Abstract
The ectonucleotide pyrophosphatase/phosphodiesterase type 2, more commonly known as autotaxin (ATX), is an ecto-lysophospholipase D encoded by the human ENNP2 gene. ATX is expressed in multiple tissues and participates in numerous key physiologic and pathologic processes, including neural development, obesity, inflammation, and oncogenesis, through the generation of the bioactive lipid, lysophosphatidic acid. Overwhelming evidence indicates that altered ATX activity leads to oncogenesis and cancer progression through the modulation of multiple hallmarks of cancer pathobiology. Here, we review the structural and catalytic characteristics of the ectoenzyme, how its expression and maturation processes are regulated, and how the systemic integration of its pleomorphic effects on cells and tissues may contribute to cancer initiation, progression, and therapy. Additionally, the up-to-date spectrum of the most frequent ATX genomic alterations from The Cancer Genome Atlas project is reported for a subset of cancers.
Keywords: metabolism, inflammation, The Cancer Genome Atlas
AUTOTAXIN ISOFORMS: THE TIMELINE OF A DISCOVERY
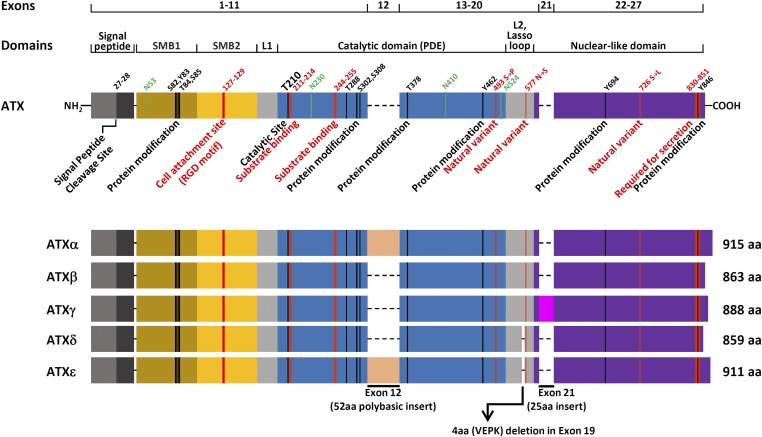
Autotaxin (ATX) is an ectonucleotide pyrophosphatase/phosphodiesterase (ENPP) encoded by the ENNP2 gene, which occupies a 116 kbp-long DNA segment of human chromosome 8. Five different alternatively spliced isoforms of the gene product have been identified (Fig. 1). In 1992, the first alternatively spliced isoform was cloned from the melanoma cell line, A2058, and characterized as a 125 kDa glycoprotein composed of 915 amino acids (1). Because it promoted chemotaxis on melanoma cells in an autocrine fashion, the protein was aptly named auto-taxin. Four years after the discovery of the first variant, now commonly referred to as ATXα, or melanoma ATX, a second isoform was cloned by the same team from the teratocarcinoma cell line, Ntera2D1. This polypeptide shared 94% identity with the melanoma protein and was immediately recognized as the alternatively spliced product of the same gene (2).
Fig. 1.
The major structural differences between ATX isoforms results from a four amino acid (VEPK) deletion in exon 19, and the alternative splicing of exons 12 and 21. Total length of each isoform and key structural features are reported. Putative functional region and natural variants are highlighted in red. Key asparagine residues are highlighted in green. Exon 12 encodes a 52 amino acid-long polybasic cleavable insert that mediates ATX recruitment to the plasma membrane through the interaction with heparan sulfate proteoglycans (11). Crystal structure representation of ATX domains and spatial relationship between functional elements are presented and discussed in great detail in several reviews and research articles (15, 16, 35, 179, 180)
The initial characterization of the first isoform revealed that ATX biological activity was sensitive to pertussis toxin treatment. Furthermore, not only did the polypeptide share close homology with the murine pyrophosphatase/type I phosphodiesterase (PDE) PC-1, including a threonine residue crucial for PDE enzymatic activity, but it was also able to hydrolyze PDE substrates in vitro (3). The confirmation that ATX was an enzyme came when a new PDE, the PDE1/nucleotide pyrophosphatase (PD-1α/PDNP 2), was cloned from a cDNA library of human retina and found to be identical to the sequence of melanoma ATXα with the exception of a missing stretch of 52 amino acids encoded by exon 12 in the central region of the open reading frame. The transcript of this variant, now frequently referred to as ATXβ, or teratoma ATX, produced a 863 amino acid polypeptide chain with a mass of 99,034 Da (4), and was independently isolated a few years later in mouse tissues (5). The third ATX isoform was detected for the first time in rat brain, but was originally designated as PD-I α, a brain-specific PDE I/nucleotide pyrophosphatase (6). Further research showed that PD-I α was identical to ATXβ teratoma protein, except for the presence of an additional stretch of 25 amino acids encoded by an alternatively spliced exon located at the 3′ end of the mRNA transcript (7). This isoform is commonly referred to as ATXγ. Recently described fourth and fifth transcript variants named ATXδ and ATXε are identical to the ATXβ isoform except for the excision of four amino acids within the L2 linker region of both isoforms and the presence of the 52 amino acid insertion in the PDE domain of ATXε (8, 9) (Fig. 1). The functional consequence of the four amino acid excision in ATXδ remains unclear, but it was reported that this variant was the only ENPP2 gene product detected in some species, suggesting that the isoform could have been selected due to a potential functional advantage. Although no specific differences in the core mechanism of catalysis or physiological activities between the five ATX variants have yet been reported, differences in enzymatic parameters, tissue expression levels, and isoform stability have been documented (7). For instance, ATXβ is the most abundant isoform found in plasma (10), whereas ATXα is unstable and less common in peripheral tissues due to a long polybasic cleavable insert that mediates ATX recruitment to the cell membrane through the interaction with heparan sulfate proteoglycans (7, 11). The identity and functional significance of factors regulating alternative splicing of exons 12 and 21, as well as the deletion of the VEPK peptide in the lasso loop, remain to be determined.
STRUCTURE AND ENZYMATIC ACTIVITY
ATX belongs to the nucleotide pyrophosphatase/PDE (NPP) family, a group of enzymes that share sequence homology with the catalytic site of bovine intestinal alkaline PDE [reviewed in (12–14)]. It contains a short transmembrane domain that separates the small N-terminus from the bulkier region of the polypeptide (Fig. 1). The short N-terminus interacts with the membrane to help regulate release of ATX into the extracellular space (see Transcriptional Control and Secretion below). The bulkier portion of the enzyme is composed of a central PDE catalytic domain and a catalytically inactive nuclease-like domain. An EF hand-like motif is positioned at the center of the nuclease-like domain, whereas two somatomedin-B (SMB)-like domains (SMB1 and SMB2) are located at the most distal region of the C terminus. SMBs are short cysteine-rich peptide sequences that share high structural homology with the SMB domain of vitronectin (15, 16). The two linker regions, L1 and L2 (lasso loop), connect the PDE domain to the SMB2 domain at the N-terminal end of the chain and to the nuclease-like domain at the C-terminal end.
In vitro substrate specificity of the ATX catalytic core is broad. ATX hydrolyzes phosphodiester bonds at the 5′-end of various oligonucleotide substrates, acts as an ATPase/GTPase by catalyzing bilateral hydrolysis of either ATP or GTP 5′ β-phosphates, hydrolyzes the AMP group from the NAD molecule, and functions as an ATP pyrophosphatase by removing the entire pyrophosphate group from nucleoside phosphate molecules (3). The threonine residue (T210) in the middle of the PDE catalytic domain serves as a point of transient binding of reaction intermediates (17) and provides a fulcrum for catalytic activity (18). All of the enzymatic activities of ATX are dependent on the concentration of divalent cations (19).
Ten years after its initial discovery, ATX was purified from human plasma and shown to have lysophospholipase D (lysoPLD) enzymatic activity, catalyzing the hydrolysis of the distal phosphoester bond that connects monoacylglycerol phosphates to the polar head group of lysophospholipids (10, 20). In this respect ATX is an atypical member of the NPP family, as none of the other paralogs efficiently hydrolyze lysophospholipids. A particularly low Michaelis constant for lysophosphatidylcholine (LPC), a lysolipid present at high concentrations in plasma and other tissues, suggests that the primary physiological function of ATX in the body is to promote the conversion of LPC to lysophosphatidic acid (LPA), a bioactive lipid involved in multiple key physiological and pathological processes (21–25). Madan et al. (26) have shown that it is possible to directly assess lysoPLD activity in vivo by measuring the enzymatic turnover of a fluorogenic LPC-like substrate in breast cancer xenografts with high ATX expression.
Numerous studies have defined several key biochemical and structural features responsible for stability, rate of catalysis, and substrate specificity of ATX. For instance, regions located in close proximity of the catalytic cleft (27) or at the edges of the polypeptide chain (28) were recognized as being critical for both substrate docking and the dynamic interplay between divalent cations and T210 during catalysis. The nuclease-like domain of ATX helps to stabilize the catalytic reaction via direct calcium binding and through formation of disulfide bridges with the PDE domain (29). Although closely related to nonspecific endonucleases, a class of enzymes that cleave internal phosphodiester bonds of polynucleotide chains, the nuclease-like domain of ATX does not contain any of the key residues involved in the catalytic reaction and, instead, appears to be important for protein stabilization and membrane targeting of ATX and other members of the NPP family (30).
Early studies showed that proteins with high carbohydrate-binding affinity bind ATX and that N-glycosidase treatment induces a shift of ATX molecular mass on SDS-PAGE, indicating the presence of glycosylated posttranslational modifications at asparagine residues (31). The carbohydrate moiety is important in secretion (see Transcriptional Control and Secretion below) and activity of the enzyme (32). Among various aminoglycoside chains, N-acetylglucosamine mannose (Man8/9GlcNAc2), a sugar moiety rather uncommon in eukaryotes, and N-acetylglucosamine (GlcNAc2) attached to asparagine residues 524 and 53, respectively, are critical for optimal enzyme function (15, 33). Molecular dynamic simulations of folding trajectories of engineered ATX lacking either the nuclease-like domain or the glycans suggested that these structural elements are essential in maintaining the T210 residue in the correct position (34).
The crystallization and extensive analysis of rat and mouse ATX, which exhibits a high degree of sequence homology with the human protein, provided the first clear glimpse into the mechanism of substrate recognition and catalysis of the enzyme (15, 16, 35). These studies showed that numerous disulfide and hydrogen bonds cooperate to bring the SMB-like motifs, the central PDE catalytic core, and nuclease-like domain together to form a lipophilic pocket adjacent to the catalytic site. It is believed that the size of the lipophilic pocket underlies the key difference between ATX and the other NPP members in terms of substrate specificity, whereby the ATX binding pocket appears to selectively accommodate fatty acid moieties of lysolipid substrates.
SMB-like domains in ATX do not appear to mediate homodimerization, as previously reported for other NPP family members (30); but rather, they preserve the characteristic disulfide-bonded knot folds typical of these structures (15). In addition, SMB2 contains an RGD motif, a tripeptide (Arg-Gly-Asp) with high binding affinity for plasminogen activator inhibitor-1 (36, 37), and integrins (38). Because SMB-like domains are in close proximity to a hydrophobic channel connecting the catalytic site to the external environment, they are believed to act as a bridge between ATX and the extracellular space where enzymatic products can directly interact with their cognate receptors (16). This view is supported by studies in platelets and mammalian cells showing that both SMB-like domains and integrins are required for the interaction of ATX with platelets, and that integrin activation increases ATX-dependent LPA production (39). The wealth of information available on both enzymatic dynamics and ATX structure is helping to establish new operative frameworks for inhibitor design which will hopefully lead to the synthesis of potent and selective molecules capable of effectively inhibiting ATX function in the context of cancer and other pathologies where its activity is dysregulated (see Role in Physiology and Cancer Pathophysiology below).
TRANSCRIPTIONAL CONTROL AND SECRETION
The regulatory mechanism(s) that controls ENPP2 gene expression has not been extensively studied. Kawagoe et al. (40) pinpointed a region of the ATX promoter located 254 nucleotides upstream of the start codon capable of binding transcription factors. These studies have shown that the ENPP2 gene is devoid of typical TATA or CAAT motifs at the 5′ region but contains putative binding sites for at least four transcription factors, including Max1, HNF-3B, AP, and Sp1, and a probable octamer binding locus in intron 2. In addition, studies in neuroblastoma cells showed that AP-1 and Sp3 transcription factors control ATX expression by binding to CRE/AP-1-like and GAbox elements at the upstream regulatory regions (41). A more recent study suggests that ATX gene transcription may be upregulated by c-Jun activity in dedifferentiated soft tissue liposarcomas, an event which has been associated with increased disease aggressiveness (42). In addition, the ATX gene promoter contains two consensus binding sites for nuclear factor of activated T-cells (NFAT) proteins (43), a family of transcription factors implicated in immune response and metastasis (44–47). Braeuer et al. (48) reported that inhibition of galectin-3 (LGALS3), a β-galactoside binding protein involved in metastatic spread (49), represses ATX expression in human melanoma cells in an NFATC2-dependent manner. NFATC1 controls cell motility by regulating ATX expression in breast cancer cells in response to the activation of aryl hydrocarbon receptor (AhR) (50) and α6β4 integrins (43). Additionally, recent work from Benesch et al. (51) suggests that both LPA and S1P inhibit ENPP2 gene expression via a PI3K-dependent inhibitory feedback loop and that treatment with pro-inflammatory cytokines rescues this inhibition.
Currently, the process that leads to ATX secretion is considerably better understood than the dynamics of its expression. Sequence similarity between ATX and NPP1, the closest related member of the NPP family, led to the initial idea that ATX is a type II transmembrane protein firmly anchored at the plasma membrane from which it is released into the extracellular space via proteolytic cleavage (52). On the contrary, careful molecular studies revealed that ATX localizes to the Golgi-apparatus and contains both a consensus-sequence for furin-dependent cleavage and a 27-amino acid residue signal peptide at the N-terminus, suggesting that the protein is synthetized as a prepro-enzyme that is proteolytically cleaved in the endomembrane system before secretion (53, 54). Interestingly, current evidence indicates that N-glycosylation and the nuclease-like domain (29) are two important factors mediating ATX secretion (32).
ATX is detected in various biological fluids and tissues, including plasma, placenta, ovary, small intestine (2), platelets (55), adipose tissue (56–59), nervous system (4, 60–63), and cerebrospinal fluid (64). In mice, both conditional gene knockout in adipose tissue and global Enpp2 heterozygosity result in a 50% decrease in ATX levels or activity in plasma (57), indicating that circulating ATX is predominately produced in adipose tissue, and further, that it is the primary enzyme responsible for maintaining circulating LPA levels.
Although it has been shown in mice that ATX can be rapidly eliminated from the circulation through a potential scavenger receptor-mediated process taking place in liver sinusoidal endothelial cells (65), the mechanisms that control ATX clearance remain poorly understood. Nonetheless, the multiorgan origin and complex regulatory dynamics controlling ATX concentration in tissues strongly suggest that aberrant ATX homeostasis may foster conditions that lead to pathological states. As discussed in the next section, there is overwhelming evidence that anomalous ATX activity leads to oncogenesis and cancer progression.
ROLE IN PHYSIOLOGY AND CANCER PATHOPHYSIOLOGY
All crucial residues and structural elements of ATX are conserved between humans and mice (35), which is arguably a testament to the fundamental importance of this gene in mammalian physiology. Although ATX can also act on sphingosylphosphorylcholine to produce sphingosine-1-phosphate, a modulator of cell motility and other biological functions (66), it is broadly accepted that the majority of biological effects are mediated through the production of the bioactive lipid mediator LPA. Although ATX is not the only source of LPA production (67), measurements in mice with either systemic or targeted heterozygous deletion of the Enpp2 gene or transgenic overexpression of the human ENPP2 gene established that ATX is a dominant physiological source of circulating bioactive LPA (68). For this reason, ATX-mediated LPA production and signal transduction through LPA-specific G protein-coupled receptors is commonly referred to as the ATX/LPA-signaling axis (69–71).
The ATX polypeptide chain contains multiple domains that could potentially modulate biological activities independent of enzymatic function. For instance, the structural homology of SMB-like domains with SMB, a short peptide derived from the proteolytic cleavage of vitronectin, suggests that these motifs may be able to regulate cell adhesion and extracellular matrix dynamics similarly to vitronectin (72, 73). In support of this hypothesis, it has been shown that SMB binds to the urokinase receptor and the plasminogen activator inhibitor-1 (36), and that SMB-like-dependent ATX binding to integrins modulates motogenic activity of both cancer and mouse aortic vascular smooth muscle cells (74).
Similarly to morphogens that modulate key aspects of both development and cancer progression as a consequence of spatiotemporal regulation (75), properly regulated expression and activity of ATX is crucial for embryogenesis (76). A number of studies have shown that ATX controls embryonic development by regulating vasculature maturation and angiogenesis. Homozygous deletion of the Enpp2 gene in mice results in embryonic lethality due to defective blood vessel formation and growth (68, 77). The effect is dependent on the integrity of the PDE domain because embryonic lethality is still observed in mice expressing ATX (T210A), a catalytically inactive version of the enzyme generated by substituting an alanine in place of the central threonine (78). It has been shown that ATX regulates both prenatal lymphatic vessel formation in mice and zebrafish (79, 80) and generates directional cues that direct primitive hematopoiesis and hemangioblast formation (81). Importantly, ATX can drive either vessel regression (82) or maturation (80) depending on the experimental context. It is likely that compartmentalization of ATX and its lipid substrates greatly influences biological function and activity because it generates high concentrations of lipid substrate in proximity to the membrane. For example, localized LPA production in plasma lipoproteins, nerves, or tissue stroma has been implicated in driving the progression of atherosclerosis (83), neuropathic pain (84–86), and T cell homing (87), respectively. In contrast to ATX, there is no evidence indicating that lipid phosphate phosphatases, the main enzymes mediating LPA degradation (88–91), have an equivalent mechanism for local action through binding to integrins or other extracellular receptors. Like other factors involved in development, fine spatiotemporal regulation of ATX expression and activity is essential not only for proper vasculogenesis but also for other key biological functions. For instance, the effects of ATX on development arise from direct regulation of mesenchymal cell migration. Ryu and Han (92) have shown that the PKC/GSK3β/β-catenin and PKC/Rho GTPase pathways are activated by the ATX/LPA axis through LPA receptor signaling, which is also required for proper nervous system growth (93) and craniofacial morphogenesis (94).
Initially cloned from melanoma and teratocarcinoma cell lines and characterized as a potent pro-migratory factor, ATX was immediately recognized as a key player in cancer pathophysiology. The first report showing ATX upregulation in a primary human cancer was published 5 years after the discovery of the gene (40). In this study, tissue samples from neuroblastoma patients showed variable expression of both ATXα and ATXβ isoforms. Since then, multiple studies have confirmed that ATX is frequently upregulated in various cancer types, including glioblastoma multiforme (95), non-small cell lung cancer (96), thyroid cancer (97, 98), follicular lymphoma (99), and peritoneal fluids of patients with either ovarian cancer, dermoid cyst, or mucinous cystadenoma (100). With the exception of non-small cell lung cancer, in which ATXβ is the most highly expressed gene variant, the tissue expression patterns of other isoforms remains largely unknown.
In two seminal papers, Robert Weinberg and Douglas Hanahan have outlined the hallmark processes that differentiate normal from dysregulated cellular growth (101, 102). Over the course of two decades of research, a large number of studies have established that in addition to cell-autonomous cancer hallmarks such as differentiation (58, 103–106), survival (107–112), proliferation (58, 113–116), and migration/metastatic behavior (50, 74, 92, 117–122), the aberrant expression/amplification of ATX activity can also dysregulate multiple cancer pathobiology systemic hallmarks including angiogenesis (119, 123), metabolic homeostasis (56–59, 104, 124, 125), and immune system function (126).
Neoplastic diseases often arise at sites of persistent inflammation and several inflammatory conditions are known to predispose to cancer (127, 128). Many studies have established ATX as a key regulator of inflammatory response at multiple levels that is required for proper maintenance of immune system homeostasis (129–131). For example, ATX binding to chemokine-activated lymphocytes is associated with increased immune cell homing to lymphoid vessels, and intravenous injection of enzymatically inactive ATX attenuates this process (132). Similarly, it has been reported that ATX both controls lymphocyte migration across the basal lamina of lymphatic high endothelial venules through a myosin II-dependent mechanism (133), and regulates the rate of in vivo T-cell transendothelial migration through lymph node vessels by localizing at the leading edge of arrested T-cells (121).
ATX activity is implicated in multiple signaling pathways linking inflammation and cancerogenesis (69). Metabolism and adipogenesis studies in mice indicate that ATX and its product, LPA, participate in energy homeostasis and obesity control (56–59, 104, 124, 125), which when dysregulated can lead to inflammation and cancerogenesis. In addition to data showing association of ATX activity with rheumatoid arthritis (134), liver inflammation (135, 136), fibrosis development (137–141), and inflammatory bowel disease (142), it has been proposed that ATX is mechanistically involved in linking both hepatitis C to hepatocellular carcinoma (143) and Epstein-Barr virus positivity to anaplastic large-cell and Hodgkin lymphoma development (107). MMTV-driven transgenic overexpression of either ATX or each of the three major LPA receptors (LPA1, -2, and -3) causes late-onset, invasive, and metastatic breast cancer development in mammary glands of mice (144). High plasma concentrations of MIP-3α and VEGF found in presymptomatic transgenic female mice lend support to the hypothesis that malignant growth in this model could be driven by a proinflammatory environment created by the amplified activity of ATX, an idea in line with previous studies showing that ATX stimulates cytokine production in multiple cell types (145, 146).
ATX hyperactivation not only predisposes to cancerogensis but also contributes to other key aspects of tumor biology, including metastatic progression. A number of studies have demonstrated that ATX activity promotes in vitro motogenic activity in ovarian (119, 147), hepatocellular (148), breast, and melanoma cell lines (118), among others, while pharmacological and genetic inhibition of ATX decreases lung metastasis in a murine orthotopic model of breast cancer (103, 149). Available evidence suggests that metastasis to bone can be promoted by ATX through multiple mechanisms, including regulation of osteoclast differentiation (103), interaction with integrin αVβ3 expressed on the surface of tumor cells (150), and bone resorption through the induction of IL6 and IL8 expression (151).
Further evidence indicates that ATX acts synergistically with other oncogenes to promote tumor progression, aggressiveness, and resistance to chemotherapeutics. Multiple genes involved in drug resistance and cellular stress response are upregulated by ATX/LPA axis activity through the stabilization of nuclear factor (erythroid-derived 2)-like 2, a basic leucine zipper transcription factor that controls the expression of genes involved in oxidative damage protection (152). In addition to synergizing with RAS signaling to promote tumor migration and metastatic dissemination in RAS-transformed cells (153), lysoPLD activity, likely mediated by ATX, has been proposed as one of the contributing factors in developing acquired resistance to the receptor tyrosine kinase inhibitor, sunitinib, in renal cell carcinoma (154) and carboplatin-induced apoptosis in ovarian cancer cells (112). Several known signaling networks implicated in ovarian cancer progression, including the Hippo-YAP axis (155), the amphiregulin/Y-box binding protein-1 positive feed-back loop (156), and EGFR signaling (157), have been shown to be regulated through LPA signaling, likely in coordination with ATX activity.
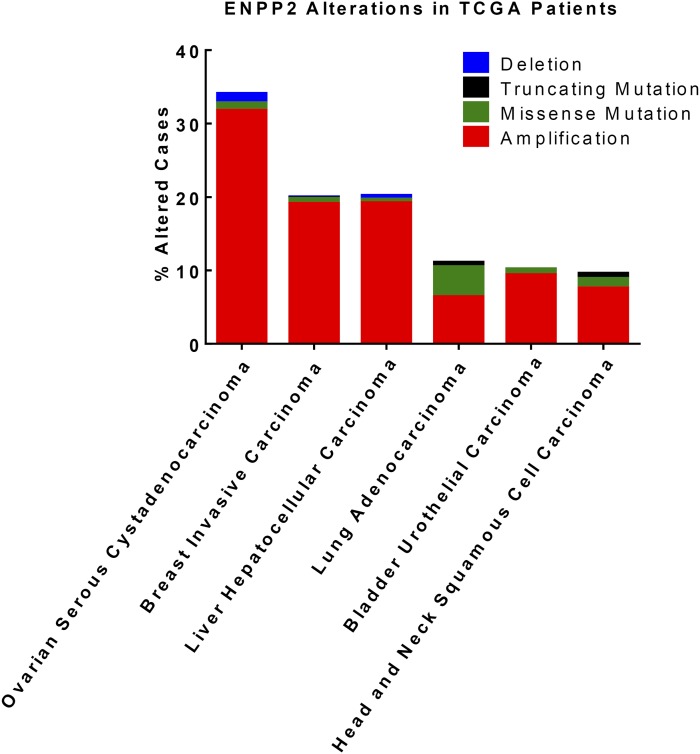
From a clinical standpoint, genomic alterations in ATX have been observed across multiple cancers. Information obtained from the cBio Cancer Genomics Portal shows that copy number alterations and mutations in ATX are present in ovarian serous cystadenocarcinoma (33% altered), breast invasive carcinoma (20% altered), liver hepatocellular carcinoma (20% altered), lung adenocarcinoma (11% altered), bladder urothelial carcinoma (10% altered), and head and neck squamous cell carcinoma (10% altered) patient tumors (Fig. 2) collected and analyzed by The Cancer Genome Atlas project (158–163). Most of these alterations are ENPP2 amplifications, which could potentially underlie dysregulation of ATX/LPA-mediated signaling pathways in these diseases. Mutations are rare and usually nonrecurrent. The greatest frequency of ENPP2 mutations is seen in lung adenocarcinoma (4.7% of all patient tumors have at least one ENPP2 mutation), followed by head and neck squamous cell carcinoma (2%), ovarian serous cystadenocarcinoma (1%), breast invasive carcinoma (0.8%), bladder urothelial carcinoma (0.8%), and liver hepatocellular carcinoma (0.5%). Follow-up studies are warranted to determine whether these mutations induce functional changes that may promote tumor development and growth. Further, additional analysis is needed to determine whether other pathway alterations may serve compensatory roles in the small set of patients with ENPP2 deletions. Together, these results indicate that the ATX/LPA signaling axis may be dysregulated in specific cancer subtypes, and that patients could potentially benefit from targeted therapies blocking ATX/LPA signaling
Fig. 2.
ATX copy number alterations and mutations in a subset of cancers from The Cancer Genome Atlas project database. Information was obtained from the cBio Cancer Genomics Portal using provisional datasets (181).
Due to the pleiotropic connection of ATX in cancer pathology, considerable effort has been made to generate small molecule inhibitors that target its enzymatic activity (120, 164–173). Using sensitive fluorescence probes, such as TG-mTMP, Kawaguchi et al. (174) identified several novel ATX inhibitor scaffolds and solved the crystal structures of ATX-compound complexes at high resolution (1.75–1.95 Å). Docking experiments with molecular isomers and high-throughput screening with compound libraries provided additional details on enzyme-inhibitor interaction mechanisms (167, 175, 176). The availability of more selective, potent, and bioavailable ATX inhibitors will expand our mechanistic understanding of ATX functions in both cellular and preclinical models of cancer. In addition to direct inhibitors of ATX function, multiple pan and selective LPA receptor inhibitors as well as immunoneutralizing antibodies to LPA are available (177). Where and how these will impact human disease either alone or in the context of ATX inhibition remains to be determined. The ultimate goal in the field is to generate a new class of anticancer molecules that either alone or in combination with traditional chemotherapeutics can complement the pharmacological tools available in the clinic and have a positive impact on patients (178).
CONCLUSIONS AND PERSPECTIVES
Overwhelming evidence suggests that anomalous ATX activity contributes to the development of favorable conditions for neoplastic disease initiation and progression. The significance of ATX in mammalian physiology is highlighted by the fact that embryos cannot develop in the absence of ENPP2 and that all key biochemical/structural features required for optimal enzymatic activity remained evolutionarily conserved since human and mouse species diverged from their common ancestor. Although much has been discovered regarding ATX structure and its pathophysiological significance, several questions remain to be answered. For example, how is the steady-state level of both ATX and LPA maintained in plasma and what is the precise role that substrate/enzyme compartmentalization dynamics play in ATX function, especially in the context of stroma-tumor interactions? Further, what are the key physiological functions that the ectoenzyme regulates? It is conceivable that the answers will come from new experimental approaches that investigate the effect of ATX on cancer biology as an emerging feature of complex systems. A mechanistic understanding of ATX enzyme function at a systems biology level will allow for the identification of genomic and proteomic network changes and signatures associated with ATX/LPA axis alteration and activity, ultimately leading to direct translation of findings into potential therapeutic opportunities.
Footnotes
Abbreviations:
- ATX
- autotaxin
- ENPP
- ectonucleotide pyrophosphatase/phosphodiesterase
- LPA
- lysophosphatidic acid
- LPC
- lysophosphatidylcholine
- lysoPLD
- lysophospholipase D
- NFAT
- nuclear factor of activated T-cells
- NPP
- nucleotide pyrophosphatase/phosphodiesterase
- PDE
- phosphodiesterase
- SMB
- somatomedin-B
REFERENCES
- 1.Stracke M. L., Krutzsch H. C., Unsworth E. J., Arestad A., Cioce V., Schiffmann E., and Liotta L. A.. 1992. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267: 2524–2529. [PubMed] [Google Scholar]
- 2.Lee H. Y., Murata J., Clair T., Polymeropoulos M. H., Torres R., Manrow R. E., Liotta L. A., and Stracke M. L.. 1996. Cloning, chromosomal localization, and tissue expression of autotaxin from human teratocarcinoma cells. Biochem. Biophys. Res. Commun. 218: 714–719. [DOI] [PubMed] [Google Scholar]
- 3.Murata J., Lee H. Y., Clair T., Krutzsch H. C., Arestad A. A., Sobel M. E., Liotta L. A., and Stracke M. L.. 1994. cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J. Biol. Chem. 269: 30479–30484. [PubMed] [Google Scholar]
- 4.Kawagoe H., Soma O., Goji J., Nishimura N., Narita M., Inazawa J., Nakamura H., and Sano K.. 1995. Molecular cloning and chromosomal assignment of the human brain-type phosphodiesterase I/nucleotide pyrophosphatase gene (PDNP2). Genomics. 30: 380–384. [DOI] [PubMed] [Google Scholar]
- 5.Piao J. H., Matsuda Y., Nakamura H., and Sano K.. 1999. Assignment of Pdnp2, the gene encoding phosphodiesterase I/nucleotide pyrophosphatase 2, to mouse chromosome 15D2. Cytogenet. Cell Genet. 87: 172–174. [DOI] [PubMed] [Google Scholar]
- 6.Narita M., Goji J., Nakamura H., and Sano K.. 1994. Molecular cloning, expression, and localization of a brain-specific phosphodiesterase I/nucleotide pyrophosphatase (PD-I alpha) from rat brain. J. Biol. Chem. 269: 28235–28242. [PubMed] [Google Scholar]
- 7.Giganti A., Rodriguez M., Fould B., Moulharat N., Coge F., Chomarat P., Galizzi J. P., Valet P., Saulnier-Blache J. S., Boutin J. A., et al. 2008. Murine and human autotaxin alpha, beta, and gamma isoforms: gene organization, tissue distribution, and biochemical characterization. J. Biol. Chem. 283: 7776–7789. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T., Okudaira S., Igarashi K., Hama K., Yatomi Y., and Aoki J.. 2012. Identification and biochemical characterization of a novel autotaxin isoform, ATXdelta, with a four-amino acid deletion. J. Biochem. 151: 89–97. [DOI] [PubMed] [Google Scholar]
- 9.Barbayianni E., Kaffe E., Aidinis V., and Kokotos G.. 2015. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog. Lipid Res. 58: 76–96. [DOI] [PubMed] [Google Scholar]
- 10.Tokumura A., Majima E., Kariya Y., Tominaga K., Kogure K., Yasuda K., and Fukuzawa K.. 2002. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 277: 39436–39442. [DOI] [PubMed] [Google Scholar]
- 11.Houben A. J., van Wijk X. M., van Meeteren L. A., van Zeijl L., van de Westerlo E. M., Hausmann J., Fish A., Perrakis A., van Kuppevelt T. H., and Moolenaar W. H.. 2013. The polybasic insertion in autotaxin alpha confers specific binding to heparin and cell surface heparan sulfate proteoglycans. J. Biol. Chem. 288: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goding J. W., Grobben B., and Slegers H.. 2003. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim. Biophys. Acta. 1638: 1–19. [DOI] [PubMed] [Google Scholar]
- 13.Stefan C., Jansen S., and Bollen M.. 2006. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signal. 2: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefan C., Jansen S., and Bollen M.. 2005. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem. Sci. 30: 542–550. [DOI] [PubMed] [Google Scholar]
- 15.Hausmann J., Kamtekar S., Christodoulou E., Day J. E., Wu T., Fulkerson Z., Albers H. M., van Meeteren L. A., Houben A. J., van Zeijl L., et al. 2011. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 18: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimasu H., Okudaira S., Hama K., Mihara E., Dohmae N., Inoue A., Ishitani R., Takagi J., Aoki J., and Nureki O.. 2011. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat. Struct. Mol. Biol. 18: 205–212. [DOI] [PubMed] [Google Scholar]
- 17.Clair T., Lee H. Y., Liotta L. A., and Stracke M. L.. 1997. Autotaxin is an exoenzyme possessing 5′-nucleotide phosphodiesterase/ATP pyrophosphatase and ATPase activities. J. Biol. Chem. 272: 996–1001. [DOI] [PubMed] [Google Scholar]
- 18.Lee H. Y., Clair T., Mulvaney P. T., Woodhouse E. C., Aznavoorian S., Liotta L. A., and Stracke M. L.. 1996. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J. Biol. Chem. 271: 24408–24412. [DOI] [PubMed] [Google Scholar]
- 19.Lee J., Jung I. D., Nam S. W., Clair T., Jeong E. M., Hong S. Y., Han J. W., Lee H. W., Stracke M. L., and Lee H. Y.. 2001. Enzymatic activation of autotaxin by divalent cations without EF-hand loop region involvement. Biochem. Pharmacol. 62: 219–224. [DOI] [PubMed] [Google Scholar]
- 20.Umezu-Goto M., Kishi Y., Taira A., Hama K., Dohmae N., Takio K., Yamori T., Mills G. B., Inoue K., Aoki J., et al. 2002. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X., Schummer M., Mao M., Yu S., Tabassam F. H., Swaby R., Hasegawa Y., Tanyi J. L., LaPushin R., Eder A., et al. 2002. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim. Biophys. Acta. 1582: 257–264. [DOI] [PubMed] [Google Scholar]
- 22.Mills G. B., and Moolenaar W. H.. 2003. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 3: 582–591. [DOI] [PubMed] [Google Scholar]
- 23.Murph M., and Mills G. B.. 2007. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev. Mol. Med. 9: 1–18. [DOI] [PubMed] [Google Scholar]
- 24.Murph M., Tanaka T., Liu S., and Mills G. B.. 2006. Of spiders and crabs: the emergence of lysophospholipids and their metabolic pathways as targets for therapy in cancer. Clin. Cancer Res. 12: 6598–6602. [DOI] [PubMed] [Google Scholar]
- 25.Panupinthu N., Lee H. Y., and Mills G. B.. 2010. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br. J. Cancer. 102: 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan D., Ferguson C. G., Lee W. Y., Prestwich G. D., and Testa C. A.. 2013. Non-invasive imaging of tumors by monitoring autotaxin activity using an enzyme-activated near-infrared fluorogenic substrate. PLoS ONE. 8: e79065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gijsbers R., Aoki J., Arai H., and Bollen M.. 2003. The hydrolysis of lysophospholipids and nucleotides by autotaxin (NPP2) involves a single catalytic site. FEBS Lett. 538: 60–64. [DOI] [PubMed] [Google Scholar]
- 28.Cimpean A., Stefan C., Gijsbers R., Stalmans W., and Bollen M.. 2004. Substrate-specifying determinants of the nucleotide pyrophosphatases/phosphodiesterases NPP1 and NPP2. Biochem. J. 381: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen S., Andries M., Derua R., Waelkens E., and Bollen M.. 2009. Domain interplay mediated by an essential disulfide linkage is critical for the activity and secretion of the metastasis-promoting enzyme autotaxin. J. Biol. Chem. 284: 14296–14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gijsbers R., Ceulemans H., and Bollen M.. 2003. Functional characterization of the non-catalytic ectodomains of the nucleotide pyrophosphatase/phosphodiesterase NPP1. Biochem. J. 371: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stracke M. L., Arestad A., Levine M., Krutzsch H. C., and Liotta L. A.. 1995. Autotaxin is an N-linked glycoprotein but the sugar moieties are not needed for its stimulation of cellular motility. Melanoma Res. 5: 203–209. [DOI] [PubMed] [Google Scholar]
- 32.Pradère J. P., Tarnus E., Grès S., Valet P., and Saulnier-Blache J. S.. 2007. Secretion and lysophospholipase D activity of autotaxin by adipocytes are controlled by N-glycosylation and signal peptidase. Biochim. Biophys. Acta. 1771: 93–102. [DOI] [PubMed] [Google Scholar]
- 33.Jansen S., Callewaert N., Dewerte I., Andries M., Ceulemans H., and Bollen M.. 2007. An essential oligomannosidic glycan chain in the catalytic domain of autotaxin, a secreted lysophospholipase-D. J. Biol. Chem. 282: 11084–11091. [DOI] [PubMed] [Google Scholar]
- 34.Koyama M., Nishimasu H., Ishitani R., and Nureki O.. 2012. Molecular dynamics simulation of Autotaxin: roles of the nuclease-like domain and the glycan modification. J. Phys. Chem. B. 116: 11798–11808. [DOI] [PubMed] [Google Scholar]
- 35.Tabchy A., Tigyi G., and Mills G. B.. 2011. Location, location, location: a crystal-clear view of autotaxin saturating LPA receptors. Nat. Struct. Mol. Biol. 18: 117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou A. 2007. Functional structure of the somatomedin B domain of vitronectin. Protein Sci. 16: 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou A., Huntington J. A., Pannu N. S., Carrell R. W., and Read R. J.. 2003. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat. Struct. Biol. 10: 541–544. [DOI] [PubMed] [Google Scholar]
- 38.Ruoslahti E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12: 697–715. [DOI] [PubMed] [Google Scholar]
- 39.Fulkerson Z., Wu T., Sunkara M., Kooi C. V., Morris A. J., and Smyth S. S.. 2011. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J. Biol. Chem. 286: 34654–34663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawagoe H., Stracke M. L., Nakamura H., and Sano K.. 1997. Expression and transcriptional regulation of the PD-Ialpha/autotaxin gene in neuroblastoma. Cancer Res. 57: 2516–2521. [PubMed] [Google Scholar]
- 41.Farina A. R., Cappabianca L., Ruggeri P., Di Ianni N., Ragone M., Merolle S., Sano K., Stracke M. L., Horowitz J. M., Gulino A., et al. 2012. Constitutive autotaxin transcription by Nmyc-amplified and non-amplified neuroblastoma cells is regulated by a novel AP-1 and SP-mediated mechanism and abrogated by curcumin. FEBS Lett. 586: 3681–3691. [DOI] [PubMed] [Google Scholar]
- 42.Sioletic S., Czaplinski J., Hu L., Fletcher J. A., Fletcher C. D., Wagner A. J., Loda M., Demetri G. D., Sicinska E. T., and Snyder E. L.. 2014. c-Jun promotes cell migration and drives expression of the motility factor ENPP2 in soft tissue sarcomas. J. Pathol. 234: 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M., and O’Connor K. L.. 2005. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene. 24: 5125–5130. [DOI] [PubMed] [Google Scholar]
- 44.Fric J., Zelante T., Wong A. Y., Mertes A., Yu H. B., and Ricciardi-Castagnoli P.. 2012. NFAT control of innate immunity. Blood. 120: 1380–1389. [DOI] [PubMed] [Google Scholar]
- 45.Pan M. G., Xiong Y., and Chen F.. 2013. NFAT gene family in inflammation and cancer. Curr. Mol. Med. 13: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin J. J., Nag S., Wang W., Zhou J., Zhang W. D., Wang H., and Zhang R.. 2014. NFAT as cancer target: mission possible? Biochim. Biophys. Acta. 1846: 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shou J., Jing J., Xie J., You L., Jing Z., Yao J., Han W., and Pan H.. 2015. Nuclear factor of activated T cells in cancer development and treatment. Cancer Lett. 361: 174–184. [DOI] [PubMed] [Google Scholar]
- 48.Braeuer R. R., Zigler M., Kamiya T., Dobroff A. S., Huang L., Choi W., McConkey D. J., Shoshan E., Mobley A. K., Song R., et al. 2012. Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res. 72: 5757–5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thijssen V. L., Heusschen R., Caers J., and Griffioen A. W.. 2015. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta. 1855: 235–247. [DOI] [PubMed] [Google Scholar]
- 50.Seifert A., Rau S., Kullertz G., Fischer B., and Santos A. N.. 2009. TCDD induces cell migration via NFATc1/ATX-signaling in MCF-7 cells. Toxicol. Lett. 184: 26–32. [DOI] [PubMed] [Google Scholar]
- 51.Benesch M. G., Zhao Y. Y., Curtis J. M., McMullen T. P., and Brindley D. N.. Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine-1-phosphate. J. Lipid Res. Epub ahead of print. April 20, 2015; doi:10.1194/jlr.M057661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moolenaar W. H. 2002. Lysophospholipids in the limelight: autotaxin takes center stage. J. Cell Biol. 158: 197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansen S., Stefan C., Creemers J. W., Waelkens E., Van Eynde A., Stalmans W., and Bollen M.. 2005. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J. Cell Sci. 118: 3081–3089. [DOI] [PubMed] [Google Scholar]
- 54.Koike S., Keino-Masu K., Ohto T., and Masu M.. 2006. The N-terminal hydrophobic sequence of autotaxin (ENPP2) functions as a signal peptide. Genes Cells. 11: 133–142. [DOI] [PubMed] [Google Scholar]
- 55.Pamuklar Z., Federico L., Liu S., Umezu-Goto M., Dong A., Panchatcharam M., Fulkerson Z., Berdyshev E., Natarajan V., Fang X., et al. 2009. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem. 284: 7385–7394. [Erratum. 2009. J. Biol. Chem. 284: 21100.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boucher J., Quilliot D., Praderes J. P., Simon M. F., Gres S., Guigne C., Prevot D., Ferry G., Boutin J. A., Carpene C., et al. 2005. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 48: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dusaulcy R., Rancoule C., Gres S., Wanecq E., Colom A., Guigne C., van Meeteren L. A., Moolenaar W. H., Valet P., and Saulnier-Blache J. S.. 2011. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 52: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferry G., Tellier E., Try A., Gres S., Naime I., Simon M. F., Rodriguez M., Boucher J., Tack I., Gesta S., et al. 2003. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J. Biol. Chem. 278: 18162–18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimura S., Nagasaki M., Okudaira S., Aoki J., Ohmori T., Ohkawa R., Nakamura K., Igarashi K., Yamashita H., Eto K., et al. 2014. ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes. 63: 4154–4164. [DOI] [PubMed] [Google Scholar]
- 60.Savaskan N. E., Rocha L., Kotter M. R., Baer A., Lubec G., van Meeteren L. A., Kishi Y., Aoki J., Moolenaar W. H., Nitsch R., et al. 2007. Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell. Mol. Life Sci. 64: 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohuchi H., Hayashibara Y., Matsuda H., Onoi M., Mitsumori M., Tanaka M., Aoki J., Arai H., and Noji S.. 2007. Diversified expression patterns of autotaxin, a gene for phospholipid-generating enzyme during mouse and chicken development. Dev. Dyn. 236: 1134–1143. [DOI] [PubMed] [Google Scholar]
- 62.Ma L., Uchida H., Nagai J., Inoue M., Aoki J., and Ueda H.. 2010. Evidence for de novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase A2 and autotaxin in nerve injury-induced neuropathic pain. J. Pharmacol. Exp. Ther. 333: 540–546. [DOI] [PubMed] [Google Scholar]
- 63.Dennis J., Nogaroli L., and Fuss B.. 2005. Phosphodiesterase-Ialpha/autotaxin (PD-Ialpha/ATX): a multifunctional protein involved in central nervous system development and disease. J. Neurosci. Res. 82: 737–742. [DOI] [PubMed] [Google Scholar]
- 64.Sato K., Malchinkhuu E., Muraki T., Ishikawa K., Hayashi K., Tosaka M., Mochiduki A., Inoue K., Tomura H., Mogi C., et al. 2005. Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J. Neurochem. 92: 904–914. [DOI] [PubMed] [Google Scholar]
- 65.Jansen S., Andries M., Vekemans K., Vanbilloen H., Verbruggen A., and Bollen M.. 2009. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 284: 216–221. [DOI] [PubMed] [Google Scholar]
- 66.Clair T., Aoki J., Koh E., Bandle R. W., Nam S. W., Ptaszynska M. M., Mills G. B., Schiffmann E., Liotta L. A., and Stracke M. L.. 2003. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 63: 5446–5453. [PubMed] [Google Scholar]
- 67.Aoki J., Inoue A., and Okudaira S.. 2008. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta. 1781: 513–518. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., and Arai H.. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281: 25822–25830. [DOI] [PubMed] [Google Scholar]
- 69.Liu S., Murph M., Panupinthu N., and Mills G. B.. 2009. ATX-LPA receptor axis in inflammation and cancer. Cell Cycle. 8: 3695–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teo K., and Brunton V. G.. 2014. The role and therapeutic potential of the autotaxin-lysophosphatidate signalling axis in breast cancer. Biochem. J. 463: 157–165. [DOI] [PubMed] [Google Scholar]
- 71.Leblanc R., and Peyruchaud O.. 2015. New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp. Cell Res. 333: 183–189. [DOI] [PubMed] [Google Scholar]
- 72.Kamikubo Y., De Guzman R., Kroon G., Curriden S., Neels J. G., Churchill M. J., Dawson P., Oldziej S., Jagielska A., Scheraga H. A., et al. 2004. Disulfide bonding arrangements in active forms of the somatomedin B domain of human vitronectin. Biochemistry. 43: 6519–6534. [DOI] [PubMed] [Google Scholar]
- 73.Felding-Habermann B., and Cheresh D. A.. 1993. Vitronectin and its receptors. Curr. Opin. Cell Biol. 5: 864–868. [DOI] [PubMed] [Google Scholar]
- 74.Wu T., Kooi C. V., Shah P., Charnigo R., Huang C., Smyth S. S., and Morris A. J.. 2014. Integrin-mediated cell surface recruitment of autotaxin promotes persistent directional cell migration. FASEB J. 28: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turing A. M. 1952. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B. 237: 37–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moolenaar W. H., Houben A. J., Lee S. J., and van Meeteren L. A.. 2013. Autotaxin in embryonic development. Biochim. Biophys. Acta. 1831: 13–19. [DOI] [PubMed] [Google Scholar]
- 77.van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., et al. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferry G., Giganti A., Coge F., Bertaux F., Thiam K., and Boutin J. A.. 2007. Functional invalidation of the autotaxin gene by a single amino acid mutation in mouse is lethal. FEBS Lett. 581: 3572–3578. [DOI] [PubMed] [Google Scholar]
- 79.Sumida H., Noguchi K., Kihara Y., Abe M., Yanagida K., Hamano F., Sato S., Tamaki K., Morishita Y., Kano M. R., et al. 2010. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 116: 5060–5070. [DOI] [PubMed] [Google Scholar]
- 80.Yukiura H., Hama K., Nakanaga K., Tanaka M., Asaoka Y., Okudaira S., Arima N., Inoue A., Hashimoto T., Arai H., et al. 2011. Autotaxin regulates vascular development via multiple lysophosphatidic acid (LPA) receptors in zebrafish. J. Biol. Chem. 286: 43972–43983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H., Yue R., Wei B., Gao G., Du J., and Pei G.. 2014. Lysophosphatidic acid acts as a nutrient-derived developmental cue to regulate early hematopoiesis. EMBO J. 33: 1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Im E., Motiejunaite R., Aranda J., Park E. Y., Federico L., Kim T. I., Clair T., Stracke M. L., Smyth S., and Kazlauskas A.. 2010. Phospholipase Cgamma activation drives increased production of autotaxin in endothelial cells and lysophosphatidic acid-dependent regression. Mol. Cell. Biol. 30: 2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siess W., Zangl K. J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., and Aepfelbacher M.. 1999. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA. 96: 6931–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inoue M., Ma L., Aoki J., Chun J., and Ueda H.. 2008. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol. Pain. 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inoue M., Xie W., Matsushita Y., Chun J., Aoki J., and Ueda H.. 2008. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 152: 296–298. [DOI] [PubMed] [Google Scholar]
- 86.Inoue M., Rashid M. H., Fujita R., Contos J. J., Chun J., and Ueda H.. 2004. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 10: 712–718. [DOI] [PubMed] [Google Scholar]
- 87.Katakai T., Kondo N., Ueda Y., and Kinashi T.. 2014. Autotaxin produced by stromal cells promotes LFA-1-independent and Rho-dependent interstitial T cell motility in the lymph node paracortex. J. Immunol. 193: 617–626. [DOI] [PubMed] [Google Scholar]
- 88.Morris A. J., and Smyth S. S.. 2014. Lipid phosphate phosphatases: more than one way to put the brakes on LPA signaling? J. Lipid Res. 55: 2195–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smyth S. S., Sciorra V. A., Sigal Y. J., Pamuklar Z., Wang Z., Xu Y., Prestwich G. D., and Morris A. J.. 2003. Lipid phosphate phosphatases regulate lysophosphatidic acid production and signaling in platelets: studies using chemical inhibitors of lipid phosphate phosphatase activity. J. Biol. Chem. 278: 43214–43223. [DOI] [PubMed] [Google Scholar]
- 90.Tanyi J. L., Morris A. J., Wolf J. K., Fang X., Hasegawa Y., Lapushin R., Auersperg N., Sigal Y. J., Newman R. A., Felix E. A., et al. 2003. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 63: 1073–1082. [PubMed] [Google Scholar]
- 91.Tomsig J. L., Snyder A. H., Berdyshev E. V., Skobeleva A., Mataya C., Natarajan V., Brindley D. N., and Lynch K. R.. 2009. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 419: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryu J. M., and Han H. J.. 2015. Autotaxin-LPA axis regulates hMSC migration by adherent junction disruption and cytoskeletal rearrangement via LPAR1/3-dependent PKC/GSK3β/β-catenin and PKC/Rho GTPase pathways. Stem Cells. 33: 819–832. [DOI] [PubMed] [Google Scholar]
- 93.Oude Elferink R. P., Bolier R., and Beuers U. H.. 2015. Lysophosphatidic acid and signaling in sensory neurons. Biochim. Biophys. Acta. 1851: 61–65. [DOI] [PubMed] [Google Scholar]
- 94.Contos J. J., Fukushima N., Weiner J. A., Kaushal D., and Chun J.. 2000. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA. 97: 13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kishi Y., Okudaira S., Tanaka M., Hama K., Shida D., Kitayama J., Yamori T., Aoki J., Fujimaki T., and Arai H.. 2006. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J. Biol. Chem. 281: 17492–17500. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y., Mou L., Liu N., and Tsao M. S.. 1999. Autotaxin expression in non-small-cell lung cancer. Am. J. Respir. Cell Mol. Biol. 21: 216–222. [DOI] [PubMed] [Google Scholar]
- 97.Kehlen A., Englert N., Seifert A., Klonisch T., Dralle H., Langner J., and Hoang-Vu C.. 2004. Expression, regulation and function of autotaxin in thyroid carcinomas. Int. J. Cancer. 109: 833–838. [DOI] [PubMed] [Google Scholar]
- 98.Seifert A., Klonisch T., Wulfaenger J., Haag F., Dralle H., Langner J., Hoang-Vu C., and Kehlen A.. 2008. The cellular localization of autotaxin impacts on its biological functions in human thyroid carcinoma cells. Oncol. Rep. 19: 1485–1491. [PubMed] [Google Scholar]
- 99.Masuda A., Nakamura K., Izutsu K., Igarashi K., Ohkawa R., Jona M., Higashi K., Yokota H., Okudaira S., Kishimoto T., et al. 2008. Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br. J. Haematol. 143: 60–70. [DOI] [PubMed] [Google Scholar]
- 100.Tokumura A., Kume T., Fukuzawa K., Tahara M., Tasaka K., Aoki J., Arai H., Yasuda K., and Kanzaki H.. 2007. Peritoneal fluids from patients with certain gynecologic tumor contain elevated levels of bioactive lysophospholipase D activity. Life Sci. 80: 1641–1649. [DOI] [PubMed] [Google Scholar]
- 101.Hanahan D., and Weinberg R. A.. 2008. Retrospective: Judah Folkman (1933–2008). Science. 319: 1055. [DOI] [PubMed] [Google Scholar]
- 102.Hanahan D., and Weinberg R. A.. 2011. Hallmarks of cancer: the next generation. Cell. 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 103.David M., Wannecq E., Descotes F., Jansen S., Deux B., Ribeiro J., Serre C. M., Gres S., Bendriss-Vermare N., Bollen M., et al. 2010. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS ONE. 5: e9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Federico L., Ren H., Mueller P. A., Wu T., Liu S., Popovic J., Blalock E. M., Sunkara M., Ovaa H., Albers H. M., et al. 2012. Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol. Endocrinol. 26: 786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mazzocca A., Dituri F., Lupo L., Quaranta M., Antonaci S., and Giannelli G.. 2011. Tumor-secreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology. 54: 920–930. [DOI] [PubMed] [Google Scholar]
- 106.Yuelling L. W., Waggener C. T., Afshari F. S., Lister J. A., and Fuss B.. 2012. Autotaxin/ENPP2 regulates oligodendrocyte differentiation in vivo in the developing zebrafish hindbrain. Glia. 60: 1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baumforth K. R., Flavell J. R., Reynolds G. M., Davies G., Pettit T. R., Wei W., Morgan S., Stankovic T., Kishi Y., Arai H., et al. 2005. Induction of autotaxin by the Epstein-Barr virus promotes the growth and survival of Hodgkin lymphoma cells. Blood. 106: 2138–2146. [DOI] [PubMed] [Google Scholar]
- 108.Brindley D. N., Lin F. T., and Tigyi G. J.. 2013. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta. 1831: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samadi N., Bekele R. T., Goping I. S., Schang L. M., and Brindley D. N.. 2011. Lysophosphatidate induces chemo-resistance by releasing breast cancer cells from taxol-induced mitotic arrest. PLoS ONE. 6: e20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Satoh Y., Ohkawa R., Nakamura K., Higashi K., Kaneko M., Yokota H., Aoki J., Arai H., Yuasa Y., and Yatomi Y.. 2007. Lysophosphatidic acid protection against apoptosis in the human pre-B-cell line Nalm-6. Eur. J. Haematol. 78: 510–517. [DOI] [PubMed] [Google Scholar]
- 111.Song J., Clair T., Noh J. H., Eun J. W., Ryu S. Y., Lee S. N., Ahn Y. M., Kim S. Y., Lee S. H., Park W. S., et al. 2005. Autotaxin (lysoPLD/NPP2) protects fibroblasts from apoptosis through its enzymatic product, lysophosphatidic acid, utilizing albumin-bound substrate. Biochem. Biophys. Res. Commun. 337: 967–975. [DOI] [PubMed] [Google Scholar]
- 112.Vidot S., Witham J., Agarwal R., Greenhough S., Bamrah H. S., Tigyi G. J., Kaye S. B., and Richardson A.. 2010. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell. Signal. 22: 926–935. [DOI] [PubMed] [Google Scholar]
- 113.Billon-Denis E., Tanfin Z., and Robin P.. 2008. Role of lysophosphatidic acid in the regulation of uterine leiomyoma cell proliferation by phospholipase D and autotaxin. J. Lipid Res. 49: 295–307. [DOI] [PubMed] [Google Scholar]
- 114.Conrotto P., Andreasson U., Kuci V., Borrebaeck C. A., and Ek S.. 2011. Knock-down of SOX11 induces autotaxin-dependent increase in proliferation in vitro and more aggressive tumors in vivo. Mol. Oncol. 5: 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ortlepp C., Steudel C., Heiderich C., Koch S., Jacobi A., Ryser M., Brenner S., Bornhäuser M., Brors B., Hofmann W. K. , et al. 2013. Autotaxin is expressed in FLT3-ITD positive acute myeloid leukemia and hematopoietic stem cells and promotes cell migration and proliferation. Exp. Hematol. 41: 444–461. [DOI] [PubMed] [Google Scholar]
- 116.Xia Q., Deng A. M., Wu S. S., and Zheng M.. 2011. Cholera toxin inhibits human hepatocarcinoma cell proliferation in vitro via suppressing ATX/LPA axis. Acta Pharmacol. Sin. 32: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dai W., Wang F., He L., Lin C., Wu S., Chen P., Zhang Y., Shen M., Wu D., Wang C., et al. 2015. Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: Partial mediation by the transcription factor NFAT1. Mol. Carcinog. 54: 301–311. [DOI] [PubMed] [Google Scholar]
- 118.Gaetano C. G., Samadi N., Tomsig J. L., Macdonald T. L., Lynch K. R., and Brindley D. N.. 2009. Inhibition of autotaxin production or activity blocks lysophosphatidylcholine-induced migration of human breast cancer and melanoma cells. Mol. Carcinog. 48: 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ptaszynska M. M., Pendrak M. L., Stracke M. L., and Roberts D. D.. 2010. Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol. Cancer Res. 8: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saunders L. P., Ouellette A., Bandle R., Chang W. C., Zhou H., Misra R. N., De La Cruz E. M., and Braddock D. T.. 2008. Identification of small-molecule inhibitors of autotaxin that inhibit melanoma cell migration and invasion. Mol. Cancer Ther. 7: 3352–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y., Chen Y. C., Krummel M. F., and Rosen S. D.. 2012. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J. Immunol. 189: 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao J., He D., Berdyshev E., Zhong M., Salgia R., Morris A. J., Smyth S. S., Natarajan V., and Zhao Y.. 2011. Autotaxin induces lung epithelial cell migration through lysoPLD activity-dependent and -independent pathways. Biochem. J. 439: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu X., and Prestwich G. D.. 2010. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 116: 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rancoule C., Dusaulcy R., Treguer K., Gres S., Attane C., and Saulnier-Blache J. S.. 2014. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie. 96: 140–143. [DOI] [PubMed] [Google Scholar]
- 125.Rancoule C., Dusaulcy R., Treguer K., Gres S., Guigne C., Quilliot D., Valet P., and Saulnier-Blache J. S.. 2012. Depot-specific regulation of autotaxin with obesity in human adipose tissue. J. Physiol. Biochem. 68: 635–644. [DOI] [PubMed] [Google Scholar]
- 126.Georas S. N. 2009. Lysophosphatidic acid and autotaxin: emerging roles in innate and adaptive immunity. Immunol. Res. 45: 229–238. [DOI] [PubMed] [Google Scholar]
- 127.Coussens L. M., Zitvogel L., and Palucka A. K.. 2013. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 339: 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coussens L. M., and Werb Z.. 2002. Inflammation and cancer. Nature. 420: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Knowlden S., and Georas S. N.. 2014. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 192: 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sevastou I., Kaffe E., Mouratis M. A., and Aidinis V.. 2013. Lysoglycerophospholipids in chronic inflammatory disorders: the PLA(2)/LPC and ATX/LPA axes. Biochim. Biophys. Acta. 1831: 42–60. [DOI] [PubMed] [Google Scholar]
- 131.Benesch M. G., Ko Y. M., McMullen T. P., and Brindley D. N.. 2014. Autotaxin in the crosshairs: taking aim at cancer and other inflammatory conditions. FEBS Lett. 588: 2712–2727. [DOI] [PubMed] [Google Scholar]
- 132.Kanda H., Newton R., Klein R., Morita Y., Gunn M. D., and Rosen S. D.. 2008. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 9: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bai Z., Cai L., Umemoto E., Takeda A., Tohya K., Komai Y., Veeraveedu P. T., Hata E., Sugiura Y., Kubo A. , et al. 2013. Constitutive lymphocyte transmigration across the basal lamina of high endothelial venules is regulated by the autotaxin/lysophosphatidic acid axis. J. Immunol. 190: 2036–2048. [DOI] [PubMed] [Google Scholar]
- 134.Nikitopoulou I., Oikonomou N., Karouzakis E., Sevastou I., Nikolaidou-Katsaridou N., Zhao Z., Mersinias V., Armaka M., Xu Y., Masu M., et al. 2012. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 209: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu J. M., Xu Y., Skill N. J., Sheng H., Zhao Z., Yu M., Saxena R., and Maluccio M. A.. 2010. Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma. Mol. Cancer. 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pleli T., Martin D., Kronenberger B., Brunner F., Koberle V., Grammatikos G., Farnik H., Martinez Y., Finkelmeier F., Labocha S., et al. 2014. Serum autotaxin is a parameter for the severity of liver cirrhosis and overall survival in patients with liver cirrhosis–a prospective cohort study. PLoS ONE. 9: e103532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Budd D. C., and Qian Y.. 2013. Development of lysophosphatidic acid pathway modulators as therapies for fibrosis. Future Med. Chem. 5: 1935–1952. [DOI] [PubMed] [Google Scholar]
- 138.Nakagawa H., Ikeda H., Nakamura K., Ohkawa R., Masuzaki R., Tateishi R., Yoshida H., Watanabe N., Tejima K., Kume Y. , et al. 2011. Autotaxin as a novel serum marker of liver fibrosis. Clin. Chim. Acta. 412: 1201–1206. [DOI] [PubMed] [Google Scholar]
- 139.Oikonomou N., Mouratis M. A., Tzouvelekis A., Kaffe E., Valavanis C., Vilaras G., Karameris A., Prestwich G. D., Bouros D., and Aidinis V.. 2012. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 47: 566–574. [DOI] [PubMed] [Google Scholar]
- 140.Tager A. M. 2012. Autotaxin emerges as a therapeutic target for idiopathic pulmonary fibrosis: limiting fibrosis by limiting lysophosphatidic acid synthesis. Am. J. Respir. Cell Mol. Biol. 47: 563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu L., Petrigliano F. A., Ba K., Lee S., Bogdanov J., McAllister D. R., Adams J. S., Rosenthal A. K., Van Handel B., Crooks G. M., et al. 2015. Lysophosphatidic acid mediates fibrosis in injured joints by regulating collagen type I biosynthesis. Osteoarthritis Cartilage. 23: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hozumi H., Hokari R., Kurihara C., Narimatsu K., Sato H., Sato S., Ueda T., Higashiyama M., Okada Y., Watanabe C. , et al. 2013. Involvement of autotaxin/lysophospholipase D expression in intestinal vessels in aggravation of intestinal damage through lymphocyte migration. Lab. Invest. 93: 508–519. [DOI] [PubMed] [Google Scholar]
- 143.Cooper A. B., Wu J., Lu D., and Maluccio M. A.. 2007. Is autotaxin (ENPP2) the link between hepatitis C and hepatocellular cancer? J. Gastrointest. Surg. 11: 1628–1634; discussion 1634–1625. [DOI] [PubMed] [Google Scholar]
- 144.Liu S., Umezu-Goto M., Murph M., Lu Y., Liu W., Zhang F., Yu S., Stephens L. C., Cui X., Murrow G., et al. 2009. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 15: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fang X., Yu S., Bast R. C., Liu S., Xu H. J., Hu S. X., LaPushin R., Claret F. X., Aggarwal B. B., Lu Y., et al. 2004. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 279: 9653–9661. [DOI] [PubMed] [Google Scholar]
- 146.Mu H., Calderone T. L., Davies M. A., Prieto V. G., Wang H., Mills G. B., Bar-Eli M., and Gershenwald J. E.. 2012. Lysophosphatidic acid induces lymphangiogenesis and IL-8 production in vitro in human lymphatic endothelial cells. Am. J. Pathol. 180: 2170–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ptaszynska M. M., Pendrak M. L., Bandle R. W., Stracke M. L., and Roberts D. D.. 2008. Positive feedback between vascular endothelial growth factor-A and autotaxin in ovarian cancer cells. Mol. Cancer Res. 6: 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park S. Y., Jeong K. J., Panupinthu N., Yu S., Lee J., Han J. W., Kim J. M., Lee J. S., Kang J., Park C. G., et al. 2011. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 30: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 149.Benesch M. G., Tang X., Maeda T., Ohhata A., Zhao Y. Y., Kok B. P., Dewald J., Hitt M., Curtis J. M., McMullen T. P., et al. 2014. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 28: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 150.Leblanc R., Lee S. C., David M., Bordet J. C., Norman D. D., Patil R., Miller D., Sahay D., Ribeiro J., Clezardin P., et al. 2014. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood. 124: 3141–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boucharaba A., Serre C. M., Gres S., Saulnier-Blache J. S., Bordet J. C., Guglielmi J., Clezardin P., and Peyruchaud O.. 2004. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Invest. 114: 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Venkatraman G., Benesch M. G., Tang X., Dewald J., McMullen T. P., and Brindley D. N.. 2015. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: implications for cancer treatment. FASEB J. 29: 772–785. [DOI] [PubMed] [Google Scholar]
- 153.Nam S. W., Clair T., Campo C. K., Lee H. Y., Liotta L. A., and Stracke M. L.. 2000. Autotaxin (ATX), a potent tumor motogen, augments invasive and metastatic potential of ras-transformed cells. Oncogene. 19: 241–247. [DOI] [PubMed] [Google Scholar]
- 154.Su S. C., Hu X., Kenney P. A., Merrill M. M., Babaian K. N., Zhang X. Y., Maity T., Yang S. F., Lin X., and Wood C. G.. 2013. Autotaxin-lysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired resistance to sunitinib in renal cell carcinoma. Clin. Cancer Res. 19: 6461–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu F. X., Zhao B., Panupinthu N., Jewell J. L., Lian I., Wang L. H., Zhao J., Yuan H., Tumaneng K., Li H., et al. 2012. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 150: 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Panupinthu N., Yu S., Zhang D., Zhang F., Gagea M., Lu Y., Grandis J. R., Dunn S. E., Lee H. Y., and Mills G. B.. 2014. Self-reinforcing loop of amphiregulin and Y-box binding protein-1 contributes to poor outcomes in ovarian cancer. Oncogene. 33: 2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jeong K. J., Cho K. H., Panupinthu N., Kim H., Kang J., Park C. G., Mills G. B., and Lee H. Y.. 2013. EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: inhibition by resveratrol. Mol. Oncol. 7: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.The Cancer Genome Atlas Research Network. 2011. Integrated genomic analyses of ovarian carcinoma. Nature. 474: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.The Cancer Genome Atlas Research Network. 2012. Comprehensive molecular portraits of human breast tumours. Nature. 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.The Cancer Genome Atlas Research Network. 2014. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 511: 543–550. [Erratum. 2014. Nature 514: 262.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.The Cancer Genome Atlas Research Network. 2014. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 507: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.The Cancer Genome Atlas Research Network. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Albers H. M., van Meeteren L. A., Egan D. A., van Tilburg E. W., Moolenaar W. H., and Ovaa H.. 2010. Discovery and optimization of boronic acid based inhibitors of autotaxin. J. Med. Chem. 53: 4958–4967. [DOI] [PubMed] [Google Scholar]
- 165.Federico L., Pamuklar Z., Smyth S. S., and Morris A. J.. 2008. Therapeutic potential of autotaxin/lysophospholipase d inhibitors. Curr. Drug Targets. 9: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fells J. I., Lee S. C., Fujiwara Y., Norman D. D., Lim K. G., Tsukahara R., Liu J., Patil R., Miller D. D., Kirby R. J., et al. 2013. Hits of a high-throughput screen identify the hydrophobic pocket of autotaxin/lysophospholipase D as an inhibitory surface. Mol. Pharmacol. 84: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fells J. I., Lee S. C., Norman D. D., Tsukahara R., Kirby J. R., Nelson S., Seibel W., Papoian R., Patil R., Miller D. D., et al. 2014. Targeting the hydrophobic pocket of autotaxin with virtual screening of inhibitors identifies a common aromatic sulfonamide structural motif. FEBS J. 281: 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.North E. J., Howard A. L., Wanjala I. W., Pham T. C., Baker D. L., and Parrill A. L.. 2010. Pharmacophore development and application toward the identification of novel, small-molecule autotaxin inhibitors. J. Med. Chem. 53: 3095–3105. [DOI] [PubMed] [Google Scholar]
- 169.Albers H. M., Dong A., van Meeteren L. A., Egan D. A., Sunkara M., van Tilburg E. W., Schuurman K., van Tellingen O., Morris A. J., Smyth S. S., et al. 2010. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. USA. 107: 7257–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.East J. E., Kennedy A. J., Tomsig J. L., De Leon A. R., Lynch K. R., and Macdonald T. L.. 2010. Synthesis and structure-activity relationships of tyrosine-based inhibitors of autotaxin (ATX). Bioorg. Med. Chem. Lett. 20: 7132–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fisher N., Hilton-Bolt T., Edwards M. G., Haxton K. J., McKenzie M., Allin S. M., and Richardson A.. 2013. Dendrimer conjugate of [4-(tetradecanoylamino)benzyl]phosphonic acid (S32826) as an autotaxin inhibitor. ACS Med. Chem. Lett. 5: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Katsamakas S., Bermperoglou E., and Hadjipavlou-Litina D.. 2015. Considering autotaxin inhibitors in terms of 2D-QSAR and 3D-Mapping: review and evaluation. Curr. Med. Chem. 22: 1428–1461. [DOI] [PubMed] [Google Scholar]
- 173.Saga H., Ohhata A., Hayashi A., Katoh M., Maeda T., Mizuno H., Takada Y., Komichi Y., Ota H., Matsumura N., et al. 2014. A novel highly potent autotaxin/ENPP2 inhibitor produces prolonged decreases in plasma lysophosphatidic acid formation in vivo and regulates urethral tension. PLoS ONE. 9: e93230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kawaguchi M., Okabe T., Okudaira S., Nishimasu H., Ishitani R., Kojima H., Nureki O., Aoki J., and Nagano T.. 2013. Screening and X-ray crystal structure-based optimization of autotaxin (ENPP2) inhibitors, using a newly developed fluorescence probe. ACS Chem. Biol. 8: 1713–1721. [DOI] [PubMed] [Google Scholar]
- 175.Albers H. M., Hendrickx L. J., van Tol R. J., Hausmann J., Perrakis A., and Ovaa H.. 2011. Structure-based design of novel boronic acid-based inhibitors of autotaxin. J. Med. Chem. 54: 4619–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Norman D. D., Ibezim A., Scott W. E., White S., Parrill A. L., and Baker D. L.. 2013. Autotaxin inhibition: development and application of computational tools to identify site-selective lead compounds. Bioorg. Med. Chem. 21: 5548–5560. [DOI] [PubMed] [Google Scholar]
- 177.Crack P. J., Zhang M., Morganti-Kossmann M. C., Morris A. J., Wojciak J. M., Fleming J. K., Karve I., Wright D., Sashindranath M., Goldshmit Y., et al. 2014. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J. Neuroinflammation. 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stoddard N. C., and Chun J.. 2015. Promising pharmacological directions in the world of lysophosphatidic acid signaling. Biomol. Ther. (Seoul). 23: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nishimasu H., Ishitani R., Aoki J., and Nureki O.. 2012. A 3D view of autotaxin. Trends Pharmacol. Sci. 33: 138–145. [DOI] [PubMed] [Google Scholar]
- 180.Moolenaar W. H., and Perrakis A.. 2011. Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 12: 674–679. [DOI] [PubMed] [Google Scholar]
- 181.cBioPortal for Cancer Genomics. The Cancer Genome Atlas Research Network. Available at: http://www.cbioportal.org/. [Google Scholar]