Abstract
Mammalian cells synthesize various sterol molecules, including the C30 sterol, lanosterol, as cholesterol precursors in the endoplasmic reticulum. The build-up of precursor sterols, including lanosterol, displays cellular toxicity. Precursor sterols are found in plasma HDL. How these structurally different sterols are released from cells is poorly understood. Here, we show that newly synthesized precursor sterols arriving at the plasma membrane (PM) are removed by extracellular apoA-I in a manner dependent on ABCA1, a key macromolecule for HDL biogenesis. Analysis of sterol molecules by GC-MS and tracing the fate of radiolabeled acetate-derived sterols in normal and mutant Niemann-Pick type C cells reveal that ABCA1 prefers newly synthesized sterols, especially lanosterol, as the substrates before they are internalized from the PM. We also show that ABCA1 resides in a cholesterol-rich membrane domain resistant to the mild detergent, Brij 98. Blocking ACAT activity increases the cholesterol contents of this domain. Newly synthesized C29/C30 sterols are transiently enriched within this domain, but rapidly disappear from this domain with a half-life of less than 1 h. Our work shows that substantial amounts of precursor sterols are transported to a certain PM domain and are removed by the ABCA1-dependent pathway.
Keywords: ATP binding cassette transporter A1, acyl-CoA:cholesterol acyltransferase, cholesterol/efflux, cholesterol/trafficking, high density lipoprotein, lipid rafts, lanosterol, Niemann-Pick disease type C
Although cholesterol is an essential lipid, its accumulation in membranes is toxic to cells. Cholesterol homeostasis is tightly controlled in cells by elaborate systems (1, 2). Impairment of cholesterol homeostasis causes various congenital and acquired human diseases (3). In peripheral cells, two pathways play major roles to prevent the excess build-up of unesterified cholesterol: 1) its esterification by ACAT1 at the endoplasmic reticulum (ER); and 2) its release from cells by several ABC transporter proteins (2, 4, 5). Evidence suggests that ACAT1 and ABCA1 work in concert to eliminate excess cellular unesterified cholesterol (6, 7).
The cholesterol biosynthetic pathway involves the biosynthesis of a series of precursor sterols in addition to cholesterol. Lanosterol, a C30 sterol having three extra methyl groups, is the first sterol synthesized in the pathway and is structurally distinct from the C27 sterol, cholesterol. The dihydro-derivative of lanosterol (dihydrolanosterol), but not cholesterol, downregulates the protein stability of HMG-CoA reductase, the key enzyme in sterol biosynthesis (8, 9). Excess build-up of lanosterol in cells causes severe cytotoxicity (10, 11). In humans, abnormal accumulation of various precursor sterols, including lanosterol, due to deficiency in a distal cholesterol biosynthetic enzyme is associated with malformation syndromes (12, 13). It is therefore essential that cells are able to minimize the cellular build-up of precursor sterols. Others and we had previously shown that upon biosynthesis at the ER, substantial amounts of precursor sterols are transported to the plasma membrane (PM) (14–17). These precursor sterols are then rapidly transported back from the PM to the ER to undergo enzymatic demethylations and other reactions to be converted to cholesterol (17, 18). Circulating lipoproteins such as HDL contain small, but significant, amounts of various precursor sterols, including lanosterol and other methylated sterols (i.e., C28, C29, and C30 sterols) (19). Also, nonhepatic cells export various C27 precursor sterols, including desmosterol and lathosterol, to HDL and LDL (15, 16).
In extrahepatic cells, cellular cholesterol release is primarily carried out by HDL. The ABC transporter, ABCA1, plays an essential role in the generation of HDL. Defective mutation in the ABCA1 gene causes Tangier disease, a familial HDL deficiency (20–22). ABCA1 mediates cellular cholesterol and phospholipid release to helical apolipoproteins, including apoA-I, which results in the formation of nascent HDL (23, 24). ABCA1 primarily resides in and mediates HDL biogenesis at the PM (25–27), while a small but significant amount of ABCA1 is also present in the endocytic compartments (28); internalized ABCA1 is degraded or recycled back to the PM (26). How ABCA1 acts to regulate lipid release has been the subject of much investigation [reviewed in (4, 29)], but the precise mechanism remains unsettled. Direct interaction between apoA-I and ABCA1 has been shown (30–32). It has also been reported that ABCA1 at the PM produces membrane deformation sites where apoA-I interacts and solubilizes lipids present in these domains to generate nascent HDL (33, 34). These results lead one to speculate that the lipid compositions of nascent HDL may reflect those of a membrane domain where nascent HDL is assembled. However, there has been controversy regarding the membrane domains where ABCA1 resides (35, 36).
In this work, we seek to determine the sterol specificity of ABCA1 as a lipid transporter. We also report the dynamic relationship between the potential membrane domain that contains ABCA1 and the newly synthesized lanosterol.
MATERIALS AND METHODS
Materials
apoA-I was prepared as previously described (37). Delipidation of FBS was performed as described (38). [3H]acetic acid and [3H]cholesterol were from American Radio Chemicals. TO901317, 9-cis retinoic acid (9cRA), and fatty acid-free BSA were from Sigma. An ACAT inhibitor, F12511, was a gift of Pierre Fabre Research (Castres Cedex, France). Other chemicals were from Fisher, Sigma, or Wako.
Cell culture and media
Mouse embryonic fibroblasts (MEFs) from Abca1+/+ (WT) and Abca1−/− mice were isolated and used for experiments, as previously described (18, 39). HEK293 cells stably expressing human ABCA1 (clone 293/2c, hereafter referred as to HEK/hABCA1) were established previously (40). WT CHO cells and 25RA CHO cells were employed as described (7, 17). 25RA cells contain a gain-of-function mutation in the cholesterol sensor, Scap (41, 42). Normal human skin fibroblast (HSF) lines were from Drs. Bzik and Brinckerhoff (Geisel School of Medicine at Dartmouth) (designated N-1, N-2, and N-3 in this work) and from Dr. Peter Pentchev, formerly at National Institutes of Health (designated N-4). The normal HSF cell line, GM00038 (designated N-5), was obtained from Coriell Institute. Niemann-Pick disease type C (NPC) patient-derived HSF cell lines, NPC1 HSF GM03123 (designated C1-1) containing P237S/I1061T mutations in NPC1 protein and GM17912 (designated C1-2) containing P1007A/T1036M mutations in NPC1 protein, were from Coriell Institute. Another NPC1 HSF cell line, NIH 93.22 (designated C1-3) (mutations are not determined), was from Dr. Pentchev. The NPC2 HSF cell line, GM17910 (designated C2-1), which contains C93F/C93F mutations in NPC2 protein, was from Coriell Institute. Another NPC2 HSF cell line (designated C2-2) (mutations are not determined) was kindly provided from Dr. Yiannis A. Ioannou (Mount Sinai School of Medicine). Niemann-Pick disease type A (NPA) HSF cell line, GM00112, which contains L302P/L302P mutations in acid sphingomyelinase protein, was from Coriell Institute. Some of the HSF lines used here were employed in our previous study (17). HSFs and MEFs were grown in DMEM with penicillin and streptomycin supplemented with 10% FBS (17, 18). The human monocyte-like THP-1 cells were differentiated to macrophage-like cells by using the procedure described in (43). All cells were grown in humidified CO2 incubators at 37°C. Media used are designated as follows: medium A contained 10 or 7.5% FBS, medium B contained 0.1% fatty acid-free BSA, medium D contained 5% delipidated FBS, and medium F contained no supplements.
Sterol release assays
Cells (CHO, HEK293, HSFs, and MEFs) were seeded into 6-well plates, as described (7, 17, 18). HSFs and MEFs were pretreated or not pretreated with 9cRA (RXR ligand) and/or the synthetic LXR agonist, TO901317, as indicated in the figure legends, to induce ABCA1 expression. Afterwards, cells were incubated with or without apoA-I (5 or 10 μg/ml) in the presence or absence of 9cRA and/or TO901317 in medium B for various times, as indicated in the figure legends. The release of endogenously synthesized sterols was monitored after incubating cells with [3H]acetate (20 or 40 μCi/ml) for either a short or long period of time, as described in the figure legends. Alternatively, cells were labeled with [3H]cholesterol (0.1 μCi/ml). ABCA1-dependent sterol release was calculated by subtracting the values obtained without apoA-I from the values obtained with apoA-I. To identify each sterol species by GC-MS, HEK/hABCA1 cells were grown in 100 mm dishes. Lipid efflux was induced by incubating cells with apoA-I in medium B for 48 h. The medium from two 100 mm dishes was pooled for sterol analysis.
Cell fractionation
Cells grown in 100 mm dishes at subconfluent stage were treated with 1.0 ml of TNE buffer [25 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA) that contained protease inhibitor cocktail (Sigma) and various detergents, according to procedures described (44), and 1.0% Triton X-100 (TX100), 1.0% Triton X-102 (TX102), 1.0% Brij 58, 1.0% Lubrol WX, 2.0% CHAPS, 1.0% CHAPSO, or 1.0% Tween 20 for 30 min at 4°C, or with 1.0% Brij 98 for 10 min at 37°C. After treatment with a detergent, cell lysates were harvested and homogenized with 10 strokes by using a Dura-Grind stainless steel dounce tissue grinder (Wheaton). Cell homogenate was spun twice at 1,000 g for 5 min; the resulting postnuclear supernatant (PNS) was subjected to OptiPrep (Axis-Shield) or sucrose gradient ultracentrifugation, as described previously (45). Briefly, the PNS was mixed with OptiPrep or with 80% sucrose (in TNE buffer) to be at 37.5 or 40% as a final concentration, respectively, and placed at the bottom of an ultracentrifuge tube. Optiprep (30%) or sucrose (30%) were layered and TNE buffer or 5% sucrose was then layered on the top. After centrifugation at 200,000 g for 3 h at 4°C in a SW41 or SW60 rotor (Beckman), eight 0.5 ml fractions or eleven 1 ml fractions were collected from the top, respectively. Alternatively, PNS was centrifuged at 100,000 g for 60 min to isolate detergent-resistant membrane (DRM) as pellet and detergent-soluble fraction as supernatant (46).
Lipid extraction and analysis
Cellular lipids were extracted by hexane/isopropanol (3:2, v/v). Lipids in medium or fractions from cell fractionation experiments were extracted by 4 vol of chloroform/methanol (2:1, v/v). Lipids extracted were dried under nitrogen gas at 40–45°C. Amounts of total cholesterol, free cholesterol, and choline-phospholipid were measured by colorimetric enzymatic assay systems, as described previously (7). Cholesteryl ester was determined by subtracting free cholesterol from total cholesterol. Cellular and medium lipids containing radio-labeled sterols were prepared by saponification, and nonsaponifiable fractions (containing labeled sterols) were separated by TLC in a solvent system of methylene chloride/ethyl acetate (97:3, v/v), as described (17). For GC-MS, extracted lipids were saponified and nonsaponifiable lipids (containing sterols) were isolated and dried under N2 gas. Sterol derivatization and GC-MS analysis were performed essentially as described previously (17). Epicoprostanol served as an internal standard.
Antibodies and immunoblot
Anti-human ABCA1 rabbit serum was produced previously (47). Other antibodies were obtained from commercial sources as follows; anti-caveolin-1 polyclonal antibodies (N-20) and anti-LAMP-2 monoclonal antibody (H4B4) from Santa Cruz Biotechnology, anti-flotillin-1 monoclonal antibody and anti-calnexin monoclonal antibody from BD Biosciences, and anti-β-actin monoclonal antibody from Sigma. Whole cell lysate was prepared by using lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP40, and protease inhibitor cocktail]. Protein concentration was determined by BCA protein assay (Pierce). Equal amounts of cell protein or equal amounts of fraction were subjected to SDS-PAGE and immunoblot analysis by using antibodies, as indicated in the figure legends.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses of results were performed using a two-tailed unpaired Student’s t-test. The difference between two sets of values was considered significant when the P value was <0.05 (*P < 0.05, **P < 0.01, ***P < 0.001).
RESULTS
Sterol specificity of ABCA1-mediated sterol release
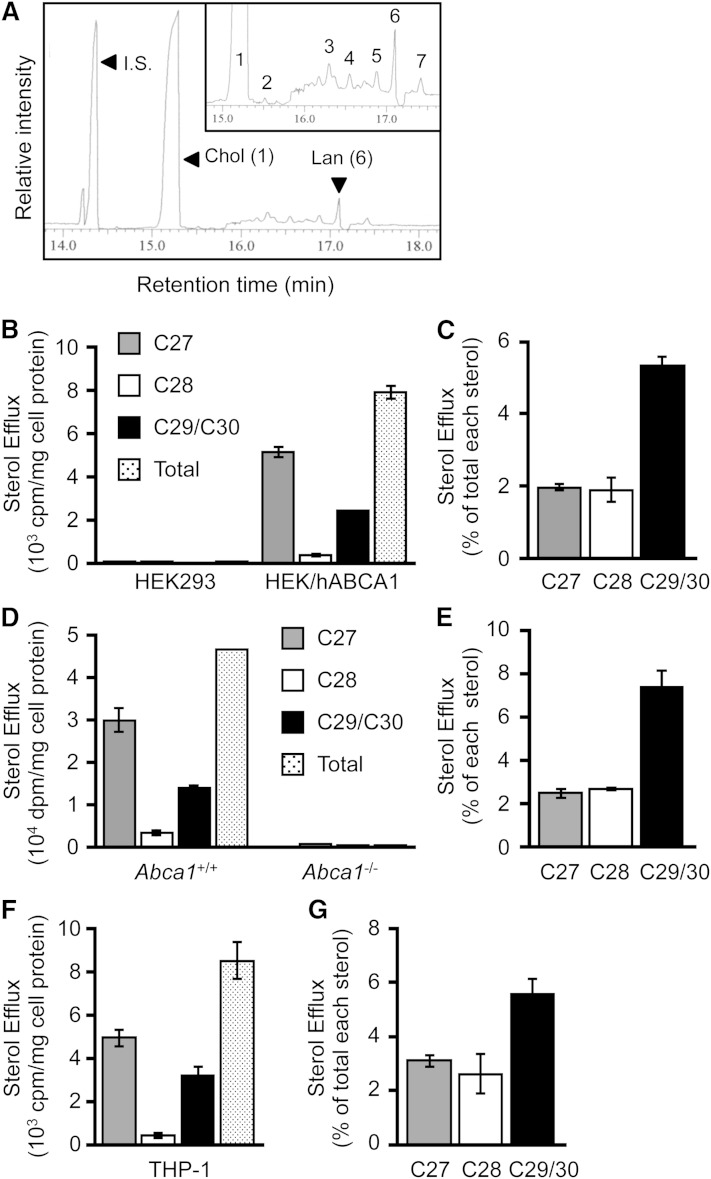
To determine the specificity of sterol molecules in the ABCA1-dependent lipid release, we first employed the HEK293 cell system (40); these cells express neither ABCA1 nor any other ABC transporters involved in cholesterol release. HEK293 cells that ectopically express ABCA1 are therefore suitable to determine the ABCA1-specific sterol release. We incubated HEK/hABCA1 cells in medium without serum, but with apoA-I, collected the conditioned medium, and analyzed its sterol composition by GC-MS. The result showed that, in addition to a peak of the internal standard, epicoprostanol (retention time of 14.4 min), we found not only a major peak with a retention time of 15.3 min, but also other minor peaks, including one having a retention time of 17.1 min (Fig. 1A). Subsequent analysis (by molecular ion and fragmentation pattern analysis) identified the major peak as cholesterol and the second major peak as lanosterol (C30) (Table 1). The amount of lanosterol released was 2.8 ± 0.1% (n = 3) of cholesterol. Other minor peaks were found to be desmosterol (C27), monomethylsterol (C28), and dimethylsterol (C29). The results indicate that, in addition to cholesterol, ABCA1 mediates the release of substantial amounts of lanosterol and other methylated sterols to apoA-I (Fig. 1A). Cellular sterol analyses showed that lanosterol content was 0.6 ± 0.2% (n = 3) of cholesterol. The results described in Fig. 1A and Table 1 suggest that lanosterol is preferentially released from cells in an ABCA1-dependent manner. To test this interpretation, we compared apoA-I-mediated release of sterols synthesized de novo between parental HEK293 and HEK/hABCA1 cells (Fig. 1B, C) and between WT and Abca1−/− MEFs (Fig. 1D, E). We incubated these cells with apoA-I for 8 h in the presence of [3H]acetate (to label newly synthesized sterols) and analyzed [3H]-labeled sterols released from cells by TLC. This TLC system efficiently separates C29/C30 sterols (including lanosterol) from the C28 and the C27 sterols (17). The results showed that HEK/hABCA1 cells and WT MEFs released labeled C27 sterols and labeled C29/C30 sterols. These cells also released labeled C28 sterols, but in much smaller quantities (Fig. 1B, D). We calculated the labeled C29/C30 sterols or the labeled C27 sterols in the medium versus the same sterol species in cells. The result showed that, indeed, the C29/C30 sterols were released more efficiently than the C27 sterols (Fig. 1C, E). In HEK293 cells and Abca1−/− MEFs, none of the labeled sterols were released, demonstrating an essential role of ABCA1 in cholesterol and lanosterol release. In macrophages, ABCA1 plays a major role in cellular sterol efflux. We performed a similar [3H]acetate-labeling experiment in THP-1 human macrophage-like cells and analyzed radioactive sterols by TLC. The result showed that the same sterol specificity in apoA-I/ABCA1-mediated sterol release was also observed in the human macrophage-like cells (Fig. 1F, G). Together, these results show that lanosterol is a preferred sterol released from cells by the apoA-I/ABCA1 pathway.
Fig. 1.
Sterol specificity of ABCA1. A: GC-MS analysis of apoA-I-mediated sterol release. HEK/hABCA1 cells were incubated with apoA-I (10 μg/ml). Lipids were extracted from medium, saponified, and analyzed by GC-MS, as described in Materials and Methods. The peaks of epicoprostanol [internal standard (I.S.)], cholesterol (Chol, peak 1), and lanosterol (Lan, peak 6) are indicated by arrowheads. Other minor peaks (2–5 and 7) are shown in the inset and described in Table 1. B–G: HEK293 and HEK/hABCA1 (B, C), and WT and Abca1−/− (D, E) MEFs were treated with 1 μg/ml of TO901317 for 24 h, and differentiated THP-1 cells (F, G) were incubated with or without apoA-I in the presence of [3H]acetate for 8 h in 6-well plates. Lipids were extracted from medium and cells, and were saponified. Sterols were analyzed by TLC. Results are shown as cpm or dpm of [3H]sterol per milligram of cell protein (B, D, F) or as percent release relative to each [3H]sterol synthesized [medium/(medium + cell) × 100] (C, E, G). Error bars represent SD (n = 3).
TABLE 1.
Identification of sterols released to apoA-I in HEK/hABCA1 cells
| Peak | Sterol (number of carbons) | Retention Time (min) | Protein (ng/mg) | Percent of Total |
| 1 | Cholesterol (C27) | 15.3 | 4,392.6 | 93.6 |
| 2 | Desmosterol (C27) | 15.5 | 10.7 | 0.2 |
| 3 | Monounsaturated monomethylsterol (C28) | 16.3 | 41.6 | 0.9 |
| 4 | Monounsaturated monomethylsterol (C28) | 16.6 | 40.3 | 0.9 |
| 5 | Monounsaturated dimethylsterol (C29) | 16.9 | 48.9 | 1.0 |
| 6 | Lanosterol (C30) | 17.1 | 119.7 | 2.6 |
| 7 | Diunsaturated dimethylsterol (C29) | 17.4 | 40.4 | 0.9 |
| C27 sterols | — | 4403.3 | 93.8 | |
| C28 sterols | — | 81.9 | 1.7 | |
| C29/C30 sterols | — | 208.9 | 4.5 |
HEK/hABCA1 cells were incubated with apoA-I. Sterols extracted from the conditioned medium were analyzed by GC-MS. The GC-MS profile is shown in Fig. 1A. The result shown is a typical result of three independent experiments.
Newly synthesized sterols, but not recycling sterols, are the preferential source for ABCA1-dependent sterol release
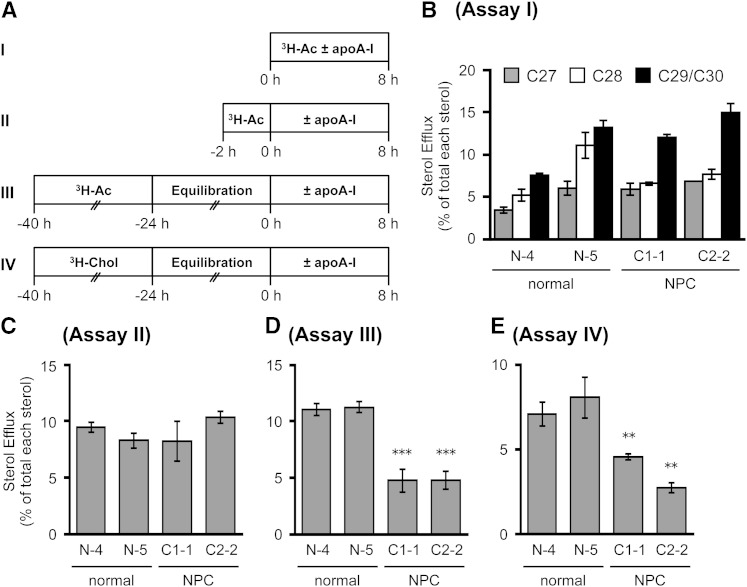
The results presented in Fig. 1 suggest that ABCA1 preferentially releases newly synthesized sterols to apoA-I, shortly after these sterols arrive at the PM. To confirm this, we made use of HSFs isolated from NPC patients. In NPC1- or NPC2-deficient cells, cholesterol transport from the endosome to the PM is defective, but ER-to-PM sterol transport is normal (17, 48). We compared the release of endogenously synthesized sterols to apoA-I in HSFs isolated from normal and NPC subjects under various conditions (Fig. 2A). When cells were incubated with apoA-I for 8 h in the presence of [3H]acetate, the labeled C29/C30, C28, and C27 sterols were all available to apoA-I-mediated release in HSFs, and the release of C29/C30 sterol was more efficient than that of the C27 sterol (Fig. 2B). As expected, the release of all sterol species was not impaired in HSFs from NPC patients. When HSFs were pulse-labeled with [3H]acetate for only 2 h followed by chase with apoA-I, the lower radioactive counts in labeled sterols (due to rapid conversion of precursor sterols) only allowed us to monitor C27 sterols reliably, but the result again showed that the release of C27 sterols was not impaired in NPC HSFs (Fig. 2C). Instead, when cells were labeled with [3H]acetate for a prolonged period (16 h) followed by a long chase period without the labeled acetate (24 h), and were then incubated with apoA-I for 8 h, the result showed that the release of labeled C27 sterol was severely affected in NPC HSFs (Fig. 2D). Under this condition, in normal HSFs, 99% of labeled sterols were C27 sterols; in NPC HSFs, only 84% of labeled sterols were C27 sterols, and C29/C30 and C28 sterols were approximately 7 and 9%, respectively. The build-up of labeled C29/C30 and C28 sterols in NPC mutant cells was previously reported (17). We then performed a third set of experiments by labeling HSFs with [3H]cholesterol for 16 h, chasing them without the label for 24 h, and then monitoring apoA-I-dependent cholesterol release. The result showed that when compared with the normal HSFs, the release of [3H]cholesterol was markedly reduced in NPC HSFs (Fig. 2E). Together, these results demonstrate that once arriving at the PM, a certain portion of all newly synthesized sterols are quickly releasable via the ABCA1/apoA-I system from the PM, with C29/C30 sterols as the preferred substrate.Later, the endogenously synthesized sterols traverse through other compartments, including the late endosome (LE)/lysosome (LS) compartment (49); their egression from the LE/LS for ABCA1/apoA-I-dependent release becomes dependent on NPC1 and NPC2 proteins. This interpretation can also explain the previous reports showing that apoA-I/ABCA1-mediated release of radiolabeled cholesterol is impaired in NPC1-deficient macrophages and fibroblasts, when these cells are incubated with radiolabeled cholesterol for a long period of time (50, 51).
Fig. 2.
Dependency of NPC1/NPC2 proteins of apoA-I-mediated release of sterols endogenously synthesized. A: Schematic procedures for pulse-chase experiments employed (assays I–IV). Cells were labeled with either [3H]acetate (3H-Ac) or [3H]cholesterol (3H-Chol). B–E: apoA-I-mediated release of labeled sterols in normal and NPC HSFs. HSFs from normal subjects (N-4 and N-5) or NPC patients (C1-1, NPC1 patient; C2-2, NPC2 patient) were seeded in 6-well plates and grown in medium A to subconfluent stage. Cells were then subjected to assay I, II, III, or IV by labeling cells with [3H]acetate or [3H]cholesterol as below. B: Cells pretreated with TO901317 (1 μg/ml) for 18 h were incubated with or without apoA-I (10 μg/ml) during the 8 h [3H]acetate (40 μCi/ml) labeling period with TO901317. C: Cells were labeled with [3H]acetate (40 μCi/ml) for 2 h followed by washing off the label and incubation with or without apoA-I (10 μg/ml) in the presence of TO901317 for 8 h. D, E: Cells were labeled with [3H]acetate (20 μCi/ml) for 16 h in medium D (D) or with [3H]cholesterol (0.1 μCi/ml) in medium A (E), followed by washing off the label and incubation with medium D with TO901317 for 24 h. The cells were then incubated with or without apoA-I (10 μg/ml) for 8 h in the presence of TO901317. Sterols were analyzed by TLC. Data shown are mean ± SD (n = 3). **P < 0.01, ***P < 0.001.
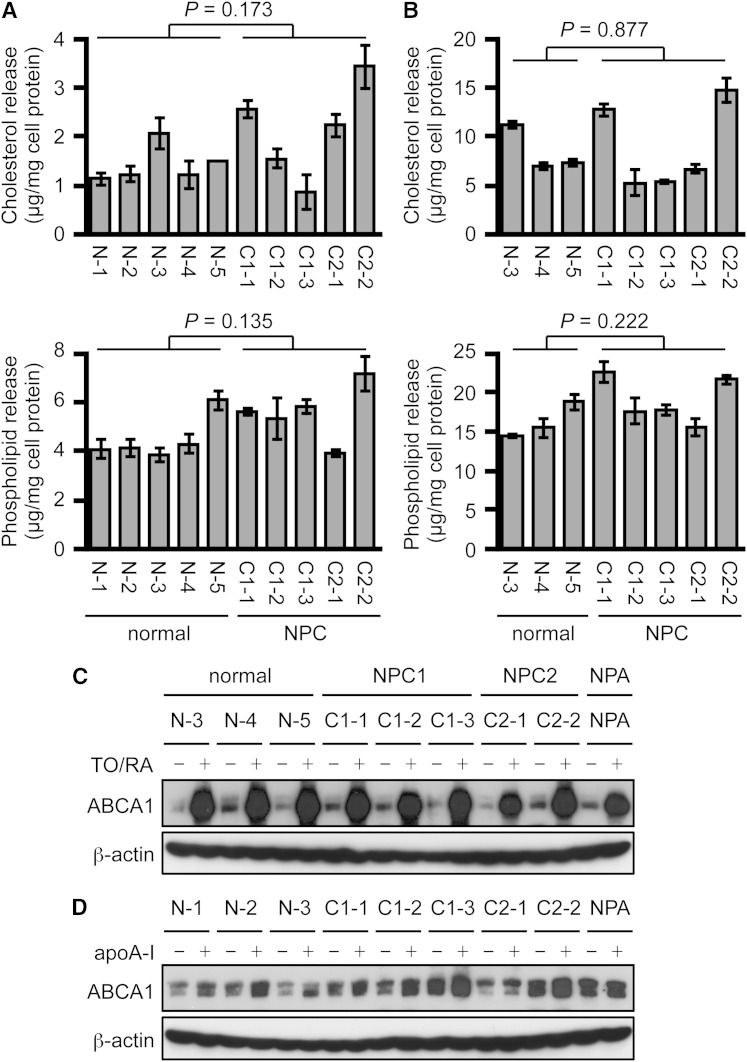
Next, lipid release from normal and NPC HSFs exposed to apoA-I was examined in the absence (Fig. 3A) or presence (Fig. 3B) of LXR and RXR ligands. Genetic heterogeneity exists among human cells of different origin. We thus used five normal and five mutant NPC (containing mutations in either NPC1 or NPC2) fibroblasts. The results showed that apoA-I-mediated release of cholesterol and phospholipid was not impaired in NPC HSFs under both conditions (Fig. 3A, B). In both normal and NPC HSFs, the treatment with LXR and RXR ligands robustly increased cholesterol and phospholipid release. Cholesterol and phospholipid release was also not impaired in HSFs from a patient with NPA (data not shown). We also examined ABCA1 protein expression in normal and NPC HSFs treated or not treated with LXR and RXR ligands (Fig. 3C) or with apoA-I (Fig. 3D). LXR and RXR ligands transcriptionally increase ABCA1 expression, whereas apoA-I protects ABCA1 protein from degradation to increase its protein level (4, 6). Consistent with lipid release, the results showed that ABCA1 expression is not impaired in NPC and NPA HSFs (Fig. 3C, D). Because cholesterol transport from the LE/LS to the PM is impaired in NPC cells (52), these results indicate that ABCA1 preferentially utilizes de novo synthesized sterols before they are eventually endocytosed to the LE/LS. To confirm this interpretation, we calculated specific activity of de novo synthesized sterol released. Normal and NPC fibroblasts were incubated with or without apoA-I in the presence of [3H]acetate, and the mass and radioactivity of sterol released to medium and remaining in cells were determined. The results showed that, in normal HSFs, specific activity of newly synthesized sterol released was 1.34-fold higher than that of labeled sterols remaining in cells (Table 2), indicating that newly synthesized sterols are the preferential source available for ABCA1/apoA-I-dependent release. On the other hand, because the size of the preexisting cholesterol pool was much larger than that of the newly synthesized sterol pool, the preexisting PM cholesterol pool also contributed substantially to the mass of sterols released. In mutant NPC HSFs, the specific activity of newly synthesized sterols released was markedly increased over that of sterols remaining in the cells, because the labeled sterols not released to apoA-I began to recycle among various cellular compartments and, with time, a significant portion of them became partially immobilized and unavailable for release in these cells. Table 2 also shows that, in NPC cells, the rate of sterol synthesis is similar to normal cells despite the large accumulation of cholesterol. Together, these results demonstrate that ABCA1 preferentially releases newly synthesized sterols to apoA-I from the PM before they are internalized to the LE/LS and other cellular compartments.
Fig. 3.
ABCA1-dependent cholesterol release to apoA-I is not impaired in NPC human fibroblasts. A, B: apoA-I-mediated release of cholesterol and phospholipid in normal and NPC HSFs. HSFs were seeded in 6-well plates as in Fig. 2. Cells were then incubated with or without 10 μg/ml of apoA-I for 24 h. Before incubation with apoA-I, the cells were pretreated with TO901317 (1 μg/ml) and 9cRA (1 μg/ml) for 18 h to induce ABCA1 expression, and TO901317 and 9cRA were included during incubation with apoA-I (B). Cholesterol (upper panels) and phospholipids (lower panels) in medium were measured by the colorimetric enzymatic assays, and apoA-I-dependent release was calculated as described in the Materials and Methods. Amounts of cholesterol and phospholipid were normalized to amounts of cellular protein. Data shown are mean ± SD. Statistical significance was evaluated by Student’s t-test between normal and NPC subjects and no significant changes were found. P values are indicated in each panel. C, D: ABCA1 protein expression. ABCA1 expression in normal and NPC HSFs was examined by immunoblot. Cells were either treated or not treated with TO901317 (TO) and 9cRA (RA) for 24 h (C) or with apoA-I (10 μg/ml) for 4 h (D).
TABLE 2.
Specific activity for newly synthesized sterol release
| Cell Line | Total {[3H]sterol (cpm)/mass cholesterol (μg)} | Specific Activity for Newly Synthesized Sterol Release (medium/cell) | |
| Medium | Cell | ||
| N-5 | 1,527/0.99 = 1,537.8 | 19,596/17.13 = 1144.1 | 1.34 |
| C1-1 | 1,512/0.88 = 1,720.1 | 19,823/30.02 = 660.2 | 2.61 |
| C2-2 | 1,432/1.58 = 931.1 | 19,296/92.75 = 208.1 | 4.47 |
Normal (N-5) and NPC (C1-1 and C2-2) HSFs pretreated with TO901317 (1 μg/ml) were incubated with or without apoA-I for 8 h in the presence or absence of [3H]acetate. Cholesterol and [3H]sterol in medium and cells were measured by an enzymatic assay or by a scintillation counter, as described in the Materials and Methods, and normalized to amounts of cellular protein. Counts per minute in [3H]sterols were divided by amounts of cholesterol in micrograms to determine specific activity for newly synthesized sterol release. Results shown are the average of triplicate assays.
ABCA1 is present in the cholesterol-rich membrane domain
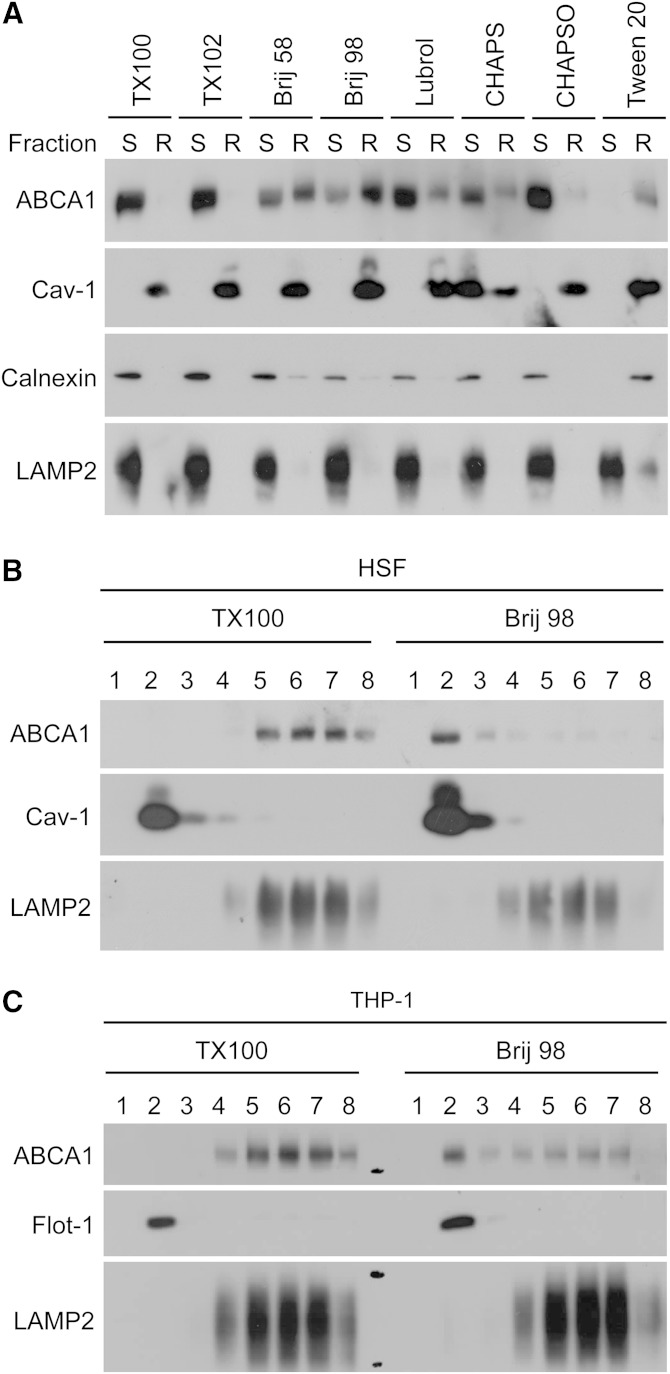
Our results suggest that newly synthesized sterols, including lanosterol, consist of a preferential sterol pool for the ABCA1/apoA-I system. Lanosterol and ABCA1 may thus colocalize at the same membrane domain; such a membrane domain has not been characterized. ABCA1 is a multi-span membrane protein and mainly acts at the PM to mediate HDL assembly (25–27, 33); however, the precise nature of the membrane domain where ABCA1 resides remains controversial. Depending on the detergents employed, ABCA1 has been reported to reside either in detergent-soluble membrane or partly in DRM; ABCA1 was recovered in detergent-soluble membrane when TX100 was used (35), while a fraction of this protein was recovered in DRM prepared using Lubrol WX (36). A recent work showed that lipid composition of nascent HDL particles generated by the ABCA1/apoA-I system is similar to that of lipid rafts (53). Heterogeneity of lipid raft domains has been reported; the protein and lipid contents/compositions of DRM vary greatly depending on the detergents used (44, 54). To further characterize membrane domain(s) where ABCA1 localizes, we employed eight different detergents (Table 3). The results showed that ABCA1 was largely soluble in most of the detergents tested, but was largely insoluble when Brij 98 or Tween 20 was used (Fig. 4A). Brij 98-DRM contained 67% of total cellular ABCA1 and the lipid raft marker caveolin-1, but the ER membrane marker, calnexin, or the LE/LS membrane marker, LAMP2, were excluded. In contrast, the Tween 20-DRM contained calnexin in addition to ABCA1 (Fig. 4A), indicating that, unlike Brij 98, Tween 20 could not completely solubilize the ER membrane. Cholesterol was highly enriched in Brij 98-DRM, with a free cholesterol/phospholipid ratio of 0.62; for TX100-DRM and for Tween 20-DRM, the values were 0.49 and 0.30, respectively (Table 3). To further confirm these results, after treating cells with Brij 98 or with TX100, cell lysate was subjected to density-gradient centrifugation. The results showed that buoyant Brij 98-DRM, but not TX100-DRM, contained ABCA1 in normal HSF (Fig. 4B). Additional results showed that, in THP-1 macrophage cell lysates, ABCA1 was also recovered in Brij 98-DRM, but not in TX100-DRM (Fig. 4C).
TABLE 3.
Characterization of DRM prepared by using different detergents in human fibroblasts
| Detergent | Concentration (%) | Condition | ABCA1 in DRMa | FC in DRMa | PL in DRMa | FC/PL in DRM |
| Triton X-100 | 1.0 | 4°C, 30 min | 4.8 | 36.2 | 22.4 | 0.49 |
| Triton X-102 | 1.0 | 4°C, 30 min | 5.4 | 75.0 | 30.2 | 0.59 |
| Brij 58 | 1.0 | 4°C, 30 min | 53.2 | 85.9 | 42.0 | 0.55 |
| Brij 98 | 1.0 | 37°C, 10 min | 67.0 | 79.8 | 32.3 | 0.62 |
| Lubrol WX | 1.0 | 4°C, 30 min | 31.1 | 84.1 | 38.1 | 0.57 |
| CHAPS | 2.0 | 4°C, 30 min | 36.0 | 13.2 | 11.3 | 0.35 |
| CHAPSO | 1.0 | 4°C, 30 min | 8.7 | 16.2 | 10.4 | 0.41 |
| Tween 20 | 1.0 | 4°C, 30 min | 89.1 | 87.8 | 75.9 | 0.30 |
Normal HSF (N-5) was treated with various detergents, as indicated. Free cholesterol (FC) and phospholipid (PL) contents in DRM (pellet) and detergent-soluble fraction (supernatant) isolated in Fig. 4A were measured by colorimetric enzymatic assays. Amounts of ABCA1 were quantified by ImageJ software.
Percent of total cellular ABCA1, free cholesterol, or phospholipid.
Fig. 4.
ABCA1 resides in a distinct membrane domain. A: Normal HSFs (N-5) grown to subconfluent stage in 100 mm dishes (one dish per treatment) were treated with the indicated detergents, as described in the Materials and Methods. Cells were homogenized, and the PNS obtained was spun at 100,000 g for 1 h. The resultant DRM pellet (R) and detergent-soluble supernatant (S) were subjected to immunoblot analysis for ABCA1, caveolin-1 (Cav-1), calnexin, and LAMP-2. The detergents used are as follows: 1.0% Triton X-100 (TX100), 1.0% Triton X-102 (TX102), 1.0% Brij 58, 1.0% Brij 98, 1.0% Lubrol WX (Lubrol), 1.0% CHAPS, 2.0% CHAPSO, and 1.0% Tween 20. B, C: Normal HSFs (N-5) (B) or differentiated THP-1 cells (C) were treated with 1.0% TX100 or with 1.0% Brij 98 as above, and PNS was subjected to density gradient centrifugation by using OptiPrep, as described in the Materials and Methods. Eight 0.5 ml fractions collected from the top were subjected to immunoblotting with antibodies to ABCA1, Cav-1, flotillin-1 (Flot-1), or LAMP-2.
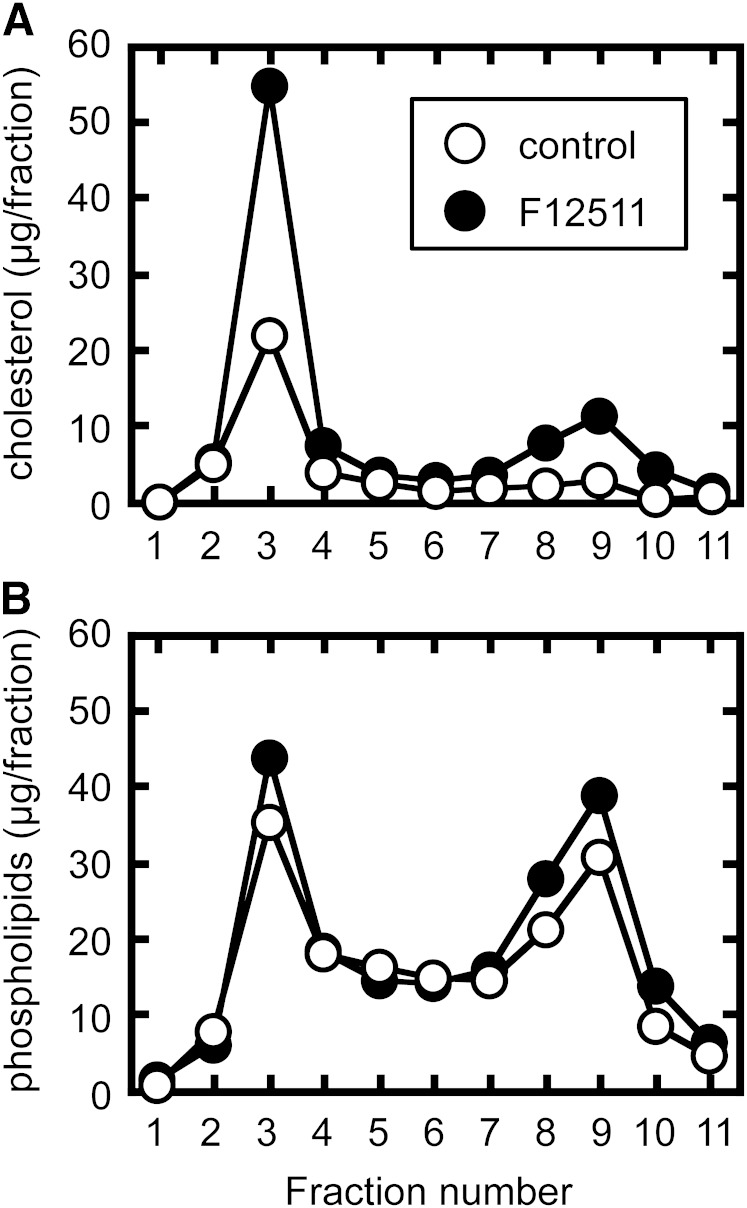
We previously reported that in CHO cells, pharmacological and genetic inactivation of ACAT1 increase ABCA1-dependent cholesterol and phospholipid release (7). The increase in cholesterol release was twice as much as the phospholipid release. Furthermore, the increase in phospholipid release was parallel to the increase in ABCA1 protein expression. These results suggested that ACAT inhibition expands a cholesterol pool in the PM that is preferentially utilized for ABCA1-dependent release. Here we sought to determine whether ACAT blockage increases cholesterol content in Brij 98-DRM. 25RA is a CHO mutant that accumulates large amounts of cholesteryl ester, as a consequence of increases in cholesterol synthesis and LDL uptake (41, 42). 25RA cells were treated or not treated with ACAT inhibitor for 6 h, and subjected to Brij 98-DRM preparation to examine changes in lipid composition in the membrane domain. The results showed that ACAT1 blockage caused a marked increase (approximately 2.5-fold) in cholesterol content in Brij 98-DRM (Fig. 5A). The treatment, however, had no effect on phospholipid content in this domain (Fig. 5B). Therefore, ACAT1 inhibition resulted in a robust increase in the cholesterol/phospholipid ratio of Brij 98-DRM. These results explain why ACAT1 blockage increases cholesterol release by 2-fold over ABCA1 protein expression and phospholipid release.
Fig. 5.
Effect of an ACAT inhibitor on cholesterol contents in Brij 98-DRM. 25RA cells were treated or not treated with an ACAT inhibitor (1 μM F12511) for 6 h. Cells were then treated with 1.0% Brij 98, and the PNS was subjected to sucrose density gradient centrifugation. Eleven 1 ml fractions were collected from the top. After extracting lipids from equal amounts of each fraction, amounts of free cholesterol (A) and phospholipid (B) were determined by enzymatic assays.
Newly synthesized C29/C30 sterols are transiently associated with the membrane domain rich in ABCA1
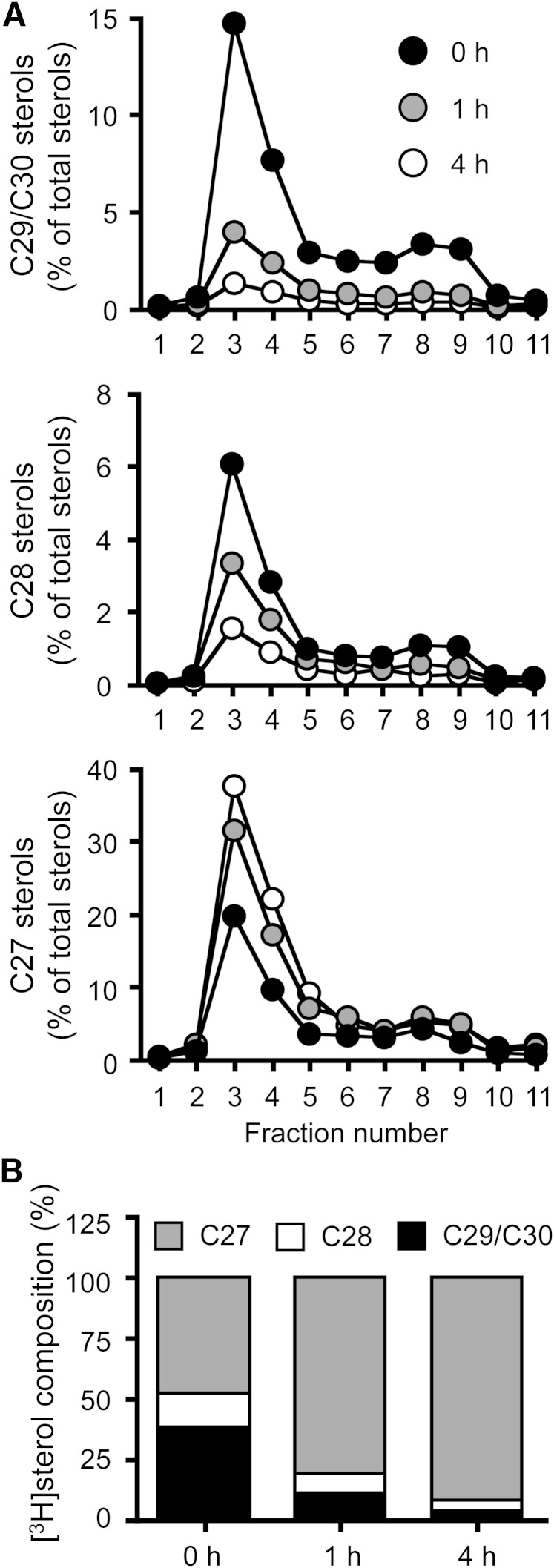
We next examined whether Brij 98-DRM is also enriched in newly synthesized lanosterol. We performed pulse/chase experiments in normal HSFs using [3H]acetate, and monitored the appearance and disappearance of newly synthesized C29/C30, C28, and C27 sterols in Brij 98-DRM. The results showed that, without the chase, the majority of the labeled C29/C30 sterols were found in Brij 98-DRM (Fig. 6A). As the chase time increased, the percentage of [3H]C29/C30 and C28 sterols rapidly decreased, while the percentage of [3H]C27 sterols increased in Brij 98-DRM and in cells (Fig. 6B). The half-life of C29/C30 sterols in the domain was much shorter than 1 h. The results thus suggest that newly synthesized sterols are rapidly transported to the PM microdomains where ABCA1 resides, and become available for ABCA1-dependent release.
Fig. 6.
Appearance and disappearance of newly synthesized lanosterol in Brij 98-DRM. A: Appearance and disappearance of precursor sterols in Brij 98-DRM. Normal HSFs (N-5) treated with TO901317 were pulse-labeled with [3H]acetate for 1 h in medium F. Afterwards, the cells were chased for 0, 1, or 4 h in medium F. PNS was subjected to sucrose density gradient analysis. Eleven 1 ml fractions were collected from the top. Lipids in each fraction were saponified, and C29/C30 (top), C28 (middle), and C27 (bottom) sterols were analyzed by TLC. Data shown are one of two representative experiments. B: Cellular compositions of newly synthesized sterols. Changes in labeled sterol compositions were plotted based on the above results.
DISCUSSION
Our current work aims to determine the sterol molecule specificity of ABCA1 as the cellular lipid release protein. The results reveal that ABCA1 prefers newly synthesized sterols, especially lanosterol, as the substrate. What determines the sterol specificity in this system is currently unknown, and we can only offer certain speculation at this point: At the PM, the ability of cholesterol to form stoichiometric complexes with phospholipids in part determines the “chemical reactivity” of cholesterol in membranes (55). Cholesterol molecules that exceed the binding capacity of phospholipids are considered more mobile, and can be depicted as “active cholesterol” (56). The structure of lanosterol is significantly different from that of cholesterol. It contains three extra methyl groups, the gem-dimethyl moieties at the C4 position, and another methyl group at the C14 position. It also contains a double bond at the C24 position. The C24 double bond in desmosterol causes weaker ability to form an ordered domain than cholesterol does (57). On the other hand, lanosterol was shown to have the ability to form liquid-ordered domains, and can induce membrane curvature formation in vitro (58). Therefore, lanosterol may not form as tight packing with phospholipids as cholesterol does (10), yet membrane domains containing this sterol may retain raft-like properties. The unique structural properties of lanosterol may thus allow it to be more mobile at the PM for ABCA1-dependent release. It was shown that ABCA1 creates a specific lipid-packing deformation site at the PM, and apoA-I recognizes and solubilizes such membrane domains to generate HDL (33, 34). HDL particles generated by ABCA1 are cholesterol-rich and have similar lipid composition to lipid rafts (53), suggesting that the ABCA1/apoA-I system generates HDL at a membrane domain containing significant amounts of cholesterol. Consistent with this notion, our detergent-based cell fractionation experiments showed that ABCA1 resides in a certain membrane domain resistant to Brij 98, a milder detergent than TX100, where cholesterol is highly enriched. This domain also contains newly synthesized lanosterol. This domain may retain a lipid raft-like nature, whereas it may be less ordered compared with TX100-DRM. Several additional lines of evidence produced in other laboratories also support the hypothesis that ABCA1 resides in a certain cholesterol-rich membrane domain. 1) A fraction of ABCA1 was recovered from DRM when Lubrol WX was used (36). 2) ABCA1 is palmitoylated, and this modification is crucial for its function (59). Palmitoylation of a transmembrane protein serves as a lipid raft-targeting signal (60). 3) Real-time tracking of single ABCA1 molecules showed that ABCA1 forms immobile homodimers in the absence of apoA-I. Upon apoA-I addition, ABCA1 rapidly became mobile (61). A GPI-anchored protein, a typical raft-associated molecule, also forms homodimers and becomes immobile, which results in the formation of homodimer rafts in a cholesterol-dependent manner (62). Based on these observations, we speculate that ABCA1 homodimers associate with lipid rafts, and cholesterol and sphingomyelin removal by apoA-I disrupts ABCA1-homodimer rafts.
Why should lanosterol be chosen as a preferred substrate for ABCA1? Because accumulation of lanosterol in the PM displays cytotoxicity (10, 11), cells need to “detoxify” lanosterol rapidly. Lanosterol is hardly a substrate of ACAT (63), because it contains the gem-dimethyl moieties at the C4 position, which produce severe steric hindrance to 3-β-OH. This property incapacitates the ability of ACAT1 to detoxify lanosterol at the ER. Therefore, the preferential release of lanosterol by ABCA1-mediated HDL biogenesis may provide a means to limit the build-up of lanosterol. We recently reported that ABCA1 also facilitates PM-to-ER lanosterol movement for its conversion to cholesterol (18). At the ER, lanosterol is first converted to dihydrolanosterol, which causes rapid degradation of HMG-CoA reductase protein to limit further biosynthesis of lanosterol (9). The potential toxicity of lanosterol at the PM can thus be minimized by the ABCA1-dependent bidirectional lanosterol transport system. It is interesting to note that another ABC transporter, ABCG1, but not ABCA1, releases 7-ketocholesterol to HDL in addition to cholesterol (64). 7-Ketocholesterol is accumulated in atherosclerotic lesions, and induces macrophage apoptosis. Thus, ABCG1 protects macrophages from cytotoxicity of 7-ketocholesterol. In addition, ABCG1 and ABCG4 export desmosterol to HDL (65). HDL contains substantial amounts of precursor sterols, including lanosterol and desmosterol. Therefore, besides cholesterol as a common substrate, ABCA1, ABCG1, and ABCG4 may have different sterol specificity. These transporters may cooperatively act to protect cells from the build-up of cytotoxic sterols.
In conclusion, the current work identifies unique substrates for ABCA1: the de novo synthesized cholesterol and methylated precursor sterols arriving at a certain PM domain where ABCA1 is enriched can be rapidly removed by the ABCA1-dependent sterol release pathway before they are transported back to the ER. Our results also support a model where the ABCA1/apoA-I system generates HDL at the PM.
Acknowledgments
The authors thank Dr. Sumiko Abe-Dohmae (Nagoya City University Medical School) for providing cells and Dr. Koichi Furukawa (Nagoya University Graduate School of Medicine) for his generous support.
Footnotes
Abbreviations:
- 9cRA
- 9-cis retinoic acid
- DRM
- detergent-resistant membrane
- ER
- endoplasmic reticulum
- HEK/hABCA1
- HEK293 cells stably expressing human ABCA1
- HSF
- human skin fibroblast
- LE
- late endosome
- LS
- lysosome
- MEF
- mouse embryonic fibroblast
- NPA
- Niemann-Pick disease type A
- NPC
- Niemann-Pick disease type C
- PM
- plasma membrane
- PNS
- postnuclear supernatant
- TX
- Triton X
This work was supported by an American Heart Association Postdoctoral Fellowship (to Y.Y.), JSPS KAKENHI from the Japan Society for the Promotion of Sciences (to Y.Y. and S.Y., respectively), a MEXT-Supported Program for the Strategic Research Foundation at Private Universities from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to S.Y.), and by a grant from the National Institutes of Health, HL060306 (to T-Y.C.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
REFERENCES
- 1.Brown M. S., and Goldstein J. L.. 2009. Cholesterol feedback: from Schoenheimer’s bottle to Scap’s MELADL. J. Lipid Res. 50(Suppl): S15–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang T. Y., Chang C. C., Ohgami N., and Yamauchi Y.. 2006. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22: 129–157. [DOI] [PubMed] [Google Scholar]
- 3.Maxfield F. R., and Tabas I.. 2005. Role of cholesterol and lipid organization in disease. Nature. 438: 612–621. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama S. 2006. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26: 20–27. [DOI] [PubMed] [Google Scholar]
- 5.Tarling E. J., de Aguiar Vallim T. Q., and Edwards P. A.. 2013. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 24: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oram J. F., and Heinecke J. W.. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85: 1343–1372. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi Y., Chang C. C. Y., Hayashi M., Abe-Dohmae S., Reid P. C., Chang T. Y., and Yokoyama S.. 2004. Intracellular cholesterol mobilization involved in the ABCA1/apolipoprotein-mediated assembly of high density lipoprotein in fibroblasts. J. Lipid Res. 45: 1943–1951. [DOI] [PubMed] [Google Scholar]
- 8.Song B. L., Javitt N. B., and DeBose-Boyd R. A.. 2005. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 1: 179–189. [DOI] [PubMed] [Google Scholar]
- 9.Lange Y., Ory D., Ye J., Lanier M., Hsu F., and Steck T.. 2008. Effectors of rapid homeostatic responses of endoplasmic reticulum cholesterol and 3-hydroxy-3-methylglutaryl-CoA reductase. J. Biol. Chem. 283: 1445–1455. [DOI] [PubMed] [Google Scholar]
- 10.Bloch K. E. 1983. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14: 47–92. [DOI] [PubMed] [Google Scholar]
- 11.Xu F., Rychnovsky S. D., Belani J. D., Hobbs H. H., Cohen J. C., and Rawson R. B.. 2005. Dual roles for cholesterol in mammalian cells. Proc. Natl. Acad. Sci. USA. 102: 14551–14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley R. I., and Herman G. E.. 2001. Inborn errors of sterol biosynthesis. Annu. Rev. Genomics Hum. Genet. 2: 299–341. [DOI] [PubMed] [Google Scholar]
- 13.Porter F. D. 2002. Malformation syndromes due to inborn errors of cholesterol synthesis. J. Clin. Invest. 110: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Echevarria F., Norton R., Nes W., and Lange Y.. 1990. Zymosterol is located in the plasma membrane of cultured human fibroblasts. J. Biol. Chem. 265: 8484–8489. [PubMed] [Google Scholar]
- 15.Johnson W. J., Fischer R. T., Phillips M. C., and Rothblat G. H.. 1995. Efflux of newly synthesized cholesterol and biosynthetic sterol intermediates from cells. Dependence on acceptor type and on enrichment of cells with cholesterol. J. Biol. Chem. 270: 25037–25046. [DOI] [PubMed] [Google Scholar]
- 16.Lusa S., Heino S., and Ikonen E.. 2003. Differential mobilization of newly synthesized cholesterol and biosynthetic sterol precursors from cells. J. Biol. Chem. 278: 19844–19851. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi Y., Reid P. C., Sperry J. B., Furukawa K., Takeya M., Chang C. C., and Chang T. Y.. 2007. Plasma membrane rafts complete cholesterol synthesis by participating in retrograde movement of precursor sterols. J. Biol. Chem. 282: 34994–35004. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi Y., Iwamoto N., Rogers M. A., Abe-Dohmae S., Fujimoto T., Chang C. C., Ishigami M., Kishimoto T., Kobayashi T., Ueda K., et al. 2015. Deficiency in the lipid exporter ABCA1 impairs retrograde sterol movement and disrupts sterol sensing at the endoplasmic reticulum. J. Biol. Chem. 290: 23464–23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koivisto P. V., and Miettinen T. A.. 1988. Increased amounts of cholesterol precursors in lipoproteins after ileal exclusion. Lipids. 23: 993–996. [DOI] [PubMed] [Google Scholar]
- 20.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 21.Bodzioch M., Orsó E., Klucken J., Langmann T., Böttcher A., Diederich W., Drobnik W., Barlage S., Büchler C., Porsch-Ozcürümez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351. [DOI] [PubMed] [Google Scholar]
- 22.Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denèfle P., et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355. [DOI] [PubMed] [Google Scholar]
- 23.Hara H., and Yokoyama S.. 1991. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J. Biol. Chem. 266: 3080–3086. [PubMed] [Google Scholar]
- 24.Francis G. A., Knopp R. H., and Oram J. F.. 1995. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier disease. J. Clin. Invest. 96: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denis M., Landry Y. D., and Zha X.. 2008. ATP-binding cassette A1-mediated lipidation of apolipoprotein A-I occurs at the plasma membrane and not in the endocytic compartments. J. Biol. Chem. 283: 16178–16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu R., Arakawa R., Ito-Osumi C., Iwamoto N., and Yokoyama S.. 2008. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler. Thromb. Vasc. Biol. 28: 1820–1824. [DOI] [PubMed] [Google Scholar]
- 27.Faulkner L. E., Panagotopulos S. E., Johnson J. D., Woollett L. A., Hui D. Y., Witting S. R., Maiorano J. N., and Davidson W. S.. 2008. An analysis of the role of a retroendocytosis pathway in ABCA1-mediated cholesterol efflux from macrophages. J. Lipid Res. 49: 1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neufeld E. B., Remaley A. T., Demosky S. J., Stonik J. A., Cooney A. M., Comly M., Dwyer N. K., Zhang M., Blanchette-Mackie J., Santamarina-Fojo S., et al. 2001. Cellular localization and trafficking of the human ABCA1 transporter. J. Biol. Chem. 276: 27584–27590. [DOI] [PubMed] [Google Scholar]
- 29.Phillips M. C. 2014. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 289: 24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oram J. F., Lawn R. M., Garvin M. R., and Wade D. P.. 2000. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 275: 34508–34511. [DOI] [PubMed] [Google Scholar]
- 31.Wang N., Silver D., Costet P., and Tall A.. 2000. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275: 33053–33058. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald M. L., Morris A. L., Chroni A., Mendez A. J., Zannis V. I., and Freeman M. W.. 2004. ABCA1 and amphipathic apolipoproteins form high-affinity molecular complexes required for cholesterol efflux. J. Lipid Res. 45: 287–294. [DOI] [PubMed] [Google Scholar]
- 33.Vedhachalam C., Duong P. T., Nickel M., Nguyen D., Dhanasekaran P., Saito H., Rothblat G. H., Lund-Katz S., and Phillips M. C.. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282: 25123–25130. [DOI] [PubMed] [Google Scholar]
- 34.Segrest J. P., Jones M. K., Catte A., Manchekar M., Datta G., Zhang L., Zhang R., Li L., Patterson J. C., Palgunachari M. N., et al. 2015. Surface density-induced pleating of a lipid monolayer drives nascent high-density lipoprotein assembly. Structure. 23: 1214–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendez A. J., Lin G., Wade D. P., Lawn R. M., and Oram J. F.. 2001. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J. Biol. Chem. 276: 3158–3166. [DOI] [PubMed] [Google Scholar]
- 36.Drobnik W., Borsukova H., Böttcher A., Pfeiffer A., Liebisch G., Schütz G. J., Schindler H., and Schmitz G.. 2002. Apo AI/ABCA1-dependent and HDL3-mediated lipid efflux from compositionally distinct cholesterol-based microdomains. Traffic. 3: 268–278. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama S., Tajima S., and Yamamoto A.. 1982. The process of dissolving apolipoprotein A-I in an aqueous buffer. J. Biochem. 91: 1267–1272. [DOI] [PubMed] [Google Scholar]
- 38.Limanek J. S., Chin J., and Chang T. Y.. 1978. Mammalian cell mutant requiring cholesterol and unsaturated fatty acid for growth. Proc. Natl. Acad. Sci. USA. 75: 5452–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu W., Abe-Dohmae S., Tsujita M., Iwamoto N., Ogikubo O., Otsuka T., Kumon Y., and Yokoyama S.. 2008. Biogenesis of HDL by SAA is dependent on ABCA1 in the liver in vivo. J. Lipid Res. 49: 386–393. [DOI] [PubMed] [Google Scholar]
- 40.Abe-Dohmae S., Ikeda Y., Matsuo M., Hayashi M., Okuhira K., Ueda K., and Yokoyama S.. 2004. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 279: 604–611. [DOI] [PubMed] [Google Scholar]
- 41.Chang T. Y., and Limanek J. S.. 1980. Regulation of cytosolic acetoacetyl coenzyme A thiolase, 3-hydroxy-3-methylglutaryl coenzyme A synthase, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and mevalonate kinase by low density lipoprotein and by 25-hydroxycholesterol in Chinese hamster ovary cells. J. Biol. Chem. 255: 7787–7795. [PubMed] [Google Scholar]
- 42.Hua X., Nohturfft A., Goldstein J., and Brown M.. 1996. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell. 87: 415–426. [DOI] [PubMed] [Google Scholar]
- 43.Sakashita N., Chang C. C., Lei X., Fujiwara Y., Takeya M., and Chang T. Y.. 2010. Cholesterol loading in macrophages stimulates formation of ER-derived vesicles with elevated ACAT1 activity. J. Lipid Res. 51: 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuck S., Honsho M., Ekroos K., Shevchenko A., and Simons K.. 2003. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA. 100: 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamauchi Y., Furukawa K., Hamamura K., and Furukawa K.. 2011. Positive feedback loop between PI3K-Akt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen. Cancer Res. 71: 4989–4997. [DOI] [PubMed] [Google Scholar]
- 46.Röper K., Corbeil D., and Huttner W. B.. 2000. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2: 582–592. [DOI] [PubMed] [Google Scholar]
- 47.Arakawa R., and Yokoyama S.. 2002. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 277: 22426–22429. [DOI] [PubMed] [Google Scholar]
- 48.Abi-Mosleh L., Infante R., Radhakrishnan A., Goldstein J., and Brown M.. 2009. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. USA. 106: 19316–19321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid P. C., Sugii S., and Chang T. Y.. 2003. Trafficking defects in endogenously synthesized cholesterol in fibroblasts, macrophages, hepatocytes, and glial cells from Niemann-Pick type C1 mice. J. Lipid Res. 44: 1010–1019. [DOI] [PubMed] [Google Scholar]
- 50.Chen W., Sun Y., Welch C., Gorelik A., Leventhal A., Tabas I., and Tall A.. 2001. Preferential ATP-binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J. Biol. Chem. 276: 43564–43569. [DOI] [PubMed] [Google Scholar]
- 51.Choi H. Y., Karten B., Chan T., Vance J. E., Greer W. L., Heidenreich R. A., Garver W. S., and Francis G. A.. 2003. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann-Pick type C disease. J. Biol. Chem. 278: 32569–32577. [DOI] [PubMed] [Google Scholar]
- 52.Karten B., Peake K. B., and Vance J. E.. 2009. Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim. Biophys. Acta. 1791: 659–670. [DOI] [PubMed] [Google Scholar]
- 53.Sorci-Thomas M. G., Owen J. S., Fulp B., Bhat S., Zhu X., Parks J. S., Shah D., Jerome W. G., Gerelus M., Zabalawi M., et al. 2012. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res. 53: 1890–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pike L. J. 2004. Lipid rafts: heterogeneity on the high seas. Biochem. J. 378: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radhakrishnan A., Anderson T. G., and McConnell H. M.. 2000. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. USA. 97: 12422–12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steck T. L., and Lange Y.. 2010. Cell cholesterol homeostasis: mediation by active cholesterol. Trends Cell Biol. 20: 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vainio S., Jansen M., Koivusalo M., Róg T., Karttunen M., Vattulainen I., and Ikonen E.. 2006. Significance of sterol structural specificity. Desmosterol cannot replace cholesterol in lipid rafts. J. Biol. Chem. 281: 348–355. [DOI] [PubMed] [Google Scholar]
- 58.Bacia K., Schwille P., and Kurzchalia T.. 2005. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA. 102: 3272–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singaraja R. R., Kang M. H., Vaid K., Sanders S. S., Vilas G. L., Arstikaitis P., Coutinho J., Drisdel R. C., El-Husseini A. D., Green W. N., et al. 2009. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ. Res. 105: 138–147. [DOI] [PubMed] [Google Scholar]
- 60.Levental I., Lingwood D., Grzybek M., Coskun U., and Simons K.. 2010. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl. Acad. Sci. USA. 107: 22050–22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagata K. O., Nakada C., Kasai R. S., Kusumi A., and Ueda K.. 2013. ABCA1 dimer-monomer interconversion during HDL generation revealed by single-molecule imaging. Proc. Natl. Acad. Sci. USA. 110: 5034–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki K. G., Kasai R. S., Hirosawa K. M., Nemoto Y. L., Ishibashi M., Miwa Y., Fujiwara T. K., and Kusumi A.. 2012. Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 8: 774–783. [DOI] [PubMed] [Google Scholar]
- 63.Tavani D. M., Nes W. R., and Billheimer J. T.. 1982. The sterol substrate specificity of acyl CoA:cholesterol acyltransferase from rat liver. J. Lipid Res. 23: 774–781. [PubMed] [Google Scholar]
- 64.Terasaka N., Wang N., Yvan-Charvet L., and Tall A.. 2007. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA. 104: 15093–15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang N., Yvan-Charvet L., Lütjohann D., Mulder M., Vanmierlo T., Kim T., and Tall A.. 2008. ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB J. 22: 1073–1082. [DOI] [PubMed] [Google Scholar]