Abstract
Low-grade chronic inflammation plays an important role in the pathogenesis of obesity-induced insulin resistance. ABCA1 is essential for reverse cholesterol transport and HDL synthesis, and protects against macrophage inflammation. In the present study, the effects of ABCA1 deficiency in hematopoietic cells on diet-induced inflammation and insulin resistance were tested in vivo using bone marrow transplanted (BMT)-WT and BMT-ABCA1−/− mice. When challenged with a high-fat high-carbohydrate diabetogenic diet with added cholesterol (HFHSC), BMT-ABCA1−/− mice displayed enhanced insulin resistance and impaired glucose tolerance as compared with BMT-WT mice. The worsened insulin resistance and impaired glucose tolerance in BMT-ABCA1−/− mice were accompanied by increased macrophage accumulation and inflammation in adipose tissue and liver. Moreover, BMT-ABCA1−/− mice had significantly higher hematopoietic stem cell proliferation, myeloid cell expansion, and monocytosis when challenged with the HFHSC diet. In vitro studies indicated that macrophages from ABCA1−/− mice showed significantly increased inflammatory responses induced by saturated fatty acids. Taken together, these studies point to an important role for hematopoietic ABCA1 in modulating a feed-forward mechanism in obesity such that inflamed tissue macrophages stimulate the production of more monocytes, leading to an exacerbation of inflammation and associated disease processes.
Keywords: cholesterol, adipose tissue, macrophages, high density lipoprotein, obesity, diabetes, ATP binding cassette transporter A1
Low-grade inflammation has emerged as an important contributor to the etiology of insulin resistance in obesity and type 2 diabetes (1). Adipose tissue macrophages appear to be responsible for much of the increase in inflammation in adipose tissue with obesity (1–3) and monocytosis plays a significant role in promoting adipose macrophage infiltration and inflammation in obesity (4–6). Interestingly, recent studies suggest that cellular cholesterol balance regulates the inflammatory status of macrophages (7, 8). The addition of a relatively small amount of dietary cholesterol results in a marked increase in the accumulation of macrophages in adipose tissue (9). Mice with macrophage-specific transgenic expression of cholesterol ester hydrolase, which results in significant cholesterol ester and free reduction in macrophages, reduces macrophage infiltration and improves insulin sensitivity (10, 11). Moreover, deficiency of ABCA1 and ABCG1 promotes monocytosis (12, 13). These studies suggest that cholesterol metabolism plays an important role in macrophage infiltration and inflammation in peripheral tissues, including adipose tissue and liver.
ABCA1 is a cell-membrane protein that is essential for HDL synthesis and reverse cholesterol transport. ABCA1 protects against cholesterol accumulation in macrophages by transporting cholesterol and phospholipids to apolipoproteins from cells (14–16). ABCA1 has been shown to be a crucial regulator of intracellular cholesterol stores and plays an important role in the functions of multiple tissues (17–19). Leukocyte ABCA1 expression is associated with increased fasting glucose concentration, and reduced ABCA1 expression in leukocytes is associated with type 2 diabetes (19–21). ABCA1 deficiency in pancreatic β cells reduces insulin release (22); whereas, hematopoietic-specific deletion of ABCA1 promotes hematopoietic stem cell proliferation, monocytosis, macrophage inflammation, and diet-induced atherosclerosis in mice (18, 23). We and others have reported that ABCA1 deficiency in macrophages increases pro-inflammatory cytokine expression in response to pro-inflammatory insults such as lipopolysaccharides (LPSs) (7, 8, 24). Considering the clear role of macrophages in the propagation of inflammatory signaling in adipose tissue and liver, we hypothesized that knockout of ABCA1 in hematopoietic-derived cells would increase monocytosis, macrophage tissue accumulation, and insulin resistance in response to a diabetogenic diet.
To address this hypothesis, we generated mice with ABCA1 deletion exclusively in hematopoietic cells using bone marrow transplantation. Our results reveal that when challenged with a high-fat high-carbohydrate diabetogenic diet with added cholesterol (HFHSC) (9), mice deficient in ABCA1 in their hematopoietic compartment exhibit increased hematopoietic stem cell proliferation, myeloid cell expansion, monocytosis, macrophage accumulation, and inflammation in adipose tissue and liver, as well as more severe diet-induced insulin resistance. Our data suggest an important role for hematopoietic ABCA1 in a feed-forward mechanism in obesity such that inflamed tissue macrophages stimulate the production of more monocytes, leading to an exacerbation of inflammation and associated disease processes.
MATERIALS and METHODS
Animals and diet
All mice used in the study were males of the DBA/2 background, housed in specific pathogen-free micro-isolators and maintained on a 12 h light/dark cycle. ABCA1−/− DBA mice were a gift from Robert Aiello, Pfizer-Wyeth, and the generation and characterization of ABCA1−/− mice has been described previously (25). For bone marrow (BM) transplantation experiments, whole BM from WT and ABCA1−/− mice was prepared by flushing the marrow cavities of the long bones with sterile saline. Cells (5 × 106) were injected retro-orbitally into lethally irradiated (11 Gy) WT recipient mice. After a 5 week recovery period, mice were placed on either standard chow or a HFHSC diet (0.15% cholesterol) (Bioserv F4997, Frenchtown, NJ) for up to 22 weeks. Body weights were measured weekly. Food intake was recorded after 10 weeks of diet. At euthanization, harvested tissues were snap-frozen in liquid nitrogen and stored at −70°C or were fixed with 10% neutral-buffered formalin and embedded in paraffin wax. All experimental procedures were undertaken with approval from the Institution Animal Care and Use Committee of the University of Washington.
Analytical procedures
Metabolic variables in the mice were measured in blood samples obtained from the retro-orbital sinus after a 5 h fast. Cholesterol and triglycerides in plasma and fast-phase LC (FPLC) fractions were measured using colorimetric assay kits. Plasma insulin levels were measured using an ELISA kit (Millipore, Billerica, MA). Intra-peritoneal glucose and insulin tolerance tests were performed after a 5 h fast at weeks 20 and 21 of diet feeding, respectively, as previously described (9). Body composition was performed on conscious immobilized mice using quantitative magnetic resonance (EchoMRI whole body composition analyzer; Echo Medical Systems, Houston, TX).
Real-time quantitative PCR
Total RNA was extracted from whole adipose, liver, or cultured cells using a commercially available RNA extraction kit according to the manufacturer’s protocol (Agilent Technologies, Santa Clara, CA). After spectroscopic quantification, 2 μg of RNA was reverse-transcribed, and the cDNA thus obtained was analyzed by real-time quantitative PCR. Primers specific for individual genes were purchased from Applied Biosystems (Assay-on-Demand; Life Technologies, Carlsbad, CA). GAPDH and L32 were used as the control housekeeping genes. Relative amounts of the target gene were calculated using the ΔΔCt formula.
Immunohistochemistry
Paraffin-embedded epididymal fat and liver were sectioned, dewaxed, and rehydrated prior to antigen retrieval by boiling in 10 mM sodium citrate buffer (pH 6.5). Tissue samples were blocked with 5% normal rabbit serum for 40 min, followed by incubation for 2 h with 10 μg/ml rat anti-mouse F4/80 antibody (AbD Serotec). Biotin-conjugated secondary anti-rat antibody was applied for 1 h, followed by a 30 min incubation with Vectastain ABC solution (Vector Laboratories). The percentage of F4/80-positive cells for each sample was calculated as the number of nuclei of F4/80-expressing cells divided by the total number of nuclei per sample.
White blood cell counts
Leukocytes and differential blood cell counts were quantified from whole blood using a hematology cell counter (HEMAVET 950FS).
Flow cytometry
Adipose macrophages were quantified using flow cytometry, as previously described (9). Briefly, epididymal white adipose tissue from mice was excised at the time of euthanization and minced in PBS. Minced samples were digested using LPS-depleted collagenase (type IV) cocktail (Sigma-Aldrich, St. Louis, MO) (1 mg/ml). The digested mixture was filtered through a 100 uM cell strainer, and stromal vascular cells were pelleted. The stromal vascular fraction (SVC) was then used for flow cytometry using anti-F4/80 antibody. Stained cells were analyzed on an LSR II flow cytometer (BD Biosciences) running FACSDiva software.
Flow cytometry was used to quantify blood monocytes and hematopoietic stem cells, as previously described (13). For all samples, cells were counted with a hemocytometer, and Fc receptors were blocked using anti-FcγRII/III antibody 2.4G2 prior to adding the fluorescent-tagged antibodies. For blood monocytes and myeloid counting, red blood cells were lysed using red blood cell lysis buffer (eBioscience). Monocytes were identified as CD45hiCD115hi and further identified into Ly6Chi and Ly6Clo subsets, myeloid cells were identified as CD11bhi/Gr-Ihi. For BM cell counting, BM cells were collected from leg bones, lysed to remove red blood cells, and filtered before use. Hematopoietic stem cells and multipotential progenitor cells (HSPCs) were identified as lineage−, Sac1+, and ckit+, and the myeloid progenitor cells (MPCs) were identified as lineage−-Sca1−- ckit+. Dead cells were excluded from all samples using fixable viability dye, eFluor 450 (eBioscience). All flow cytometry data were collected on the LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo software 10.0 (Tree Star).
Cell culture
Thioglycollate-elicited peritoneal macrophages were obtained, as previously described (26), and cultured in DMEM with 10% fetal bovine serum for 2 h. Unseeded cells were washed away, and the medium was changed to DMEM with 0.1% fatty acid-free bovine albumin. Cells were loaded with 50 μg/ml acetylated LDL overnight. For cytokine expression experiments, cells were pretreated with or without 10 μg/ml of apoA-I in DMEM and 0.1% fatty acid-free bovine albumin for 3 h, washed twice, treated with or without the indicated fatty acids (250 μM palmitate or mixed fatty acids) conjugated to 2% (w/v) fatty acid-free and low endotoxin BSA (Sigma-Aldrich) overnight, and then processed for RNA extractions.
In vitro proliferation assay
BM cells were collected from leg bones, lysed to remove red blood cells, and cultured in IMDM (Gibco; Invitrogen) for 2 h to remove adherent cells in order to enrich progenitor cells. The nonadherent cells were then cultured for 72 h in IMDM medium containing 6 ng/ml interleukin (IL)-3 (R&D Systems), 100 ng/ml stem cell factor (CSF; R&D Systems), and 2 ng/ml GM-CSF (R&D Systems). For proliferation measurements, cells were further incubated with 2 μCi/ml [3H]thymidine for 4 h and the radioactivity incorporated into the cells was determined by liquid scintillation counting.
Statistical analyses
Data were analyzed using the GraphPad Prism 5 program (GraphPad Software Inc., La Jolla, CA) and are represented as means and SD. Student’s t-test was used to detect differences within groups when applicable. One-way ANOVA was used to compare differences among all groups, and Bonferroni post hoc testing was used to detect differences among mean values of the groups. P < 0.05 was considered to be statistically significant.
RESULTS
Hematopoietic ABCA1 deletion worsens diet-induced insulin resistance in vivo without affecting body mass and adiposity
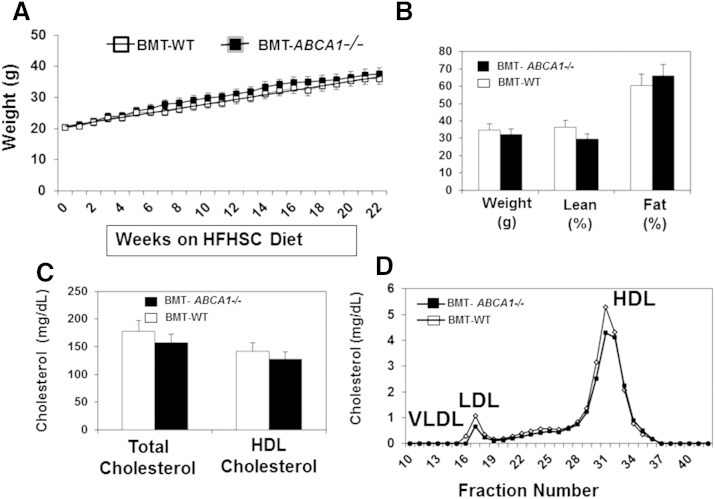
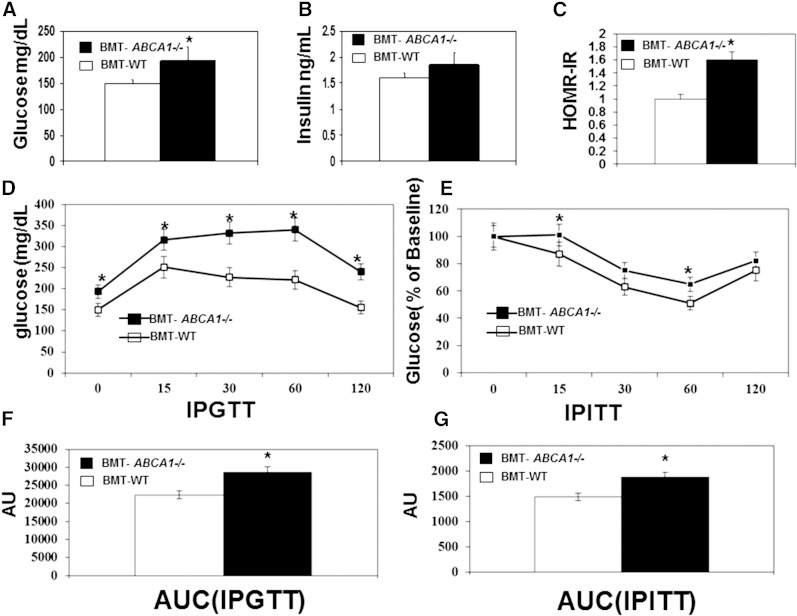
To assess the potential role of ABCA1 in regulating obesity-induced inflammation and insulin resistance in vivo, we generated bone marrow transplanted (BMT)-WT and BMT-ABCA1−/− chimeras by transplanting BM from ABCA1−/− and WT littermates into lethally irradiated WT recipients. After a 5 week recovery period, mice were either maintained on chow diet or switched to the HFHSC diet for up to 22 weeks. We found that there were no differences in body mass, adiposity, and macrophage accumulation between BMT-WT and BMT-ABCA1−/− mice (data not shown) on the control diet. While the HFHSC diet increased both body mass and adiposity, there were no significant differences between BMT-WT and BMT-ABCA1−/− mice (Fig. 1A, B). Consistent with the literature (18, 19), plasma total cholesterol and HDL cholesterol were not significantly different between HFHSC diet-fed BMT-WT and BMT-ABCA1−/− mice (Fig. 1C). FPLC analysis indicated that the lipid profiles were similar between HFHSC diet-fed BMT-WT and BMT-ABCA1−/− mice (Fig. 1D). However, despite similar body mass, adiposity, and plasma lipid profiles, fasting plasma glucose levels (Fig. 2A) and the calculated HOMA_IR index (Fig. 2C) were significantly higher for the HFHSC diet-fed BMT-ABCA1−/− mice compared with those of HFHSC diet-fed BMT-WT mice. These data suggest that HFHSC diet-fed BMT-ABCA1−/− mice were more insulin resistant.
Fig. 1.
Hematopoietic ABCA1 deletion did not affect diet-induced body weight or plasma lipid profiles. BMT-WT and BMT-ABCA1−/− mice were fed the HFHSC diet for 22 weeks. A: Body weight was recorded weekly. B: Body composition was determined at week 20. Total and HDL cholesterol (C) and FPLC lipid profile (D) were determined at week 22. Data are presented as means ± SD, n = 12 mice per group.
Fig. 2.
Hematopoietic ABCA1 deletion worsened insulin resistance. BMT-WT and BMT-ABCA1−/− mice were fed the HFHSC diet for 22 weeks. Plasma fasting glucose (A) and plasma fasting insulin (B) were measured at week 21. C: Calculated HOMR-IR. D: Intraperitoneal glucose tolerance test. E: Intraperitoneal insulin tolerance test. F: Area under the curve (AUC) was calculated to measure the degree of the glucose tolerance impairment. G: AUC was calculated to measure the degree of the insulin tolerance impairment. *P < 0.05 versus BMT-WT mice; n = 10–15 per group.
We further performed intraperitoneal glucose and insulin tolerance tests in HFHSC diet-fed BMT-WT and BMT-ABCA1−/− mice. As shown in Fig. 2D–G, significantly worse glucose and insulin tolerance were noted in HFHSC diet-fed BMT-ABCA1−/− mice as compared with HFHSC diet-fed BMT-WT mice.
Hematopoietic ABCA1 deletion results in higher macrophage accumulation and inflammatory cytokine expression in adipose tissue
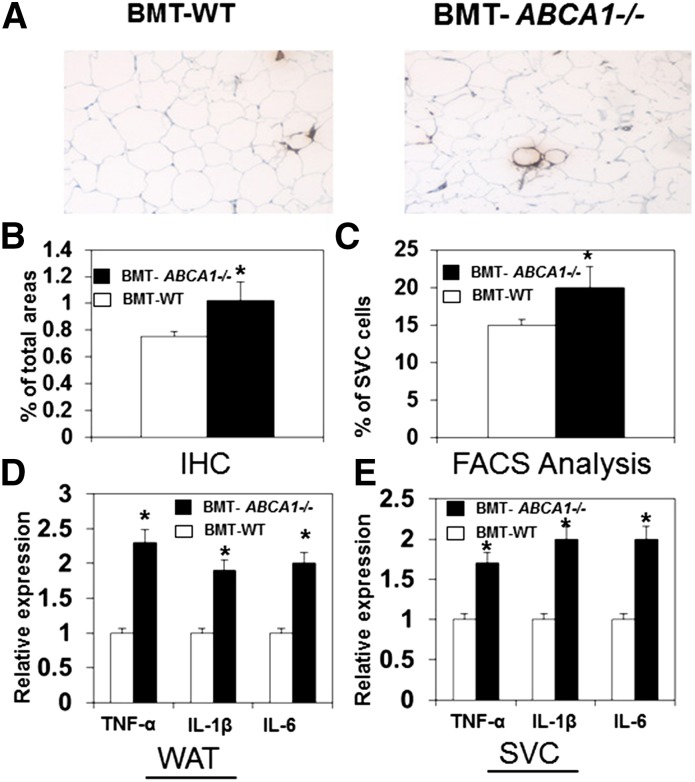
In obesity, macrophages accumulated within adipose tissue promote insulin resistance (27). To determine whether hematopoietic ABCA1 deficiency affects macrophage accumulation in adipose tissue, we examined the macrophage content of white adipose tissue. We found that immunohistochemical staining of F4/80 (Fig. 3A, B) and mRNA expression (data not shown) were significantly greater in adipose tissue of HFHSC diet-fed BMT-ABCA1−/− mice. Moreover, fluorescence-activated cell sorting analysis of the SVC showed that the percentage of F4/80-positive cells (Fig. 3C) was significantly higher in adipose tissue of HFHSC diet-fed BMT-ABCA1−/− mice, indicating increased macrophage accumulation. We subsequently examined the inflammatory profile of adipose tissue and found that adipose tissue and the SVC from HFHSC diet-fed BMT-ABCA1−/− mice had significantly higher expression of TNF-α, IL-1β, and IL-6 (Fig. 3D).
Fig. 3.
Adipose tissue macrophage accumulation and inflammation was enhanced in HFHSC diet-fed BMT- ABCA1−/− mice. A: Adipose tissue sections were stained with the macrophage-specific antibody, F4/80. B: Quantification of macrophage F4/80 staining is shown. C: F4/80-positive cells were determined in SVC using fluorescence-activated cell sorting analysis. Expression of genes in whole adipose tissue (WAT) (D) and SVC (E). *P < 0.05 versus BMT-WT; n = 5–10 per group.
Hematopoietic ABCA1 deletion results in elevated macrophage accumulation and inflammatory cytokine expression in the liver
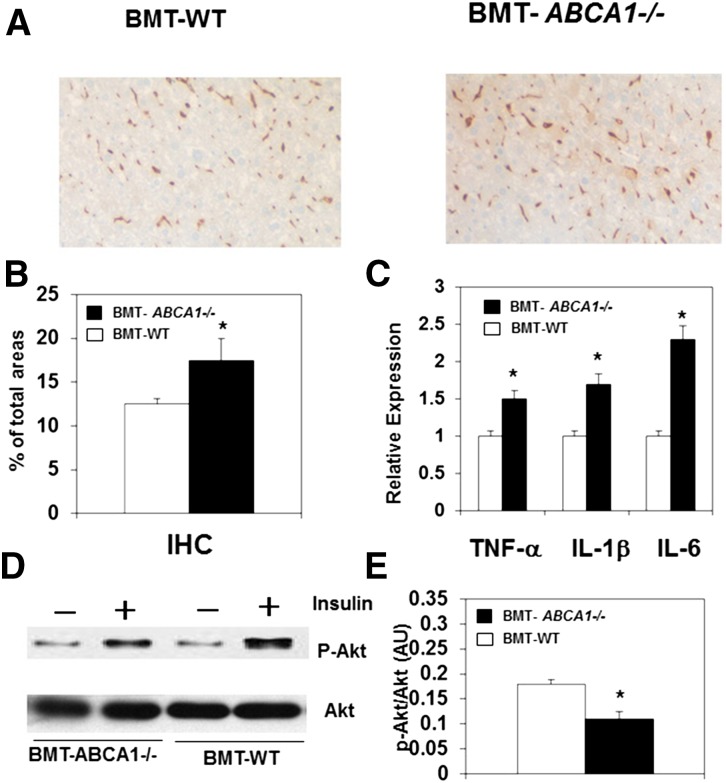
Liver is another tissue that contributes to insulin resistance in obesity and contains a significant content of macrophages (Kupffer cells). We next examined the macrophage content of liver. We found that when fed the HFHSC diet, BMT-ABCA1−/− mice had significantly greater expression of F4/80 and CD68 in the liver (data not shown). Consistent with the increase of mRNA transcripts, immunohistochemical staining of F4/80 was significantly greater in the liver of HFHSC diet-fed BMT-ABCA1−/− mice (Fig. 4A, B), indicating higher macrophage accumulation. We subsequently examined the expression of inflammatory genes in the liver. In agreement with the observed increase in macrophage accumulation in the liver of HFHSC diet-fed BMT-ABCA1−/− mice, we also found a marked increase in the expression of inflammatory cytokines, TNF-α, IL-1β, and IL-6 (Fig. 4C). Consistent with the changes in insulin sensitivity, we also observed significantly decreased Akt phosphorylation in the liver of BMT-ABCA1−/− mice as compared with BMT-WT controls after stimulation with insulin (Fig. 4D).
Fig. 4.
Hematopoietic ABCA1 deletion increased macrophage accumulation and inflammation and worsened insulin resistance in the liver. BMT-WT and BMT-ABCA1−/− mice were fed the HFHSC diet for 22 weeks. A: Liver sections were stained with the macrophage-specific antibody, F4/80. B: Quantification of macrophage F4/80 staining is shown. C: Expression of inflammatory genes in the liver. D: Liver Akt phosphorylation after insulin stimulation in vivo in mice. *P < 0.05 versus BMT-WT; n = 5–10 per group.
Hematopoietic ABCA1 deletion results in myelopoiesis and monocytosis when challenged with the HFHSC diet
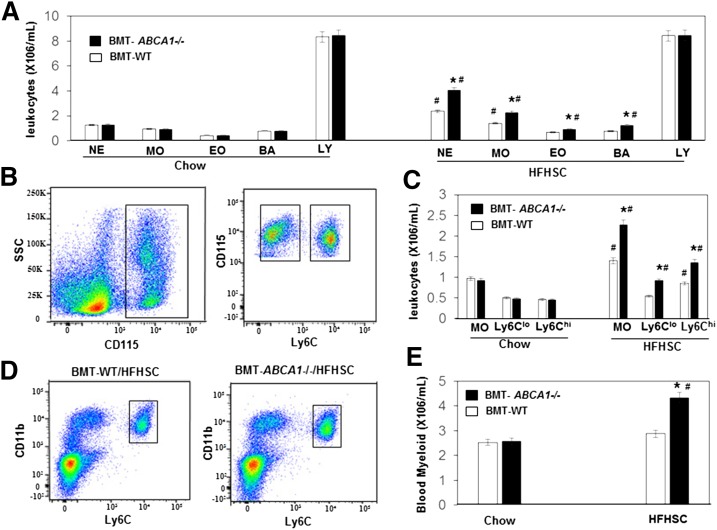
There is a strong association between obesity and leukocytosis, particularly of the myeloid lineage (6, 28). A recent pioneering study has shown that MPC proliferation and monocytosis drive macrophage accumulation and insulin resistance in obesity (4). A possible explanation for the increased macrophage accumulation and insulin resistance in the HSHFC-fed BMT-ABCA1−/− mice is that they may have higher blood levels of monocytes and elevated MPC proliferation. Leukocytes and differential blood counts were quantified from whole blood using a hematology cell counter, and monocyte subset and BM progenitor cells were quantified using flow cytometry. As shown in Fig. 5A, on the control chow diet there were no differences in leukocytes, blood counts, monocyte subset, or BM progenitor cells between BMT-WT and BMT-ABCA1−/− mice. Consistent with previous findings (4), diet-induced obese BMT-WT mice showed increases in the numbers of circulating monocytes and neutrophils (Fig. 5A, diet effect). Despite similar body weights as HFHSC diet-fed BMT-WT mice, blood counting indicated that HFHSC diet-fed BMT-ABCA1−/− mice further increased the number of peripheral leukocytes, monocyte counts, neutrophils, and eosinophils, but had similar lymphocytes (Fig. 5A). Flow cytometry analysis indicated that there was a significant increase in blood myeloid cells with a balanced increase in the “inflammatory” Ly6Chi and “patrolling” Ly6Clo monocyte subsets in the HFHSC diet-fed BMT-ABCA1−/− mice compared with HFHSC diet-fed BMT-WT mice (Fig. 5B–E).
Fig. 5.
Hematopoietic ABCA1 deletion led to leukocytosis and monocytosis with HFHSC diet feeding. Analysis of peripheral blood by hematology cell counter and flow cytometry of BMT-WT and BMT-ABCA1−/− mice fed a chow or HFHSC diet for 21 weeks. A: White blood cell counts. MO, monocytes; NE, neutrophils; EO, eosinophils; LY, lymphocytes. B: Flow cytometry gating strategy for blood monocytes and subsets and representative histograms. C: Total monocytes were defined as CD115hi, divided into Ly6clo and Ly6chi subsets and quantified. D: Representative histogram of myeloid cells in peripheral blood. E: Myeloid cells were defined as CD11bhi and Ly6chi and quantified in peripheral blood. Results are means ± SD of five mice per group. *P < 0.05 versus BMT-WT; #P < 0.05 HFHSC diet effect.
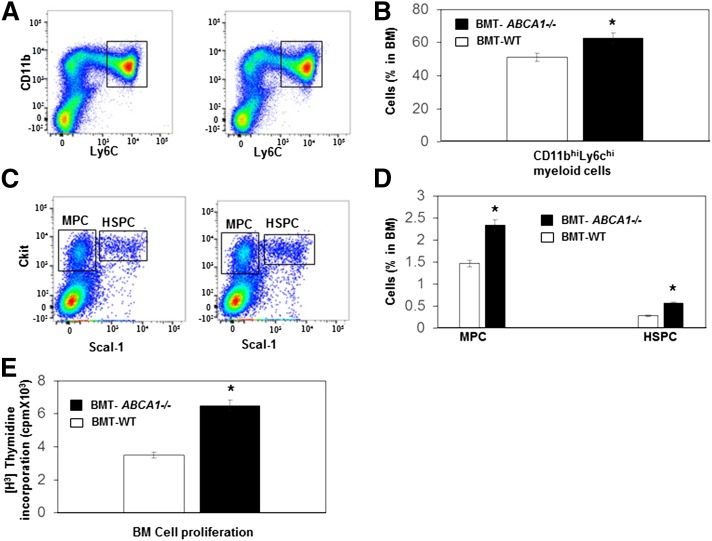
The phenotype of the HFHSC diet-fed BMT-ABCA1−/− mice suggested a myeloproliferative disorder phenotype, thus we quantified BM hematopoietic stem cells and HSPCs and other MPCs in those mice. Importantly, both myeloid (Fig. 6A, B) and MPCs (Fig. 6C, D) were significantly increased in HFHSC diet-fed BMT-ABCA1−/− mice as compared with HFHSC diet-fed BMT-WT mice. Moreover, the lin−Sca+cKit+ population representing HSPCs showed a 2-fold increase in HFHSC diet-fed BMT-ABCA1−/− mice (Fig. 6C, D). We further performed an in vitro BM proliferation assay and found that HFHSC diet-fed BMT-ABCA1−/− progenitors displayed significantly increased proliferation compared with BMT-WT progenitors in response to IL-3/GM-CSF (Fig. 6E). These findings suggest that increased proliferation and expansion of HSPCs may be responsible for the monocytosis and neutrophilia that develops in the BMT-ABCA1−/− mice fed the HFHSC diet.
Fig. 6.
Hematopoietic ABCA1 deletion led to myeloid hyperplasia and hyper-HSPC proliferation with HFHSC diet feeding. Analysis of BM cells by flow cytometry of BMT-WT and BMT-ABCA1−/− mice fed the HFHSC diet for 21 weeks. A: Representative histogram of myeloid cell in BM. B: Myeloid cells were defined as CD11bhi and Ly6chi and quantified in BM. C: Representative histogram of MPCs and HSPCs in BM. D: MPCs were identified as Sca1− and ckit+ and HSPCs were identified as Sac1+ and ckit+ and were quantified and shown as percentage of total BM cells. E: BM was isolated from HFHSC diet-fed BMT-WT and BMT-ABCA1−/− mice and incubated with IL-3 and GM-CSF, and proliferation was assessed by [H3]thymidine incorporation. Results are means ± SD of five mice per group. *P < 0.05 versus BMT-WT.
The ABCA1 pathway in macrophages reduces fatty acid-induced pro-inflammatory cytokine expression
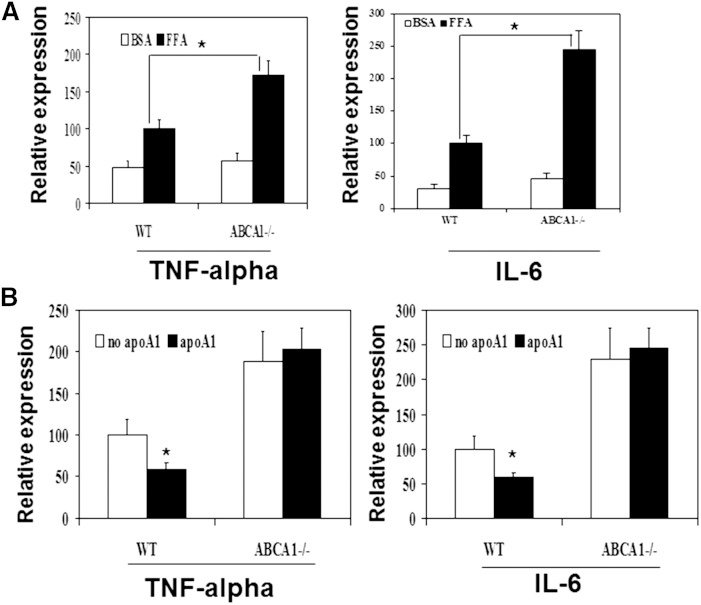
Obesity and type 2 diabetes are characterized by elevated fatty acid levels, and fatty acids can activate inflammatory pathways, which have been suggested to contribute to insulin resistance. We and others have shown that macrophage ABCA1 deficiency increases LPS-induced pro-inflammatory cytokine expression. However, the role of the ABCA1 pathway in fatty acid-induced inflammation in macrophages is unknown. Therefore, we investigated whether ABCA1 deficiency in macrophages alters fatty acid-induced pro-inflammatory cytokine expression, which may in turn influence insulin resistance. Peritoneal macrophages from ABCA1−/− and WT mice were cholesterol loaded by acetylated LDL treatment and then incubated for 24 h with free fatty acids (250 μM palmitate or mixed fatty acids). The expression levels of inflammatory cytokines were measured by real-time quantitative PCR. As showed in Fig. 7A, ABCA1 deficiency significantly increased the fatty acid-induced expression of TNF-α and IL-6.
Fig. 7.
Macrophage ABCA1 pathway protected against fatty acid-induced pro-inflammatory cytokine expression. Thioglycollate-elicited peritoneal macrophages from ABCA1−/− mice and their WT littermates were collected, cultured, and cholesterol loaded. A: Cells were then incubated without (BSA) or with fatty acids (FFA; 250 μM palmitate) overnight. B: Cells were pretreated without (no apoA-I) or with 10 μg/ml apoA-I (apoA-I) and then incubated with fatty acids (FFA; 250 μM palmitate) overnight. Gene expressions were then determined using real-time quantitative PCR. Experiments were repeated at least three times. *P < 0.05 WT versus ABCA1−/−.
To further test whether stimulating the ABCA1 pathway attenuates fatty acid-induced pro-inflammatory cytokine expression, we pretreated cholesterol-loaded peritoneal macrophages with apoA-I for 3 h, washed the cells, and then incubated the cells with free fatty acids for 24 h. As shown in Fig. 7B, pretreating cells with apoA-I, which activates ABCA1-mediated cholesterol efflux and the anti-inflammatory pathways, significantly attenuated fatty acid-induced TNF-α and II-6 expression in WT but not in ABCA1−/− cells. These findings strongly suggest that activation of the ABCA1 pathway can significantly reduce fatty acid-induced macrophage inflammation.
DISCUSSION
We demonstrate that deletion of ABCA1 in hematopoietic cells worsens diet-induced hyperglycemia and insulin resistance. Importantly, the worsened insulin action in adipose tissue and liver of those mice occurs in conjunction with increased macrophage accumulation and enhanced inflammatory cytokine expression in these tissues. In addition, deletion of ABCA1 in hematopoietic cells results in significantly higher hematopoietic stem cell proliferation, myeloid cell expansion, and monocytosis when challenged with the HFHSC diet. We further demonstrate that ABCA1 deficiency increases free fatty acid-induced pro-inflammatory gene expression in macrophages. Our findings are consistent with the important roles of macrophage inflammation and monocytosis in promoting insulin resistance in diet-induced obesity (4, 29, 30). Our results suggest that hematopoietic ABCA1 is an important regulator of monocytosis and macrophage accumulation in diet-induced obesity.
Macrophages play a central role in diet-induced obesity and subsequent development of systemic insulin resistance. Adipose tissue macrophages from lean mice express many genes characteristic of alternatively activated macrophages that may protect adipose tissue from inflammation; whereas, diet-induced obesity leads to a shift in the activation state to a pro-inflammatory state contributing to insulin resistance (3, 30, 31). There is also a strong association between obesity and leukocytosis, particularly of the myeloid lineage (6, 28). Pioneering studies by others (4) have shown that MPC proliferation and monocytosis drive macrophage accumulation and insulin resistance in obesity through a feed-forward mechanism, such that inflamed tissue macrophages stimulate the production of more monocytes, leading to an exacerbation of inflammation and associated disease processes (4). Our results are consistent with this feed-forward mechanism, based on a model in which deficiency of ABCA1 in macrophages increases tissue macrophage inflammation, likely by increasing free-fatty acid-induced inflammatory responses. This induces proliferation of the hyper-responsive BM hematopoietic progenitor cells due to ABCA1 deficiency in those cells, ultimately resulting in monocytosis, more macrophage accumulation, and insulin resistance. Consistent with this model, we and others have shown that deletion of ABCA1 results in an increased macrophage inflammatory response to LPS (7, 8, 24) and free fatty acids (Fig. 7). Human studies also suggest an association between ABCA1 expression and chronic inflammation (32). Further, it has been shown that hematopoietic cell-specific deletion of ABCA1 promotes hematopoietic stem cell proliferation and monocytosis (18, 23). Taken together, these studies suggest that hematopoietic ABCA1 deficiency may act as an enhancer of the feed-forward mechanism that promotes diet-induced insulin resistance. We are currently studying to determine whether increasing hematopoietic ABCA1 will weaken this malicious feed-forward mechanism and reduce diet-induced insulin resistance.
ABCA1−/−-BM-derived macrophage mice demonstrated increased macrophage infiltration, impairment in glucose tolerance, and increased insulin resistance on a HFHSC diet. This suggests that hematopoietic ABCA1 is crucial in preventing macrophage accumulation and insulin resistance in response to dietary fat and cholesterol. Our observations also indicate that loss of hematopoietic ABCA1 and the subsequent increase in macrophage accumulation in the liver and adipose tissue are sufficient to influence whole-body glucose tolerance. However, in contrast to our study, a study by Zhu et al. (33) found no change in insulin sensitivity in mice lacking macrophage ABCA1, using the myeloid-specific M lysozyme promoter. Our BM transplantation studies involve the depletion of ABCA1 in all hematopoietic cells. In contrast, the myeloid-specific M lysozyme promoter used to drive Cre expression is restricted to macrophages and neutrophils (33). This suggests that the increased inflammation and decreased insulin sensitivity observed in mice receiving ABCA1-null BM is attributable to loss of ABCA1 in other cell types or to loss of ABCA1 in cells in addition to macrophages. We did not observe any change in lymphocytes; instead, we found significantly higher hematopoietic stem cell proliferation, myeloid cell expansion, and monocytosis. These data suggest that deletion of ABCA1 in mature myeloid cells or macrophages is not sufficient, and that ABCA1 deletion in hematopoietic progenitor cells is required to promote diet-induced insulin resistance. Interestingly, the same divergent results were observed in studies of atherosclerosis between BM transplantation and myeloid-specific M lysozyme promoter-driven mice. There was no change in atherosclerosis in mice using myeloid-specific M lysozyme promoter, while BM transplantation showed increased atherosclerosis in lethally irradiated recipients of ABCA1-deficient BM (18, 19, 34, 35). These studies indicate a critical role for hematopoietic progenitor cell ABCA1 and its associated monocytosis in atherogenesis as well as insulin resistance.
In summary, our results demonstrate that knockout of ABCA1 in hematopoietic cells appears to have no apparent impact on adipose and liver macrophage inflammation in lean mice. However, when mice are made obese and there is a dramatic need to buffer against lipids, ABCA1 is very important for controlling monocytosis, adipose and liver macrophage inflammation, and, in turn, insulin sensitivity. Thus, ABCA1 provides an important line of defense to prevent the deleterious effects of nutrient overload during obesity. This raises the exciting possibility of raising ABCA1 in hematopoietic-derived cells as a potential target for the therapeutic treatment of insulin resistance as well as atherosclerosis.
Acknowledgments
The authors are grateful for the excellent insights and critical discussions of this manuscript by Dr. Alan Chait (Department of Medicine, University of Washington).
Footnotes
Abbreviations:
- BM
- bone marrow
- BMT
- bone marrow transplanted
- CSF
- stem cell factor
- FPLC
- fast-phase LC
- HFHSC
- high-fat high-carbohydrate diabetogenic diet with added cholesterol
- HSPC
- multipotential progenitor cell
- IL
- interleukin
- LPS
- lipopolysaccharide
- MPC
- myeloid progenitor cell
- SVC
- stromal vascular fraction
This work was supported by the National Institutes of Health Grant HL055362 (R.C.L., C.T.), National Institutes of Health/National Heart, Lung, and Blood Institute Grant HL121214 (C.T.), American Heart Association Grant SDG3860011 (C.T.), and the Diabetes Research Center Pilot and Feasibility Award (P30 DK17047; C.T.). The authors declare no financial conflicts of interest. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hotamisligil G. S., Shargill N. S., and Spiegelman B. M.. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 2.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., et al. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., and Ferrante A. W. Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagareddy P. R., Kraakman M., Masters S. L., Stirzaker R. A., Gorman D. J., Grant R. W., Dragoljevic D., Hong E. S., Abdel-Latif A., Smyth S. S., et al. 2014. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 19: 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luft V. C., Schmidt M. I., Pankow J. S., Couper D., Ballantyne C. M., Young J. H., and Duncan B. B.. 2013. Chronic inflammation role in the obesity-diabetes association: a case-cohort study. Diabetol. Metab. Syndr. 5: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohshita K., Yamane K., Hanafusa M., Mori H., Mito K., Okubo M., Hara H., and Kohno N.. 2004. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care. 27: 491–496. [DOI] [PubMed] [Google Scholar]
- 7.Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., and Tall A. R.. 2008. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 118: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X., Lee J. Y., Timmins J. M., Brown J. M., Boudyguina E., Mulya A., Gebre A. K., Willingham M. C., Hiltbold E. M., Mishra N., et al. 2008. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283: 22930–22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian S., Han C. Y., Chiba T., McMillen T. S., Wang S. A., Haw A. 3rd, Kirk E. A., O’Brien K. D., and Chait A.. 2008. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bie J., Zhao B., Song J., and Ghosh S.. 2010. Improved insulin sensitivity in high fat- and high cholesterol-fed Ldlr-/- mice with macrophage-specific transgenic expression of cholesteryl ester hydrolase: role of macrophage inflammation and infiltration into adipose tissue. J. Biol. Chem. 285: 13630–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B., Song J., Chow W. N., St. Clair R. W., Rudel L. L., and Ghosh S.. 2007. Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr mice. J. Clin. Invest. 117: 2983–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy A. J., Dragoljevic D., and Tall A. R.. 2014. Cholesterol efflux pathways regulate myelopoiesis: a potential link to altered macrophage function in atherosclerosis. Front. Immunol. 5: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yvan-Charvet L., Pagler T., Gautier E. L., Avagyan S., Siry R. L., Han S., Welch C. L., Wang N., Randolph G. J., Snoeck H. W., et al. 2010. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 328: 1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oram J. F. 2002. ATP-binding cassette transporter A1 and cholesterol trafficking. Curr. Opin. Lipidol. 13: 373–381. [DOI] [PubMed] [Google Scholar]
- 15.Oram J. F. 2008. The ins and outs of ABCA. J. Lipid Res. 49: 1150–1151. [DOI] [PubMed] [Google Scholar]
- 16.Oram J. F., and Heinecke J. W.. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85: 1343–1372. [DOI] [PubMed] [Google Scholar]
- 17.Oram J. F., and Vaughan A. M.. 2006. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 99: 1031–1043. [DOI] [PubMed] [Google Scholar]
- 18.van Eck M., Bos I. S., Kaminski W. E., Orso E., Rothe G., Twisk J., Bottcher A., Van Amersfoort E. S., Christiansen-Weber T. A., Fung-Leung W. P., et al. 2002. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. USA. 99: 6298–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunham L. R., Singaraja R. R., Duong M., Timmins J. M., Fievet C., Bissada N., Kang M. H., Samra A., Fruchart J. C., McManus B., et al. 2009. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29: 548–554. [DOI] [PubMed] [Google Scholar]
- 20.Patel D. C., Albrecht C., Pavitt D., Paul V., Pourreyron C., Newman S. P., Godsland I. F., Valabhji J., and Johnston D. G.. 2011. Type 2 diabetes is associated with reduced ATP-binding cassette transporter A1 gene expression, protein and function. PLoS One. 6: e22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht C., Simon-Vermot I., Elliott J. I., Higgins C. F., Johnston D. G., and Valabhji J.. 2004. Leukocyte ABCA1 gene expression is associated with fasting glucose concentration in normoglycemic men. Metabolism. 53: 17–21. [DOI] [PubMed] [Google Scholar]
- 22.Brunham L. R., Kruit J. K., Pape T. D., Timmins J. M., Reuwer A. Q., Vasanji Z., Marsh B. J., Rodrigues B., Johnson J. D., Parks J. S., et al. 2007. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13: 340–347. [DOI] [PubMed] [Google Scholar]
- 23.Yvan-Charvet L., Ranalletta M., Wang N., Han S., Terasaka N., Li R., Welch C., and Tall A. R.. 2007. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 117: 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang C., Liu Y., Kessler P. S., Vaughan A. M., and Oram J. F.. 2009. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J. Biol. Chem. 284: 32336–32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeish J., Aiello R. J., Guyot D., Turi T., Gabel C., Aldinger C., Hoppe K. L., Roach M. L., Royer L. J., de Wet J., et al. 2000. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA. 97: 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang C., and Oram J. F.. 2009. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim. Biophys. Acta. 1791: 563–572. [DOI] [PubMed] [Google Scholar]
- 27.Osborn O., and Olefsky J. M.. 2012. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18: 363–374. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt M. I., Duncan B. B., Sharrett A. R., Lindberg G., Savage P. J., Offenbacher S., Azambuja M. I., Tracy R. P., and Heiss G.. 1999. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 353: 1649–1652. [DOI] [PubMed] [Google Scholar]
- 29.Edgel K. A., McMillen T. S., Wei H., Pamir N., Houston B. A., Caldwell M. T., Mai P. O., Oram J. F., Tang C., and Leboeuf R. C.. 2012. Obesity and weight loss result in increased adipose tissue ABCG1 expression in db/db mice. Biochim. Biophys. Acta. 1821: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumeng C. N., Bodzin J. L., and Saltiel A. R.. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng C. N., Deyoung S. M., Bodzin J. L., and Saltiel A. R.. 2007. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 56: 16–23. [DOI] [PubMed] [Google Scholar]
- 32.Bochem A. E., van der Valk F. M., Tolani S., Stroes E. S., Westerterp M., and Tall A. R.. 2015. Increased systemic and plaque inflammation in ABCA1 mutation carriers with attenuation by statins. Arterioscler. Thromb. Vasc. Biol. 35: 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X., Chung S., Bi X., Chuang C. C., Brown A. L., Liu M., Seo J., Cuffe H., Gebre A. K., Boudyguina E., et al. 2013. Myeloid cell-specific ABCA1 deletion does not worsen insulin resistance in HF diet-induced or genetically obese mouse models. J. Lipid Res. 54: 2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aiello R. J., Brees D., Bourassa P. A., Royer L., Lindsey S., Coskran T., Haghpassand M., and Francone O. L.. 2002. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vasc. Biol. 22: 630–637. [DOI] [PubMed] [Google Scholar]
- 35.Bi X., Zhu X., Gao C., Shewale S., Cao Q., Liu M., Boudyguina E., Gebre A. K., Wilson M. D., Brown A. L., et al. 2014. Myeloid cell-specific ATP-binding cassette transporter A1 deletion has minimal impact on atherogenesis in atherogenic diet-fed low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 34: 1888–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]