Abstract
This study aimed at analysing the erosive potential of 30 substances (drinks, candies, and medicaments) on deciduous enamel, and analyse the associated chemical factors with enamel dissolution. We analysed the initial pH, titratable acidity (TA) to pH 5.5, calcium (Ca), inorganic phosphate (Pi), and fluoride (F) concentration, and degree of saturation ((pK -pI)HAP, (pK -pI)FAP, and (pK−pI)CaF2) of all substances. Then, we randomly distributed 300 specimens of human deciduous enamel into 30 groups (n = 10 for each of the substances tested. We also prepared 20 specimens of permanent enamel for the sake of comparison between the two types of teeth, and we tested them in mineral water and Coca-Cola®. In all specimens, we measured surface hardness (VHN: Vickers hardness numbers) and surface reflection intensity (SRI) at baseline (SHbaseline and SRIbaseline), after a total of 2 min (SH2min) and after 4 min (SH4min and SRI4min) erosive challenges (60 ml of substance for 6 enamel samples; 30°C, under constant agitation at 95 rpm). There was no significant difference in SHbaseline between deciduous and permanent enamel. Comparing both teeth, we observed that after the first erosive challenge with Coca-Cola®, a significantly greater hardness loss was seen in deciduous (−90.2±11.3 VHN) than in permanent enamel (−44.3±12.2 VHN; p = 0.007), but no differences between the two types of teeth were observed after two challenges (SH4min). After both erosive challenges, all substances except for mineral water caused a significant loss in relative surface reflectivity intensity, and most substances caused a significant loss in surface hardness. Multiple regression analyses showed that pH, TA and Ca concentration play a significant role in initial erosion of deciduous enamel. We conclude that drinks, foodstuffs and medications commonly consumed by children can cause erosion of deciduous teeth and erosion is mainly associated with pH, titratable acidity and calcium concentration in the solution.
Introduction
Dental erosion is the acid dissolution of dental hard tissues caused by multiple factors. One of these factors are acidic substances in the diet (nutrition-related factors) [1]. Erosion can occur in both deciduous and permanent teeth [2–5]. It starts with a softening of the tooth surface (enamel) and progresses to extensive loss of tooth substance when contact with the acids continues [6–8]. Various dietary substances and medicaments have been associated with dental erosion [9–15], and many studies have investigated which chemical factors are most significantly associated with enamel dissolution [16–23]. However, many studies have focused on permanent teeth, and more detailed investigations should be carried out to find out the effect of different dietary substances on deciduous enamel, and which chemical factors will play a role on erosive demineralization of these teeth.
Deciduous enamel is histologically different to permanent enamel. Basically, prism arrangements in deciduous and permanent enamel are similar [24], but the prisms in deciduous enamel are smaller, with more complete boundaries, and are more widely spread than those in permanent enamel [25]. Also, the prisms in deciduous enamel are more gently curved, and have slightly less pronounced Hunter-Schreger bands [25]. Deciduous enamel is considerably less mineralized [26], has greater total carbonate content [27], and a higher organic content [28] than permanent enamel. These histological differences could also lead to different erosion patterns in deciduous and permanent enamel, so it is important to fully investigate the effect of different dietary substances on deciduous enamel.
Moreover, in a study by Ganss et al. [29], children who initially presented with erosive lesions in deciduous teeth had a significantly greater risk (3.9-fold) of having erosive lesions in their permanent teeth. Similar results were also reported by Harding et al. [30], who showed that 5-year-old children who present with severe erosive tooth wear in deciduous teeth are 5 times more likely to present erosive tooth wear in permanent teeth at the age of 12 years. It is, thus, suggested that tooth wear in deciduous teeth ought to be regarded as a predictive factor for wear in permanent teeth, and health professionals should be fully aware of the erosive effect of different dietary substances on deciduous enamel in order to be able to give children and parents the best oral health recommendations. Consequently, the aim of this study was to analyse the potential of different substances to cause erosion of deciduous enamel, and to determine which chemical factors are most strongly associated with enamel dissolution in deciduous teeth.
Material and Methods
Preparation of enamel specimens
From a pool of extracted teeth, we randomly selected 150 caries-free human deciduous molars and 20 (permanent) premolars. The teeth were extracted by dental practitioners in Switzerland. Before the extraction, the patients and their parents were informed about the use of their teeth for research purposes and their oral consent was obtained. Because we are using teeth from a pooled bio-bank, the local ethics committee categorized the samples as “irreversibly anonymised”, and no previous approval was necessary. The crowns of all teeth were separated from the roots, and cut in two halves (into buccal and lingual surfaces). The enamel slabs were embedded in acrylic resin blocks (Paladur®, Bad Homburg, Germany) using two planar parallel moulds of 8 mm and 0.2 mm. The latter mould was removed and the blocks were then serially ground (LaboPol-21 rotating polishing machine, Struers, Ballerup, Denmark) with silicon carbide paper discs (grade 18 μm for 30 s, 8 μm grade for 30 s, 5 μm grade for 1 min, 3 μm diamond abrasive paste for 1 min), removing 200 μm of enamel from each specimen. After each polishing step, the resin blocks were rinsed and sonicated for 2 min in tap water and all specimens were then stored in a saturated mineral solution (1.5 mM CaCl2, 1.0 mM KH2PO4, 50 mM NaCl, pH 7.0 [31]) until the time of the experiment. The 300 deciduous enamel samples were randomly distributed into 30 groups (n = 10 for each of the substances tested). The permanent enamel samples were divided into two groups (n = 10).
Substances tested
In the present study, we tested 30 substances, ranging from drinks, candies, and medicaments frequently used by children and young adolescents (Table 1). For the experiment, all carbonated drinks, candies, and medicaments were pre-treated as follows. The carbonated drinks were degassed by stirring at room temperature (10 min). The candy was dissolved in deionized water (5.2 g candy / 10 ml water), under constant mixing at 45°C; the resulting candy solution was then cooled and used at 30°C for the experiments. The medicaments and concentrated drinks were all prepared with deionized water according to the manufacturer’s instructions. The chewing gum was ground for 5 min (2 g chewing gum in 10 ml of deionized water) using a mortar and pestle, and the resulting solution was used in the experiment. The fruits were squeezed/crushed and the juice was then passed through a sieve (1.0 x 1.0 mm).
Table 1. Basic information on the substances tested and their chemical parameters: pH, titratable acidity to pH 5.5 (mmol OH−/l to pH 5.5), calcium [Ca], inorganic phosphate [Pi], and fluoride [F] concentrations, degree of saturation with respect to hydroxyapatite ((pK−pI)HAP), with respect to fluorapatite ((pK−pI)FAP), and with respect to calcium fluoride ((pK−pI)CaF2).
| Substance | Brand name/producer | Flavour | Erosion-related ingredients* | pH | mmol OH−/l to pH 5.5 | [Ca] (mmol/l) | [Pi] (mmol/l) | [F] (ppm) | (pK−pI)HAP | (pK−pI)FAP | (pK−pI)CaF2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MINERAL WATER | |||||||||||
| Mineral water | Valser®, Coca-Cola Company | – | – | 6.53 | – | 10.57 | < 0.01 | 0.58 | −0.35 | 3.47 | –0.82 |
| SOFT DRINKS | |||||||||||
| Coca-Cola® | Coca-Cola®, Coca-Cola Company | Cola | Phosphoric acid, carbonic acid, | 2.55 | 9.32 | 0.53 | 5.39 | 0.05 | −20.59 | −14.31 | –5.45 |
| Pepsi Cola® | Pepsi Cola®, PepsiCo | Cola | Phosphoric acid, citric acid, carbonic acid, and flavours | 2.51 | 8.30 | 0.22 | 5.38 | <0.05 | −22.83 | −17.09 | −7.00 |
| Fanta® Regular | Fanta®, Coca−Cola Company | Orange | Orange fruit, citric acid, carbonic acid, and flavours | 2.59 | 36.19 | 0.56 | 0.14 | <0.05 | −24.76 | −18.65 | −5.64 |
| Sprite® | Sprite®, Coca−Cola Company | Lemon | Carbonic acid, citric acid, acidity regulator, and flavours | 2.57 | 31.56 | 0.47 | < 0.01 | <0.05 | −34.71 | −28.78 | −6.12 |
| Guaraná Antártica® | Antártica | Guaraná | Citric acid and carbonic acid | 2.62 | 15.55 | 0.03 | < 0.01 | <0.05 | −36.96 | −31.02 | −7.21 |
| Rivella® Red | Rivella | NA | Milk serum, carbonic acid, citric acid, and flavours | 3.28 | 32.88 | 2.95 | 2.72 | 0.07 | −12.52 | −6.41 | −3.61 |
| Ice tea | NA, Coop (supermarket in Switzerland) | NA | Black tea extract, citric and ascorbic acids | 2.43 | 24.36 | 0.03 | 0.06 | 0.88 | −33.58 | −26.06 | −4.45 |
| Ice tea peach | Lipton, Unilever | Peach | Black tea extract and peach juice | 2.65 | 25.15 | 0.08 | 0.13 | 0.55 | −28.39 | −21.12 | −4.01 |
| FRUITS, JUICES AND SMOOTHIES | |||||||||||
| Kiwi (fruit) | NA | NA | NA | 3.24 | 159.81 | 1.06 | 3.40 | <0.05 | −14.53 | −9.93 | −7.12 |
| Orange (fruit) | NA | NA | NA | 3.93 | 71.93 | 1.50 | 1.18 | <0.05 | −10.22 | −5.22 | −4.77 |
| Orange juice | Hohes C, Eckes AG | Orange | Orange juice | 3.63 | 83.56 | 2.11 | 1.58 | <0.05 | −11.32 | −5.89 | −4.38 |
| Apple juice | Ramseier Premium, Ramseier Suisse AG | Apple | Apple juice and pear juice | 3.24 | 70.30 | 1.17 | 1.62 | <0.05 | −15.23 | −9.44 | −4.68 |
| Apple juice for babies | Nestlè | Apple and pear | Apple juice, pear juice, vitamin C | 3.59 | 48.19 | 2.55 | 1.96 | 0.17 | −10.98 | −4.70 | −2.68 |
| Ribena® | Lucozade Ribena Suntory | Blackcurrant | Blackcurrant juice concentrate, citric acid, and vitamin C | 2.51 | 27.94 | 0.36 | 0.17 | 0.01 | −26.06 | −20.42 | −6.93 |
| Fruit smoothie | innocent | Kiwi, apple and limes | Apple juice, grape juice, kiwi juice, lime juice, and pineapple juice | 3.27 | 82.44 | 2.10 | 0.27 | <0.05 | −16.13 | −10.62 | −4.94 |
| YOGHURT | |||||||||||
| Forest berries yoghurt | NA, Migros (Supermarket in Switzerland) | Berries | Forest Berries; | 4.13 | 62.86 | 37.39 | 10.72 | <0.05 | −0.55 | 4.63 | −2.86 |
| SOUR CANDIES | |||||||||||
| Candy spray | Mega Mouth® Candy Spray, Bazooka Candy Brands International Ltd | NA | Citric acid | 2.14 | 441.75 | 0.12 | 0.16 | <0.05 | −31.67 | −26.76 | −9.65 |
| Sour candy | Haribo® Pommes, Haribo GmbH & Co.,Germany | Apple | Citric, malic, and tartaric acids | 2.46 | 88.10 | 0.07 | 0.12 | <0.05 | −30.57 | −24.64 | −7.18 |
| Sour chewing gum | Trident® Senses, Modelez | Mega Mystery | Citric acid, malic acid | 2.74 | 22.57 | 0.37 | 0.03 | <0.05 | −26.56 | −21.57 | −7.76 |
| SPORTS AND ENERGY DRINKS | |||||||||||
| Monster Energy Drink® | Monster Energy Drink®, Vertrieb Spar GmbH, Austria | NA | Citric, sorbic, carbonic, and benzoic acids, vitamin B, taurine | 3.35 | 62.39 | 0.07 | 0.03 | <0.05 | −25.05 | −19.38 | −5.82 |
| Red Bull® Energy Drink | Red Bull®, Red Bull GmbH, Austria | NA | Sodium citrate, carbonic acid, taurine, vitamin B | 3.35 | 67.76 | 1.41 | < 0.01 | 0.13 | −25.72 | −19.38 | −3.27 |
| Gatorade® | Gatorade®, PepsiCo | NA | Citric acid, flavours | 2.89 | 37.38 | 0.05 | 2.98 | 0.05 | −23.94 | −17.74 | −5.97 |
| MEDICAMENTS | |||||||||||
| Dafalgan® syrup for children | Bristol−Myers Squibb | NA | NA | 5.26 | 7.91 | 0.07 | < 0.01 | <0.05 | −15.16 | −11.65 | −6.37 |
| Mucosolvon® for children | Boehringer Ingelheim | NA | Benzoic acid | 3.13 | 14.43 | 0.01 | 0.01 | <0.05 | −31.47 | −26.41 | −8.21 |
| Fluimucil® Effervescent | Zambon Schweiz | NA | NA | 4.48 | 14.04 | 0.01 | < 0.01 | <0.05 | −29.35 | −25.55 | −8.26 |
| Tossamin® sugar free syrup | Novartis Consumer Health Schweiz | NA | Sorbic acid | 4.43 | 19.46 | 0.01 | 1.46 | <0.05 | −16.42 | −12.59 | −8.12 |
| Ventolin® syrup | Glaxo Smith Kline | NA | NA | 3.19 | 56.08 | 0.02 | < 0.01 | <0.05 | −36.98 | −32.35 | −8.85 |
| Claritine® syrup | MSD Merk Sharp & Dohme AG | NA | Peach aroma | 2.98 | 74.34 | 0.07 | < 0.01 | <0.05 | −37.13 | −32.23 | −8.74 |
| Maltofer® syrup | Vifor (International) AG | NA | NA | 4.90 | 5.48 | 0.12 | < 0.01 | <0.05 | −20.68 | −17.47 | −7.45 |
* Erosion-related ingredients are those listed on the packaging of each substance.
NA = not available.
When [Pi] values were <0.01mmol/l, exact values of 0.0001 mmol/l were used in the (pK−pI) calculations.
Chemical analysis of the substances
For the chemical analyses [22], we used 10 g of each solution at 30°C to measure the initial pH and the titratable acidity to pH 5.5 (total amount of base needed to raise the pH of the substance to 5.5). An automatic titrator (Toledo DL 53, Mettler Toledo, Electrode DG 101-SC, Software: LabX pro, Schwerzenbach, Switzerland) established the initial pH of the solutions, which were then individually titrated with 0.5 mol/l NaOH in steps of 0.02 ml [23]. Titratable acidity was calculated as the amount of base (mmol/L of sample) required to raise the pH to 5.5. Calcium (Ca) concentration was measured with the standard atomic absorption method, using an atomic absorption spectrometer with an air/acetylene flame. Lanthanum was added to all the products and standards (final end concentration 0.2%) to suppress interference from inorganic phosphates (Pi). Total Pi concentration was analysed by the ammonium molybdate method of Chen et al. (1956) [32]. Fluoride (F) concentration was determined using an F ion-specific electrode (Orion 960900, Boston, MA, USA). Before F measurement, we added total ionic strength adjustment buffer (TISAB) to all products and standard solutions (1:1 ratio), without previously neutralizing the substances. The concentrations of Ca and Pi are expressed in mmol/l and those of F in ppm. The degree of saturation (pK−pI) with respect to hydroxyapatite (HAP), fluorapatite (FAP), and calcium fluoride (CaF2) was calculated from the pH and the concentrations of Ca, Pi and F using a computer program [33]. This program assumes a solubility product for HAP of 10−58.5 and for FAP of 10−59.6 [34, 35]. The concentrations of Ca, Pi and F, the pH, and the titratable acidity were measured in duplicate.
Surface hardness measurement
The present method describes hardness measurements using nanoindentations. Surface hardness (SH) of each enamel specimen was determined with a Vickers diamond under a pressure of 50 mN for 15 s (Fischerscope HM 2000 XYp; Helmut Fischer, Hünenberg, Switzerland). A total of six baseline indentations were made at intervals of 50 μm. Further indentations next to the previous indentations were made following the experimental procedure. Vickers hardness was automatically calculated from the depth of the indentations by the computer program. The load resolution was ≤ 0.04 mN and the indentation depth was 600 nm for sound enamel and < 1000 nm for most softened specimens. The device allowed fully automatic measurements using a programmable x, y stage. The WIN-HCU software calculated SH. The SH value for each enamel slab was determined by calculating the average of six indentations.
Surface reflection intensity
For the surface reflection intensity (SRI) measurements, we used a recently developed table-top reflection device [36–38]. The device was connected to a computer running a specific software that registers the point of highest reflection intensity, which is expressed as a SRI value. We measured SRI initially (SRIbaseline) and after the second challenge (SRI4min), and from these SRI values, we calculated the relative percentage decrease in reflection intensity (rSRI) using the formula rSRIi = (100×(SRI4min−SRIbaseline)) / SRIbaseline. In practical terms, more negative rSRI values represent greater decrease in reflection intensity, which, in turn, represent more erosion of the enamel surface.
Study design
Immediately prior to the experimental procedures, the resin blocks were further polished with 1 μm diamond abrasive for 1 min (LaboPol-6, DP-Mol Polishing, DP-Stick HQ; Struers, Copenhagen, Denmark) to ensure the removal of possible remnants from storage. Initially, the samples were incubated in freshly collected human saliva (20 ml / 6 enamel samples, 3 h, 37°C, under constant shaking). For that, stimulated saliva was collected from one healthy adult donor (stimulated salivary flow rate 2.32 ml/min) by chewing on a piece of paraffin pellets (Fluka; Sigma-Aldrich Chemie GmbH, Munich, Germany) for 30 min. An approval from the institutional review board is not necessary for collecting saliva samples, so the local Ethical Committee (Kantonale Ethikkommission) waived the need for ethical approval. In the eyes of the Ethical Committee, when collecting saliva samples, we are only required to obtain the consent from the saliva expeditor, which can be done verbally. In our study, the saliva donor gave a verbal consent, since written consent was not required. The saliva was collected in an ice-cooled tube at least 1 h after the donor had consumed any food or drink [39, 40]. The samples were then carefully rinsed with tap water (50 s) and with deionized water (10 s), then dried with oil-free air (5 s). All enamel samples had their baseline SH and SRI individually measured (SHbaseline and SRIbaseline), after which they were subjected to two consecutive erosive challenges. Each erosive challenge consisted of individually immersing the specimens into the respective test substance (10 ml / sample) for 2 min at 30°C, under constant agitation (95 rpm). The samples were then taken out of the solution, washed (10 s) and dried (5 s), and a second SH measurement was performed (SH2min). Subsequently, the samples were submitted to another erosive challenge (2 min), rinsed, dried, and a final SH and SRI measurement was carried out (SH4min and SRI4min). A total of 10 deciduous enamel specimens were tested per substance (5 buccal and 5 lingual surfaces randomly chosen). In addition, the two groups containing the permanent enamel samples were also submitted to the same experimental protocol, and were treated with mineral water (n = 10) or Coca-Cola® (n = 10).
Statistical analyses
Wilcoxon’s signed rank tests were used to compare the SH and SRI values before and after immersion in the respective drink or solution. Changes in SH (ΔSH) were calculated as follows: for the first 2-min erosive challenge ΔSH2–0 = SH2min − SHbaseline; for the second 2-min erosive challenge ΔSH4–2 = SH4min − SH2min; and for the total 4-min erosive challenge ΔSH4–0 = SH4min−SHbaseline. Associations between the changes in SRI (rSRI, denoted as dependent variable), ΔSH (denoted as the dependent variable) and pH, titratable acidity, and Ca, Pi and F concentrations, HAP saturation, FAP saturation, CaF2 saturation (independent variables) were investigated using Spearman’s Correlation Coefficients. Since HAP and FAP saturation are not independent of pH, titratable acidity, and Ca, Pi and F concentrations, care was taken not to include them in the regression analyses. Multiple linear regression analyses were carried out to verify the association of ΔSH2−0 and ΔSH4−0 with pH, titratable acidity, Ca, Pi and F concentrations. Association between ΔSH4−0 and rSRI were investigated using spearman’s correlation coefficient and linear regression analysis. Furthermore, additional differences between deciduous and permanent enamel were verified using the Mann-Whitney U test. The significance level was set at 0.05 for all analyses.
Results
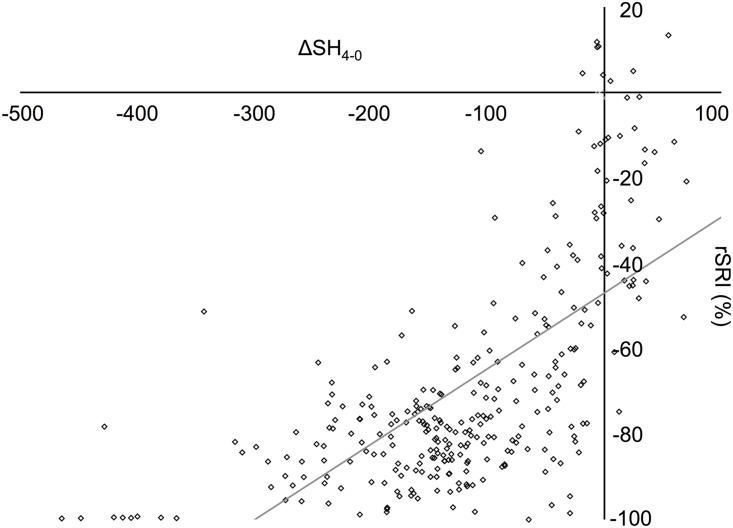
Table 1 presents the 30 substances and their chemical parameters. The SH values at baseline (SHbaseline), the mean SH loss (ΔSH) after the first (ΔSH2−0) and second (ΔSH4−2) erosive challenges, as well as the relative surface reflection intensity, are presented in Table 2. Most of the substances caused a significant decrease in SH after the first erosive challenge (p<0.05), with the exception of mineral water (negative control), ice tea peach, apple juice for babies, and some medicaments. Interestingly, during the second erosive challenge, only mineral water, yogurt and some medicaments caused no further loss of SH. After both erosive challenges, all substances caused significant loss in relative surface reflectivity intensity, except for mineral water (Table 2). There was a significant correlation (p < 0.001; ρ = 0.66) between loss in surface hardness (ΔSH4−0) and relative percentage decrease in reflection intensity (rSRI; Fig 1), with regression Eq (1) fitting the data:
| (1) |
Table 2. Mean and standard error of the mean (SEM) for surface hardness at baseline (SHbaseline), the difference in surface hardness between baseline and the first erosive challenge (ΔSH2−0), the difference in surface hardness between the first and the second erosive challenges (ΔSH4−2), and the relative difference in surface reflection intensity between baseline and the second erosive challenge (rSRI4−0).
| SHbaseline | ΔSH2−0 | ΔSH4−2 | rSRI4−0 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | p-value | Mean | SEM | p-value | Mean | SEM | p-value | |
| MINERAL WATER | |||||||||||
| Mineral water | 509.5 | 19.6 | −5.0 | 7.7 | 0.695 | −6.1 | 5.5 | 0.375 | 15.6 | 11.0 | 0.301 |
| SOFT DRINKS | |||||||||||
| Coca−Cola® | 501.0 | 12.7 | −90.2 | 11.3 | 0.002 | −79.1 | 10.3 | 0.002 | −83.0 | 2.0 | 0.002 |
| Pepsi−Cola® | 497.6 | 10.3 | −60.7 | 7.6 | 0.002 | −86.4 | 8.8 | 0.002 | −87.7 | 1.2 | 0.004 |
| Fanta® Regular | 491.2 | 10.9 | −100.6 | 9.8 | 0.002 | −105.1 | 14.8 | 0.002 | −85.8 | 1.5 | 0.002 |
| Sprite® | 511.0 | 13.0 | −124.4 | 4.7 | 0.002 | −134.1 | 8.2 | 0.002 | −85.3 | 1.4 | 0.002 |
| Guaraná Antártica® | 502.5 | 14.4 | −32.3 | 8.6 | 0.014 | −58.5 | 7.8 | 0.002 | −77.1 | 2.1 | 0.002 |
| Rivella® Red | 491.1 | 14.2 | −44.8 | 15.0 | 0.002 | −112.9 | 11.9 | 0.002 | −78.1 | 2.3 | 0.002 |
| Ice tea | 500.8 | 10.5 | −63.7 | 5.4 | 0.002 | −84.1 | 8.4 | 0.002 | −82.8 | 2.5 | 0.004 |
| Ice tea Peach | 483.6 | 10.0 | −25.5 | 11.2 | 0.106 | −101.2 | 9.8 | 0.002 | −82.2 | 1.6 | 0.004 |
| FRUITS, JUICES AND SMOOTHIES | |||||||||||
| Kiwi (fruit) | 498.9 | 10.3 | −60.8 | 15.9 | 0.004 | −142.3 | 13.5 | 0.002 | −94.3 | 1.9 | 0.002 |
| Orange (fruit) | 502.0 | 11.5 | −16.2 | 5.1 | 0.014 | −43.4 | 7.3 | 0.002 | −60.7 | 2.1 | 0.004 |
| Orange juice | 499.4 | 13.3 | −19.2 | 5.2 | 0.006 | −30.0 | 5.6 | 0.002 | −72.4 | 6.1 | 0.002 |
| Apple juice | 480.2 | 7.6 | −37.5 | 13.6 | 0.027 | −107.4 | 17.7 | 0.004 | −93.4 | 1.1 | 0.002 |
| Apple juice for babies | 494.6 | 9.8 | −15.4 | 7.6 | 0.065 | −48.6 | 6.0 | 0.002 | −71.0 | 3.7 | 0.004 |
| Ribena® | 506.8 | 11.3 | −50.1 | 7.0 | 0.002 | −91.4 | 14.8 | 0.004 | −84.6 | 2.1 | 0.002 |
| Fruit smoothie | 532.6 | 15.5 | −38.8 | 10.8 | 0.006 | −77.2 | 5.3 | 0.002 | −71.6 | 3.2 | 0.002 |
| YOGHURT | |||||||||||
| Forest berries yoghurt | 494.5 | 6.2 | 24.7 | 11.4 | 0.037 | 1.6 | 12.8 | 0.922 | −23.9 | 7.6 | 0.006 |
| SOUR CANDIES | |||||||||||
| Candy spray | 509.9 | 13.1 | −301.7 | 11.3 | 0.002 | −110.7 | 12.5 | 0.002 | −97.2 | 2.4 | 0.004 |
| Sour candy | 525.7 | 9.0 | −74.1 | 14.3 | 0.002 | −110.7 | 15.1 | 0.002 | −84.0 | 2.3 | 0.002 |
| Sour chewing gum | 490.3 | 13.6 | −53.9 | 7.0 | 0.002 | −81.5 | 6.7 | 0.002 | −80.7 | 1.6 | 0.002 |
| SPORTS AND ENERGY DRINKS | |||||||||||
| Monster Energy Drink® | 509.9 | 14.9 | −51.6 | 6.7 | 0.002 | −77.1 | 14.0 | 0.004 | −75.4 | 2.1 | 0.002 |
| Red Bull® Energy Drink | 515.5 | 15.3 | −52.6 | 9.3 | 0.004 | −92.2 | 8.7 | 0.002 | −74.9 | 2.4 | 0.002 |
| Gatorade® | 541.8 | 18.4 | −115.4 | 20.2 | 0.002 | −89.1 | 9.2 | 0.002 | −71.7 | 3.0 | 0.002 |
| MEDICAMENTS | |||||||||||
| Dafalgan syrup | 478.9 | 13.1 | 17.1 | 8.4 | 0.049 | 18.7 | 15.6 | 0.232 | −20.6 | 6.1 | 0.006 |
| Mucosolvon cough syrup | 520.1 | 13.7 | −7.8 | 9.2 | 0.625 | −41.6 | 7.5 | 0.002 | −69.9 | 3.8 | 0.002 |
| Fluimucil effervescent | 496.4 | 6.8 | −11.9 | 4.7 | 0.020 | −36.1 | 2.6 | 0.002 | −46.4 | 4.3 | 0.006 |
| Tossamin sugar free syrup | 510.5 | 10.3 | 15.8 | 14.0 | 0.492 | −13.5 | 10.6 | 0.193 | −49.5 | 6.7 | 0.004 |
| Ventolin syrup | 512.9 | 9.4 | −54.2 | 5.9 | 0.002 | −85.3 | 6.7 | 0.002 | −74.0 | 3.7 | 0.004 |
| Claritine syrup | 527.9 | 15.9 | −10.8 | 5.8 | 0.106 | −13.8 | 6.4 | 0.065 | −40.8 | 3.5 | 0.002 |
| Maltofer syrup | 501.7 | 8.2 | 9.9 | 5.7 | 0.131 | −5.4 | 6.1 | 0.432 | −19.8 | 4.3 | 0.002 |
Fig 1. Association between relative surface reflection intensity (rSRI) and change in surface hardness (ΔSH4−0).
The solid line represents the regression line (Eq 1).
By far the most erosive substance was candy spray, which caused a loss of SH of more than 300 Vickers Hardness Numbers after the first erosive challenge, and caused the greatest relative change in SRI with a decrease of more than 95% in the SRI of the samples. Kiwi fruit caused the greatest decrease in SH during the second erosive challenge. Regarding the chemical parameters, we see that candy spray had the lowest pH and the highest titratable acidity, whereas kiwi exhibited the second-highest titratable acidity.
Analysing the effect of the different chemical properties of the drinks on dental erosion in deciduous enamel, we see that pH showed a moderate positive correlation with ΔSH and rSRI, whereas all other parameters showed a weak correlation (Table 3). This was also shown by the results of the multivariate linear regression analyses (Table 4), where, despite the weak correlation values observed in Table 3, not only pH, but also titratable acidity, Ca concentration, and, to a lesser extent, Pi concentration all play a role in initial enamel erosion. Table 4 shows that lower pH values and Ca concentration, and higher titratable acidity values are significantly related to more loss of SH during erosion.
Table 3. Spearman’s correlation coefficients between the chemical properties of the substances and the difference in surface hardness between baseline and the first erosive challenge (ΔSH2−0), the total difference in surface hardness after all erosive challenges (ΔSH4−0), and the relative difference in surface reflection intensity between baseline and the second erosive challenge (rSRI4−0).
| ΔSH2−0 | ΔSH4−0 | rSRI4−0 | |
|---|---|---|---|
| pH | 0.635*** | 0.667*** | 0.644*** |
| Titratable acidity | −0.197*** | −0.275*** | −0.256*** |
| [Ca] | −0.018 | −0.094 | −0.155** |
| [Pi] | −0.094 | −0.158** | −0.273*** |
| [F] | −0.165** | −0.232*** | −0.126* |
| (pK−pI)HAP ± | 0.306*** | 0.268*** | 0.153* |
| (pK−pI)FAP † | 0.289*** | 0.245*** | 0.119* |
| (pK−pI)CaF2 ‡ | 0.029 | −0.033 | −0.061 |
[Ca], [Pi], [F]: calcium, phosphate and fluoride concentrations, respectively;
* significant at p<0.05;
** significant at p<0.005;
*** significant at p<0.001;
± Degree of saturation with respect to hydroxyapatite;
† Degree of saturation with respect to fluorapatite.
‡ Degree of saturation with respect to CaF2.
Table 4. Multiple linear regression analysis of the changes in surface hardness (ΔSH) of all specimens after immersion in all substances.
| Intercept | pH | Titratable acidity | [Ca] | [Pi] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔSH | β | p | β | p | β | p | β | p | β | p |
| ΔSH2−0 | −135.70 | <0.001 | 34.45 | <0.001 | −0.46 | <0.001 | 2244.0 | <0.001 | ns | ns |
| ΔSH4−0 | −314.70 | <0.001 | 69.32 | <0.001 | −0.53 | <0.001 | 3885.0 | <0.001 | −5457.0 | 0.023 |
β-estimates and p-values are listed only for variables with a significant impact on ΔSH;
[Ca] and [Pi]: calcium and phosphate concentrations, respectively;
ns = not significant.
Comparing permanent enamel with deciduous enamel treated with the same substances (Table 5), we observed no significant differences in initial hardness between the two kinds of teeth. However, a significant difference was observed in the change in SH when the samples were immersed in Coca-Cola®. After the first erosive challenge (ΔSH2−0), deciduous enamel exhibited significantly greater hardness loss (−90.2 ± 11.3 VHN) than permanent enamel (−44.3 ± 12.2 VHN; p = 0.007). However, no differences between the two types of teeth were observed in the total change in SH after both challenges (ΔSH4−0), or in the surface reflection intensity.
Table 5. Mean ± SEM (standard error of the mean) for surface hardness at baseline (SHbaseline), difference in surface hardness after the first (ΔSH2−0) and both (ΔSH4−0) erosive challenges, and the relative change in surface reflectivity (rSRI4−0), for deciduous and permanent enamel samples.
| Substance | Deciduous | Permanent | p-value | |
|---|---|---|---|---|
| Mineral water (negative control) | SHBaseline | 509.5±19.6 | 517.7±11.7 | 0.280 |
| ΔSH2−0 | −5.0±7.7 | 27.8±13.1 | 0.089 | |
| ΔSH4−0 | −11.1±12.0 | 19.1±12.6 | 0.089 | |
| rSRI4−0 | −15.6±11.0 | 1.6±5.2 | 0.436 | |
| Coca-Cola® | SHBaseline | 501.0±12.7 | 514.8±13.3 | 0.579 |
| ΔSH2−0 | −90.2±11.3 | −44.3±12.2 | 0.007* | |
| ΔSH4−0 | −169.3±11.2 | −139.8±10.7 | 0.075 | |
| rSRI4−0 | −83.0±2.0 | −86.6±1.4 | 0.143 |
* Significant difference between deciduous and permanent enamel;
SHbaseline: surface hardness at baseline;
ΔSH2−0: surface hardness decrease between baseline and the first erosive challenge;
ΔSH4−0: surface hardness decrease between baseline and the second erosive challenge;
rSRI4−0: relative difference in surface reflection intensity between baseline and the second erosive challenge.
Discussion
Despite the great number of studies on dental erosion, there is still a lack of information regarding the erosive dissolution of deciduous teeth. In the present study, we show the erosive effect of various substances on deciduous enamel. Moreover, we analysed the effect of different chemical factors on the initial erosion process in deciduous teeth. In line with the previous studies, we observed that several soft drinks, fruit juices and smoothies, sour candies, and medicaments can cause significant erosion. This is not surprising given their degree of saturation with respect to HAP and FAP.
Dental enamel is mostly made up of calcium (Ca2+), phosphate (PO4 3+), hydroxide (OH−), and, to a lesser extent, fluoride (F−) ions [41]. In the oral cavity, the teeth are surrounded by saliva, and the enamel crystals are in a constant equilibrium with the saliva. In other words, there is a continuous exchange of Ca2+, PO4 3+, OH−, and F− between saliva and enamel. When the teeth are exposed to substances that have a low concentration of these ions, there is a tendency for enamel to release more of these ions to the environment in order to attain a new state of equilibrium [41]. Acidic substances with low pH values can exacerbate this process and lead to further demineralization. Therefore, the solubility of enamel is highly dependent on the pH of the surrounding substance, as well as the substance’s Ca2+, PO4 3+, and (to a lesser extent) F− concentrations [12, 16, 17, 42–44]. These parameters are, therefore, used to calculate the degree of saturation (pK−pI) of the substances with respect to hydroxyapatite (HAP) and fluorapatite (FAP) [33].
The degree of saturation values essentially indicate whether a substance is more or less likely to cause dissolution of enamel. When a substance has (pK−pI)HAP and (pK−pI)FAP values below zero, it is said that the substance is undersaturated with respect to HAP and FAP, and this will cause enamel to dissolve until equilibrium is reached. However, if the substance has positive (pK−pI)HAP and (pK−pI)FAP values, it is considered supersaturated with respect to HAP and FAP, and will cause ions to deposit on the tooth mineral until a new equilibrium is reached [41]. Interestingly, in the present study, the vast majority of the substances had low pH values (varying from 2.14 to 6.70) and negative (pK−pI)HAP and (pK−pI)FAP values, which prompted enamel to demineralize.
Although the (pK−pI)HAP and (pK−pI)FAP values are good indicators of whether enamel demineralization occurs, they are calculated based on the ionic composition of HAP and FAP of permanent enamel. Deciduous enamel, however, has a slightly different histological composition, so the (pK−pI)HAP and (pK−pI)FAP values presented in Table 1 can only serve as a guide to deciduous enamel dissolution. We therefore carried out the multiple regression analyses to verify which specific variables play a significant role in erosive demineralization of deciduous enamel.
Our results suggest that pH, titratable acidity, Ca2+ concentration and, to a lesser extent, Pi concentration in the substances can significantly influence erosion in deciduous enamel. Many studies have demonstrated how Ca concentrations in erosive solutions can modulate enamel demineralization [1, 12, 17, 44]. Higher Ca concentration in a given solution will increase its degree of saturation, thus lessening its erosive effect [45]. This is in line with our results, which showed that higher concentrations of Ca in the tested substances prompted significantly less erosive demineralization. Pi concentration, on the other hand, was not significant during the first erosive challenge (ΔSH2−0), but only became significant after 4 min immersion in the substances (ΔSH4−0). Similar results were also observed by Hemingway, Parker (46], who suggested that calcium ions are dissolved from the hydroxyapatite before phosphate ions, thus explaining the relationship between calcium concentration and erosion, and the lack of association between phosphate concentration and erosion. In addition, Lussi, Megert (22] argue that there are four species of Pi (H3PO4, H2PO4 −, HPO4 2− and PO4 3−) that could be present in a solution, but their concentrations are strongly influenced by the pH of the solution. At acidic pH, most Pi species are in the form of H2PO4 −, and only a minute fraction is in the form of PO4 3-, which is the only species of importance in the ion activity of enamel [22, 46]. Therefore, at low pH, extremely high amounts of Pi would be necessary to increase the degree of saturation of a given solution to a level at which it would effectively hinder enamel demineralization [22]. In contrast to what was expected, the multivariate analysis in the present study shows that higher Pi concentrations are associated with a greater loss of SH. This is probably because, within the substances we have tested, the highest [Pi] values were measured in the highly erosive substances, such as Coca-Cola®, Pepsi®, Rivella® Red, kiwi fruit and Gatorade®, and this may be an expression that in some of these substances, like Coca-Cola® and Pepsi®, there is a high phosphoric acid content, and, consequently, high Pi concentrations. It is, therefore, possible to conclude that (similarly to permanent enamel) Pi concentration does not play a significant role in erosive dissolution of deciduous enamel. Dissolution of deciduous enamel is, thus, strongly influenced by the Ca concentration, pH and titratable acidity of the substance.
Titratable acidity is a measure of the buffering of a solution, and it is directly related to the concentration of the undissociated form of the acid in a given substance [41]. The undissociated form of the acid is of considerable importance because this species has no charge and it is able to diffuse more readily into the near-surface layer of enamel. Once there, this species then dissociates acting as a proton (H+) carrier into the enamel mineral, and it maintains the acidic (undersaturated) condition that promotes further dissolution [23, 47]. So, higher titratable acidity values are strong indicators of higher concentrations of the undissociated species of the acid, which, in turn, lead to more enamel erosion.
Besides the effect of specific chemical factors associated with erosion in deciduous enamel, we also compared the effect of two substances (mineral water and Coca-Cola®) on both permanent and deciduous teeth. Our results showed no significant differences between the two types of teeth when the specimens were treated with mineral water. Treatment with Coca-Cola®, however, caused a significantly greater loss of SH in deciduous enamel than in permanent enamel within the first 2 min (ΔSH2−0), but no differences were observed in the total loss of SH after two erosive challenges (ΔSH4−0). We, therefore, suggest that the initial erosive process may start differently in the two kinds of teeth, but also the lack of difference after the second erosive challenge could be due to the small sample size in the present study. In any case, conflicting results have been reported from studies on the dissolution pattern of deciduous and permanent enamel [4, 5, 48–52], so these differences should be further investigated.
In the present study, we show that various soft drinks, sour candies, sports drinks and energy drinks, and some fruits and fruit juices are able to cause enamel erosion. Thus, the excessive consumption of such substances can lead to substantial dental erosion, which may compromise patients’ dentition for their entire lifetime [5].
It is important to note that, although the enamel samples were kept in saliva for 3 h to allow the formation of the salivary pellicle, all erosion challenges were made without saliva. More specifically, the sour candy and sour chewing gum were both diluted in water, and the tests on the erosive effect of these substances did not take into account the buffering effect of saliva. In a preliminary experiment carried out in our laboratory, we also dissolved sour chewing gum in human saliva and did the erosive challenge following the methods used in the present study. Dissolving the substance in 10 ml saliva caused no loss in enamel SH after 2 min or 4 min erosive challenge. However, Lagerlof and Dawes [53] showed that the maximum volume of saliva in the mouth before swallowing is 1.19 ml or 0.96 ml for males and females, respectively. So, when the sour chewing gum was dissolved in only 2 ml saliva in the preliminary experiment, we observed that even one drop of the solution was able to considerably decrease enamel SH after 2 min and 4 min challenge, which was probably related to the low pH (3.47) of the solution (unpublished results).
In this experiment, we used two parameters to measure enamel erosion: change in surface hardness (ΔSH) and surface reflection intenstiy (rSRI). Previous studies have shown that SRI is a viable additional method to measure the erosive demineralization of permanent enamel [37, 38, 54], because it highly correlates with Knoop surface microhardness, calcium release, and surface roughness [36]. In the present study, we were able to further demonstrate that SRI is significantly associated with surface hardness measured with Vickers nanoindentations. Moreover, we also show that SRI is a suitable viable option to measure erosive demineralization on deciduous enamel.
In conclusion, we were able to corroborate the erosive potential of a broad range of drinks, foodstuffs and medications commonly consumed/used by children and young adolescents, and we show that erosive dissolution of deciduous enamel is significantly associated with pH, titratable acidity and calcium concentration in the solution. This study is an extensive overview, and it can be used to judge the erosive potential of many dietary substances and medications used by children.
Acknowledgments
The present study was supported by the Deutsche Gesellschaft für Zahnerhaltung (DGPZM-Wissenschaftsfonds). The authors show their gratitude towards G. Fischer and Prof. Häusler, Institute of Mathematical Statistics, University of Bern, for the statistical analyses, and to Brigitte Megert for her appreciated efforts in the laboratory. We also thank Dr. R.P. Shellis for his valuable comments on this manuscript.
Data Availability
The data underlying the findings are fully available and all relevant data are presented within the manuscript.
Funding Statement
This study was funded by the Deutsche Gesellschaft für Zahnerhaltung (DGPZM-Wissenschaftsfonds) to AL and TSC.
References
- 1. Lussi A, Carvalho TS. Erosive tooth wear: a multifactorial condition of growing concern and increasing knowledge. Monogr Oral Sci. 2014;25:1–15. 10.1159/000360380 . [DOI] [PubMed] [Google Scholar]
- 2. Hunter ML, West NX, Hughes JA, Newcombe RG, Addy M. Erosion of deciduous and permanent dental hard tissue in the oral environment. Journal of dentistry. 2000;28:257–63. doi: S0300-5712(99)00079-2 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3. Rios D, Magalhães AC, Honório HM, Buzalaf MA, Lauris JR, Machado MA. The prevalence of deciduous tooth wear in six-year-old children and its relationship with potential explanatory factors. Oral Health Prev Dent. 2007;5:167–71. . [PubMed] [Google Scholar]
- 4. Kreulen CM, Van 't Spijker A, Rodriguez JM, Bronkhorst EM, Creugers NH, Bartlett DW. Systematic review of the prevalence of tooth wear in children and adolescents. Caries research. 2010;44:151–9. 10.1159/000308567 . [DOI] [PubMed] [Google Scholar]
- 5. Carvalho TS, Lussi A, Jaeggi T, Gambon DL. Erosive tooth wear in children. Monogr Oral Sci. 2014;25:262–78. 10.1159/000360712 . [DOI] [PubMed] [Google Scholar]
- 6. Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion—an overview with emphasis on chemical and histopathological aspects. Caries research. 2011;45 Suppl 1:2–12. doi: 000325915 [pii] 10.1159/000325915 . [DOI] [PubMed] [Google Scholar]
- 7. Shellis RP, Ganss C, Ren Y, Zero DT, Lussi A. Methodology and models in erosion research: discussion and conclusions. Caries research. 2011;45 Suppl 1:69–77. doi: 000325971 [pii] 10.1159/000325971 . [DOI] [PubMed] [Google Scholar]
- 8. Ganss C, Lussi A. Diagnosis of erosive tooth wear. Monogr Oral Sci. 2014;25:22–31. 10.1159/000359935 [DOI] [PubMed] [Google Scholar]
- 9. Lussi A, Jaeggi T, Jaeggi-Schärer S. Prediction of the erosive potential of some beverages. Caries research. 1995;29:349–54. . [DOI] [PubMed] [Google Scholar]
- 10. Järvinen VK, Rytömaa II, Heinonen OP. Risk factors in dental erosion. Journal of dental research. 1991;70:942–7. . [DOI] [PubMed] [Google Scholar]
- 11. Hughes JA, West NX, Parker DM, Newcombe RG, Addy M. Development and evaluation of a low erosive blackcurrant juice drink in vitro and in situ. 1. Comparison with orange juice. Journal of dentistry. 1999;27:285–9. . [DOI] [PubMed] [Google Scholar]
- 12. Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries research. 1999;33(1):81–7. . [DOI] [PubMed] [Google Scholar]
- 13. O'Sullivan EA, Curzon ME. A comparison of acidic dietary factors in children with and without dental erosion. ASDC J Dent Child. 2000;67:186–92, 60 . [PubMed] [Google Scholar]
- 14. Hunter ML, Patel R, Loyn T, Morgan MZ, Fairchild R, Rees JS. The effect of dilution on the in vitro erosive potential of a range of dilutable fruit drinks. International journal of paediatric dentistry / the British Paedodontic Society [and] the International Association of Dentistry for Children. 2008;18:251–5. 10.1111/j.1365-263X.2008.00917.x . [DOI] [PubMed] [Google Scholar]
- 15. Hunter L, Patel S, Rees J. The in vitro erosive potential of a range of baby drinks. International journal of paediatric dentistry / the British Paedodontic Society [and] the International Association of Dentistry for Children. 2009;19:325–9. 10.1111/j.1365-263X.2009.00975.x . [DOI] [PubMed] [Google Scholar]
- 16. Lussi A, Jäggi T, Schärer S. The influence of different factors on in vitro enamel erosion. Caries research. 1993;27:387–93. . [DOI] [PubMed] [Google Scholar]
- 17. Barbour ME, Parker DM, Allen GC, Jandt KD. Enamel dissolution in citric acid as a function of calcium and phosphate concentrations and degree of saturation with respect to hydroxyapatite. European journal of oral sciences. 2003;111(5):428–33. . [DOI] [PubMed] [Google Scholar]
- 18. Dugmore CR, Rock WP. A multifactorial analysis of factors associated with dental erosion. British dental journal. 2004;196:283–6; discussion 73. doi: 4811041 [pii] 10.1038/sj.bdj.4811041 . [DOI] [PubMed] [Google Scholar]
- 19. Johansson AK, Lingström P, Imfeld T, Birkhed D. Influence of drinking method on tooth-surface pH in relation to dental erosion. European journal of oral sciences. 2004;112:484–9. 10.1111/j.1600-0722.2004.00172.x . [DOI] [PubMed] [Google Scholar]
- 20. Shellis RP, Finke M, Eisenburger M, Parker DM, Addy M. Relationship between enamel erosion and liquid flow rate. European journal of oral sciences. 2005;113(3):232–8. 10.1111/j.1600-0722.2005.00210.x . [DOI] [PubMed] [Google Scholar]
- 21. Jensdottir T, Bardow A, Holbrook P. Properties and modification of soft drinks in relation to their erosive potential in vitro. Journal of dentistry. 2005;33(7):569–75. 10.1016/j.jdent.2004.12.002 . [DOI] [PubMed] [Google Scholar]
- 22. Lussi A, Megert B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Br J Nutr. 2012;107:252–62. doi: S0007114511002820 [pii] 10.1017/S0007114511002820 . [DOI] [PubMed] [Google Scholar]
- 23. Shellis RP, Barbour ME, Jesani A, Lussi A. Effects of Buffering Properties and Undissociated Acid Concentration on Dissolution of Dental Enamel in Relation to pH and Acid Type. Caries research. 2013;47(6):601–11. 10.1159/000351641 . [DOI] [PubMed] [Google Scholar]
- 24. Radlanski RJ, Renz H, Willersinn U, Cordis CA, Duschner H. Outline and arrangement of enamel rods in human deciduous and permanent enamel. 3D-reconstructions obtained from CLSM and SEM images based on serial ground sections. Eur J Oral Sci. 2001;109(6):409–14. . [DOI] [PubMed] [Google Scholar]
- 25. Shellis RP. Variations in growth of the enamel crown in human teeth and a possible relationship between growth and enamel structure. Arch Oral Biol. 1984;29(9):697–705. . [DOI] [PubMed] [Google Scholar]
- 26. Wilson PR, Beynon AD. Mineralization differences between human deciduous and permanent enamel measured by quantitative microradiography. Arch Oral Biol. 1989;34(2):85–8. . [DOI] [PubMed] [Google Scholar]
- 27. Sønju Clasen AB, Ruyter IE. Quantitative determination of type A and type B carbonate in human deciduous and permanent enamel by means of Fourier transform infrared spectrometry. Adv Dent Res. 1997;11(4):523–7. . [DOI] [PubMed] [Google Scholar]
- 28. STACK MV. Variation in the organic content of deciduous enamel and dentine. Biochem J. 1953;54(2):xv . [PubMed] [Google Scholar]
- 29. Ganss C, Klimek J, Giese K. Dental erosion in children and adolescents—a cross-sectional and longitudinal investigation using study models. Community Dent Oral Epidemiol. 2001;29(4):264–71. . [DOI] [PubMed] [Google Scholar]
- 30. Harding MA, Whelton HP, Shirodaria SC, O'Mullane DM, Cronin MS. Is tooth wear in the primary dentition predictive of tooth wear in the permanent dentition? Report from a longitudinal study. Community Dent Health. 2010;27(1):41–5. . [PubMed] [Google Scholar]
- 31. Zero DT, Rahbek I, Fu J, Proskin HM, Featherstone JD. Comparison of the iodide permeability test, the surface microhardness test, and mineral dissolution of bovine enamel following acid challenge. Caries research. 1990;24:181–8. . [DOI] [PubMed] [Google Scholar]
- 32. Chen PS, Toribara TY, Warner H. Microdetermination of phosphorus. Analytical chemistry. 1956;28:1756–58. [Google Scholar]
- 33. Larsen MJ. An investigation of the theoretical background for the stability of the calcium-phosphate salts and their mutual conversion in aqueous solutions. Archives of oral biology. 1986;31:757–61. . [DOI] [PubMed] [Google Scholar]
- 34. McCann HG. The solubility of fluorapatite and its relationship to that of calcium fluoride. Archives of oral biology. 1968;13(8):987–1001. . [DOI] [PubMed] [Google Scholar]
- 35. McDowell H, Gregory TM, Brown E. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 25 and 37°C. J Res Natl Bur Stand. 1977;81:273–81. [Google Scholar]
- 36. Rakhmatullina E, Bossen A, Hoöschele C, Wang X, Beyeler B, Meier C, et al. Application of the specular and diffuse reflection analysis for in vitro diagnostics of dental erosion: correlation with enamel softening, roughness, and calcium release. Journal of biomedical optics. 2011;16:107002 10.1117/1.3631791 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lussi A, Bossen A, Höschele C, Beyeler B, Megert B, Meier C, et al. Effects of enamel abrasion, salivary pellicle, and measurement angle on the optical assessment of dental erosion. Journal of biomedical optics. 2012;17:97009–1. 10.1117/1.JBO.17.9.097009 . [DOI] [PubMed] [Google Scholar]
- 38. Brevik SC, Lussi A, Rakhmatullina E. A new optical detection method to assess the erosion inhibition by in vitro salivary pellicle layer. Journal of dentistry. 2013;41(5):428–35. 10.1016/j.jdent.2013.02.011 . [DOI] [PubMed] [Google Scholar]
- 39. Wetton S, Hughes J, West N, Addy M. Exposure time of enamel and dentine to saliva for protection against erosion: a study in vitro. Caries research. 2006;40(3):213–7. 10.1159/000092228 . [DOI] [PubMed] [Google Scholar]
- 40. Stiefel DJ. Characteristics of an in vitro dental pellicle. Journal of dental research. 1976;55(1):66–73. . [DOI] [PubMed] [Google Scholar]
- 41. Shellis RP, Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. Monogr Oral Sci. 2014;25:163–79. 10.1159/000359943 . [DOI] [PubMed] [Google Scholar]
- 42. ten Cate JM, Buijs MJ, Damen JJ. pH-cycling of enamel and dentin lesions in the presence of low concentrations of fluoride. European journal of oral sciences. 1995;103:362–7. . [DOI] [PubMed] [Google Scholar]
- 43. Hughes JA, West NX, Parker DM, van den Braak MH, Addy M. Effects of pH and concentration of citric, malic and lactic acids on enamel, in vitro. Journal of dentistry. 2000;28:147–52. . [DOI] [PubMed] [Google Scholar]
- 44. Attin T, Meyer K, Hellwig E, Buchalla W, Lennon AM. Effect of mineral supplements to citric acid on enamel erosion. Archives of oral biology. 2003;48:753–9. . [DOI] [PubMed] [Google Scholar]
- 45. Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69:722–4. . [PubMed] [Google Scholar]
- 46. Hemingway CA, Parker DM, Addy M, Barbour ME. Erosion of enamel by non-carbonated soft drinks with and without toothbrushing abrasion. British dental journal. 2006;201(7):447–50; discussion 39; quiz 66. 10.1038/sj.bdj.4814073 . [DOI] [PubMed] [Google Scholar]
- 47. Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. Monogr Oral Sci. 2006;20:66–76. . [DOI] [PubMed] [Google Scholar]
- 48. Amaechi BT, Higham SM, Edgar WM. Factors influencing the development of dental erosion in vitro: enamel type, temperature and exposure time. J Oral Rehabil. 1999;26:624–30. . [DOI] [PubMed] [Google Scholar]
- 49. Hunter ML, West NX, Hughes JA, Newcombe RG, Addy M. Relative susceptibility of deciduous and permanent dental hard tissues to erosion by a low pH fruit drink in vitro. Journal of dentistry. 2000;28:265–70. doi: S0300-5712(99)00074-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 50. Johansson AK, Sorvari R, Birkhed D, Meurman JH. Dental erosion in deciduous teeth—an in vivo and in vitro study. Journal of dentistry. 2001;29:333–40. . [DOI] [PubMed] [Google Scholar]
- 51. Lippert F, Parker DM, Jandt KD. Susceptibility of deciduous and permanent enamel to dietary acid-induced erosion studied with atomic force microscopy nanoindentation. European journal of oral sciences. 2004;112:61–6. . [DOI] [PubMed] [Google Scholar]
- 52. Magalhães AC, Rios D, Honório HM, Delbem AC, Buzalaf MA. Effect of 4% titanium tetrafluoride solution on the erosion of permanent and deciduous human enamel: an in situ/ex vivo study. J Appl Oral Sci. 2009;17:56–60. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lagerlof F, Dawes C. The volume of saliva in the mouth before and after swallowing. Journal of dental research. 1984;63(5):618–21. . [DOI] [PubMed] [Google Scholar]
- 54. Rakhmatullina E, Bossen A, Bachofner KK, Meier C, Lussi A. Optical pen-size reflectometer for monitoring of early dental erosion in native and polished enamels. Journal of biomedical optics. 2013;18(11):117009 10.1117/1.JBO.18.11.117009 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying the findings are fully available and all relevant data are presented within the manuscript.