonia or acute decompensated heart failure), systemic inflammatory disease (such as rheumatoid arthritis), cancer actively being treated, hematologic disorders affecting platelet function, prior heart transplantation, hematocrit <30%, ST segment elevation myocardial infarction (STEMI), end-stage liver disease, end-stage renal disease on dialysis, and systemic immunosuppressive medication use (such as corticosteroid use). Eligible participants provided written informed consent. The study protocol was approved by the UNC Biomedical Institutional Review Board.
Whole blood was drawn in the catheterization lab from the indwelling arterial sheath after it had been placed as part of the coronary angiography procedure, but before initiation of a revascularization procedure (if indicated). Plasma was immediately separated by centrifugation and stored at −80°C.
Classification of CAD extent
The extent of CAD was classified based on the results of the coronary angiography. Obstructive CAD was defined according to the anatomical or physiological “criteria for revascularization” from recent clinical practice guidelines (12). Briefly, the anatomic criteria for revascularization [percutaneous coronary intervention (PCI) or coronary artery bypass grafts (CABGs)] are the presence of ≥50% stenosis in the left main coronary artery or ≥70% stenosis in one or more of the nonleft main coronary arteries. The classification of nonobstructive CAD included those without obstructive CAD that had mild stenosis (10–69% in one or more nonleft main coronary arteries and/or 10–49% stenosis in the left main coronary artery), whereas the classification of no apparent CAD included those with no angiographic evidence of CAD (<10% stenosis in all coronary arteries). Only unprotected lesions (lesions not bypassed by collateral vessels, bypass grafts, or stents) in native coronary arteries were considered for classification in order to reflect the current extent of coronary artery stenosis at the time of blood sampling that was unrelated to a prior revascularization procedure.
Quantification of eicosanoid metabolite concentrations
Free plasma eicosanoid metabolite concentrations were quantified using an LC/MS/MS method with optimized sensitivity and specificity for EET quantification, as previously described with minor modifications (13, 14). Briefly, plasma (0.25 ml) was diluted in 0.1% acetic acid/5% methanol solution (0.25 ml) containing 0.009 mM butylated hydroxytoluene (BHT), spiked with internal standards [3 ng each of prostaglandin E2-d4 (Cayman Chemical, Ann Arbor, MI), 10,11-dihydroxynondecanoic acid, and 10,11-epoxyheptadecanoid acid (kindly provided by Dr. Bruce Hammock, University of California, Davis; Ref. 15)], and processed by liquid-liquid extraction (0.1% acetic acid/5% methanol and ethyl acetate) to isolate lipids. Extracts in ethyl acetate were then dried by centrifugation under vacuum, covered in argon, and stored at −80°C for future processing. Dried extracts were reconstituted in 80% methanol (containing 10 mg/ml BHT) for elution through phospholipid removal columns (Phree; Phenomenex, Torrance, CA), as directed by the manufacturer. Phospholipid removal enhances purification of the extracts, thereby reducing matrix ion suppression and improving signal-to-noise ratio (16). Phospholipid-free extracts were then dried a second time by centrifugation under vacuum and stored at −80°C. At the time of analysis, extracts were reconstituted in 50 μl of 30% ethanol, and a panel of 34 eicosanoid metabolites (supplementary Table 1) was quantified by targeted LC/MS/MS, as previously described (13, 14).
Concentration values falling below the lower limit of quantitation were imputed as a concentration equal to half of the lowest standard. Analytes with >50% of the values below or above the lower or upper limit of quantitation, respectively, were not included in the analysis (supplementary Table 1) (13). Among the 28 metabolites that met these criteria, 24 of 28 (86%) had <10% values out of the quantitation range. Free plasma concentrations ranged from nanomolar to micromolar.
Gene expression in peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated in 98 participants enrolled between July 2013 and February 2014. Briefly, blood (5 ml) was centrifuged over a density gradient medium (Lymphoprep; Axis Shield, Oslo, Norway). Cells were collected at the interface, washed with cold PBS, and homogenized. Total RNA was isolated using the RNeasy Miniprep kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Expression of CYP2J2 (Hs00356035_m1) and EPHX2 (Hs00157403_m1) was quantified using Taqman Assays on Demand (Applied Biosystems, Foster City, CA) and calculated relative to GAPDH (Hs02758991_g1) using the 2-ΔCt method, as previously described (17).
Time-to-event analysis of cardiovascular outcomes
The incidence of future cardiovascular events was abstracted from the electronic medical record from the time of the index angiography through August 2014. Those who did not present to the UNC health care system for routine follow-up or emergent care after the index coronary angiography visit were considered to be lost to follow-up and were excluded from the analysis. The prespecified primary end point was the time to the first occurrence of death due to a cardiovascular cause, hospitalization for a nonfatal acute coronary syndrome (ACS) event (unstable angina, non-STEMI, STEMI), or hospitalization for a coronary revascularization procedure (PCI or CABG). Clinician-reported outcomes were verified from the electronic medical records by two individuals.
Statistical analysis
Data are presented as mean ± standard deviation, median (interquartile range), or count (%). All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC), and P < 0.05 was considered statistically significant, unless otherwise indicated. Study population characteristics were compared across CAD extent using one-way ANOVA for continuous data and Chi-square test or Fisher’s exact test for categorical data as appropriate.
Metabolite levels and epoxide/diol ratios were not normally distributed and thus log-transformed prior to statistical analysis. Pearson correlations between individual metabolites were evaluated. Significant correlations were observed among the EET as well as the DHET regioisomers (supplementary Figure 1). Thus, to minimize redundancy, the sum of the 8,9-, 11,12-, and 14,15-EET regioisomers (sum EETs) and the 5,6-, 8,9-, 11,12-, and 14,15-DHET regioisomers (sum DHETs) were calculated (13). The sum EETs served as the primary end point for comparison across CAD extent. The sum of the EET and DHET regioisomers (sum EETs + DHETs) and the ratio of 14,15-EET to 14,15-DHET (14,15-EET:14,15-DHET ratio) (secondary end points) were calculated as biomarkers of CYP epoxygenase and sEH metabolic function, respectively (18).
The sum EETs, sum EETs + DHETs, and 14,15-EET:14,15-DHET ratio were compared across CAD extent by ANOVA (unadjusted). Comparisons across CAD extent were also completed after adjusting for demographic factors (age, African American race, sex) and clinical factors that were independently associated with CAD extent (P < 0.15) in a multivariable model (diabetes, prior acute myocardial infarction, beta blocker use, angiotensin-converting enzyme inhibitor use). Hyperlipidemia status was also included in the adjusted model due to the potential impact of lipoprotein levels on free EET concentrations in plasma. Post hoc pairwise comparisons were conducted using Fisher’s least significant difference (LSD) test. In order to obtain additional insight into these analyses, the following comparisons across CAD extent were explored: sum DHETs, individual EET and DHET regioisomers, other biomarkers of sEH metabolic function (11,12-EET:11,12-DHET; 8,9-EET:8,9-DHET; sum EETs:sum DHETs; 12,13-epoxyoctadecaenoic acid to dihydroxyoctadecaenoic acid [EpOME:DHOME]), and PBMC expression of CYP2J2 and EPHX2.
To determine the magnitude of the association between sum EETs and CAD extent relative to other demographic and clinical factors, multiple regression analysis was also performed. Potential covariates included demographic factors (age, gender, African American race), cardiovascular risk factors (obesity, hypertension, diabetes, cigarette smoking, hyperlipidemia, peripheral artery disease, prior acute myocardial infarction, prior stroke), comorbidities (lung disease, heart failure), body mass index, and chronic medication use preangiography (angiotensin-converting enzyme inhibitor, angiotensin receptor blockers, aspirin, beta blockers, statins, calcium channel blockers, P2Y12 inhibitors, metformin, nitrates, nonstatin lipid modifiers, fish oil, proton pump inhibitors), and indices of CAD extent (current ACS, presence of collaterals, maximum coronary artery stenosis, postangiography revascularization procedure). All covariates associated with sum EETs (P < 0.15) in a univariate analysis were carried forward into a stepwise multivariate analysis that included forward selection and backward elimination. Although hyperlipidemia status did not meet these criteria (P = 0.35), this covariate was also considered in the modeling. Collinear variables (variance inflation factor >10 or condition index >30) were removed, and covariates with P < 0.15 were retained in the final adjustment model. A model was also constructed for sum EETs + DHETs levels.
Metabolomic analyses [Student’s t-tests for each individual metabolite followed by false discovery rate (FDR) analyses and quantitative enrichment analysis (QEA)] were performed with Metaboanalyst 3.0 (19). For these analyses, biomarker comparisons were conducted between those with obstructive CAD and all other patients (no apparent CAD and nonobstructive CAD groups were combined and served as a single reference group). Metabolites with P < 0.05 and FDR q < 0.05 were considered significantly different across obstructive CAD status. Metabolites within the same pathway tended to be positively correlated with each other (supplementary Figure 1). In order to determine the most enriched eicosanoid metabolic pathways across obstructive CAD status, metabolites were then assigned to nine distinct eicosanoid metabolic pathways according to their parent substrate and biosynthesis enzyme. QEA was conducted to determine the relative impact of obstructive CAD on the nine eicosanoid metabolic pathways. P values for each pathway were generated from observed Q statistics. The Q statistic for each pathway was determined by averaging the Q statistics for each metabolite within that pathway. Pathways were considered significantly enriched when P < 0.05 and corresponding FDR q < 0.05.
The relationship between baseline CAD extent and time to occurrence of the primary end point (cardiovascular death, nonfatal ACS event, coronary revascularization) was assessed with Cox proportional hazards regression. Due to a low number of events in those with no apparent (0 events) or nonobstructive (4 events) CAD, the association between baseline EET levels (tertiles) and the primary end point was explored exclusively within the obstructive CAD patients. Due to the low number of events, time-to-event analyses were unadjusted. Kaplan-Meier curves were generated using GraphPad Prism 6.0.
RESULTS
Study population
The demographic and clinical characteristics of the study population are shown in Table 1. Of 162 participants, 72 (44%) were diagnosed with obstructive CAD, 51 (31%) were diagnosed with nonobstructive CAD, and 39 (24%) exhibited no apparent CAD. Several indices of CAD burden were significantly more prevalent with increasing CAD extent including history of a prior revascularization procedure (P = 0.009) and diagnosis of an ACS during the index visit (P < 0.001). The prevalence of CAD risk factors and medication use were comparable to previously reported coronary angiography cohorts and reflected clinical practice guidelines (12, 20). Cholesterol and triglyceride levels did not significantly differ across CAD extent.
TABLE 1.
Characteristics of the total study population and across the angiographic extent of CAD
| Characteristic | Total (N = 162) | No Apparent CAD (N = 39) | Nonobstructive CAD (N = 51) | Obstructive CAD (N = 72) | Pa |
| Demographics | |||||
| Age (years) | 61.8 ± 10.3 | 58.2 ± 10.4 | 62.1 ± 9.9 | 63.6 ± 10.2 | 0.028 |
| Female | 70 (43.2%) | 18 (46.2%) | 22 (43.1%) | 30 (41.7%) | 0.901 |
| African American | 34 (21.0%) | 10 (25.6%) | 11 (21.6%) | 13 (18.1%) | 0.640 |
| Body mass index (kg/m2) | 31.8 ± 7.5 | 34.0 ± 8.0 | 30.8 ± 8.8 | 31.4 ± 6.0 | 0.110 |
| Past medical history | |||||
| Current/recent (<1 year) smoker | 46 (28.4%) | 11 (28.2%) | 15 (29.4%) | 20 (27.8%) | 0.980 |
| History of hypertension | 130 (80.3%) | 28 (71.2%) | 41 (80.4%) | 61 (84.7%) | 0.263 |
| History of diabetes | 51 (31.5%) | 9 (23.1%) | 14 (27.5%) | 28 (38.9%) | 0.174 |
| History of hyperlipidemia | 111 (68.5%) | 23 (59.0%) | 34 (66.7%) | 54 (75.0%) | 0.209 |
| Prior acute myocardial infarction | 24 (14.8%) | 1 (2.6%) | 6 (11.8%) | 17 (23.6%) | 0.006 |
| Prior revascularization | 56 (34.6%) | 6 (15.4%) | 18 (35.3%) | 32 (44.4%) | 0.009 |
| Medication use | |||||
| ACE inhibitor use | 73 (45.1%) | 14 (35.9%) | 18 (35.3%) | 41 (56.9%) | 0.025 |
| ARB use | 33 (20.4%) | 10 (25.6%) | 13 (25.5%) | 10 (13.9%) | 0.187 |
| Aspirin use | 122 (75.3%) | 29 (74.4%) | 37 (72.6%) | 56 (77.8%) | 0.793 |
| Beta blocker use | 99 (61.1%) | 16 (41.0%) | 32 (62.8%) | 51 (70.8%) | 0.009 |
| Statin use | 123 (75.9%) | 27 (69.2%) | 39 (76.5%) | 57 (79.2%) | 0.502 |
| Calcium channel blocker use | 46 (28.4%) | 13 (33.3%) | 14 (27.5%) | 19 (26.4%) | 0.729 |
| Cardiac catheterization laboratory | |||||
| ACS on presentation | 29 (17.9%) | 3 (7.7%) | 3 (5.9%) | 23 (31.9%) | <0.001 |
| Presence of collateral | 20 (12.4%) | 5 (12.8%) | 4 (7.8%) | 11 (15.3%) | <0.001 |
| Stenosis in most severe vessel (%) | 50 (80) | 0 (0) | 40 (25) | 90 (15) | <0.001 |
| Serum lipoprotein levels, nb | 82 | 12 | 22 | 48 | |
| Total cholesterol (mg/dl) | 150.5 (60) | 157 (53) | 160.5 (60) | 147 (58.5) | 0.835 |
| LDL cholesterol (mg/dl) | 82 (42) | 83.5 (35.5) | 83 (44) | 80 (39) | 0.625 |
| HDL cholesterol (mg/dl) | 38.5 (13) | 35.5 (12) | 41 (11) | 38.5 (14.5) | 0.329 |
| Triglycerides (mg/dl) | 140.5 (128) | 164 (164.5) | 162 (128) | 130.5 (119.5) | 0.521 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker. Data presented as mean ± standard deviation, median (interquartile range), or count (%).
One-way ANOVA across CAD extent was performed for continuous variables, and Chi-square test or Fisher’s exact test was performed for categorical variables.
A fasting lipid panel obtained for clinical purposes was available in 82 participants; comparisons of lipid levels were completed using log-transformed data.
Association of EET metabolic pathway biomarkers and CAD extent
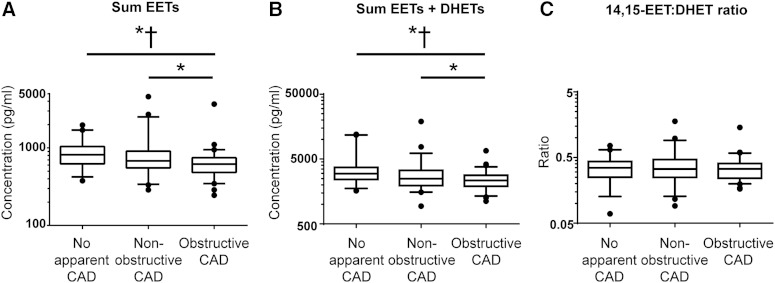
The sum of free EET levels in plasma were inversely associated with CAD extent, such that EET levels in obstructive CAD patients were lower than EET levels in patients with either no apparent CAD or nonobstructive CAD (Fig. 1A; Table 2). Sum EET levels significantly differed between no apparent CAD and obstructive CAD patients (P < 0.05), whereas the observed difference in EET levels between nonobstructive CAD and obstructive CAD patients was not statistically significant after adjusting for covariates (P = 0.063). No difference in EET levels was observed between patients with no apparent CAD and nonobstructive CAD. Consistent results were observed with the individual 8,9-, 11,12-, and 14,15-EET regioisomers and the DHET metabolites (Table 2). Furthermore, higher maximum coronary artery stenosis (another indicator of CAD extent: the percent narrowing of the most obstructed coronary artery) was significantly associated with lower sum EETs (Pearson’s r = −0.26, P = 0.001).
Fig. 1.
Plasma biomarkers of CYP-mediated EET biosynthesis and hydrolysis across CAD extent. A: Sum EETs were associated with CAD extent across no apparent CAD (N = 39), nonobstructive CAD (N = 51), and obstructive CAD (N = 72) patients (ANOVA: unadjusted P = 0.003, adjusted P = 0.043). B: A significant association was also observed with sum EETs + DHETs (ANOVA: unadjusted P = 0.001, adjusted P = 0.007). C: In contrast, the 14,15-EET:DHET ratio was not associated with CAD extent (ANOVA: unadjusted P = 0.693, adjusted P = 0.747). Data presented as median (midline), interquartile range (box), and 95% confidence intervals (whiskers). * P < 0.05 in unadjusted post hoc pairwise comparisons. † P < 0.05 in adjusted pairwise comparisons.
TABLE 2.
Comparison of free plasma CYP epoxygenase-derived and sEH-derived AA metabolite biomarkers across CAD extent
| Analyte | No Apparent CAD(N = 39) | Nonobstructive CAD(N = 51) | Obstructive CAD(N = 72) | Pa |
| AA-CYP epoxides (pg/ml) | ||||
| 14,15-EET | 368 (170) | 295 (170) | 286 (117)d,e | 0.006 |
| 11,12-EET | 192 (89) | 171 (99) | 152 (85)d,e | 0.003 |
| 8,9-EET | 235 (125) | 205 (112) | 189 (87)d,e | 0.007 |
| Sum EETsb | 815 (417) | 678 (356) | 618 (258)d,e | 0.003 |
| AA-sEH diols (pg/ml) | ||||
| 14,15-DHET | 1,007 (568) | 971 (449) | 845 (442)d | 0.006 |
| 11,12-DHET | 657 (393) | 633 (367)d | 563 (257)d | 0.005 |
| 8,9-DHET | 300 (193) | 229 (206) | 217 (110)d | 0.039 |
| 5,6-DHET | 290 (145) | 244 (198) | 229 (122)d | 0.028 |
| Sum DHETs | 2,324 (1,305) | 2,084 (1,605) | 1,956 (875)d | 0.004 |
| CYP epoxygenase function (pg/ml) | ||||
| Sum EETs + DHETs | 3,321 (1,441) | 2,737 (1,670) | 2,593 (997)d,e | 0.001 |
| sEH function (ratio) | ||||
| 14,15-EET:14,15-DHET | 0.35 (0.18) | 0.33 (0.22) | 0.34 (0.16) | 0.693 |
| 11,12-EET:11,12-DHET | 0.28 (0.19) | 0.29 (0.19) | 0.27 (0.16) | 0.413 |
| 8,9-EET:8,9-DHET | 0.80 (0.44) | 0.81 (0.51) | 0.78 (0.31) | 0.880 |
| Sum EETs:DHETsc | 0.37 (0.22) | 0.38 (0.22) | 0.36 (0.18) | 0.649 |
Data are presented as median (interquartile range).
One-way ANOVA was performed on log-transformed data.
Plasma concentrations of the 5,6-EET regioisomer were below the limit of quantification and not included in the calculation.
5,6-DHET was removed from ratio values to determine its impact on analysis because 5,6-EET could not be included.
P < 0.05 versus no apparent CAD (Fisher’s LSD).
P < 0.05 versus nonobstructive CAD (Fisher’s LSD).
A similar inverse relationship was observed between CAD extent and free EETs+DHETs (Fig. 1B; Table 2). In addition, mRNA levels of the CYP epoxygenase enzyme CYP2J2 in PBMCs were inversely associated with CAD extent (P = 0.042; supplementary Figure 2A). In contrast, the 14,15-EET:DHET ratio was not significantly associated with CAD extent (Fig. 1C; Table 2). Similarly, no association was observed between CAD extent and either the sum EET:DHET ratio (Table 2) or the 12,13-EpOME:DHOME ratio biomarkers of sEH metabolic function (P = 0.992), or EPHX2 mRNA levels in PBMCs (P = 0.320; supplementary Figure 2B).
The multivariate analysis demonstrated that CAD extent was a significant independent predictor of EET levels (Table 3). The presence of collaterals, compensatory anastomotic bypasses between or within coronary arteries without an intervening capillary bed (21), was also associated with lower EET levels. Moreover, use of certain medications was associated with higher (statins, calcium channel blockers) and lower (beta blockers, angiotensin-converting enzyme inhibitors) EET levels (Table 3). CAD extent was also a significant independent predictor of sum EETs + DHETs levels (Table 4).
TABLE 3.
Multivariate relationships between clinical factors and free plasma EETs
| Predictora | Parameter Estimate | SE | Pb | Partial R2 |
| CAD extent | −0.042 | 0.017 | 0.013 | 0.067 |
| Male | 0.106 | 0.027 | <0.001 | 0.049 |
| Presence of collaterals | −0.099 | 0.041 | 0.017 | 0.032 |
| Statin use | 0.143 | 0.040 | <0.001 | 0.024 |
| Beta blocker use | −0.082 | 0.029 | 0.006 | 0.036 |
| History of hyperlipidemia | −0.073 | 0.035 | 0.040 | 0.025 |
| Calcium channel blocker use | 0.057 | 0.030 | 0.060 | 0.017 |
| ACE inhibitor use | −0.050 | 0.028 | 0.071 | 0.016 |
| Full model | <0.001 | 0.265 |
CAD extent was coded as an ordinal variable (1 = no apparent CAD; 2 = nonobstructive CAD; 3 = obstructive CAD). The remaining factors were coded as nominal variables (0 = no; 1 = yes).
Factors with P < 0.15 were retained in the multivariate model.
TABLE 4.
Multivariate relationships between clinical factors and free plasma sum EETs + DHETs
| Predictora | Parameter Estimate | SE | Pb | Partial R2 |
| CAD extent | −0.055 | 0.016 | <0.001 | 0.080 |
| Male | 0.092 | 0.025 | <0.001 | 0.060 |
| Beta blocker use | −0.071 | 0.028 | 0.012 | 0.022 |
| Statin use | 0.119 | 0.037 | 0.002 | 0.032 |
| History of hyperlipidemia | −0.056 | 0.034 | 0.098 | 0.014 |
| Full model | <0.001 | 0.208 |
CAD extent was coded as an ordinal variable (1 = no apparent CAD; 2 = nonobstructive CAD; 3 = obstructive CAD). The remaining factors were coded as nominal variables (0 = no; 1 = yes).
Factors with P < 0.15 were retained in the multivariate model.
Identification of altered eicosanoid pathways across obstructive CAD status
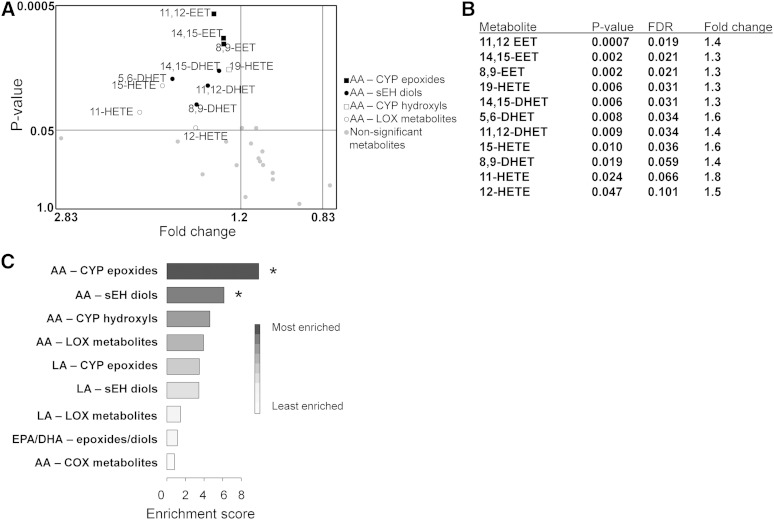
Among all of the individual eicosanoid metabolites quantified, 11 were altered (P < 0.05) between obstructive CAD patients (N = 72) and all other participants (N = 90) (Fig. 2A, B; Table 5). The three EET and four DHET regioisomers were among the 11 analytes associated with CAD extent. QEA revealed that only the AA-derived CYP epoxide and AA-derived sEH diol pathways were significantly associated with the presence of obstructive CAD (Fig. 2C; Table 6).
Fig. 2.
Enrichment of eicosanoid pathways across obstructive CAD status. Twenty-eight eicosanoid metabolites were assigned to 9 distinct eicosanoid metabolic pathways according to their parent substrate [AA, linoleic acid (LA), and EPA/DHA fatty acids] and biosynthesis enzyme (CYP, LOX, COX, and sEH), and compared between obstructive CAD patients (N = 72) and all other patients (N = 90). The EPA- and DHA-derived metabolites (all derived from CYP or sEH biosynthesis) were combined into one pathway due to the low number of metabolites in that group. A: A volcano plot shows how each of the 28 individual metabolites within pathways differs between obstructive CAD patients and all other patients. Metabolites in the upper left box [fold change (FC) >1.2 and P < 0.05] were considered to be significantly altered. P value decreases as y-axis value increases. B: A list (rank ordered by P value with accompanying FDR and FC) of the top metabolites that significantly differed by obstructive CAD status is provided. C: QEA revealed that AA-derived epoxides (P = 0.001; FDR q = 0.010) and AA-derived diols (P = 0.006; FDR q = 0.028) were enriched across obstructive CAD status relative to parallel pathways. *QEA P < 0.05 and FDR q < 0.05.
TABLE 5.
Comparison of free plasma eicosanoid metabolites by obstructive CAD status
| Analyte (pg/ml) | No Obstructive CAD (n = 90) | Obstructive CAD (n = 72) | Pa | FDR q |
| AA-CYP epoxides | ||||
| 14,15-EET | 312 (164) | 286 (117) | 0.002 | 0.021 |
| 11,12-EET | 181 (99) | 152 (85) | <0.001 | 0.019 |
| 8,9-EET | 211 (100) | 189 (87) | 0.002 | 0.021 |
| AA-sEH diols | ||||
| 14,15-DHET | 981 (490) | 845 (442) | 0.006 | 0.031 |
| 11,12-DHET | 642 (367) | 563 (257) | 0.009 | 0.034 |
| 8,9-DHET | 252 (194) | 217 (110) | 0.019 | 0.059 |
| 5,6-DHET | 268 (177) | 229 (122) | 0.008 | 0.034 |
| AA-CYP hydroxyls | ||||
| 19-HETE | 694 (408) | 588 (267) | 0.006 | 0.031 |
| 20-HETE | 1,270 (827) | 1,087 (664) | 0.076 | 0.134 |
| AA-LOX metabolites | ||||
| 15-HETE | 1,021 (620) | 847 (382) | 0.010 | 0.036 |
| 11-HETE | 6,361 (3,944) | 5,361 (3,222) | 0.024 | 0.066 |
| 12-HETE | 70 (42) | 53 (38) | 0.047 | 0.101 |
| 8-HETE | 13,028 (8,100) | 12,094 (5,639) | 0.068 | 0.134 |
| 5-HETE | 2,426 (1,707) | 2,068 (1,478) | 0.076 | 0.134 |
| LA-CYP epoxides | ||||
| 12,13-EpOME | 1,786 (918) | 1,652 (1,127) | 0.083 | 0.135 |
| 9,10-EpOME | 4,350 (2,562) | 3,817 (2,921) | 0.046 | 0.101 |
| LA-sEH diols | ||||
| 12,13-DHOME | 2,911 (1,916) | 2,466 (1,929) | 0.109 | 0.170 |
| 9,10-DHOME | 1,961 (1,162) | 1,432 (1,158) | 0.046 | 0.101 |
| LA-LOX metabolites | ||||
| 13-HODE | 21,839 (10,222) | 19,661 (12,561) | 0.141 | 0.208 |
| 9-HODE | 15,489 (8,711) | 14,728 (10,611) | 0.180 | 0.230 |
| 9,12,13-TriHOME | 718 (742) | 668 (829) | 0.240 | 0.292 |
| 9,10,13-TriHOME | 9,478 (742) | 8,406 (13,552) | 0.304 | 0.340 |
| EPA/DHA-epoxides/diols | ||||
| 19,20-EpDPE | 1,666 (1,477) | 1,641 (888) | 0.253 | 0.295 |
| 19,20-DiHDPA | 1,206 (741) | 1,188 (596) | 0.581 | 0.603 |
| 17,18-DHET | 2,806 (2,184) | 2,671 (1,740) | 0.181 | 0.230 |
| AA-COX metabolites | ||||
| 8-iso-PGF2α | 29 (11) | 28 (12) | 0.157 | 0.220 |
| 6-keto-PGF1α | 51 (20) | 50 (21) | 0.748 | 0.748 |
| PGF2α | 229 (539) | 326 (788) | 0.379 | 0.409 |
DiHDPA,dihydroxydocosapentaenoic acid; EpDPE, epoxydocosapentaenoic acid; PGF, prostaglandin F; TriHOME, trihydroxyoctadecenoic acid. Data presented as median (interquartile range).
Student’s t-test was performed on log-transformed data followed by FDR calculations to account for multiple testing.
TABLE 6.
QEA results on eicosanoid metabolic pathways
| Metabolites (N) | Observed Q | Expected Q | Pa | FDR q | |
| AA-CYP epoxides | 3 | 6.14 | 0.62 | 0.001 | 0.010 |
| AA-sEH diols | 4 | 3.82 | 0.62 | 0.006 | 0.028 |
| AA-CYP hydroxyls | 2 | 2.87 | 0.62 | 0.023 | 0.070 |
| AA-LOX metabolites | 5 | 2.45 | 0.62 | 0.041 | 0.090 |
| LA-CYP epoxides | 2 | 2.17 | 0.62 | 0.058 | 0.090 |
| LA-sEH diols | 2 | 2.14 | 0.62 | 0.060 | 0.090 |
| LA-LOX metabolites | 4 | 0.91 | 0.62 | 0.221 | 0.284 |
| EPA/DHA-epoxides/diols | 3 | 0.70 | 0.62 | 0.303 | 0.341 |
| AA-COX metabolites | 3 | 0.51 | 0.62 | 0.374 | 0.374 |
QEA was performed on log-transformed data, and P values were generated from observed Q statistics. Bold indicates QEA P < 0.05 and FDR q < 0.05.
Risk of future cardiovascular events
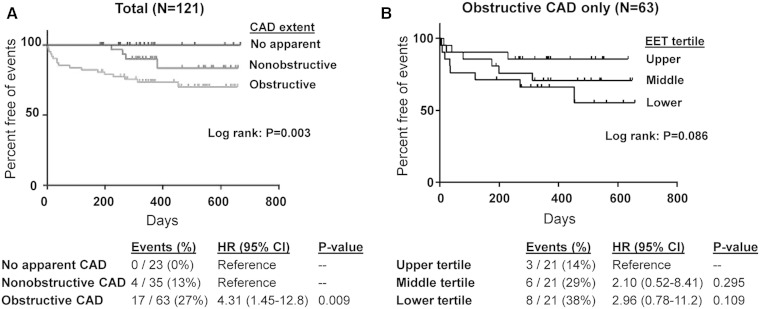
Follow-up data were available in 121 (75%) of the enrolled participants, and the median length of follow-up was 1.0 years. Cardiovascular death, a nonfatal ACS event, or a coronary revascularization procedure occurred in 21 participants (0, 10, and 11 events, respectively). CAD extent at baseline was significantly associated with incidence of a cardiovascular event, with 17 of the 21 events occurring in those with obstructive CAD (Fig. 3A), similar to previous studies (20).
Fig. 3.
CAD extent, EETs, and risk of subsequent cardiovascular events. Kaplan-Meier curves were generated for incidence of the primary end point (time to the occurrence of death from cardiovascular causes, ACS event, or coronary revascularization procedure) according to CAD extent at baseline in all patients with reliable follow-up data (N = 121) (A) and free plasma EET levels at baseline (tertiles) exclusively within the subset of patients with obstructive CAD (N = 63) (B). The log rank P value (unadjusted) for each Kaplan-Meier curve is provided. Below the curves, the number and frequency of events within each CAD extent and EET tertile group, along with the unadjusted hazard ratio (HR), 95% confidence interval (CI), and P value, are provided. Due to the lack of events in those with no apparent CAD at baseline, the no apparent CAD and nonobstructive CAD groups were combined and served as a single reference group.
Although the presence of obstructive CAD is associated with lower EET levels, considerable interindividual variability in EET levels existed within the obstructive CAD group [median (interquartile range): 639 (286) pg/ml]. Consequently, we explored the relationship between baseline EET levels and risk of a future cardiovascular event exclusively within the subset of patients with obstructive CAD who had follow-up data (N = 63). An inverse relationship between EET levels and cardiovascular event incidence appeared to exist, such that the highest number of events was observed in those in the lowest EET level tertile (Fig. 3B); however, this relationship was not statistically significant (P = 0.086).
DISCUSSION
This is the first study that reports a relationship between circulating EET metabolite levels and the angiographic extent of CAD in humans. We observed that the presence of obstructive CAD is significantly and independently associated with lower plasma free (unesterified) EET levels. Furthermore, the association between obstructive CAD and lower EET levels a) correlates with lower CYP epoxygenase metabolic function, but not higher sEH metabolic function and b) is significantly more pronounced than other eicosanoid metabolism pathways. Collectively, these findings suggest that the presence of obstructive CAD is associated with lower EET metabolite levels secondary to suppressed EET biosynthesis. Based on these findings, the authors suggest that novel strategies that promote the effects of EETs may have therapeutic promise in patients with obstructive CAD.
It has become increasingly clear that CYPs metabolize AA into bioactive eicosanoids with potent cellular and physiological effects in the cardiovascular system (22). Most notably, CYP epoxygenase-derived EETs elicit protective effects in preclinical models (5, 6), including vasodilation by hyperpolarizing vascular smooth muscle cells (23), anti-inflammation by attenuating nuclear factor κB signaling (24), and antiapoptosis by promoting phosphoinositide-3-kinase/Akt signaling (25). Consequently, promoting the effects of EETs, most notably by inhibiting sEH and increasing endogenous EET levels, has emerged as a cardiovascular protective therapeutic strategy with potential clinical utility (26). Due in part to the technical complexity of measuring EETs, which are not quantified on traditional metabolomic or eicosanoid panels (11), major gaps in knowledge surrounding the biological and therapeutic importance of this pathway in human cardiovascular disease remain (7). Importantly, characterizing the relationship between EET metabolite levels and CAD extent in humans is central to a better understanding of the functional role of this pathway in the initiation and progression of CAD. Through application of a targeted LC/MS/MS assay, the current study identified a significant inverse relationship between plasma EET levels and angiographic extent of CAD. Pathway analyses revealed that these differences were significantly more pronounced than metabolites derived from CYP hydroxylase, LOX, and COX pathways. Taken together, these findings suggest that CYP-derived EETs may play an important role in the pathogenesis and progression of CAD in humans.
In the current study, CAD extent was also associated with lower sum EETs + DHETs in plasma and CYP2J2 expression in PBMCs, but not altered plasma EET:DHET ratios or EPHX2 expression. These data suggest that the inverse association between EET levels and CAD extent was mediated by suppression of EET biosynthesis (CYP epoxygenase metabolism), and not an increase in EET hydrolysis (sEH metabolism). Accordingly, it is well established that inflammatory stimuli suppress CYP-mediated xenobiotic metabolism through a variety of mechanisms, including cytokine-mediated transcriptional downregulation of CYP expression (27); fittingly, inflammatory stimuli also drive the development and progression of CAD (28). Multiple preclinical studies have also demonstrated that CYP epoxygenase expression and EET biosynthesis are suppressed in the presence of pathological inflammation. Notably, hepatic CYP epoxygenase expression, hepatic CYP epoxygenase metabolic function, and plasma EET levels were suppressed in an atherogenic diet mouse model of inflammatory fatty liver disease (29). In contrast, no changes in sEH expression or EET hydrolysis were observed. Although most studies have investigated the impact of inflammation on hepatic CYP expression and function, cytokines have also been reported to downregulate CYP epoxygenase expression in endothelial cells (30). Moreover, hepatic, renal, pulmonary, and myocardial Cyp2c/2j expression and EET biosynthesis were suppressed in an endotoxin mouse model of acute inflammation (17). Interestingly, 19-HETE was among the metabolites that differed across obstructive CAD status. This metabolite is primarily biosynthesized by CYP2E1, and to a lesser extent CYP4A11 (31). 19-HETE is also derived from isoforms within the CYP2J subfamily (32, 33). In contrast, 20-HETE (which did not significantly differ across CAD extent) is predominantly derived from CYP4A11 and CYP4F2 in humans (31), suggesting that suppression of CYP-mediated eicosanoid biosynthesis may be isoform specific.
Altogether, these preclinical and clinical findings suggest that suppression of CYP epoxygenase expression and EET biosynthesis may be, at least in part, a key pathological consequence of the inflammation-mediated development and progression of CAD. Although the aforementioned cellular and physiological effects have been attributed to free EETs, and sEH rapidly hydrolyzes free EETs to their corresponding DHET metabolites, it is well-established that cellular EETs are readily esterified to membrane phospholipids (6, 34). Furthermore, >90% of plasma EETs are esterified to lipoprotein phospholipids in rats and humans (35–37). The direct functional role of cellular and circulating esterified EETs, however, remains unclear and requires further investigation (34, 35). It is important to note that free plasma oxylipins were quantified in the current investigation, and that changes in circulating lipoprotein levels have been reported to impact the relative extent of oxylipin esterification into lipoprotein phospholipids (36, 37). Consequently, the presence of hyperlipidemia and higher lipoprotein levels could have enhanced EET esterification to lipoprotein phospholipids and thus contributed to the observed decrease in free EET levels in patients with obstructive CAD. Importantly, circulating lipoprotein levels did not significantly differ across CAD extent. Furthermore, the observed differences in free EET and EET + DHET levels across CAD extent persisted after adjusting the analysis for hyperlipidemia status. Taken together, these data suggest that differences in lipoprotein levels and hyperlipidemia status did not markedly impact the association between obstructive CAD and lower free plasma EET and EET + DHET levels. However, quantification of free EET concentrations, as opposed to both free and total concentrations, is a limitation of the current study because we cannot conclusively determine whether increased EET esterification to lipoprotein phospholipids contributed, at least in part, to the observed results. Indeed, an improved understanding of the biological and therapeutic relevance of EET incorporation into phospholipids is of considerable interest to the field (35). Future studies that quantify free and total EET levels are needed to further elucidate the mechanisms underlying the relationship between advanced CAD and lower EET levels in humans.
A few additional studies have quantified plasma EET levels in patients at risk for or with established cardiovascular disease. Ramirez et al. (38) found that presence of the metabolic syndrome was associated with lower circulating EET levels. Furthermore, Minuz et al. (39) reported that patients with renovascular disease had lower circulating EETs compared with both healthy volunteer and essential hypertension controls. In contrast, we previously reported that a cohort of patients with established CAD (defined as history of coronary artery stenosis ≥50% or a revascularization procedure) had higher plasma EET levels compared with a population of healthy volunteer controls (40). Higher EET levels appeared to be driven by suppressed sEH metabolic function, such that the median 14,15-EET:DHET ratio was significantly higher in CAD cases compared with healthy volunteers (0.37 vs. 0.18, respectively). In the present study, however, no differences in sEH metabolic function, or EPHX2 mRNA levels in PBMCs, were observed across those with no apparent (0.35), nonobstructive (0.33), and obstructive CAD (0.34). Direct comparison of the prior and present study should be completed with caution because the controls in the prior study were healthy volunteers that had no CAD risk factors and were not receiving medication for any chronic conditions. Consequently, multiple potential confounding factors may have driven the apparent suppression of sEH metabolic function in CAD cases. Statins, for example, were taken by 93% of CAD cases and none of the controls (40), and may have directly increased EET levels. Statins have been reported to increase 14,15-EET levels in human neutrophils and bronchial epithelial cells, potentially due to suppression of sEH activity (41), and increase CYP epoxygenase expression in endothelial cells (42). Moreover, in the present study, statin use was positively associated with sum EET levels in the multivariable analysis. Furthermore, participants in the present study were recruited from a single population and the reference subpopulation with no apparent angiographic evidence of CAD had similar CAD risk factors, circulating lipoprotein levels, and medication use compared with those with nonobstructive and obstructive CAD. Thus, there was reduced potential for confounding factors between these clinically relevant stages of CAD. Moreover, the observed association between EET levels and CAD extent was independent of clinical factors and medication use in an adjusted model. Future prospective studies are needed to delineate the direct effects of medication use such as statins and comorbid conditions such as hyperlipidemia on EET biosynthesis and hydrolysis in patients at risk for or with CAD.
The relationship between metabolite biomarkers of the EET metabolic pathway and prognosis in patients with established CAD has not been studied to date. Therefore, we explored the relationship between baseline EET levels and risk of a future adverse cardiovascular event in patients with obstructive CAD at baseline. We observed an inverse relationship across the EET tertiles where the highest event incidence occurred in those with the lowest EET levels at baseline. This relationship was not statistically significant and should be interpreted with caution due to the small number of events. These findings, however, are biologically plausible considering the anti-inflammatory and protective effects of EETs in numerous preclinical models of cardiovascular disease. Furthermore, these data are consistent with a previously reported inverse association between circulating EET and monocyte chemoattractant protein 1 (MCP-1) concentrations [a proinflammatory chemokine predictive of poor prognosis in CAD (43)], in which patients with stable CAD in the lowest and middle EET tertiles had significantly higher MCP-1 levels compared with those in the highest EET tertile (18). These preliminary observations underscore the need to rigorously evaluate the relationship between EET levels and the risk of cardiovascular events in larger populations.
Although the current study is the largest to date evaluating the relationship between CYP-derived eicosanoid metabolites and CAD in humans, our analysis has limitations that must be acknowledged. Due to the cross-sectional design of the present study, causality between plasma EET levels and CAD extent cannot be established. Consequently, we are unable to discern whether low EET levels result from the presence of obstructive CAD or whether low EET levels are a causal factor in the development of obstructive CAD. Additionally, metabolomic analyses of a panel of 28 metabolites included multiple statistical comparisons. To account for the possibility of false-positive findings, an FDR was calculated for each comparison. Associations were deemed significant if FDR q < 0.05, which enhanced confidence in our results. Validation of the observed relationships in an independent cohort will ultimately be necessary. Finally, numerous potentially important eicosanoids were either below the limit of quantitation or not evaluated by the used LC/MS/MS method. In particular, COX-derived prostaglandins are naturally found in low abundance in human plasma, and their quantification in peripheral blood is complicated by rapid metabolism and artifactual ex vivo formation (44). Alternative strategies, such as quantification in peripheral blood following ex vivo stimulation or in urine, were beyond the scope of the current investigation. Thus, future studies that integrate multiple analytical platforms and sampling strategies will be needed to more precisely discern the association between EETs and CAD extent relative to prostaglandins and a broader array of bioactive lipids.
Recent failures in CAD drug development suggest that innovative approaches are needed to mitigate increasing attrition rates and more successfully translate novel therapies into clinical practice (45). Compared with the conventional “one size fits all” methodology to drug development, a precision medicine approach has the potential to increase the probability of success for promising therapeutic candidates (46). Although targeted therapies are routinely used in oncology, this strategy has not been readily adopted in CAD. Biomarkers offer considerable promise to prospectively identify subsets of CAD patients at high risk of experiencing a cardiovascular event that exhibit dysfunction in a specific pathway (putative responders), thereby enabling novel therapies that target the pathway to maximize their therapeutic effect and improve outcomes. Our findings offer important insight into the potential therapeutic utility of increasing EETs in obstructive CAD patients predisposed to low EET levels. Importantly, agents that promote the effects of EETs are in early development for a variety of therapeutic indications (47–49). In parallel, technology for the high-throughput quantification of CYP-derived eicosanoids in the clinical setting is advancing (50). Consequently, our results set the foundation for future clinical research in this area, including the rational design of prospective, biomarker-guided interventional studies in targeted subsets of the CAD population (low EET levels) with enriched potential to derive clinical benefit from emerging EET-promoting therapies.
Supplementary Material
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ACS
- acute coronary syndrome
- CAD
- coronary artery disease
- COX
- cyclooxygenase
- CYP
- cytochrome P450
- DHET
- dihydroxyeicosatrienoic acid
- DHOME
- dihydroxyoctadecaenoic acid
- EET
- epoxyeicosatrienoic acid
- EpOME
- epoxyoctadecaenoic acid
- FDR
- false discovery rate
- LA
- linoleic acid
- LOX
- lipoxygenase
- LSD
- least significant difference
- PBMC
- peripheral blood mononuclear cell
- QEA
- quantitative enrichment analysis
- sEH
- soluble epoxide hydrolase
- STEMI
- ST segment elevation myocardial infarction
- UNC
- University of North Carolina at Chapel Hill
This work was supported by a University of North Carolina at Chapel Hill Royster Society of Fellows Chancellor’s Fellowship and an American Heart Association Predoctoral Fellowship (13PRE16470017; A.O-O.), as well as funds from the Intramural Research Program of the National Institutes of Health/National Institute of Environmental Health Science (Z01 ES025034; D.C.Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., de Ferranti S., Després J. P., Fullerton H. J., Howard V. J., et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . 2015. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.Waldman S. A., and Terzic A.. 2014. Molecular insights provide the critical path to disease mitigation. Clin. Pharmacol. Ther. 95: 3–7. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt R. 2006. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 124: 128–145. [DOI] [PubMed] [Google Scholar]
- 4.Zeldin D. C. 2001. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 276: 36059–36062. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y., Theken K. N., and Lee C. R.. 2010. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J. Mol. Cell. Cardiol. 48: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imig J. D. 2012. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 92: 101–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oni-Orisan A., Alsaleh N., Lee C. R., and Seubert J. M.. 2014. Epoxyeicosatrienoic acids and cardioprotection: the road to translation. J. Mol. Cell. Cardiol. 74: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiecker M., Darius H., Hankeln T., Soufi M., Sattler A. M., Schaefer J. R., Node K., Borgel J., Mugge A., Lindpaintner K., et al. . 2004. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 110: 2132–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee C. R., North K. E., Bray M. S., Couper D. J., Heiss G., and Zeldin D. C.. 2007. CYP2J2 and CYP2C8 polymorphisms and coronary heart disease risk: the Atherosclerosis Risk in Communities (ARIC) study. Pharmacogenet. Genomics. 17: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C. R., North K. E., Bray M. S., Fornage M., Seubert J. M., Newman J. W., Hammock B. D., Couper D. J., Heiss G., and Zeldin D. C.. 2006. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Hum. Mol. Genet. 15: 1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köfeler H. C., Fauland A., Rechberger G. N., and Trötzmüller M.. 2012. Mass spectrometry based lipidomics: an overview of technological platforms. Metabolites. 2: 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine G. N., Bates E. R., Blankenship J. C., Bailey S. R., Bittl J. A., Cercek B., Chambers C. E., Ellis S. G., Guyton R. A., Hollenberg S. M., et al. ; American College of Cardiology Foundation, American Heart Association Task Force on Practice Guidelines, and Society for Cardiovascular Angiography and Interventions. 2013. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter. Cardiovasc. Interv. 82: E266–E355. [DOI] [PubMed] [Google Scholar]
- 13.Zha W., Edin M. L., Vendrov K. C., Schuck R. N., Lih F. B., Jat J. L., Bradbury J. A., DeGraff L. M., Hua K., Tomer K. B., et al. . 2014. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J. Lipid Res. 55: 2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edin M. L., Wang Z., Bradbury J. A., Graves J. P., Lih F. B., DeGraff L. M., Foley J. F., Torphy R., Ronnekleiv O. K., Tomer K. B., et al. . 2011. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 25: 3436–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman J. W., Watanabe T., and Hammock B. D.. 2002. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J. Lipid Res. 43: 1563–1578. [DOI] [PubMed] [Google Scholar]
- 16.Lim C. W., Lai K. Y., Yeo J. F., Tai S. H., and Chan S. H.. 2015. Quantitative assessment of moniliformin in cereals via alternative precipitation pathways, aided by LC–LIT-MS and LC-Q-TOF-MS. Food Chem. 174: 372–379. [DOI] [PubMed] [Google Scholar]
- 17.Theken K. N., Deng Y., Kannon M. A., Miller T. M., Poloyac S. M., and Lee C. R.. 2011. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab. Dispos. 39: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuck R. N., Theken K. N., Edin M. L., Caughey M., Bass A., Ellis K., Tran B., Steele S., Simmons B. P., Lih F. B., et al. . 2013. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis. 227: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia J., Mandal R., Sinelnikov I. V., Broadhurst D., and Wishart D. S.. 2012. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 40: W127–W133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddox T. M., Stanislawski M. A., Grunwald G. K., Bradley S. M., Ho P. M., Tsai T. T., Patel M. R., Sandhu A., Valle J., Magid D. J., et al. . 2014. Nonobstructive coronary artery disease and risk of myocardial infarction. J. Am. Med. Assoc. 312: 1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koerselman J., van der Graaf Y., de Jaegere P. P., and Grobbee D. E.. 2003. Coronary collaterals: an important and underexposed aspect of coronary artery disease. Circulation. 107: 2507–2511. [DOI] [PubMed] [Google Scholar]
- 22.Roman R. J. 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 23.Campbell W. B., Gebremedhin D., Pratt P. F., and Harder D. R.. 1996. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 78: 415–423. [DOI] [PubMed] [Google Scholar]
- 24.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., and Liao J. K.. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhanasekaran A., Gruenloh S. K., Buonaccorsi J. N., Zhang R., Gross G. J., Falck J. R., Patel P. K., Jacobs E. R., and Medhora M.. 2008. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am. J. Physiol. Heart Circ. Physiol. 294: H724–H735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imig J. D., and Hammock B. D.. 2009. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 8: 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan E. T. 2001. Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab. Dispos. 29: 207–212. [PubMed] [Google Scholar]
- 28.Hansson G. K. 2005. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 29.Schuck R. N., Zha W., Edin M. L., Gruzdev A., Vendrov K. C., Miller T. M., Xu Z., Lih F. B., DeGraff L. M., Tomer K. B., et al. . 2014. The cytochrome P450 epoxygenase pathway regulates the hepatic inflammatory response in fatty liver disease. PLoS One. 9: e110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler P., Popp R., Busse R., and Schini-Kerth V. B.. 1999. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP-dependent mechanism. Circulation. 99: 1878–1884. [DOI] [PubMed] [Google Scholar]
- 31.Powell P. K., Wolf I., Jin R., and Lasker J. M.. 1998. Metabolism of arachidonic acid to 20-hydroxy-5,8,11,14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J. Pharmacol. Exp. Ther. 285: 1327–1336. [PubMed] [Google Scholar]
- 32.Qu W., Bradbury J. A., Tsao C. C., Maronpot R., Harry G. J., Parker C. E., Davis L. S., Breyer M. D., Waalkes M. P., Falck J. R., et al. . 2001. Cytochrome P450 CYP2J9, a new mouse arachidonic acid omega-1 hydroxylase predominantly expressed in brain. J. Biol. Chem. 276: 25467–25479. [DOI] [PubMed] [Google Scholar]
- 33.Wu S., Chen W., Murphy E., Gabel S., Tomer K. B., Foley J., Steenbergen C., Falck J. R., Moomaw C. R., and Zeldin D. C.. 1997. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J. Biol. Chem. 272: 12551–12559. [DOI] [PubMed] [Google Scholar]
- 34.Spector A. A., Fang X., Snyder G. D., and Weintraub N. L.. 2004. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 43: 55–90. [DOI] [PubMed] [Google Scholar]
- 35.Shearer G. C., and Newman J. W.. 2009. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr. Atheroscler. Rep. 11: 403–410. [DOI] [PubMed] [Google Scholar]
- 36.Shearer G. C., and Newman J. W.. 2008. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot. Essent. Fatty Acids. 79: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman J. W., Pedersen T. L., Brandenburg V. R., Harris W. S., and Shearer G. C.. 2014. Effect of omega-3 fatty acid ethyl esters on the oxylipin composition of lipoproteins in hypertriglyceridemic, statin-treated subjects. PLoS One. 9: e111471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez C. E., Shuey M. M., Milne G. L., Gilbert K., Hui N., Yu C., Luther J. M., and Brown N. J.. 2014. Arg287Gln variant of EPHX2 and epoxyeicosatrienoic acids are associated with insulin sensitivity in humans. Prostaglandins Other Lipid Mediat. 113–115: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minuz P., Jiang H., Fava C., Turolo L., Tacconelli S., Ricci M., Patrignani P., Morganti A., Lechi A., and McGiff J. C.. 2008. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension. 51: 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theken K. N., Schuck R. N., Edin M. L., Tran B., Ellis K., Bass A., Lih F. B., Tomer K. B., Poloyac S. M., Wu M. C., et al. . 2012. Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis. 222: 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Planagumà A., Pfeffer M. A., Rubin G., Croze R., Uddin M., Serhan C. N., and Levy B. D.. 2010. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal Immunol. 3: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisslthaler B., Michaelis U. R., Randriamboavonjy V., Busse R., and Fleming I.. 2003. Cytochrome P450 epoxygenases and vascular tone: novel role for HMG-CoA reductase inhibitors in the regulation of CYP 2C expression. Biochim. Biophys. Acta. 1619: 332–339. [DOI] [PubMed] [Google Scholar]
- 43.de Lemos J. A., Morrow D. A., Blazing M. A., Jarolim P., Wiviott S. D., Sabatine M. S., Califf R. M., and Braunwald E.. 2007. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J. Am. Coll. Cardiol. 50: 2117–2124. [DOI] [PubMed] [Google Scholar]
- 44.Tsikas D., and Zoerner A. A.. 2014. Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: a historical retrospect and a discussion. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 964: 79–88. [DOI] [PubMed] [Google Scholar]
- 45.Pammolli F., Magazzini L., and Riccaboni M.. 2011. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 10: 428–438. [DOI] [PubMed] [Google Scholar]
- 46.Pacanowski M. A., Leptak C., and Zineh I.. 2014. Next-generation medicines: past regulatory experience and considerations for the future. Clin. Pharmacol. Ther. 95: 247–249. [DOI] [PubMed] [Google Scholar]
- 47.Shen H. C., and Hammock B. D.. 2012. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J. Med. Chem. 55: 1789–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falck J. R., Koduru S. R., Mohapatra S., Manne R., Atcha R., Manthati V. L., Capdevila J. H., Christian S., Imig J. D., and Campbell W. B.. 2014. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates: carboxylate modifications. J. Med. Chem. 57: 6965–6972. [Erratum. 2014. J. Med. Chem. 57: 9218.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podolin P. L., Bolognese B. J., Foley J. F., Long E. III, Peck B., Umbrecht S., Zhang X., Zhu P., Schwartz B., Xie W., et al. . 2013. In vitro and in vivo characterization of a novel soluble epoxide hydrolase inhibitor. Prostaglandins Other Lipid Mediat. 104–105: 25–31. [DOI] [PubMed] [Google Scholar]
- 50.Zhu P., Peck B., Licea-Perez H., Callahan J. F., and Booth-Genthe C.. 2011. Development of a semi-automated LC/MS/MS method for the simultaneous quantitation of 14,15-epoxyeicosatrienoic acid, 14,15-dihydroxyeicosatrienoic acid, leukotoxin and leukotoxin diol in human plasma as biomarkers of soluble epoxide hydrolase activity in vivo. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879: 2487–2493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.