Abstract
Gram positive mycobacteria with a high GC content, such as the etiological agent of tuberculosis Mycobacterium tuberculosis, possess an outer membrane mainly composed of mycolic acids (MAs), the so-called mycomembrane, which is essential for the cell. About thirty genes are involved in the biosynthesis of MAs, which include the hadA, hadB and hadC genes that encode the dehydratases Fatty Acid Synthase type II (FAS-II) known to function as the heterodimers HadA-HadB and HadB-HadC. The present study shows that M. smegmatis cells remain viable in the absence of either HadA and HadC or both. Inactivation of HadC has a dramatic effect on the physiology and fitness of the mutant strains whereas that of HadA exacerbates the phenotype of a hadC deletion. The hadC mutants exhibit a novel MA profile, display a distinct colony morphology, are less aggregated, are impaired for sliding motility and biofilm development and are more resistant to detergent. Conversely, the hadC mutants are significantly more susceptible to low- and high-temperature and to selective toxic compounds, including several current anti-tubercular drugs.
Introduction
Mycobacterium tuberculosis, the etiological agent of tuberculosis, infects one-third of the world population with 9 million new cases and 1.5 million people dying each year from this disease [1]. This failure is primarily due to the capability of the pathogen to develop a non-replicating persistent drug-tolerant form [2,3,4] and to the outbreak of multi-drug and total-drug resistant strains [5,6,7]. The main reason to the development of resistance is the poor compliance with medical treatments due to the side-effects on everyday wellbeing and the duration of treatments [8]. There is therefore an urgent need finding new drugs with highly short-time efficiency [9] or increasing the efficiency of existing drugs [10,11].
M. tuberculosis has a unique cell envelope, with a highly efficient permeability outer membrane barrier crucial to its viability and virulence. This so-called mycomembrane is composed of long-chain (up to C100) fatty acids, called mycolic acids (MAs), whose biosynthesis is targeted by several major anti-tubercular drugs [12,13]. In mycobacteria, the synthesis of MAs involved two distinct Fatty Acid Synthases (FAS), i.e. the eukaryote-like multifunctional single protein FAS-I and the bacterial-like multi protein complex FAS-II [13]. The HadA-HadB and HadB-HadC heterodimers of the FAS-II complex perform a key dehydration reaction [14,15]. Enzymatic assays have shown that HadA and HadC subunits are involved in the substrate specificity, bringing either short-/medium- or long-size substrates to the catalytic activity of HadB, respectively [14]. The hadABC knock-out mutant in M. tuberculosis was shown to be non-viable [14] and comprehensive transposon mutagenesis has concluded that hadA and hadB, but not hadC, are essential for cell viability [16,17], although so far only the essentiality of hadB in M. smegmatis has been confirmed [15], while the non-essentiality of hadC has been shown in M. tuberculosis [18]. Nevertheless, the key dehydration step for the synthesis of MAs has stimulated the search for drugs that would target the Had enzymes. Indeed, two anti-tubercular drugs used in the sixties, Thioacetazone and Isoxyl, have recently been shown to target HadC and HadA [19]. Although both drugs are barely used because of either a low efficacy (for Isoxyl [20] or toxic side-effects (for Thioacetazone [21]) they underscore the fact that the proteins Had are druggable targets for fighting tuberculosis.
M. smegmatis, a fast-growing and safer-to-handle species, has been widely used as a surrogate of the highly pathogenic M. tuberculosis. This is based on the assumption of the conservation of the basic functions in both species, such as the building of the mycomembrane. As a consequence, a better understanding of M. smegmatis physiology would benefit to the knowledge of M. tuberculosis physiology. Notwithstanding M. smegmatis belongs to the nontuberculous mycobacteria (NTM) complex and as such per se is also an opportunistic pathogen for humans and animals [22,23,24,25]. Therefore any further understanding of M. smegmatis might also give new hints to better fight against hardly-cured diseases due to NTM. In this study, we decipher the respective biological role of the HadABC dehydratase subunits and show that M. smegmatis hadA and hadC genes are not essential for cell viability but play a major role in the physiology and adaptive response of the bacteria.
Materials and Methods
Bacterial strains, plasmids and growth conditions
Strains and Plasmids used in this study are listed in Table 1. For liquid cultures, mycobacteria strains were grown in Middlebrook 7H9 medium (Difco) containing 0.05% Tween-80, 0.2% glycerol, 10% ADC (Difco) and the appropriate antibiotics (Kanamycin 37.5 μg/ml, Hygromycin 150 μg/ml). For solid medium, Tween-less Middlebrook 7H10 broth supplemented by 0.5% glycerol and 10% OADC (Difco) was used. When required, Zeocin was added at 15 μg/ml. For E. coli growth, Luria-Bertani medium (Invitrogen) was used with antibiotics when required (Kanamycin 37.5 μg/ml, Hygromycin 150 μg/ml). To induce the tetROp promoter from the pGBT plasmid and its derivatives, Tetracycline (20 ng/ml) was also added to the liquid and solid media.
Table 1. List of strains and plasmids.
| Bacterial strains | description | sources |
|---|---|---|
| mc2 155 | M. smegmatis mc 2 strain | [26] |
| ΔhadC | native copy of MSMEG_1342 replaced by Sh ble (ZeoR) | This work |
| ΔhadABC pABC | native copy of MSMEG_1340/1341/1342 operon replaced by Sh ble (ZeoR), and carrying the pABC plasmid | This work |
| MC1061 recA | E. coli cloning strain | Our collection |
| Plasmids | ||
| pJV53 | KanR, Plasmid expressing the recombineering proteins | [27] |
| pJV53::hyg | HygR, Hygromycin resistant derivative of Plasmid pJV53 | V. Malaga |
| pGBT | KanR, C. glutamicum tetRO promoter cloned in pGB9.2 | [28] |
| pMVZ261 | ZeoR, source of the Sh ble gene | G. Etienne |
| pC | KanR, native copy of MSMEG_1342 cloned in pGBT | This work |
| pABC | KanR, native copy of MSMEG_1340/1341/1342 in pGBT | This work |
| pAB | KanR, native copy of MSMEG_1340/1341 cloned in pGBT | This work |
| pBC | KanR, native copy of MSMEG_1341/1342 cloned in pGBT | This work |
| pB | KanR, native copy of MSMEG_1341 cloned in pGBT | This work |
DNA manipulation
Molecular biology materials were used as recommended by the manufacturers: DNA purification (Quiagen), enzyme restrictions and T4 DNA ligase (Fermentas and Biolabs), PCR with the phusion polymerase (Finnzyme), and pJET1.2 cloning kit (Fermentas). DNA inserts were checked by sequencing (MillGen).
Construction of deletion mutants
KO-mutants were generated with the recombineering system [27,28], with slight modifications [28]. To delete the whole hadABC cluster, co-transformation was done with 100 ng of plasmid DNA along with 100 ng of AES (allelic exchange sequence), and selection made on Zeocin, Kanamycin and Tetracycline containing medium. PCR on lysates of recovered clones were performed to check for the replacement of the target sequence by the Zeocin resistant cassette.
Drugs and temperature susceptibility assays
Cultures at OD590 ~4–5 of the different strains were adjusted to the same OD then serially diluted. 5 μl of each dilution (starting OD590 0.2) was spotted on 7H10-based medium containing OADC, glycerol, Tetracycline (20 ng/ml) and Kanamycin (37.5 μg/ml). When required, drugs were added to the medium: Rifampicin (2 μg/ml), Isoniazid (5 μg/ml), Ethionamid (10 μg/ml), Ethambutol (5 μg/ml) and Vancomycin (1 μg/ml). After 4–5 days at 37°C (for drugs testing) or at 30°C, 37°C and 42°C (for temperature testing), CFUs were counted.
Susceptibility to SDS
Cultures were grown to OD590 ~ 0.6–0.8 in 7H9 medium + ADC + glycerol + tween + Kanamycin + Tetracycline, harvested, washed once with 7H9 + tween, and suspended in an equal volume of 7H9 + glycerol + Kanamycin + Tetracycline + tween. Then, each preparation was adjusted to OD590 0.2 and SDS added to 0.1% final. After 65 min, aliquots were serial diluted and spotted on growth plates. Survival rate was estimated by counting the CFUs after incubation for 3–4 days at 37°C.
Sedimentation assays
Cultures at OD590 ~4–5 of the different strains in 7H9 + ADC + glycerol + tween + Kanamycin + Tetracycline, were adjusted in triplicate to OD590 ~ 1 and kept unshaken at 37°C. At 3 and 22 hours, the upper 1 ml was removed for OD measurements.
Biofilm formation
Exponential phase cultures (OD590 ~1) in 7H9 medium + ADC + glycerol + tween + Kanamycin + Tetracycline, were diluted (1:100) in standard Sauton’s media [29] containing Kanamycin and Tetracycline. Then 4.5 ml samples were dispensed into 12 wells plates (3 wells/strain). After being wrapped three times with parafilm, the plates were incubated at 37°C for one week.
Nile Red accumulation
The experiment was performed as previously described with slight modification [30]. Strains were grown as a cell layer on solid 7H10 medium containing glycerol, ADC, Kanamycin and Tetracycline. Cells were scrapped and resuspended in PBS buffer (containing 25 mM glucose). Each suspension was adjusted to OD590 0.5, then Nile Red added (4 μM final). Fluorescence at 610 nm (excitation at 533 nm) was measured in technical triplicate in a 96 wells plate (incubated at 37°C) with the Clariostar reader (BMG).
Total RNAs preparation
Cultures were grown to OD590 ~ 0.6–0.8 in 7H9 medium + ADC + glycerol + tween + Kanamycin + Tetracycline. Total RNA was extracted using the RNeasy kit (Qiagen) following manufacturer’s instructions with slight modifications. Briefly, 15 ml of cultures were centrifuged for 5 min at 1,600 g, the pellet suspended in 1.2 ml of 0.1% β-mercaptoethanol containing RLT lysis buffer along with 0.1 mm-diameter glass beads. Cells were lysed by two 120 sec pulses at full speed in a bead-beater device. The sample was centrifuged 30 sec at 14,800 g. One volume of absolute ethanol was added to the filtrate, and total RNA purified with an RNeasy column following the manufacturer’s procedure. RNA sample was treated twice for 45 min with successively 3U and 2U of Turbo DNase (Turbo DNA free kit-Ambion). Biological triplicates were performed for each condition.
Analysis of Mycolic Acids. Cultures were grown to stationary phase in 7H9 medium + glycerol + Kanamycin + Tetracycline
Whole cells or bacterial residues obtained after lipid extraction with organic solvents [31] were saponified by a mixture of 40% KOH and methoxyethanol (1:7, v/v) at 110°C for 3 h in a screw-capped tube. After acidification, fatty acids were extracted with diethyl ether and methylated with an ethereal solution of diazomethane[32]. The mycolate patterns of the strains were determined by HPTLC (High Performance Thin-Layer Chromatography) on HPTLC Silica Gel 60 (Merck), using a mixture of petroleum ether/diethyl ether (9:1, v/v, five runs) as eluent. Revelation of lipid spots was performed by immersion of the plate in a solution of rhodamine. The various classes of mycolates, alpha-, alpha’, epoxy-mycolates (α, α', E, respectively) and the compound X were quantified by absorption measurement at the specific wavelength with TLC Scanner 3 using wincats software.
Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) was performed in reflectron mode, using the 5800 MALDI-TOF/TOF Analyzer (Applied Biosystems/ABsciex) equipped with a Nd:YAG laser (349 nm wavelength). A total of 2,500 shots were accumulated in positive ion mode. Lipid samples were dissolved in chloroform and were directly spotted onto the target plate as 0.5 μl droplets, followed by the addition of 0.5 μl of matrix solution (10 mg of 2,5-dihydroxybenzoic acid [Sigma-Aldrich]/ml in CHCl3/CH3OH, 1/1 [vol/vol]). Samples were allowed to crystallize at room temperature. Mass spectrometry data were acquired using the instrument default calibration [33].
For Nuclear Magnetic Resonance (NMR) analyses, compounds were dissolved in CDCl3/CD3OD (1/1, v/v, 99.8% purity, Euriso-top, CEA Saclay, France). 1D and 2D 1H- COSY 1H/1H (COrrelation SpectroscopY) experiments were conducted in the 600 MHz Bruker NMR spectrometer equipped with cryosonde. 1H chemical shifts are given in parts/million downfield from internal tetramethylsilane at 0 ppm. All experiments were recorded at 295° K without sample spinning. The Bruker pulse programs were used and optimized (pulse lengths and delays) for each one- or two-dimensional experiments. Data were analyzed using the TopSpin (Bruker BioSpin) software.
Results
The HadA and HadC subunits are dispensable to cell viability
In M. tuberculosis, the hadA, hadB and hadC genes may be co-transcribed from five promoters [28]. The synteny observed between M. tuberculosis and M. smegmatis in this region suggests that the operon organization is also conserved in M. smegmatis [28]. Comprehensive transposon mutagenesis in M. tuberculosis has concluded that hadC is not essential [16,17]. Accordingly we were able to delete hadC in M. smegmatis, by replacing the gene by a zeocin resistance cassette (Fig 1A–1C). To get further insights into the contribution of each of the hadA, hadB and hadC genes to the bacterial physiology, we attempted to generate mutants bearing various deletion combinations of the three genes. To avoid any polar effect of inactivating genes organized into an operon, this was done by co-transforming the wt strain with an allelic exchange sequence fragment to delete the whole hadABC operon and a plasmid expressing different combinations of the had genes. The set of plasmids included pGBT, the cloning vector, and pABC, pBC, pAB, pB expressing from a tetROp promoter either the whole operon or hadB and hadC or hadA and hadB or only hadB, respectively. Each co-transformation was independently repeated at least twice and consistently gave reproducible results. As expected, no co-transformant was obtained with the vector alone (pGBT), hadB being essential [15], whereas complementing with the pABC plasmid gave 10–15 colonies. With the same efficiency, plasmid pAB (ΔhadC) also gave co-transformants, in agreement with our ability to delete the chromosomal copy of the hadC gene. Interestingly, transformants were also obtained with plasmid pBC (ΔhadA), indicating that hadA was not essential either in M. smegmatis. More surprisingly, a comparable co-transformation efficiency was observed with plasmid pB (ΔhadAC), indicating that cells were viable despite the inactivation of both hadA and hadC (Fig 1). Therefore HadB was the only essential subunit of the HadA-HadB and HadB-HadC dehydratase complexes.
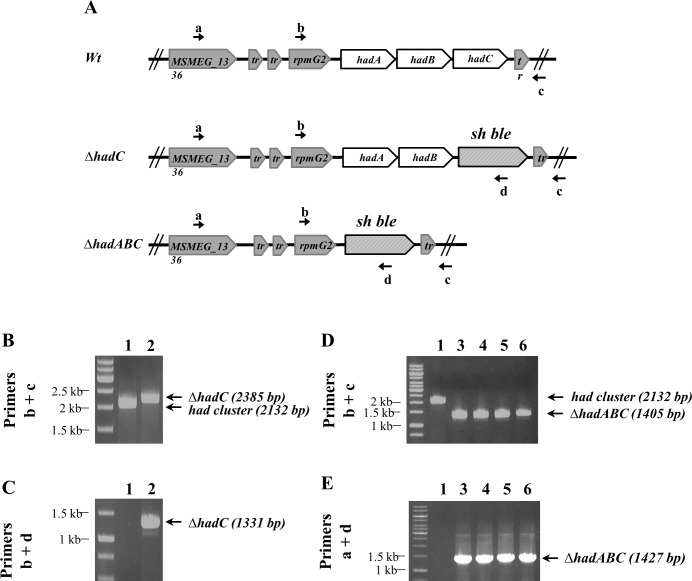
Fig 1. Construction of M. smegmatis ΔhadC and ΔhadABC strains by recombineering.
(A) Genetic organization showing the replacement of the hadC gene and hadABC operon by a zeocin resistance cassette (Sh ble) and the primers a, b, c, d used for PCR verification of the constructions. (b+c) primer couple confirms the deletion of the hadC gene (B) and hadABC operon (D). Amplification with the (b+d) and (a+d) primer couples confirms the presence of the zeocin cassette in the ΔhadC (C) and ΔhadABC (E) strains, respectively. PCR was performed on cell lysates: wt (lane 1), ΔhadC (lane 2), ΔhadABC (lanes 3–6) carrying either the plasmid pABC (lane 3) or pAB (lane 4) or pBC (lane 5) or pB (lane 6).
Impact of HadC on the adaptive response to cold shock
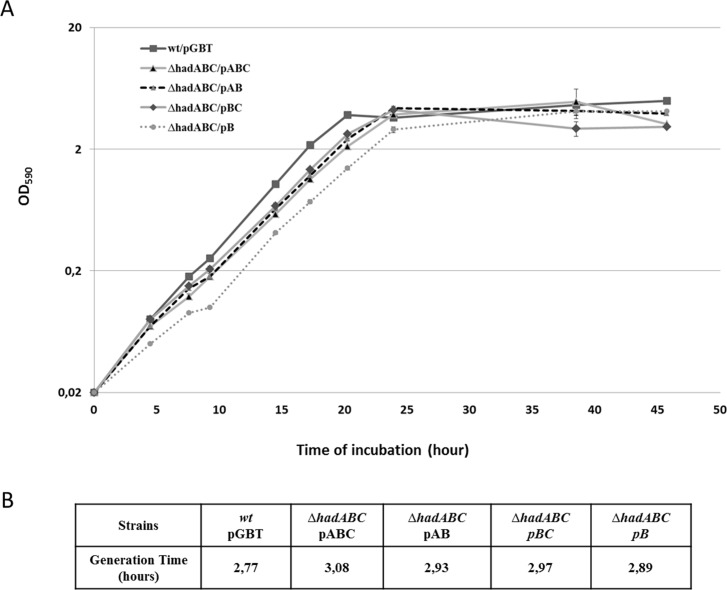
As a first step to evaluate the contribution of each of the had genes to the fitness of the bacterium, the growth of the mutants was followed in 7H9-based liquid cultures. As shown in Fig 2A and 2B, the growth curves of the single (ΔhadC and ΔhadA, i.e. ΔhadABC/pAB and ΔhadABC/pBC, respectively) and double mutants (ΔhadAC i.e ΔhadABC/pB) were comparable to that of the wt reference strains (wt/pGBT and ΔhadABC/pABC), each strain reaching a similar plateau value with similar growth rates (Fig 2B). In contrast, when a cold-shock was applied, although a lag was observed for all strains before the growth resumed, the lag displayed by the ΔhadC (ΔhadABC/pAB) and ΔhadAC (ΔhadABC/pB) mutants lasted twenty hours longer (Fig 3). As the growth profile of the ΔhadA (ΔhadABC/pBC) was similar to that of the control wt strains, it was concluded that the longer lag phenotype of the ΔhadC and ΔhadAC strains was likely due to the inactivation of the hadC gene. Expectedly, the simple hadC deletion mutant (ΔhadC/pGBT) displayed a growth profile similar to that of ΔhadABC/pAB, a phenotype fully complemented by a plasmid expressing hadC (ΔhadC/pC) (data not shown). Therefore a functional HadC, although not essential, is required for the adaptive response of the bacterium to a cold-shock.
Fig 2. The inactivation of either hadA, or hadC or hadAC does not affect the growth of M. smegmatis.
(A) Growth curves at 37°C in 7H9-based medium. Error bars represent standard deviations from three biological replicates. (B) Calculated generation time for each strain during exponential growth.
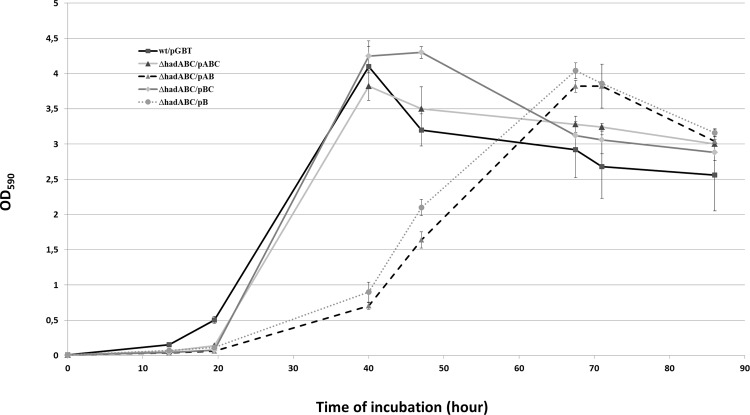
Fig 3. hadC is required for the adaptive response to cold-shock.
Exponential phase cultures at 37°C were diluted 100-fold in 7H9-based medium kept at room temperature (and not in a 37°C pre-warmed medium as in Fig 2) then re-incubated at 37°C (time 0). Error bars represent standard deviations from three biological replicates.
Colony morphology and sedimentation velocity in the hadA/hadC mutants
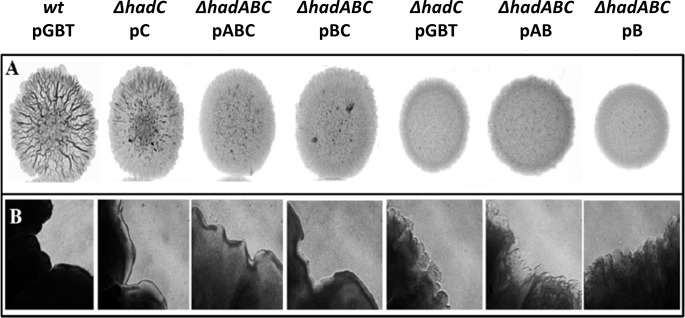
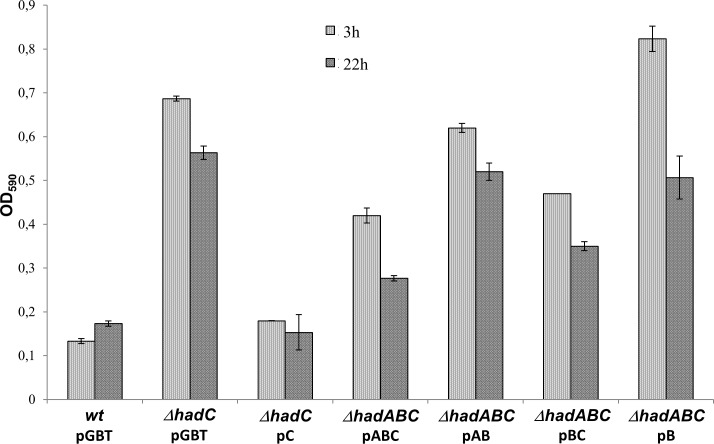
The colony morphology of some of the mutants was readily distinguishable from that of the wt, as illustrated in Fig 4. The wt strain (wt/pGBT) formed rough colonies with ridges and well-delimited borders whereas the hadC mutant (ΔhadC/pGBT) formed smooth colonies with unstructured border. The introduction of a plasmid expressing the wild-type hadC allele from a TetR-regulated promoter (pC) partially restored the wild-type morphology (ΔhadC/pC). In the ΔhadABC background, the expression of the hadABC genes from the tetRO promoter (pABC) led to the formation of rough colonies with well delimited border, although the roughness was less pronounced than that of the wt strain (Fig 4). The absence of a full complementation in both ΔhadC/pC and ΔhadABC/pABC strains was likely due to a lower expression of the had genes from the tetRO promoter as shown by RT-qPCR (S1 Fig). The morphology of the ΔhadABC/pAB colonies (ΔhadC) as well as that of the double mutant ΔhadABC/pB (ΔhadAC) was smoother with more unstructured border than that of the appropriate reference strain (ΔhadABC/pABC). Considering that the morphology of the ΔhadABC/pBC colony (ΔhadA) was similar to that of the control ΔhadABC/pABC strain, we concluded that the alteration of the colony morphology was mainly due to the inactivation of hadC. Smooth colony morphology might be indicative of the alteration of the cell surface proprieties. Because cell sedimentation velocity is inversely correlated to the hydrophobicity of the envelope [34], measuring the rate of sedimentation could be a mean of underscoring the alteration of the envelope. When shacked cultures at OD590 1 were kept unshaken for 3 h and 24 h, the ODs of the upper layer of the cultures were 0.7 and 0.6 respectively for the ΔhadC mutant, whereas those for the wt cultures dropped to OD590 ~0.15. The complementation of the mutant with the plasmid expressing hadC fully restored the wt phenotype (Fig 5). In the ΔhadABC background, similar results were obtained, i.e. the sedimentation velocity of the ΔhadC was slower (Fig 5, ΔhadABC/pAB). In agreement with the colony morphology phenotype, the sedimentation velocity of the ΔhadA mutant was similar to that of the wt strain (Fig 5, ΔhadABC/pBC), whereas that of the double ΔhadAC mutant (ΔhadABC/pB) was slower. Of note, the velocity of the double mutant was even slower than that of the single mutant ΔhadC at 3 h (ΔhadABC/pAB), indicating an additive effect of the hadA mutation on the phenotype of the ΔhadC strain. Therefore, altogether these results indicated that the HadC protein plays a major role in keeping the wild-type characteristics of the envelope, notably its hydrophobicity.
Fig 4. The colony morphology of the had mutants is altered.
(A) Colony morphology of the various strains grown on 7H10-based medium. (B) Corresponding borders of the colony (10X-magnification), representative of five pictures taken at different locations.
Fig 5. The inactivation of hadC reduces the cell sedimentation rate.
Cultures at OD590 ~ 4–5 were adjusted at OD590 ~ 1 (in triplicate) in a 7H9 liquid medium containing Tween (0.05%) and Tetracycline (20 ng/ml) to induce the tetROp promoter, then kept at 37°C without agitation. Sedimentation rates were estimated by measuring the OD of 1 ml sample taken from the upper most layers at 3 and 22 hours. Error bars represent standard deviations.
The development of biofilms and the sliding motility are negatively affected by the inactivation of HadC
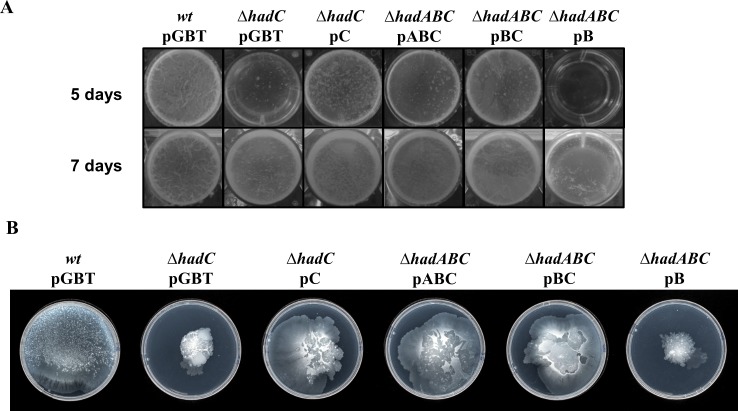
Lowering the hydrophobicity of the cell surface would negatively interfere with both the formation of biofilms and the sliding motility of M. smegmatis [35]. As shown on Fig 6A, the hadC mutation dramatically delayed the development of biofilms. At day 5 the wt (wt/pGBT, ΔhadABC/pABC) as well as the ΔhadA (ΔhadABC/pBC) strains displayed a visible pellicle, in contrast to any of the ΔhadC deletion bearing mutants (ΔhadC/pGBT, ΔhadABC/pAB and ΔhadABC/pB strains). Complementation with a plasmid expressing hadC restored the wild-type rate of biofilms development (ΔhadC/pC,ΔhadABC/pBC). At day 7, biolfims could eventually be observed in the ΔhadC mutants, but barely in the ΔhadAC mutant (ΔhadABC/pB), indicating that, the loss of a functional HadA, if it has no visible effect by itself, could again have an additive effect when combined with the inactivation of hadC. Fig 6B displays the results of the sliding motility assays. After seven days of incubation, the wt strain cells (wt/pGBT, ΔhadABC/pABC) covered the whole surface of the Petri dish, whereas cells bearing a ΔhadC deletion (ΔhadC/pGBT and ΔhadABC/pB strains) remained concentrated at the center of the Petri dish. Plasmids expressing hadC did restore a wild-type phenotype (ΔhadC/pC, ΔhadABC/pBC). Again the loss of a functional HadA (ΔhadABC/pBC) has no visible effect by itself, but aggravates the ΔhadC phenotype (ΔhadABC/pB). These results therefore underscore the major role of the accessory gene hadC and strengthened the notion that HadC contributes to the hydrophobicity potential of the envelope.
Fig 6. The inactivation of hadC compromises the development of biofilms and the sliding motility.
(A) Biofilm formation was monitored after 5 and 7 days of growth. The picture is representative of 3 biological replicates. (B) The sliding motility was monitored after 7 days of incubation on a semi-solid 7H9 medium without any carbon source.
Resistance to SDS and permeability to the lipophilic Nile Red molecule
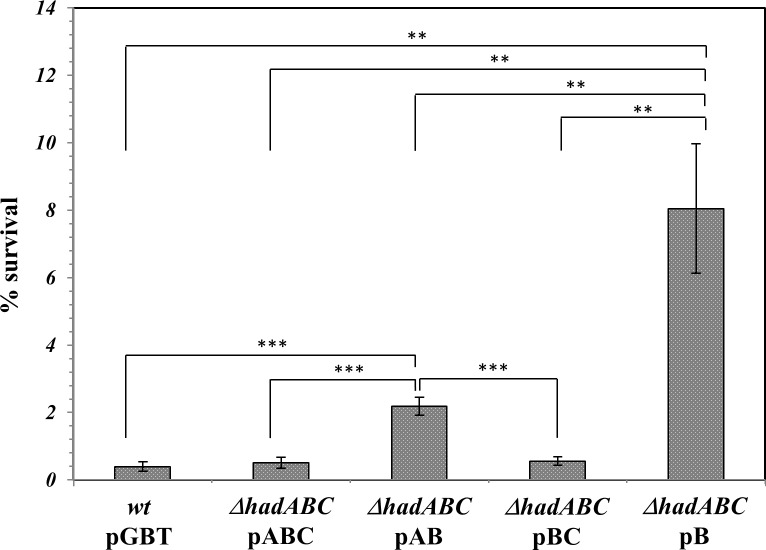
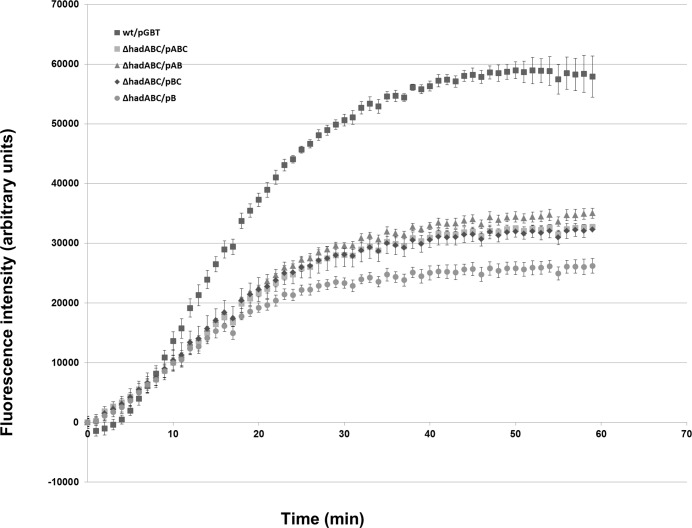
Alteration of the hydrophobicity of the envelope may be detected by measuring the sensitivity of bacteria to detergents [36,37]. The cell fraction surviving a 65-min challenge to 0.1% SDS was then measured (Fig 7). The survival rate of wt strains (wt/pGBT, ΔhadABC/pABC), as well as that of the ΔhadA strain (ΔhadABC/pBC), was similar and closed to 0.4%. In contrast the deletion of hadC (ΔhadABC/pAB) rendered the bacteria 6-fold more resistant to SDS. When the deletion of hadA was combined to that of hadC (ΔhadABC/pB), the resistance of the bacteria was even higher, with a survival rate of 8%. These results are in agreement with a lowering of the hydrophobicity of the envelope associated with the inactivation of hadC and again confirmed the synergistic effect of the simultaneous loss of hadA. With the affected hydrobobicity of the ΔhadC envelope, the mutant is expected to accumulate less the lipophilic molecule Nile Red [30]. Accordingly, although the wt-like reference strain (ΔhadABC/pABC) did accumulate the Nile Red molecule less efficiently that the real wt strain (wt/pGBT), the accumulation kinetics of the ΔhadAC double mutant (ΔhadABC/pB) was much less efficient than those of the two former strains (Fig 8). Although with this assay the phenotype of the single mutants (ΔhadABC/pBC or ΔhadABC/pAB) could not be distinguished from that of the control strain (ΔhadABC/pABC), the results reinforced the notion that HadA exacerbates the effect of HadC and that both proteins are required for maintaining the hydrophobic potential of the envelope.
Fig 7. The hadC and hadAC mutant strains are more resistant to SDS.
The survival rates were estimated by counting the CFUs (in duplicate). Error bars represent standard deviation from three biological replicates. P-values < 0.05 (*), < 0.01 (**), < 0.001 (***).
Fig 8. The hadAC double mutant envelope is more permissive to the lipophilic Nile Red.
This is a representative experiment of two biological repeats giving similar results. Data and error bars from technical triplicate represent the means and standard deviation, respectively.
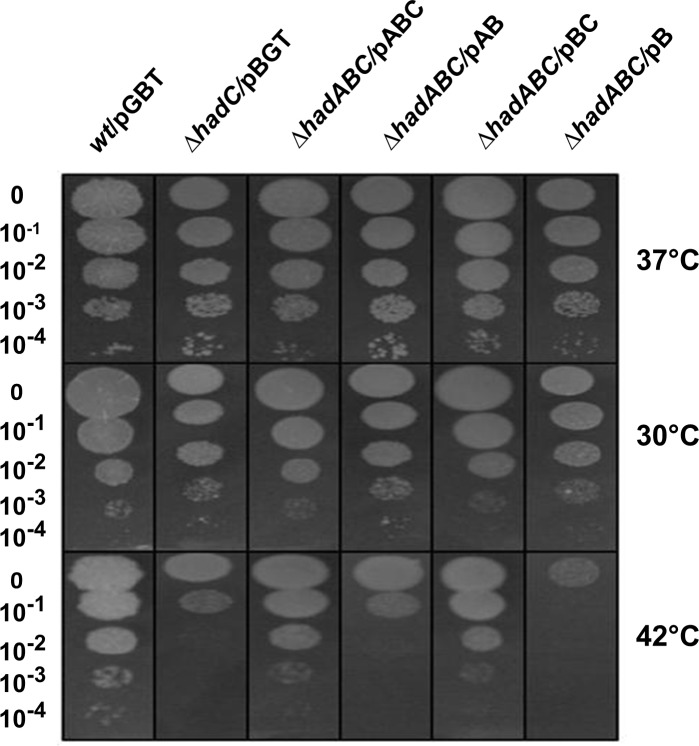
The fitness of the ΔhadC and ΔhadAC mutants is compromised at high temperature
Considering the importance of the thermal regulation of the fluidity of the biological membrane and the contribution of the MA structure to this fluidity in mycobacteria [38,39], we challenged the fitness of the had mutants at different temperatures. As shown in Fig 9, the efficiency of colony formation of the hadC mutants (ΔhadC/pGBT, ΔhadABC/pAB) was similar to that of the appropriate wt reference strains (wt/pGBT,ΔhadABC/pABC) at 30°C and 37°C, but was three orders of magnitude lower at 42°C. The temperature sensitivity of the mutant was fully complemented by the hadC-expressing plasmid (ΔhadABC/pABC). A functional HadC is therefore required for optimal growth at high temperature. In contrast the fitness of the hadA mutant (ΔhadABC/pBC) was not compromised at 42°C. Moreover, when the hadA mutation was combined with the hadC deletion (ΔhadABC/pB), the sensitivity of the double mutant to high temperature was one order of magnitude even higher than that of the single hadC mutation (ΔhadC/pGBT, ΔhadABC/pAB). Again a phenotype associated to the inactivation of hadA was only visible in a ΔhadC background.
Fig 9. The fitness of the hadC and hadAC mutants is compromised at high temperature.
CFUs were recorded at day 4 for the 37°C and 42°C plates and at day 5 for the 30°C plates.
Sensitivity of the ΔhadC mutant to selective drugs
The very low permeability of the envelope is a hallmark of mycobacteria [40]. This characteristic contributes to the resistance of mycobacteria to a wide range of antibiotics [41]. As MAs, the major constituent of the mycomembrane, play an essential role to this barrier [42], we measured the susceptibility of the had mutant to various drugs. The results are shown in Table 2. The efficiency of colony formation of the hadC mutant (ΔhadC/pGBT) towards Rifampicin (RIF), a large lipophilic anti tubercular drug targeting the transcription apparatus was at least three orders of magnitude below that of the wt strain (wt/pGBT). This susceptibility phenotype was fully complemented by the hadC expressing plasmid pC (ΔhadC/pC). The loss of HadA neither impaired the susceptibility of the strain (ΔhadABC/pBC) nor exacerbated the susceptibility of a hadC mutant (ΔhadABC/pB) to RIF. The ΔhadC mutant (ΔhadC/pGBT) was also two orders of magnitude more susceptible to Isoniazid (INH) and Ethionamid (ETH), a first- and a second-line anti tubercular small hydrophilic drugs, respectively, both targeting the FAS-II InhA enzyme [43]. The pC complementing plasmid fully restored the wild-type susceptibility level (ΔhadC/pC). Again the loss of a functional HadA was neutral, in both the single (ΔhadABC/pBC) and in the double mutants (ΔhadABC/pB). Importantly, when challenged against Ethambutol (EMB), another small hydrophilic drug that targets the arabinogalactan biosynthesis machinery [44], no effect of the various mutations was observed. The susceptibility of the mutants was also tested against Vancomycin (VAN), a drug that cannot diffuse across outer membranes, including the mycomembrane [45], due to its size (1450 Da) and its hydrophilicity. Interestingly, the hadC mutant was more susceptible to VAN by two orders of magnitude than was the wt and the complemented hadC/pC strains. This result therefore suggested a dramatic lowering of the hydrophobicity of the mycomembrane. Again the hadA mutation alone (ΔhadABC/pBC) had no visible effect on the susceptibility to VAN. However when the mutation was combined with the hadC deletion (ΔhadABC/pBC) the efficiency of plating decreased further down by two orders of magnitude when compared to that of the ΔhadC strain.
Table 2. Drug hyper-susceptibility of the had mutants.
CFUs were recorded at day 4 of incubation at 37°C. Relative values to wt’s are shown.
| Drug (μg/ml) | wt/pGBT | ΔhadC/pGBT | ΔhadC/pC | ΔhadABC/pABC | ΔhadABC/pBC | ΔhadABC/pB |
|---|---|---|---|---|---|---|
| Ethambutol (5) | 1 | 1 | 1 | 1 | 1 | 1 |
| Isoniazid (5) | 1 | 10 −2 | 1 | 1 | 1 | 10 −2 |
| Ethionamid (10) | 1 | 10 −2 | 1 | 1 | 1 | 10 −2 |
| Rifampicin (2) | 1 | < 10 −3 | 1 | 1 | 1 | < 10 −3 |
| Vancomycin (1) | 1 | 10 −2 | 1 | 1 | 1 | 10 −4 |
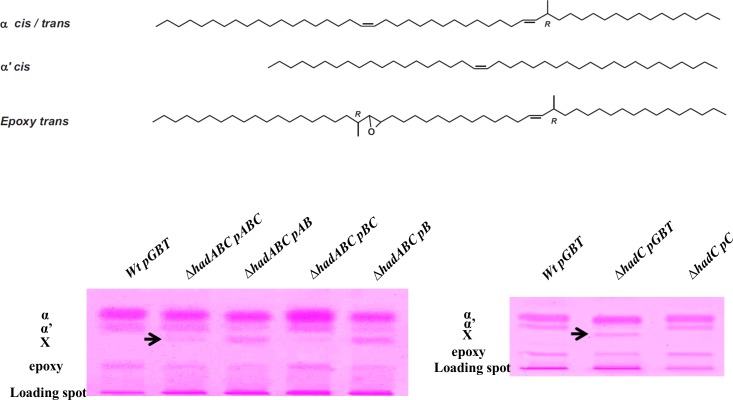
Mycolic acids profile of the hadC mutants
Mycolic acids (MAs) are 2-alkyl, 3-hydroxyl fatty acids, composed of a so-called meromycolic chain synthesized by the FAS-II complex, which is condensed with a shorter fatty acid chain that generates the so-called α-branch synthesized by FAS-I. Each mycobacterium species is defined by specific types of meromycolic chains depending on the length of the chain and the types of decorations that are carried by the chains. In order to evaluate the impact of the mutations of the had genes on the MA structures, lipids were extracted from the different mutants and analyzed. Similar amounts of lipids were extracted from the strains (roughly 17% of the dry weight). Saponification of the bacterial residues, followed by extraction and methylation of MAs yielded comparable amounts of lipid material (8–9% of the delipidated cell walls), which were quantitatively analyzed by high-performance thin layer chromatography (HPTLC). The M. smegmatis wt displayed the expected ɑ-, ɑ’-, and epoxy-MAs (Fig 10) [31]. In the ΔhadABC background, the complemented ΔhadABC/pABC as well as the ΔhadA (ΔhadABC/pBC) strains displayed a profile similar to that of the wt (wt/pGBT), with traces of an additional spot (named X on Fig 10). These data are in agreement with the phenotypes described above, viz (i) that the pABC plasmid did not fully complement the ΔhadABC deletion and (ii) that the single inactivation of HadA had no obvious impact on the bacterial physiology. In contrast, the deletion of hadC, both in the ΔhadC (ΔhadABC/pAB) and ΔhadAC (ΔhadABC/pB) mutants, clearly altered the HPTLC profile, with a decrease of the amount of epoxy-MAs and a concomitant increase of compound X. A similar altered profile was observed for the simple ΔhadC mutant (ΔhadC/pGBT) that was complemented by the pC plasmid (ΔhadC/pC) (Fig 10).
Fig 10. The inactivation of HadC alters the MA profile.
(Top) Structures of the major species of the meromycolic chains in M. smegmatis. α, α’ and epoxy refer to the types of mycolic acids produced by the wt strain. cis and trans correspond to the configurations of the double bonds. R refers to the absolute configuration of the asymmetric carbon bearing the methyl branch. (Bottom) High-performance thin layer chromatography (HPTLC) of total fatty acid methyl esters derived from the saponification of crude cells from the wt, isogenic mutant and complemented strains.
As shown in Table 3, the relative amount of α-, α’- and epoxy-MAs in the wt strain was 63%, 23% and 14%, respectively. In the ΔhadC mutant, whereas the sum of α- and α’-MAs (hardly separated by HPTLC) remained similar to that in the wt strain (84%), the relative amount of epoxy-MAs decreased to 5% with a concomitant emergence of the compound X representing 11% of the total. Complementing with the plasmid pC (ΔhadC/pC) has kept the relative amounts of the α- and α’-MAs close to the wt values and has partially restored percentage of epoxy-MAs (9%) concomitantly to a decrease of compound X (4%).
Table 3. Inverse quantitative relationship between the epoxy-MAs and compound X.
Lipids were quantitated by high-performance thin layer chromatography (HPTLC). Values are average percentages of technical quadruplicates of total extractable lipids ± σ; nd = not detected.
| α | α’ | epoxy | X | |
|---|---|---|---|---|
| wt | 63.14 ± 1.89 | 22.50 ± 1.47 | 14.36 ± 0.79 | nd |
| ΔhadC | 84.09 ± 0.24 a | 4.94 ± 0.74 | 11.02 ± 0.83 | |
| ΔhadC/pC | 68.44 ± 2.08 | 19.27 ± 1.09 | 8.57 ± 0.91 | 3.73 ± 0.52 |
a Additive percentage of both α- and α’-mycolates; α, α’, epoxy and X as in Fig 10.
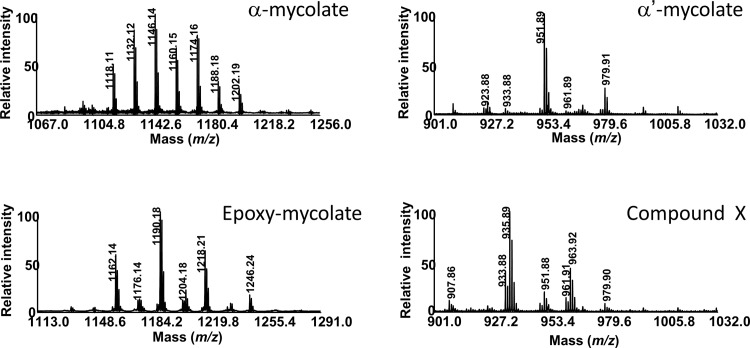
MALDI-TOF MS comparative analyses indicated that the mass spectra of α-, α’- and epoxy-MAs from the wt and ΔhadC mutants displayed similar signals with some differences in the odd/even ratios (data not shown), that may reflect a disruption in the protein-protein interactions between the dehydratases of FAS-II and methyltransferases that introduce unsaturations (double bonds, cyclopropanes) in MAs [28], as recently proposed for M. tuberculosis ΔhadC mutant [18]. As shown in Fig 11, the mass spectrum of the purified compound X showed [M+Na]+ ion-peaks in the mass region of α’-MAs, at m/z 907.8 (C59:2), 933. 8 (C61:3), 935.8 (C61:2), 951.8 (C62:1), 961.9 (C63:3), 963.9 (C63:2). NMR analyses indicated similar structures, with the expected resonances [31] for the α-, α’- and epoxy-MAs, whereas the NMR spectrum of compound X did not exhibit the characteristic O-methyl signal from the ester group (at 3.7 ppm), and those at 2.4 and 3.6 ppm, assignable to the proton resonances at C-2 and C-3 positions in MAs, respectively (data not shown). These data therefore indicated the absence of a “mycolic motif” in compound X found chemically highly unstable. Nevertheless, the decrease of the amount of epoxy-MAs observed for the ΔhadC mutant (ΔhadC/pGBT), which was partially complemented by the pC plasmid (ΔhadC/pC), and which was correlated with a concomitant increase of compound X (Fig 10, Table 3) suggests a biosynthetic relationship between the two compounds.
Fig 11. MALDI-TOF mass spectra of the purified compound X, α-, α’- and epoxy-mycolates of M.smegmatis ΔhadC mutant.
Values indicate the masses of sodium adducts (M+23).
Discussion
Mycolic acids (MAs), the major components of the mycomembrane, are essential for the viability and/or virulence of pathogenic mycobacteria [13,46]. The essential dehydratase step in the biosynthesis of MAs is performed by the HadA-HadB and HadB-HadC complexes [14,15]. The HadB subunit bears the catalytic domain whereas HadA and HadC determine the substrate specificity [14,47]. In this work we showed, that in M. smegmatis, the inactivation of hadC and hadA, both separately and together, was compatible with cell viability. Therefore, either in vivo HadB, as a homodimer, could perform the essential dehydration step for the biosynthesis of mycolates or there may be protein(s) that would possess(es) function redundant to those of HadA and/or HadC. The later explanation is supported by phylogenetic studies indicating that Rv0504c (in M. tuberculosis) and MSMEG_0948 (in M. smegmatis) are paralogs of HadA and HadC ([28], our unpublished data). Interestingly, a gene encoding a functional HadAB-like complex (MSMEG_6754) has been recently identified in M. smegmatis, whose overexpression suppresses the lethality of a hadB mutation [48]. We have measured the expression of both MSMEG_0948 and MSMEG_6745 in the mutant backgrounds (S1 Fig). Although both genes were actually found down-regulated, one cannot exclude that this level of expression was sufficient to compensate for the loss of HadA and HadC.
Although not essential, the inactivation of hadC dramatically impacts the physiology and fitness of the bacterium. The inactivation of hadA alone has generally little or no impact in the tested conditions but when combined with the hadC deletion, the deletion of hadA could exacerbate the phetotype of the hadC mutant. The effects were both unspecific and specific. One of the unspecific effects caused by the absence of HadC is the lowering of the hydrophobicity of the cell. Accordingly mutant cells did not aggregate as much as wt cells and did give a smooth colony morphotype, both characteristics usually correlate with an avirulent phenotype in pathogenic mycobacteria species [49,50,51,52]. Similarly, the ability of the hadC mutant cells to slide on the surface of growth medium [53] was also impaired, likely because the more hydrophilic envelope of the mutant would interact more with the substratum, preventing the sliding [35]. Similarly, the ability of the hadC mutant to develop biofilms was also affected, probably due to the reduced capacity of bacteria cells to aggregate. Although the biological role of biofilms for the pathogenicity in mycobacteria is still an issue [54,55,56], there is a correlation between the structure of MAs, the ability of forming biofilms and the virulence potential [57,58]. Accordingly in M. tuberculosis H37Rv genetic background we recently observed [18] that the virulence of a hadC mutant was compromised as that of the avirulent M. tuberculosis H37Ra strain that naturally bears a frameshift mutation inactivating the hadC [59].
Thermal regulation of the fluidity of biological membranes allows optimal membrane function at different temperatures. This can be done by changing the relative amount and structures of various lipids [39]. Indeed there are several reports showing that MAs structures are regulated by temperature with the ratio of saturation/unsaturation and the relative presence of functional groups in the MAs as well as the chain lengths of MAs being critical determinants of the fluidity of the mycomembrane [38,60,61,62,63]. Our data indicated a change in the sensitivity of the hadC and hadAC mutants to either cold- or high-temperatures. Expectedly, the MA profile of the hadC mutant was clearly altered, with notably the reduction of the synthesis of epoxy-MAs and the concomitant emergence of an unstable molecule (compound X), suggesting a possible biosynthetic filiation between the two compounds. Consistent with our findings, both HadC and HadA subunits have proved to be essential for the solubility and the stability of the HadB-containing enzymes in mycobacteria in vivo [14]. Furthermore, there are evidences for a functional interdependence between methyltransferases involved in the addition of functional groups and that of HadAB/HadBC enzymes [19,28,64,65]. Therefore, in the absence of HadC and HadA subunits, structural modifications that might be crucial to adapt to stresses, such as temperature changes, would no longer be introduced. Alternatively or additionally, as physical interactions between the proteins of the FAS-II system have been reported [65,66], the absence of HadC and HadA subunits might affect the stability of the whole biosynthesis complex in such a way that the synthesis of MAs would be compromised at high temperature.
The alteration of the permeability barrier in the hadA and hadC mutants was expected to impact the susceptibility of the bacteria to exogenous toxic compounds. Indeed the hadC mutant was more susceptible to Isoniazid (INH), Ethionamid (ETH) and Rifampicin (RIF) three anti-tubercular drugs currently used, and to Vancomycin (VAN). The susceptibility of the hadC mutant to RIF and VAN was exacerbated by a simultaneous deletion of hadA. In contrast, the susceptibility of the mutants to the anti-tubercular drug Ethambutol (EMB) was not changed. VAN is a large hydrophilic molecule that cannot diffuse through the lipophilic outer membrane lipid bilayer and as such cannot be used against gram negative pathogens. The higher susceptibility of the hadC and hadAC mutants to VAN was likely due to the lower hydrophobicity of their mycomembrane, allowing a better diffusion of the drug to reach its target, the growing peptidoglycan layer [67]. The higher susceptibility to RIF, a large hydrophobic molecule, might be explained by a higher fluidity of the mycomembrane in the mutants, permitting a better global diffusion of the drug [42,68]. Indeed, the inactivation of the hadC gene in M. tuberculosis results in the production of shorter MAs with higher unsaturation degree, two characteristics promoting an increased fluidity of the mycomembrane [18]. Interestingly, although EMB, INH and ETH are all small hydrophilic molecules, the hadC mutant was more susceptible to INH and ETH only. As this type of molecules likely diffuses through porins [42,69,70], the efficiency of their penetration into the cell would be less sensitive to the variation of hydrophobicity of the mycomembrane. Therefore the differential response of the mutant regarding the three drugs is probably more related to their modes of action. EMB targets the arabinogalactan biosynthesis pathway [44] whereas INH and ETH target InhA, an enzyme of the FAS-II complex [43]. The absence of HadC might affect the overall structure of the FAS-II complex allowing a better accessibility of InhA to the drugs.
The low fitness as well as the higher susceptibility to drugs of the hadC and hadAC mutants suggest that targeting HadC and HadA could make the bacterium more susceptible to the natural host defenses and potentiate the activity of current anti-tubercular drugs. The outcome would be the possibility of reducing the drug dosage, limiting therefore the toxic side-effects as well as a shortening of the treatment. In fine that should improve the poor compliance with medical treatments, the main reason for the occurrence of resistant strains [8,71]. Actually, Thioacetazone and Isoxyl drugs both target HadAB enzyme and possibly HadBC [64,72,73]. They were both used as anti-tubercular agents but barely now because of a low efficacy (for Isoxyl [20] or toxic side-effects (for Thioacetazone [21]). However, more efficient and less toxic derivatives of both drugs have been reported [72,74,75]. In addition using the hadC and/or hadAC mutant(s) instead of the wt strain in an exhaustive drug screening might lead to the discovery of not only new anti-tubercular drugs but also to drugs already used against other pathogens that might also be now efficient against M. tuberculosis. Such a strategy based on the targeting of non-essential genes to weaken bacteria might be applied to any pathogens, as well.
Supporting Information
Error bars are SEM from three biological triplicates. The expression (log2) in the different mutants was relative to the wt strain values (wt/pGBT) and measured by RT-qPCR. For each sample, 1 μg of RNA was reverse transcribed using random hexamers and Supercript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. qPCR on purified cDNA was performed using KAPA SYBRFAST qPCR Master Mix universal (CliniSciences) and appropriate primer sets (S1 Table). qPCR in technical duplicate was performed in a Bio-Rad CFX96 thermocycler with the following protocol: denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 3 s and annealing/elongation with data collection at 60°C for 20 s. Standard curves and melting curves were drawn to check for the amplification efficiency and the specificity of each primer pairs, respectively. The mean threshold cycle (CT) value was normalized against sigA CT. The fold difference of expression was calculated using the Pfaffl method (Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29: e45).
(TIF)
(DOCX)
Acknowledgments
We are grateful to Graham F. Hatfull for providing the mycobacteria Recombineering system, Marcus A. Horwitz for the pGB9.2 plasmid, Brian D. Robertson for the pMIND plasmid and Wladimir Malaga for the pJV53::hyg plasmid. We thank the members of the Daffé’s team for their constructive criticisms.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
SJ Ministère de l'Enseignement Supérieur et de la Recherche, JD a European Respiratory Society/Marie Curie Fellowship, AP a Marie Curie International Incoming Fellowship. This work was supported by institutional grants from the Centre National de la Recherche Scientifique.
References
- 1. WHO (2014) Global tuberculosis report 2013 World Health Organization. [Google Scholar]
- 2. Balaban NQ, Gerdes K, Lewis K, McKinney JD (2013) A problem of persistence: still more questions than answers? Nat Rev Microbiol 11: 587–591. [DOI] [PubMed] [Google Scholar]
- 3. Gengenbacher M, Kaufmann SH (2012) Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev 36: 514–532. 10.1111/j.1574-6976.2012.00331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young DB, Gideon HP, Wilkinson RJ (2009) Eliminating latent tuberculosis. Trends Microbiol 17: 183–188. 10.1016/j.tim.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 5. Falzon D, Jaramillo E, Wares F, Zignol M, Floyd K, Raviglione MC (2013) Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect Dis 13: 690–697. 10.1016/S1473-3099(13)70130-0 [DOI] [PubMed] [Google Scholar]
- 6. Migliori GB, Sotgiu G, Gandhi NR, Falzon D, DeRiemer K, Centis R, et al. (2013) Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur Respir J 42: 169–179. 10.1183/09031936.00136312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, et al. (2009) Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest 136: 420–425. 10.1378/chest.08-2427 [DOI] [PubMed] [Google Scholar]
- 8. D.A. M (1998) How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. INT J TUBERC LUNG DIS 2: 10–15. [PubMed] [Google Scholar]
- 9. Fattorini L, Piccaro G, Mustazzolu A, Giannoni F (2013) Targeting dormant bacilli to fight tuberculosis. Mediterranean journal of hematology and infectious diseases 5: e2013072 10.4084/MJHID.2013.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willand N, Dirie B, Carette X, Bifani P, Singhal A, Desroses M, et al. (2009) Synthetic EthR inhibitors boost antituberculous activity of ethionamide. Nature medicine 15: 537–544. 10.1038/nm.1950 [DOI] [PubMed] [Google Scholar]
- 11. Lechartier B, Rybniker J, Zumla A, Cole ST (2014) Tuberculosis drug discovery in the post-post-genomic era. EMBO molecular medicine 6: 158–168. 10.1002/emmm.201201772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daffe M, Draper P (1998) The envelope layers of mycobacteria with reference to their pathogenicity. Advances in microbial physiology 39: 131–203. [DOI] [PubMed] [Google Scholar]
- 13. Marrakchi H, Laneelle MA, Daffe M (2014) Mycolic acids: structures, biosynthesis, and beyond. Chem Biol 21: 67–85. 10.1016/j.chembiol.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 14. Sacco E, Covarrubias AS, O'Hare HM, Carroll P, Eynard N, Jones TA, et al. (2007) The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 104: 14628–14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown AK, Bhatt A, Singh A, Saparia E, Evans AF, Besra GS (2007) Identification of the dehydratase component of the mycobacterial mycolic acid-synthesizing fatty acid synthase-II complex. Microbiology 153: 4166–4173. [DOI] [PubMed] [Google Scholar]
- 16. Sassetti CM, Boyd DH, Rubin EJ (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Molecular microbiology 48: 77–84. [DOI] [PubMed] [Google Scholar]
- 17. Zhang YJ, Ioerger TR, Huttenhower C, Long JE, Sassetti CM, Sacchettini JC, et al. (2012) Global assessment of genomic regions required for growth in Mycobacterium tuberculosis. PLoS Pathog 8: e1002946 10.1371/journal.ppat.1002946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slama N, Jamet S, Frigui W, Pawlik A, Bottai D, Laval F, et al. (2015) The changes in mycolic acid structures caused by hadC mutation have a dramatic effect on the virulence of Mycobacterium tuberculosis. Molecular microbiology. [DOI] [PubMed] [Google Scholar]
- 19. Grzegorzewicz AE, Kordulakova J, Jones V, Born SE, Belardinelli JM, Vaquie A, et al. (2012) A common mechanism of inhibition of the Mycobacterium tuberculosis mycolic acid biosynthetic pathway by isoxyl and thiacetazone. The Journal of biological chemistry 287: 38434–38441. 10.1074/jbc.M112.400994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Urbancik B (1970) Clinical experience with thiocarlide (Isoxyl). Antibiotica et chemotherapia Fortschritte Advances Progres 16: 117–123. [DOI] [PubMed] [Google Scholar]
- 21. Caminero JA, Sotgiu G, Zumla A, Migliori GB (2010) Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. The Lancet Infectious diseases 10: 621–629. 10.1016/S1473-3099(10)70139-0 [DOI] [PubMed] [Google Scholar]
- 22. Wallace RJ Jr., Nash Donald R., Tsukamura Michio, Blacklock Zeta M., and Silcox Vella A. (1988) Human disease due to Mycobacterium smegmatis. The Journal of infectious diseases 158: 52–58. [DOI] [PubMed] [Google Scholar]
- 23. Bercovier H, Vincent V (2001) Mycobacterial infections in domestic and wild animals due to Mycobacterium marinum, M. fortuitum, M. chelonae, M. porcinum, M. farcinogenes, M. smegmatis, M. scrofulaceum, M. xenopi, M. kansasii, M. simiae and M. genavense. Revue scientifique et technique 20: 265–290. [DOI] [PubMed] [Google Scholar]
- 24. Brown-Elliott BA, Wallace RJ Jr. (2002) Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clinical microbiology reviews 15: 716–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gutierrez MC, Supply P, Brosch R (2009) Pathogenomics of mycobacteria. Genome Dyn 6: 198–210. 10.1159/000235772 [DOI] [PubMed] [Google Scholar]
- 26. Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR Jr., (1990) Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4: 1911–1919. [DOI] [PubMed] [Google Scholar]
- 27. van Kessel JC, Hatfull GF (2007) Recombineering in Mycobacterium tuberculosis. Nat Methods 4: 147–152. [DOI] [PubMed] [Google Scholar]
- 28. Jamet S, Quentin Y, Coudray C, Texier P, Laval F, Daffe M, et al. (2015) Evolution of Mycolic Acid Biosynthesis Genes and Their Regulation during Starvation in Mycobacterium tuberculosis. Journal of bacteriology 197: 3797–3811. 10.1128/JB.00433-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kulka K, Hatfull G, Ojha AK (2012) Growth of Mycobacterium tuberculosis biofilms. Journal of visualized experiments: JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu WX, Zhang L, Mai JT, Peng RC, Yang EZ, Peng C, et al. (2014) The Wag31 protein interacts with AccA3 and coordinates cell wall lipid permeability and lipophilic drug resistance in Mycobacterium smegmatis. Biochemical and biophysical research communications 448: 255–260. 10.1016/j.bbrc.2014.04.116 [DOI] [PubMed] [Google Scholar]
- 31. Laval F, Haites R, Movahedzadeh F, Lemassu A, Wong CY, Stoker N, et al. (2008) Investigating the function of the putative mycolic acid methyltransferase UmaA: divergence between the Mycobacterium smegmatis and Mycobacterium tuberculosis proteins. The Journal of biological chemistry 283: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 32. Daffe M, Laneelle MA, Asselineau C, Levy-Frebault V, David H (1983) [Taxonomic value of mycobacterial fatty acids: proposal for a method of analysis]. Annales de microbiologie 134B: 241–256. [PubMed] [Google Scholar]
- 33. Laval F, Laneelle MA, Deon C, Monsarrat B, Daffe M (2001) Accurate molecular mass determination of mycolic acids by MALDI-TOF mass spectrometry. Analytical chemistry 73: 4537–4544. [DOI] [PubMed] [Google Scholar]
- 34. Dahl JL, Lau Bonilla D (2011) The wag31 gene of Mycobacterium tuberculosis is positively regulated by the stringent response. FEMS microbiology letters 319: 153–159. 10.1111/j.1574-6968.2011.02278.x [DOI] [PubMed] [Google Scholar]
- 35. Recht J, Martinez A, Torello S, Kolter R (2000) Genetic analysis of sliding motility in Mycobacterium smegmatis. Journal of bacteriology 182: 4348–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camacho LR, Constant P, Raynaud C, Laneelle MA, Triccas JA, Gicquel B, et al. (2001) Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. The Journal of biological chemistry 276: 19845–19854. [DOI] [PubMed] [Google Scholar]
- 37. Nikaido H, Vaara M (1985) Molecular basis of bacterial outer membrane permeability. Microbiological reviews 49: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu J, Barry CE 3rd, Besra GS, Nikaido (1996) Mycolic acid structure determines the fluidity of the mycobacterial cell wall. The Journal of biological chemistry 271: 29545–29551. [DOI] [PubMed] [Google Scholar]
- 39. Magnuson K, Jackowski S, Rock CO, Cronan JE Jr., (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiological reviews 57: 522–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jarlier V, Nikaido H (1990) Permeability barrier to hydrophilic solutes in Mycobacterium chelonei. Journal of bacteriology 172: 1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen L, Pieters J (2009) Mycobacterial subversion of chemotherapeutic reagents and host defense tactics: challenges in tuberculosis drug development. Annual review of pharmacology and toxicology 49: 427–453. 10.1146/annurev-pharmtox-061008-103123 [DOI] [PubMed] [Google Scholar]
- 42. Brennan PJ, Nikaido H (1995) The envelope of mycobacteria. Annual review of biochemistry 64: 29–63. [DOI] [PubMed] [Google Scholar]
- 43. Vilcheze C, Jacobs WR Jr. (2007) The mechanism of isoniazid killing: clarity through the scope of genetics. Annual review of microbiology 61: 35–50. [DOI] [PubMed] [Google Scholar]
- 44. Takayama K, Kilburn JO (1989) Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrobial agents and chemotherapy 33: 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Danilchanka O, Pavlenok M, Niederweis M (2008) Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrobial agents and chemotherapy 52: 3127–3134. 10.1128/AAC.00239-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Daffe M (2015) The cell envelope of tubercle bacilli. Tuberculosis. [DOI] [PubMed] [Google Scholar]
- 47. Biswas R, Dutta A, Dutta D, Hazra D, Banerjee DR, Basak A, et al. (2015) Crystal structure of dehydratase component HadAB complex of mycobacterial FAS-II pathway. Biochemical and biophysical research communications 458: 369–374. 10.1016/j.bbrc.2015.01.119 [DOI] [PubMed] [Google Scholar]
- 48. Carrere-Kremer S, Blaise M, Singh VK, Alibaud L, Tuaillon E, Halloum I, et al. (2015) A new dehydratase conferring innate resistance to thiacetazone and intra-amoebal survival of Mycobacterium smegmatis. Molecular microbiology 96: 1085–1102. 10.1111/mmi.12992 [DOI] [PubMed] [Google Scholar]
- 49. Kansal RG, Gomez-Flores R, Mehta RT (1998) Change in colony morphology influences the virulence as well as the biochemical properties of the Mycobacterium avium complex. Microbial pathogenesis 25: 203–214. [DOI] [PubMed] [Google Scholar]
- 50. Catherinot E, Clarissou J, Etienne G, Ripoll F, Emile JF, Daffe M, et al. (2007) Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infection and immunity 75: 1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Belisle JT, Brennan PJ (1989) Chemical basis of rough and smooth variation in mycobacteria. Journal of bacteriology 171: 3465–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van der Woude AD, Sarkar D, Bhatt A, Sparrius M, Raadsen SA, Boon L, et al. (2012) Unexpected link between lipooligosaccharide biosynthesis and surface protein release in Mycobacterium marinum. The Journal of biological chemistry 287: 20417–20429. 10.1074/jbc.M111.336461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martinez A, Torello S, Kolter R (1999) Sliding motility in mycobacteria. Journal of bacteriology 181: 7331–7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marsollier L, Brodin P, Jackson M, Kordulakova J, Tafelmeyer P, Carbonnelle E, et al. (2007) Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS pathogens 3: e62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lenaerts AJ, Hoff D, Aly S, Ehlers S, Andries K, Cantarero L, et al. (2007) Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrobial agents and chemotherapy 51: 3338–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Islam MS, Richards JP, Ojha AK (2012) Targeting drug tolerance in mycobacteria: a perspective from mycobacterial biofilms. Expert review of anti-infective therapy 10: 1055–1066. 10.1586/eri.12.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sambandan D, Dao DN, Weinrick BC, Vilcheze C, Gurcha SS, Ojha A, et al. (2013) Keto-mycolic acid-dependent pellicle formation confers tolerance to drug-sensitive Mycobacterium tuberculosis. mBio 4: e00222–00213. 10.1128/mBio.00222-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ojha A, Anand M, Bhatt A, Kremer L, Jacobs WR Jr., Hatfull GF (2005) GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 123: 861–873. [DOI] [PubMed] [Google Scholar]
- 59. Zheng H, Lu L, Wang B, Pu S, Zhang X, Zhu G, et al. (2008) Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS One 3: e2375 10.1371/journal.pone.0002375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kremer L, Guerardel Y, Gurcha SS, Locht C, Besra GS (2002) Temperature-induced changes in the cell-wall components of Mycobacterium thermoresistibile. Microbiology 148: 3145–3154. [DOI] [PubMed] [Google Scholar]
- 61. Stratton HM, Brooks PR, Carr EL, Seviour RJ (2003) Effects of culture conditions on the mycolic acid composition of isolates of Rhodococcus spp. from activated sludgefoams. Systematic and applied microbiology 26: 165–171. [DOI] [PubMed] [Google Scholar]
- 62. Baba T, Kaneda K, Kusunose E, Kusunose M, Yano I (1989) Thermally adaptive changes of mycolic acids in Mycobacterium smegmatis. Journal of biochemistry 106: 81–86. [DOI] [PubMed] [Google Scholar]
- 63. Alibaud L, Alahari A, Trivelli X, Ojha AK, Hatfull GF, Guerardel Y, et al. (2010) Temperature-dependent regulation of mycolic acid cyclopropanation in saprophytic mycobacteria: role of the Mycobacterium smegmatis 1351 gene (MSMEG_1351) in CIS-cyclopropanation of alpha-mycolates. The Journal of biological chemistry 285: 21698–21707. 10.1074/jbc.M110.125724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grzegorzewicz AE, Eynard N, Quemard A, North EJ, Margolis A, Lindenberger JJ, et al. (2015) Covalent modification of the FAS-II dehydratase by Isoxyl and Thiacetazone. ACS infectious diseases 1: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cantaloube S, Veyron-Churlet R, Haddache N, Daffe M, Zerbib D (2011) The Mycobacterium tuberculosis FAS-II dehydratases and methyltransferases define the specificity of the mycolic acid elongation complexes. PLoS One 6: e29564 10.1371/journal.pone.0029564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Veyron-Churlet R, Guerrini O, Mourey L, Daffe M, Zerbib D (2004) Protein-protein interactions within the Fatty Acid Synthase-II system of Mycobacterium tuberculosis are essential for mycobacterial viability. Mol Microbiol 54: 1161–1172. [DOI] [PubMed] [Google Scholar]
- 67. Healy VL, Lessard IA, Roper DI, Knox JR, Walsh CT (2000) Vancomycin resistance in enterococci: reprogramming of the D-ala-D-Ala ligases in bacterial peptidoglycan biosynthesis. Chemistry & biology 7: R109–119. [DOI] [PubMed] [Google Scholar]
- 68. Liu J, Nikaido H (1999) A mutant of Mycobacterium smegmatis defective in the biosynthesis of mycolic acids accumulates meromycolates. Proceedings of the National Academy of Sciences of the United States of America 96: 4011–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stahl C, Kubetzko S, Kaps I, Seeber S, Engelhardt H, Niederweis M (2001) MspA provides the main hydrophilic pathway through the cell wall of Mycobacterium smegmatis. Molecular microbiology 40: 451–464. [DOI] [PubMed] [Google Scholar]
- 70. Trias J, Benz R (1994) Permeability of the cell wall of Mycobacterium smegmatis. Molecular microbiology 14: 283–290. [DOI] [PubMed] [Google Scholar]
- 71. Weiss LA, Stallings CL (2013) Essential roles for Mycobacterium tuberculosis Rel beyond the production of (p)ppGpp. Journal of bacteriology 195: 5629–5638. 10.1128/JB.00759-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coxon GD, Craig D, Corrales RM, Vialla E, Gannoun-Zaki L, Kremer L (2013) Synthesis, antitubercular activity and mechanism of resistance of highly effective thiacetazone analogues. PLoS One 8: e53162 10.1371/journal.pone.0053162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American journal of respiratory and critical care medicine 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 74. Shahab FM, Kobarfard F, Shafaghi B, Dadashzadeh S (2010) Preclinical pharmacokinetics of KBF611, a new antituberculosis agent in mice and rabbits, and comparison with thiacetazone. Xenobiotica; the fate of foreign compounds in biological systems 40: 225–234. 10.3109/00498250903461411 [DOI] [PubMed] [Google Scholar]
- 75. Phetsuksiri B, Baulard AR, Cooper AM, Minnikin DE, Douglas JD, Besra GS, et al. (1999) Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis. Antimicrobial agents and chemotherapy 43: 1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Error bars are SEM from three biological triplicates. The expression (log2) in the different mutants was relative to the wt strain values (wt/pGBT) and measured by RT-qPCR. For each sample, 1 μg of RNA was reverse transcribed using random hexamers and Supercript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. qPCR on purified cDNA was performed using KAPA SYBRFAST qPCR Master Mix universal (CliniSciences) and appropriate primer sets (S1 Table). qPCR in technical duplicate was performed in a Bio-Rad CFX96 thermocycler with the following protocol: denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 3 s and annealing/elongation with data collection at 60°C for 20 s. Standard curves and melting curves were drawn to check for the amplification efficiency and the specificity of each primer pairs, respectively. The mean threshold cycle (CT) value was normalized against sigA CT. The fold difference of expression was calculated using the Pfaffl method (Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research 29: e45).
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.