Abstract
Nutrition in older adults on peritoneal dialysis is an important aspect of a patient's clinical management as well as being influenced by their overall well-being, both mental and physical. This is especially pertinent as individuals age, since the potential impact of life changes and physical changes contribute to the development of protein-energy wasting and potentially exacerbating sarcopenia and wasting. This article provides an outline of the nutritional issues to consider in older adults on peritoneal dialysis (PD).
Keywords: Nutrition, older age, peritoneal dialysis
Older people can be vulnerable to experiencing nutritional issues due to age-related changes manifested in the physical, social, and psychological domains. Physically, one's resources change over time, with a natural decline in muscle strength and physical functioning, taste changes, and problems with dentition. Aging also brings with it an increase in the occurrence of life-changing events such as bereavement of loved ones, a sense of loss of independence, living with 1 or more long-term conditions, and changes in one's living environment and home. Although psychologically, older people may have developed a resilience brought on by life experience (1), the changes highlighted above can precede the development of a dampening of mood in the absence of meaningful social support. All this can have a profound effect on one's nutritional intake and status. Using the brief framework set up above in the context of renal disease, the following practical based guidance will describe the nutritional needs and support required in older people on peritoneal dialysis (PD).
Existing Guidelines
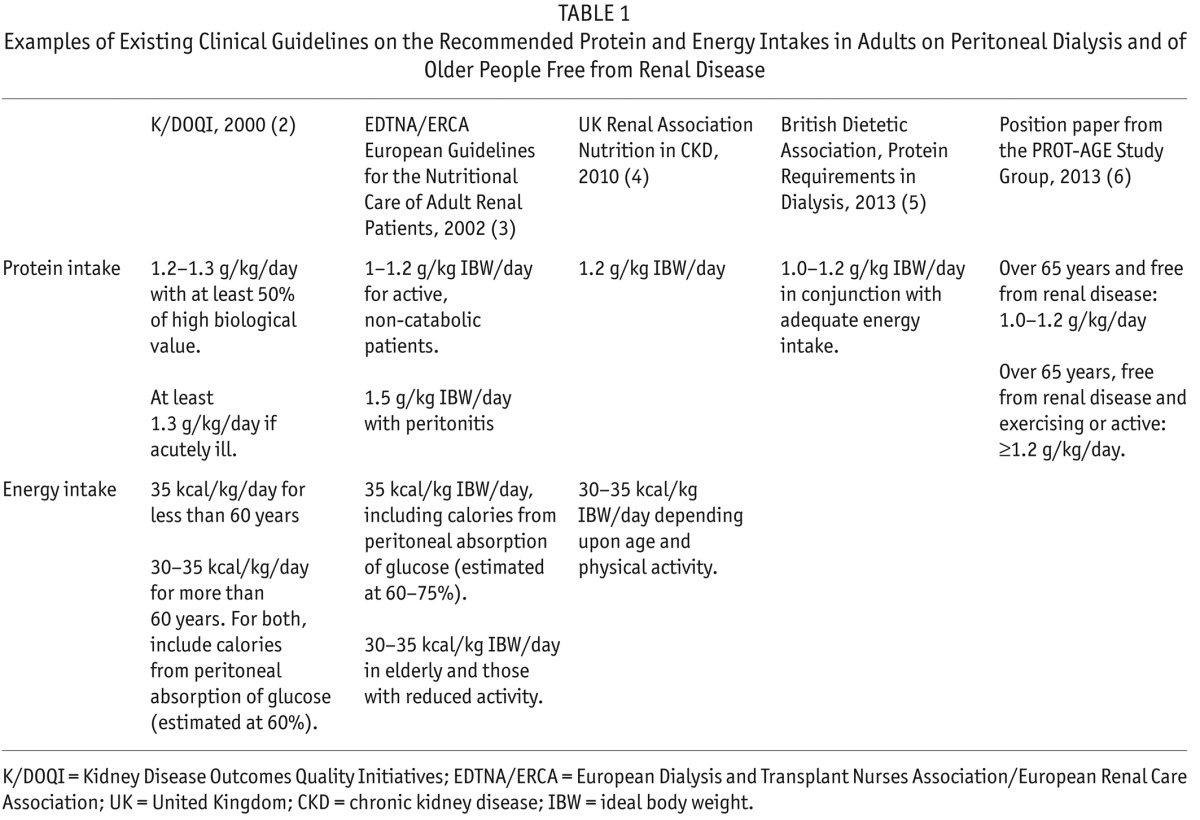
Guidelines that focus on nutrition in PD do, in some cases, reference older people on PD (Table 1). The guidelines offer structured direction on how to nutritionally manage a patient on PD in relation to protein and energy consumption but fail to acknowledge the wider social issues and practicalities that could help nutritionally support the comorbid elderly, who may also be frail.
TABLE 1.
Examples of Existing Clinical Guidelines on the Recommended Protein and Energy Intakes in Adults on Peritoneal Dialysis and of Older People Free from Renal Disease
Nutritional Costs and Benefits of Peritoneal Dialysis
Peritoneal dialysis is a treatment that can incur nutritional costs yet provide some benefits, depending on the individual.
It has been established that PD leads to the absorption of glucose, the calorific value of which can range from 300 to 450 kcals per day depending on the type of PD undertaken, dwell time, and peritoneal membrane transport status (7). This side effect offers a nutritional advantage to anyone who is unable to meet their energy requirements through diet alone, for example an older person with a poor appetite. However, this is a feature that can also promote undesirable fat distribution. Longitudinal body composition analysis showed that the visceral to subcutaneous fat ratio was increased in incident patients on PD compared with those on in-center hemodialysis (8). In addition, obesity at the start of PD was found to be associated with risk of death and technique failure (9).
The main nutritional cost of PD is the regular and significant loss of protein into the dialysate. Westra et al. demonstrated that automated PD (APD) incurs a loss of approximately 10 g of protein per 24 hours and is increased by dwell time and number of night-time exchanges (10). Since older patients frequently undertake APD, especially in the context of having assisted APD, protein losses into the dialysate can be significant. Protein intake in older people on PD was already found to be suboptimal, at 0.95 ± 0.29 g/kg/day (compared with recommendations in Table 1), without taking into account this protein loss (11). Episodes of peritonitis further exacerbate the loss of protein into the dialysate (12).
The impact of protein loss is evident in people on PD through the consistently lower albumin levels observed compared with those in people on hemodialysis (HD). A large cross-sectional study showed that the equivalent mortality risk in people on PD compared with HD occurred at different albumin thresholds, with albumin levels being 2 g/L lower in the population on PD (13). It is possible, therefore, that protein may be used more effectively in PD patients than in those on HD. One possible mechanism could be the greater correction and stabilization of metabolic acidosis (as determined by bicarbonate levels) in PD, which is only corrected intermittently in HD. This may prevent the muscle catabolism associated with metabolic acidosis (14,15) by potentially influencing muscle protein synthesis, breakdown, or both.
Recent ESPEN guidelines (European Society of Parenteral and Enteral Nutrition) recommend that healthy older adults need higher protein intakes than previously thought to counteract the processes in aging that result in a decline in protein synthesis (16). This is thought to be especially relevant in inflammatory conditions which lead to increased catabolism. In people on PD, chronic inflammation can arise as a result of the PD solutions, catheter, and presence of peritonitis. Therefore, ESPEN has recommended that all healthy older adults (65 years or older) aim for a protein intake of 1.0 – 1.2 g/kg/day. This further highlights that protein is critical for older adults, especially in a treatment such as PD, to maintain muscle integrity and help prevent sarcopenia and the loss of function which can ensue.
Practically, achieving a protein intake of 1.0 – 1.2 g/kg/day can be challenging for older adults. This is especially difficult when the diet is predominantly vegetarian or vegan, which can commonly occur amongst populations from certain cultural backgrounds. Supplementing protein in the diet can occur either through commercial preparations such as protein powders or through home-made preparations such as using skimmed milk powder to fortify foods or drinks. The nutritional value of the entire supplement, however, must be considered. For example, 20 g of skimmed milk powder contains approximately 7 g of protein (with a good compliment of essential amino acids); however, it also contains approximately 360 mg/9 mmol of potassium and 200 mg/6.5 mmol of phosphorous. In contrast, 2 large egg whites contain the same amount of protein yet only 120 mg/3 mmol potassium and 26 mg/0.9 mmol phosphorous. Therefore the choice of protein supplement must be made on an individual basis on what is acceptable to and clinically appropriate for the patient.
Nutrineal (Baxter, Compton, UK) (1.1% amino acid containing dialysate), a bag designed to improve protein balance, as well as being free from glucose, can be used as a single bag and has shown to improve albumin levels when hypoalbuminemia is present (17). About 18 g of amino acids are absorbed from a single 2-L bag. Calorie consumption during the exchange helps to enhance a positive protein balance as it inhibits protein degradation (18). The use of Nutrineal is not widespread due to a trend towards metabolic acidosis and an increase in uremic symptoms (usually with the use of 2 bags) and due to the difficulties in consuming the additional calories required during the exchange—often a challenging prospect for patients who have a poor appetite and intake.
Nutritional Status of Older Adults on PD
The prevalence of states of under- and over-nutrition in older people on PD is unlikely to be solely related to the influence of renal disease and its treatment but will also depend on the patients' cognitive and physical functioning as well as their social, physical, and cultural environment, all of which influence eating habits. Malnutrition is common in dialysis patients. Using subjective global assessment (SGA) as a nutritional assessment tool (which is often regarded as the gold standard), the cross-sectional prevalence of malnutrition in Italian older patients on dialysis (both PD and HD) was 51%, compared with only 27 – 31% in younger age groups. No differences were noted between dialysis modalities (19). This equal distribution of malnutrition in older people on either dialysis modality was confirmed in an English cross-sectional study where only 22% of the 106 participants aged 65 – 89 years were found to be at risk of malnutrition. This same study noted a potential for over-nutrition, as 52% had a BMI of > 25 kg/m2 (indicative of being overweight). Interestingly, 35% of people in this overweight group were found to be at risk of malnutrition (11). Therefore, being overweight, especially in parts of the world where obesity is increasingly prevalent, may mask the presence of malnutrition. In older people, this is termed sarcopenic overweight or obesity, where the reduction of lean mass is occluded by increasing fat mass and worsening malnutrition, which in turn negatively influences survival. In cases where the treatment (i.e. PD) results in the reduction of available protein, sarcopenic obesity is a potential occurrence.
Assessment of nutritional status is therefore important, as malnutrition is not always visually apparent. The latest diagnostic test to establish protein-energy wasting, developed by Fouque et al. (20), uses a combination of factors: biochemical criteria; low body weight, reduced total body fat, or weight loss; decrease in muscle mass; and low protein or energy intakes. The latter 2 criteria are not readily accessible and require skilled assessments, usually by dietitians. Screening tools can be used to determine the potential presence of risk factors for protein-energy wasting. However, at present, there are none that have been validated for older people on PD. We therefore rely on assessing patients based on their own self-reported intake and factors influencing this.
Salt Intake and Volume Control in Older People on PD
The use of salt restriction (together with volume control) can be overlooked as an effective method to control hypertension in patients on PD. Many patients on PD are fluid overloaded when compared with those on HD. This can contribute significantly to the presence of hypertension. Studies have shown that there is a relationship between salt intake and blood pressure, either through the addition of salt to the diet (21) or through implementing salt restrictions (22) and observing the impact on blood pressure in patients on PD. Salt intake is restricted to less than 6 g per day with the aim of mediating increased fluid intake. Restrictions lower than this may have a negative impact on intake, especially in older people who may be more reliant on ready-made processed foods.
Symptoms in Older Adults on PD and Impact on Nutrition
Patients on PD experience more gastrointestinal symptoms than gender- and age-matched medical outpatients without renal failure, in particular abdominal pain, laxative use, and irritable bowel syndrome (23). Further detailed assessment of gastrointestinal symptoms found that patients on PD experienced significantly more dyspepsia, epigastric burning, bloating, postprandial fullness, and early satiety than patients on HD (24). These symptoms are likely to compromise nutritional intake in patients on PD. The nutritional intake of older patients on HD compared with PD was found to be the same (11). This may be because older patients on HD have a compromised intake due to reasons such as time away from home and prolonged recovery time post-HD. This suggests that although the spectrum and etiology of symptoms might vary depending on dialysis modality, the impact on nutritional intake remains similar. Being aware of these gastrointestinal symptoms can allow for their management which in turn may help improve nutritional intake in older people on PD.
Psychosocial Influences on Eating in Older Adults on PD
The causes of protein-energy wasting are multifactorial, and in older people, there is a greater probability that these are related to the social environment. The National Institute of Health-sponsored Hemodialysis (HEMO) study showed that half of approximately 1,900 people (of all ages) treated with HD relied on others for shopping and food preparation (25). The presence of others during meal times can significantly increase calorie intake in homebound older people (26). Johansson et al. showed that older adults, regardless of dialysis modality, had lower energy intakes with fewer social networks (11). In addition, there was an independent relationship between worsening nutritional intake and lower mood (depression), worse physical functioning, and poorer socio-economic status. Although some of these factors are not modifiable, some may be, such as the logistics of ensuring accessibility to adequate meals in the absence of an existing social network.
Diabetes and Nutrition in Older Adults on PD
There is a high prevalence of diabetes amongst older people on PD. In chronic kidney disease, there is an increased potential for hypoglycemic events due to the prolonged action of hypoglycemic agents including insulin caused by decreased clearance, as well as decreased gluconeogenesis by the kidney. The glucose absorbed from the dialysate during PD has been found to lead to an increase in blood glucose concentrations (27), leading to an increase in insulin requirements (unlike that seen when commencing HD, which often leads to a reduction in insulin requirements). This increase in blood glucose concentrations may prove beneficial for older people because it mediates the risk of episodes of hypoglycemia and consequently of events such as falls. Diabetes organizations recommend this approach where older adults are vulnerable, i.e. physically frailer, are experiencing cognitive dysfunction, or have a shorter life expectancy (28).
Summary
In summary, older people may be more vulnerable to nutritional deficits due to social and emotional issues. In addition, gastrointestinal symptoms and the nutritional consequences of dialysis can promote malnutrition. Protein energy malnutrition is more prevalent in older than in younger people. The effect of dialysis on protein losses might enhance sarcopenia and therefore should be addressed through appropriate dietary interventions. The absorption of calories from the dialysate can positively enhance the energy intake of individuals whose diet is compromised. Salt intake can be reduced to less than 6 g per day (although decreasing to levels significantly below 6 g per day can compromise nutritional intake). Diabetes control may change positively on commencement of PD with a lessening of hypoglycemic events.
Key Points
Older adults are more vulnerable to malnutrition than younger individuals due to many non-nutritional factors such as mood and social circumstances.
Additional calories from the dialysate can benefit those whose calorie intake is compromised.
Preserving muscle integrity is important in older adults. Therefore aim for adequate protein intake and prevention of metabolic acidosis.
Gastrointestinal-related symptoms are likely to compromise nutritional intake in older people on peritoneal dialysis.
Limiting salt intake to less than 6 g per day can help with volume control.
Disclosures
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. van Kessel G. The ability of older people to overcome adversity: a review of the resilience concept. Geriatric Nursing 2013; 34:122–7. [DOI] [PubMed] [Google Scholar]
- 2. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation Am J Kidney Dis 2000; 35(6 Suppl 2):S1–140. [DOI] [PubMed] [Google Scholar]
- 3. European Dialysis and Transplantation Nurses Association/European Renal Care Association-Dietitians Special Interest Group European Guidelines for the Nutritional Care of Adult Renal Patients. 2002.
- 4. UK Renal Association Clinical Practice Guidelines on Nutrition in CKD, 5th Edition 2009–2010. http://www.renal.org/guidelines/modules/nutrition-in-ckd#downloads Accessed on 25 March 2015.
- 5. Naylor HL, Jackson H, Walker GH, Macafee S, Magee K, Hooper L, et al. on behalf of the Renal Nutrition Group of the British Dietetic Association British Dietetic Association evidence-based guidelines for the protein requirements of adults undergoing maintenance haemodialysis or peritoneal dialysis. J Hum Nutr Diet 2013; 26:315–28. [DOI] [PubMed] [Google Scholar]
- 6. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc 2013; 14:542–59. [DOI] [PubMed] [Google Scholar]
- 7. Burkart J. Metabolic consequences of peritoneal dialysis. Semin Dial 2004; 17(6):498–504. [DOI] [PubMed] [Google Scholar]
- 8. Pellicano R, Strauss BJ, Polkinghorne KR, Kerr PG. Longitudinal body composition changes due to dialysis. Clin J Am Soc Nephrol 2011; 6:1668–75. [DOI] [PubMed] [Google Scholar]
- 9. McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol 2003; 14:2894–901. [DOI] [PubMed] [Google Scholar]
- 10. Westra WM, Kopple JD, Krediet RT, Appel M, Mehrotra R. Dietary protein requirements and dialysate protein losses in chronic peritoneal dialysis patients. Perit Dial Int 2007; 27:192–5. [PubMed] [Google Scholar]
- 11. Johansson L, Hickson M, Brown EA. Influence of psychosocial factors on energy and protein intake of older people on dialysis. J Ren Nutr 2013; 23(5):348–55. [DOI] [PubMed] [Google Scholar]
- 12. Blumenkrantz MJ, Gahl GM, Kopple JD, Kamdar AV, Jones MR, Kessel M, et al. Protein losses during peritoneal dialysis. Kidney Int 1981; 19:593–602. [DOI] [PubMed] [Google Scholar]
- 13. Mehrotra R, Duong U, Jiwakanon S, Kovesdy CP, Moran J, Kopple JD, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with haemodialysis. Am J Kidney Dis 2011; 58(3):418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J. Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int 2002; 61:1286–92. [DOI] [PubMed] [Google Scholar]
- 15. Vashistha T, Kalantar-Zadeh K, Molnar MZ, Torlén K, Mehrotra R. Dialysis modality and correction of uremic metabolic acidosis: relationship with all-cause and cause-specific mortality. Clin J Am Soc Nephrol 2013; 8:254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deutz NEP, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from ESPEN Expert Group. Clin Nutr 2014; 33(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor GS, Patel V, Spencer S, Fluck RJ, McIntyre CW. Long-term use of 1.1% amino acid dialysis solution in hypoalbuminemic continuous ambulatory peritoneal dialysis patients. Clin Nephrol 2002; 58:445–50. [DOI] [PubMed] [Google Scholar]
- 18. Delarue J, Maingourd C, Objois M, Pinault M, Cohen R, Couet C, et al. Effects of an amino acid dialysate on leucine metabolism in continuous ambulatory peritoneal dialysis patients. Kidney Int 1999; 56(5):1934–43. [DOI] [PubMed] [Google Scholar]
- 19. Cianciaruso B, Brunori G, Traverso G, Panarello G, Enia G, Strippoli P, et al. Nutritional status in the elderly patient with uraemia. Nephrol Dial Transplant 1995; 10(Suppl 6):65–8. [DOI] [PubMed] [Google Scholar]
- 20. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008; 73:391–8. [DOI] [PubMed] [Google Scholar]
- 21. Fine A, Fontaine B, Ma M. Commonly prescribed salt intake in continuous ambulatory peritoneal dialysis is too restrictive: results of a double-blind crossover study. J Am Soc Nephrol 1997; 8:1311–4. [DOI] [PubMed] [Google Scholar]
- 22. Gunal AI, Duman S, Ozkahya M, Toz H, Asci G, Akcicek F, et al. Strict volume control normalizes hypertension in peritoneal dialysis patients. Am J Kidney Dis 2001; 37:588–93. [PubMed] [Google Scholar]
- 23. Cano AE, Neil AK, Kang JY, Barnabas A, Eastwood JB, Nelson SR, et al. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by haemodialysis or peritoneal dialysis. Am J Gastroenterol 2007; 102:1990–7. [DOI] [PubMed] [Google Scholar]
- 24. Strid H, Fjell A, Simren M, Bjornsson ES. Impact of dialysis on gastroesophageal reflux, dyspepsia, and proton pump inhibitor treatment in patients with chronic renal failure. Eur J Gastroenterol Hepatol 2009; 21:137–42. [DOI] [PubMed] [Google Scholar]
- 25. Burrowes JD, Larive B, Cockram DB, Dwyer J, Kusek JW, McLeroy S, et al. Effects of dietary intake, appetite, and eating habits on dialysis and non-dialysis treatment days in hemodialysis patients: cross-sectional results from the HEMO study. J Ren Nutr 2003; 13:191–8. [DOI] [PubMed] [Google Scholar]
- 26. Locher JL, Robinson CO, Roth DL, Ritchie CS, Burgio KL. The effect of the presence of others on caloric intake in homebound older adults. J Gerontol A Biol Sci Med Sci 2005; 60:1475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mori T, Chida M, Oba I, Koizuma K, Furusho M, Tanno M, et al. Diurnal variations of blood glucose by continuous blood glucose monitoring in peritoneal dialysis patients with diabetes. Adv Perit Dial 2014; 30:54–9. [PubMed] [Google Scholar]
- 28. Kirkman S, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60;2342–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


