Abstract
In the present study, the wastewater sample collected from the Dongming discharging river in Shijiazhuang city was analysed using both chemical analysis and biological assays including the Salmonella mutagenicity test, micronucleus test and single-cell gel electrophoresis. Chemical analysis of the sample was performed using gas chromatography mass spectrometry and inductively coupled plasma mass spectrometry. The Salmonella mutagenicity test was performed on Salmonella typhimurium TA97, TA98, TA100 and TA102 strains with and without S9 mixture. The mice received the wastewater in natura through drinking water at concentrations of 25%, 50%, and 100%. One group of mice was exposed for 2 consecutive days, and the other group of mice was exposed for 15 consecutive days. To establish the levels of primary DNA damage, single-cell gel electrophoresis was performed on treated mouse liver cell. The concentrations of chromium and lead in the sample exceeded the national standard (GB20922-2007) by 0.78 and 0.43-fold, respectively. More than 30 organic compounds were detected, and some of the detected compounds were mutagens, carcinogens and environmental endocrine disrupters. A positive response for Salmonella typhimurium TA98 strain was observed. Mouse exposure via drinking water containing 50% and 100% of wastewater for 15 consecutive days caused a significant increase of MN frequencies in a dose-response manner. Mouse exposure via drinking water containing 50% and 100% of wastewater for 15 consecutive days caused a significant increase of the Olive tail moments in a dose-response manner. All the results indicated that the sample from the Dongming discharging river in Shijiazhuang city exhibited genotoxicity and might pose harmful effects on the local residents.
Introduction
To alleviate the shortage of water resources, wastewater irrigation is a widespread practice with a long tradition in the arid and semi-arid regions of the world, especially in developing countries such as China, Mexico, Peru, Egypt, Lebanon, Morocco, India and Vietnam[1]. Globally, approximately 20 million ha of land is reported to be irrigated with wastewater, and at least 10% of the world's population is estimated to consume foods produced by irrigation with wastewater [2]. Farm irrigation with wastewater is reported to exhibit both beneficial and harmful effects [3–4]. Wastewater contains large amounts of nitrogen and phosphorus, which reduces or eliminates the need for supplementary fertilisation for crop growth. However, lots of researches in and abroad have confirmed that a variety of xenobiotics are present in wastewater. These xenobiotics include lead, chromium, cadmium, polychlorinated biphenyls, phthalates, polycyclic aromatic hydrocarbons, organochlorine pesticide and heterocyclic compounds, to name a few[5–8]. The application of wastewater on agricultural land may cause accumulation of metals and persistent organic chemicals in soils and agricultural products, which can potentially harm human and animal health. The contamination of soils and crops due to wastewater irrigation are widely reported in countries such as Germany, France and India [4, 9]. Metals such as cadmium and lead can be sequestered in the soils and absorbed by crops, which serve as the transmission route in the human chain. The persistent organic contaminants accumulated in soil can also be transferred through the food chains and cause adverse effects on human health after long-term exposure[3].
China has a long history of using wastewater for irrigation since the 1940s [10]. The wastewater currently used for farm irrigation in China is mostly untreated and of poor quality. A survey in 1994 found that approximately 85% of the wastewater used for farm irrigation did not meet the nation’s standards for reuse [11]. With urban development, the presence of comprehensive wastewater collection and treatment systems has increased gradually. However, the treatment rate and treatment level of wastewater remain low in China. According to the reports of the Ministry of Environmental Protection of China, the average disposal rate of urban wastewater was 82.3%, and the disposal rate of domestic wastewater was 72.9% in China in 2010. Approximately 45% of the wastewater irrigated areas in China were seriously contaminated with heavy metals [12].
Shijiazhuang City is one of the major wastewater irrigation areas in China, including several areas located in south of the city with a total area covering approximately 438.8 km2. Approximately 3.04 x 108 m3 of wastewater, which is mainly industrial wastewater and domestic wastewater, is annually discharged through the Dongming discharging channel in Shijiazhuang city. The sources of the wastewater include textile mills, pharmaceutical factories, machinery, electronics, chemical plants, food processing plants, and domestic wastewater from household. The primary pollutants are chlorides, lead, chromium and arsenic [13]. To study the effects of wastewater irrigation on human health, two retrospective studies on the cause of death in wastewater irrigation areas were performed by the Department of Epidemiology and Hygienic Statistics, School of Public Health, Hebei Medical University [14, 15]. Both of the studies found that all-cause standardised mortality and cancer-standardised mortality in a wastewater irrigation area were significantly higher compared with the control area. The results of these studies showed that wastewater irrigation might be an important factor leading to the increased cancer mortality of people living in wastewater irrigation areas. Based on the results of the epidemiological surveys, it is important to evaluate the possible genotoxicity of the irrigative wastewater to effectively manage wastewater irrigation.
Many studies have focused on the genotoxicity of industrial wastewater and domestic wastewater [16–19], while fewer studies have focused on the genotoxicity of irrigative wastewater. The aim of the present research was to investigate the genotoxicity of the irrigative wastewater from Shijiazhuang city in China. More than 200 short-term genotoxicity and mutagenicity assays have been developed for screening potentially carcinogenic chemicals. In summary, most assays were able to detect carcinogens or noncarcinogens with an efficiency of approximately 70% compared with the outcome of 2-year cancer bioassays [20]. Because no single genetic assay can cover all the genetics endpoints, a battery of genetic assays should be used to evaluate the genotoxicity of xenobiotics.
A battery of genotoxicity assays with different genetic endpoints was used in this study: the Salmonella mutagenicity test (TA97, TA98, TA100 and TA102; with or without metabolic activation) for base mutation, the micronucleus (MN) test with polychromatic erythrocytes (PCEs) in mice bone marrow for chromosome damage and single-cell gel electrophoresis (SCGE) assays with mice hepatocytes in vivo for primary DNA damage. SCGE assays enable the detection of single-strand and double-strand DNA breaks and apurinic sites [21–24]. The assay is based on the quantification of DNA damage in an electric field and is increasingly used by genetic toxicologists to test individual chemicals and environmental samples.
Materials and Methods
2.1 Reagents and chemicals
Chemicals were obtained from the following sources: glucose-6-phosphate, L-histine, D-botine, nicotinamide adenine dinucleotide phosphate (NADP), dimethyl sulphoxide (DMSO) and XAD-2 resins were obtained from Sigma Chemical Co.; low melting-point agarose was obtained from Biotech; cyclophosphamide (CP) was obtained from Jiangsu Heng Rui Medical Co., Ltd.; phenobarbitol sodium was obtained from Beijing Shuang He Medical Co., Ltd. The chemicals used as positive controls in the Salmonella mutagenicity test were supplied by the Center for Disease Prevention and Control of Hebei Province in China. Other solvents and chemicals were of analytical grade.
2.2 Sample collection and chemical analysis
Wastewater samples were collected directly upstream (114°31′E, 37°56′N), midstream (114°35′E, 37°51′N) and downstream (114°59′E, 37°41′N) of the Dongming discharging river from Shijiazhuang city during spring (20 April, 2010). No specific permissions were required for these locations. Wastewater samples were collected using an automatic sampling device in a time-proportional manner over a period of 24 h and were stored in precleaned brown glass flasks. The composite sample was filtered with an 11.0 μm pore-size filter and sterilised through a 0.22 μm Millipore filter. The composite sample was stored at -20°C in the dark for chemical analyses and bioassays. Ten liters of the composite sample was extracted using solid-phase extraction with XAD-2 resins according to a previous method [25]. The extracts were redissolved in 1 ml of dimethyl sulfoxide (DMSO). The wastewater samples were collected following the standard method of chemical analysis [26]. Chemical analysis of the extract was performed using gas chromatography mass spectrometry (GC-MS) (Agilent 6890/5973N, USA)). The metal concentrations in the composite sample were determined using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7500a, USA).
2.3 The Salmonella mutagenicity test
To assess the mutagenicity of the sample, the Salmonella mutagenicity test was performed using the standard plate-incorporation method and the preincubation procedure as described [27, 28]. A set of test strains, TA97, TA98, TA100 and TA102, were supplied by the Center for Disease Prevention and Control of Hebei Province in China. A mixture containing 0.1 ml of the sample with different dilution concentrations and 0.1 ml of the test strain was initially incubated at 37°C for 20 min in culture tubes. If metabolic activation was required, 0.5 ml of an S9 mixture was added. The mixture was added to a tube containing 2 ml of top agar with 0.5 mM biotin-histidine. The tube was gently vortexed and poured onto a minimal glucose plate. After incubation at 37°C for 48 h in the dark, the number of revertant colonies was counted and compared to the number of spontaneous revertant colonies on solvent control plates. Four dilution concentrations were examined, including 25 μl, 50 μl, 75 μl, and 100 μl /plate. The samples were tested with or without an S9 mixture. The S9 mixture was prepared from livers of Sprague-Dawley rats pretreated with a polychlorinated biphenyl mixture (Aroclor 1254) and was used in the assay to simulate the metabolic activation occurring in the liver of eukaryotes. Negative and positive controls were also conducted at the same time. Distilled water served as a negative control. Diagnostic mutagens, including 9-fluorenone (0.2 μg/plate), sodium azide (2.5 μg/plate), methylsulfonic methylester (30 μl/plate), 2-aminoflurene (10 μg/plate) and 1,8-dihydroxyanthraquinone (50 μg/plate) were served as positive control chemicals. Triplicate plates were performed for each dose, and the assay was repeated twice.
The sample was considered to be a mutagen when (a) the number of revertant colonies in the assay was at least twice the number of revertant colonies in negative control and (b) a dose-response increase in the number of revertants colonies was observed for one or more strains.
2.4 Micronucleus test
Approximately 4-week-old Kunming mice weighing between 20 and 25 g were supplied by the Division of Laboratory Animals of Hebei Medical University. The mice were acclimated for a period of 1 week before the beginning of the experiments. The animals were maintained in a room under controlled conditions of temperature (22±2°C), humidity (50±10%), and a 12 h light/dark cycle. The mice were fed a standard rodent pellet diet (purchased from the Division of Laboratory Animals, Hebei Medical University). During the acclimatisation period, the animals were observed once daily. Mice were accepted for the study upon absence of disease as demonstrated by good physical condition.
Mice were randomly divided into five groups according to weight with 10 animals per group with five males and five females. In a preliminary toxicity assay, neither death nor clinical signs were observed in mice at the maximum recommended volume of 20 ml/kg.bw by gavage. Three dilution concentrations (25%, 50%, and 100%) were performed and administered ad libitum by drinking water. One group of mice was exposed for 2 consecutive days, and the other group of mice was exposed for 15 consecutive days. Distilled water administered to the negative group ad libitum by drinking water for the same period. The positive control group received an intraperitoneal injection of cyclophosphamide (40 mg/kg). At the end of the experimental period, mice were deprived of food for 24 h and prepared for the experimental procedure. The animals were sacrificed by an overdose of CO2. At the time of necropsy, the breastbone of each mouse was collected and cleaned of the surrounding muscle tissue. The bone marrow fluids were squeezed out with a hemostat, dropped in the fetal calf serum on one end of the clean slide and mixed carefully. At least two thin smears were prepared by pulling bone-marrow fluids behind a cover glass held at a 45° angle. All the slides were air-dried, fixed in methanol for 10 min, and stained according to the method developed by Schmid et al. [29, 30]. These slides were coded for blind analysis. At least 1000 polychromatic erythrocytes per animal were scored for the presence of micronuclei under immersion objective (1000×) using an Olympus BH-2 microscope. The following criteria were applied for the identification of micronuclei: no connection with the main nucleus, same color and intensity as the main nucleus and an area smaller than one-third of the main nucleus. The ratio of PCE/NCE was also determined by counting a total of 1000 erythrocytes.
A compound can be considered as a mutagen if the induced MN frequencies were statistically significant compared with those induced by the negative control, and a dose-response increase in the number of MN frequencies was observed. This study was carried out in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, Ministry of Health of China that seeks to minimize both the number of animals used and any suffering that they might experience. The protocol was approved by the Ethics Committee for Animal Experiments of Hebei Medical University (approval number: HEBMU -2010-03; approval date: March 25, 2010). Studies comply with Animal Research: Reporting In Vivo Experiment guidelines (S1 Checklist).
2.5 SCGE
Kunming mice were randomly divided into five groups according to weight with 8 animals per group, with four males and four females. Three groups received the samples with three dilution concentrations (25%, 50%, and 100%) ad libitum by drinking water for 15 consecutive days. The negative group received distilled water for the same period of time, and the positive group controls received an intraperitoneal injection of phenobarbital sodium (140 mg/kg), which can induce primary DNA lesions [31]. The water intake was recorded every day, and the water intake of each mouse was calculated. At the time of necropsy, a liver-cell suspension with a cell density of 104 ~ 105/ml was prepared. Cell viability was determined using trypan blue dye exclusion [32]. The number of trypan blue negative cells was considered to be the number of viable cells and was superior to 95%. The slides were prepared using the conventional comet assay method. The slides were immersed in a cold, freshly prepared lysing solution. The slides were protected from light and maintained at 4°C for 1 h and placed in electrophoresis buffer at 4°C for 30 min to allow the DNA to unwind before electrophoresis. Electrophoresis was performed at 25 V and 300 mA for 20 min in the dark at room temperature. After electrophoresis, the slides were neutralised in Tris 400 mM (pH 7.5), rinsed three times in distilled water, and left to dry overnight at room temperature. The dry slides were stained according to the method described by Santos et al. [33]. For each animal, 50 cells were evaluated. The Olive tail moments (OTMs, Olive tail moment = percent of DNA in the tail × the distance between the center of gravity of DNA in the tail and the center of gravity of DNA in the head) were observed using the CASP comet analysis software [34].
2.6 Statistical analysis
Values were expressed as the mean ± SD. The dose-response relationships were analysed by Spearman correlation using SPSS 18.0. The statistical analysis was performed by one-way ANOVA for the analysis of MN frequencies; the OTMs data of the SCGE were analysed using the nonparametric Kruskal–Wallis test followed by a post-hoc multiple-comparison test. A medullar toxicity analysis involving the PCE/NCE ratio was statistically analysed by Student’s t-test, and P< 0.05 was considered statistically significant for the assays.
Results
3.1 Chemical analysis
The following concentrations of heavy metals were measured in the composite sample: Pb, 0.285 mg/l; Cr, 0.178 mg/l; Mn, 0.128 mg/l; Ni, 0.047 mg/l; Be, 0.00044 mg/l; As, 0.028 mg/l; and Cd, 0.002 mg/l. More than 30 organic compounds were detected by GC-MS, including phthalates, heterocyclic compounds, polycyclic aromatic hydrocarbon compounds, aniline compounds, etc. The main organic pollutants are shown in Table 1.
Table 1. Main organic pollutions in the irrigative wastewater taken from the Dongming discharging river in Shijiazhuang city determinated by GC-MS.
| Name | Molecular formula | Molecular weight |
|---|---|---|
| Mono(2-ethylhexyl) phthalate | C16H22O4 | 278.34 |
| Dibutyl phthalate | C16H22O4 | 278.34 |
| Dihexyl phthalate | C20H30O4 | 334.21 |
| Diisobutyl phthalate | C16H22O4 | 278.15 |
| Phenylacetic acid | C8H8O2 | 136.05 |
| Dipropyl phthalate | C14H18O4 | 250.19 |
| Di(2-ethylhexyl)phthalate | C24H38O4 | 390.56 |
| 2-(Methylthio)- benzothiazole | C8H7NS2 | 181.00 |
| 1-Methylfluorene | C14H12 | 180.09 |
| Benzothiazole | C7H5NS | 135.01 |
| N-methyl-N-phenyl- Formamide | C8H9NO | 135.07 |
| Indole | C8H7N | 117.06 |
| N-(2-Naphthyl)aniline | C16H13N | 219.28 |
| Benzylidene malonaldehyde | C10H8O2 | 160.05 |
3.2 The Salmonella mutagenicity test
The results from the Salmonella mutagenicity test are shown in Table 2. With and without an S9 mixture, the number of revertant colonies of the TA98 strain induced by the samples of different dilution concentrations was more than twice the negative control. With and without the S9 mixture, the number of revertant colonies of TA97, TA100, and TA102 strains induced by the samples with different dilution concentrations was less than twice the negative control. With and without the S9 mixture, there was no significant dose-response relationship with the number of revertant colonies of TA97, TA100, and TA102 strains induced by the samples with different dilution concentrations. Significant dose-response relationships were observed with the number of revertant colonies of the TA98 strain (r s = 0.976, P = 0.000; r s = 0.954, P = 0.000), and the dose-response relationship curves could be fitted well with the equations: ŷ = 34.833+113.133x and ŷ = 42.833+124.133x.
Table 2. The Salmonella bioassay of the irrigative wastewater taken from the Dongming discharging river in Shijiazhuang city (n = 6).
| TA97 (-S9) | TA97 (+S9) | TA98 (-S9) | TA98 (+S9) | |||||
| Dose (μl/plate) | MI a | Revertant colonies | MI a | Revertant colonies | MI a | Revertant colonies | MI a | Revertant colonies |
| NC b | 1 | 97.2±10.2 | 1 | 100.7±14.7 | 1 | 30.2±2.3 | 1 | 30.5±1.4 |
| 25 | 1.2 | 116.0±15.7 | 1.1 | 111.2±13.2 | 2.3 d | 67.5±7.3* | 3.0 d | 90.0±14.4* |
| 50 | 1.2 | 119.7±15.6 | 1.2 | 121.8±7.0 | 3.2 d | 97.2±4.1* | 3.4 d | 102.3±12.4* |
| 75 | 1.2 | 118.0±10.3 | 1.3 | 126.8±5.3 | 3.7 d | 111.7±7.6* | 4.5 d | 137.3±4.7* |
| 100 | 1.3 | 129.0±6.4 | 1.3 | 130.0±7.2 | 5.0 d | 150.0±7.2* | 5.3 d | 163.2±9.3* |
| PC | 21.5 d | 2078.5±118.0 | 12.9 d | 1274.5±108.8 | 98.2 d | 2950.7±83.3 | 85.9 d | 2618.5±268.1 |
| TA100 (-S9) | TA100 (+S9) | TA102 (-S9) | TA102 (+S9) | |||||
| Dose (μl/plate) | MI a | Revertant colonies | MI a | Revertant colonies | MI a | Revertant colonies | MI a | Revertant colonies |
| NC b | 1 | 116.3±6.6 | 1 | 120.0±7.3 | 1 | 221.3±11.9 | 1 | 220.0±8.8 |
| 25 | 1.1 | 132.3±10.3 | 1.1 | 134.8±8.0 | 1.1 | 238.3±11.6 | 1.1 | 242.3±12.7 |
| 50 | 1.1 | 130.0±4.7 | 1.1 | 133.5±11.0 | 1.1 | 250.8±7.8 | 1.2 | 263.8±10.0 |
| 75 | 1.1 | 130.0±7.1 | 1.2 | 144.8±13.7 | 1.2 | 258.3±12.0 | 1.2 | 263.5±11.0 |
| 100 | 1.2 | 139.3±5.9 | 1.1 | 131.3±8.8 | 1.1 | 242.7±7.4 | 1.1 | 242.7±7.4 |
| PC c | 20.3 d | 2368.3±206.9 | 12.4 d | 1475.5±41.5 | 9.1 d | 2005.3±120.7 | 5.3 d | 1158.3±163.0 |
a: Mutagenic index (MI): number of revertant colonies induced in the sample/number of spontaneous revertant colonies in the negative control.
b: Negative control (NC) for all strains: sterile distilled water
c: Positive controls (PC) in experiments: Without S9: 0.2 μg/plate 9-fluorenone was used for strain TA97 and TA98, 2.5 μg/plate sodium azide for strain TA100, 30 μl/plate methylsulfonic methylester was used for strain TA102; With S9: 10 μg/plate 2-aminoflurene was used for strain TA97, TA98, and TA100; 50 μg/plate 1,8-dihydroxyanthraquinone was used for strain TA102
d: MI was more than twice that of the negative control.
*: Significant dose-response relationships were observed
3.3 Micronucleus test
The micronucleus are shown in Fig 1. The results from the MN test are presented in Table 3. The MN frequencies of the PCEs in mouse bone marrow induced by the samples with different dilution concentrations for two consecutive days were not significantly different from the MN frequencis induced by the negative control. After the mice were exposed to the samples for 15 consecutive days, the MN frequencies of the PCEs in mouse bone marrow induced with the concentrations of 50% and 100% were significantly increased, and a dose-response relationship was observed (ŷ = 0.003+0.007x, r s = 0.814, P = 0.000). The sample did not affect the proliferation of normal bone marrow erythrocytes, and medullar toxicity was not observed.
Fig 1. Micronucleus of mice bone polychromatic erythrocytes induced by the irrigative wastewater in Shijiazhuang city.
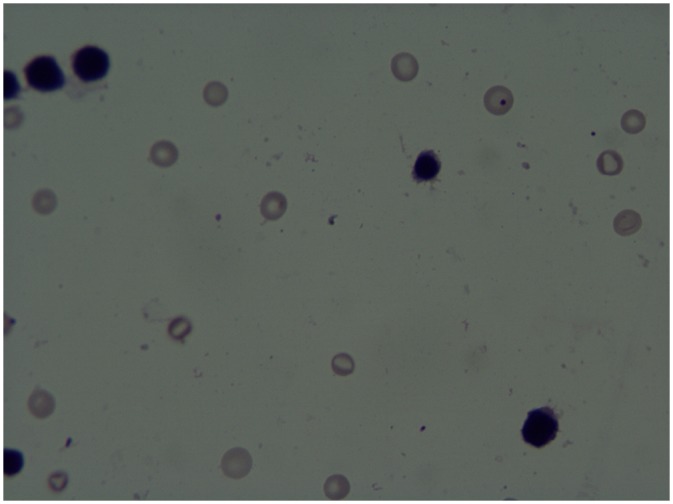
Table 3. Micronucleus frequencies of PCEs in mouse bone marrow induced by the irrigative wastewater taken from the Dongming discharging river in Shijiazhuang city (n = 10).
| 2 consecutive days exposure | 15 consecutive days exposure | |||
|---|---|---|---|---|
| Dose | MN (‰±SD) | PCE/NCE ( ±SD) | MN (‰±SD) | PCE/NCE ( ±SD) |
| NC a | 3.40±1.35 | 1.03±0.07 | 3.30±1.16 | 1.02±0.06 |
| 25% | 3.80±1.48 | 1.03±0.11 | 4.00±1.41 | 1.01±0.07 |
| 50% | 4.10±1.37 | 1.00±0.10 | 7.60±2.17** | 1.03±0.07 |
| 100% | 4.50±1.08 | 1.00±0.12 | 9.40±2.27** | 0.96±0.06 |
| PC b | 17.70±2.00** | 0.91±0.08** | 16.20±2.35** | 0.89±0.06** |
a: Negative control, Sterile distilled water
b: Positive control, cyclophosphamide (40 mg/kg)
** P<0.01 versus negative control
3.4 SCGE
The figures from SCGE are shown in Fig 2 (A for negative control group; B for 25% wastewater group; C for 50% wastewater group; D for 100% wastewater group; E for positive control group). The results from SCGE are presented in Table 4. After the mice were exposed to the sample for 15 consecutive days, the OTMs of the mouse hepatocytes induced with the concentrations of 50% and 100% were significantly increased compared with the negative control, and a dose-response relationship was observed (ŷ = 0.298+4.055x, r s = 0.905, P = 0.000). No significant difference was observed concerning the body weight and water intake.
Fig 2. The result of single-cell gel electrophoresis. (A): negative control; (B)25% wastewater; (C) 50% wastewater; (D) 100% wastewater; (E) positive control.
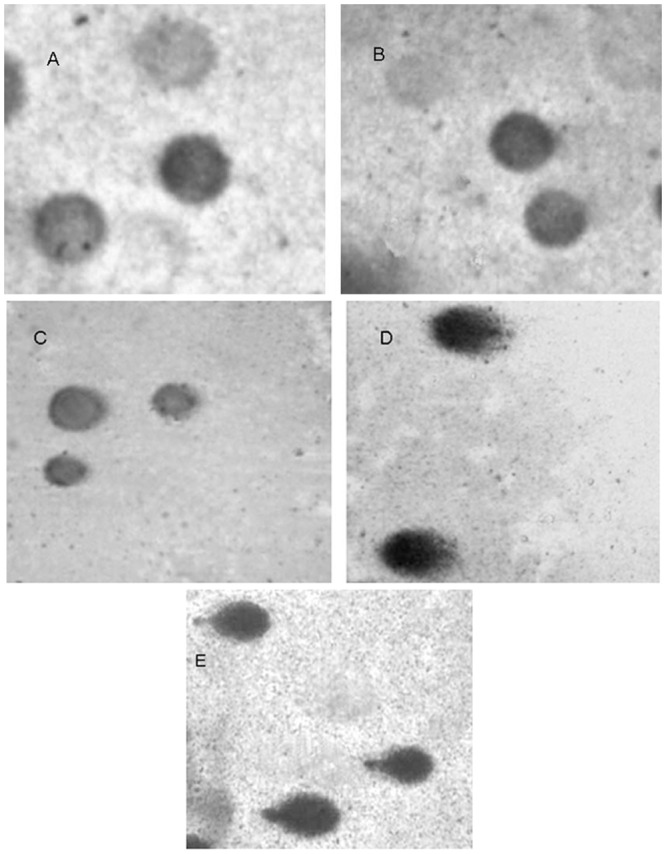
Table 4. Results from SCGE in mouse hepatocytes induced by the irrigative wastewater taken from the Dongming discharging river in Shijiazhuang city (n = 8, ±SD).
| Dose | Body weight (g) | Water intake c (ml/day) | Olive tail moment |
|---|---|---|---|
| NC a | 34.41±3.00 | 5.68±0.66 | 0.65±0.09 |
| 25% | 32.30±3.57 | 6.30±0.51 | 0.68±0.10 |
| 50% | 33.45±3.61 | 6.44±0.49 | 2.58±0.18** |
| 100% | 33.01±3.65 | 5.96±0.70 | 4.38±0.58** |
| PC b | 34.37±2.92 | 6.18±0.47 | 20.18±1.30** |
a: Negative control, Sterile distilled water
b: Positive control, phenobarbital sodium (140 mg/kg)
c Water intake (ml/day) for each mouse
** P<0.01 versus negative control
Discussion
Many national and international studies have focused on the genotoxicity of urban wastewater, industrial wastewater, river and drinking water [16–18, 35, 36]. In these studies, two main methods of sample pretreatment were commonly adopted: organic pollutants may be enriched by solid-phase extraction or liquid-liquid extraction methods to improve the sensitivity of the test substance, or the water sample may be sterilised through a Millipore filter (0.22 μM) and directly used for biological detection. The organic extracts obtained by solid-phase extraction methods commonly induced genetic damage in vivo and in vitro [36–38]. The water samples were concentrated before biological detection for the first method, which may reduce the environmental relevance of the biological exposure concentrations. Each enrichment method can concentrate parts of the composition in the water sample, and some pollutants might be lost during the enrichment process. Other contaminants such as heavy metals were present in wastewater in addition to organic pollutants. Therefore, the organic extracts, which were obtained in the enrichment process and served as the test substance, were not suitable. Therefore, the second sample pretreatment method was recommended because the results from the genotoxicity test using this method were more realistic and reliable. In the study by Durgo et al. [39], the wastewater sample was sterilised through a Millipore filter (0.22 μM), and the results of SCGE indicated significant DNA damaging potential for human leukocytes. Wastewater samples from an oncological ward of the general hospital of Vienna in Austria filtered with an 11.0 μm filter and a 0.22 μm filter were tested using the SCGE assay with primary rat hepatocytes. A significant and dose-dependent induction of DNA damage (up to two-fold over the background) was observed [40]. In the present study, the irrigative wastewater sample from Shijiazhuang city was filtered with an 11.0 μm filter and sterilised with a 0.22 μm Millipore filter.
Two main methods have been used for studies on wastewater. One method uses chemical analysis of the main composition, and the other method uses biological methods (such as SCGE) to detect the total genotoxicity. For research on environmental samples, chemical analysis and biological methods are equally important. The chemical analysis shows the composition of the environmental samples (qualitative analysis) and the concentration of each compound (quantitative analysis), and the biological assays shows the total toxicity on the organisms. Combining biological assays with chemical analyses is a good method for research on environmental samples and contributes to the prevention and control of environmental pollution.
The major metals in the wastewater sample were analysed by ICP-MS. The concentrations of chromium and lead in the sample exceeded the national standards (GB20922-2007) by 0.78 and 0.43-fold, respectively. Positive results for chromium were observed in nine mutation experiments including the Salmonella mutagenicity test, SCGE, MN assay, sister chromatid exchange assay, etc. [41, 42]. American researchers found that a cumulative hexavalent chromium exposure showed a strong dose-dependent relationship for lung cancer in a cohort study of 2,357 workers in chromate production facilities [43]. Several studies on the genotoxicity of lead acetate were conducted in rodents using the MN test, and the results showed an increase in MN frequencies [44–47].
The GC-MS analysis of the organic extracts in the composite sample identified more than 30 organic compounds, and some of the compounds were mutagens, carcinogens and environmental endocrine disrupters. Dibutyl phthalate, diisobutyl phthalate, and mono (2-ethylhexyl) phthalate, which are mutagens, were identified in the samples [48, 49]. Some organic pollutants that are not considered to be mutagens may generate mutagenic effects after metabolic activation in the body, such as di(2-ethylhexyl)-phthalate, benzothiazole, and N-(2-Naphthyl)aniline [50–52].
Chemical analysis of complex mixtures offers limited information concerning biological toxicity. Based on the chemical analysis, a battery of genotoxicity assays with different genetic endpoints were used in the research to evaluate the biological toxicity. The combined use of these three bioassays with different genetic endpoints increases the confidence level of genotoxicity estimation. The results of the Salmonella mutagenicity test showed that direct and indirect frameshift-type mutagens were present in the sample. Furthermore, the sample could induce DNA damage in a dose-dependent manner in mice hepatocytes in vivo. With increasing exposure duration, the results of the MN test in PCEs of mouse bone marrow changed from negative to positive. Our results were consistent with the findings of earlier investigations. The MN test collaborative research group (CSGMT) reported MN test results of 11 test substances and found that the MN frequencies of multiple exposures were higher compared with a single exposure in most cases, and individual results changed from negative to positive [53]. Genotoxicity can be a consequence of long-term exposure to low levels of chemicals and can exhibit a hereditary and delayed-onset nature that may lead to major consequences at the population level [54]. None of the chemicals detected in the chemical analysis were present at concentrations individually cause genotoxicity; therefore, the potential for component interactions (additive or synergistic) was likely the cause of the genotoxicity of the total sample.
In summary, all the results indicated that the samples from the Dongming discharging river in Shijiazhuang city exhibited genotoxicity and might pose potential harmful effects on the local residents. To standardise the wastewater irrigation management and to maximise the protection of the local residents’ health, the wastewater emissions of lead, chromium and organic pollutants from the upstream factories should be strictly controlled. Wastewater treatment plants should improve the wastewater treatment rate and enhance the removal efficiency of heavy metals and organic pollutants.
Conclusions
In the present study, the wastewater collected from the discharging river in Shijiazhuang city was analysed using both chemical analysis and biological assays. The concentrations of chromium and lead in the sample exceeded the national standards (GB20922-2007) by 0.78 and 0.43-fold, respectively. More than 30 organic compounds were detected, and some of the compounds were mutagens, carcinogens and environmental endocrine disrupters. The present results from the biological assays conformed that the irrigative wastewater sample exhibited genotoxicity by causing base mutation, chromosomal damage and DNA damage. In summary, the results from both the chemical analysis and biological assays imply that the genotoxic chemicals contained in the irrigative wastewater may harm organisms in the ecosystem and humans as a result of accumulation in the food chain. However, the present work is a preliminary report. Further studies are needed to confirm the carcinogenetic risk of irrigative wastewater on humans.
Supporting Information
(PDF)
Funding Statement
This research was supported by the Health Department Science Research Foundation of Hebei Province (Project No.20090057).
References
- 1. Jiménez B, Asano T. Water Reuse: An International Survey of Current Practice, Issues and Needs. London: IWA Publishing; 2008. [Google Scholar]
- 2. Hamilton A, Stagnitti F, Xiong X, Kreidl S, Benke K. Wastewater irrigation: the state of play. Vadose Zone Journal 2007;6: 823–40. [Google Scholar]
- 3. Chen Y, Wang C, Wang Z. Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants. Environ Int. 2005;31:778–83. 10.1016/j.envint.2005.05.024 . [DOI] [PubMed] [Google Scholar]
- 4. Singh S, Kumar M. Heavy metal load of soil, water and vegetables in peri-urban Delhi. Environ Monit Assess. 2006;120:79–91. 10.1007/s10661-005-9050-3 . [DOI] [PubMed] [Google Scholar]
- 5. Kabdasli I, Arslan T, Arslan-Alaton I, Olmez-Hanci T, Tunay O. Organic matter and heavy metal removals from complexed metal plating effluent by the combined electrocoagulation/Fenton process. Water science and technology: a journal of the International Association on Water Pollution Research. 2010;61:2617–24. 10.2166/wst.2010.202 . [DOI] [PubMed] [Google Scholar]
- 6. Kobori H, Ham YS, Saito T. Influence of treated sewage effluent on organic pollution assessment in the Sakai River basin in Central Japan. Environ Monit Assess. 2009;151:243–9. 10.1007/s10661-008-0265-y . [DOI] [PubMed] [Google Scholar]
- 7. Lefebvre O, Moletta R. Treatment of organic pollution in industrial saline wastewater: a literature review. Water research. 2006;40:3671–82. 10.1016/j.watres.2006.08.027 . [DOI] [PubMed] [Google Scholar]
- 8. Tu J, Zhao Q, Wei L, Yang Q. Heavy metal concentration and speciation of seven representative municipal sludges from wastewater treatment plants in Northeast China. Environ Monit Assess. 2012;184:1645–55. 10.1007/s10661-011-2067-x . [DOI] [PubMed] [Google Scholar]
- 9. Ingwersen J, Streck T. Modeling the environmental fate of cadmium in a large wastewater irrigation area. J Environ Qual. 2006;35:1702–14. 10.2134/jeq2005.0412 . [DOI] [PubMed] [Google Scholar]
- 10. Wei N, Cheng XR, Liu YP. Overview of main reuse approaches of municipal wastewater. Water-Saving Irrigation. 2006;1:31–4, 6. [Google Scholar]
- 11. He P, Phan L, Gu G, Hervouet G. Reclaimed municipal wastewater—a potential water resource in China. Water Sci Technol. 2001;43:51–8. . [PubMed] [Google Scholar]
- 12. Xu J, Wu L, Chang AC, Zhang Y. Impact of long-term reclaimed wastewater irrigation on agricultural soils: a preliminary assessment. J Hazard Mater. 2010;183:780–6. 10.1016/j.jhazmat.2010.07.094 . [DOI] [PubMed] [Google Scholar]
- 13. Khan MU, Malik RN, Muhammad S. Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere. 2013;93:2230–8. 10.1016/j.chemosphere.2013.07.067 . [DOI] [PubMed] [Google Scholar]
- 14. Huang C, Hu W, Li R. Investigation and analysis of cancer spectrum in the city of wastewater irrigation. Environmental Protection.1984;16:34. [Google Scholar]
- 15. Ma J. Investigation of malignant tumor mortality in irrigation drainage area. 2008;dissertation. [Google Scholar]
- 16. Krishnamurthi K, Saravana Devi S, Hengstler JG, Hermes M, Kumar K, Dutta D, et al. Genotoxicity of sludges, wastewater and effluents from three different industries. Arch Toxicol. 2008;82:965–71. 10.1007/s00204-008-0380-0 . [DOI] [PubMed] [Google Scholar]
- 17. Oliveira-Martins CR, Grisolia CK. Toxicity and genotoxicity of wastewater from gasoline stations. Genet Mol Biol. 2009;32:853–6. 10.1590/S1415-47572009005000094 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radic S, Stipanicev D, Vujcic V, Rajcic MM, Sirac S, Pevalek-Kozlina B. The evaluation of surface and wastewater genotoxicity using the Allium cepa test. Sci Total Environ. 2010;408(5):1228–33. 10.1016/j.scitotenv.2009.11.055 . [DOI] [PubMed] [Google Scholar]
- 19. Thewes MR, Junior DE, Droste A. Genotoxicity biomonitoring of sewage in two municipal wastewater treatment plants using the Tradescantia pallida var. purpurea bioassay. Genet Mol Biol. 2011;34:689–93. 10.1590/S1415-47572011005000055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Curieux F, Marzin D, Erb F. Comparison of three short-term assays: results on seven chemicals. Potential contribution to the control of water genotoxicity. Mutat Res. 1993;319:223–36. . [DOI] [PubMed] [Google Scholar]
- 21. Brendler-Schwaab S, Hartmann A, Pfuhler S, Speit G. The in vivo comet assay: use and status in genotoxicity testing. Mutagenesis. 2005;20:245–54. 10.1093/mutage/gei033 . [DOI] [PubMed] [Google Scholar]
- 22. Moller P. The alkaline comet assay: towards validation in biomonitoring of DNA damaging exposures. Basic Clin Pharmacol Toxicol. 2006;98:336–45. 10.1111/j.1742-7843.2006.pto_167.x . [DOI] [PubMed] [Google Scholar]
- 23. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. . [DOI] [PubMed] [Google Scholar]
- 24. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21. . [DOI] [PubMed] [Google Scholar]
- 25. Junk GA, Richard JJ, Grieser MD, Witiak D, Witiak JL, Arguello MD, et al. Use of macroreticular resins in the analysis of water for trace organic contaminants. J Chromatogr. 1974;99(0):745–62. Epub 1974/11/06. . [DOI] [PubMed] [Google Scholar]
- 26. Standard Methods for the Examination of Water and Wastewater. 22th ed American Public Health Association Press; Washington; 2012. [Google Scholar]
- 27. Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975;31:347–64. . [DOI] [PubMed] [Google Scholar]
- 28. Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. . [DOI] [PubMed] [Google Scholar]
- 29. Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. . [DOI] [PubMed] [Google Scholar]
- 30. von Ledebur M, Schmid W. The micronucleus test. Methodological aspects. Mutat Res. 1973;19:109–17. . [DOI] [PubMed] [Google Scholar]
- 31. Sasaki YF, Izumiyama F, Nishidate E, Matsusaka N, Tsuda S. Detection of rodent liver carcinogen genotoxicity by the alkaline single-cell gel electrophoresis (Comet) assay in multiple mouse organs (liver, lung, spleen, kidney, and bone marrow). Mutat Res. 1997;391:201–14. . [DOI] [PubMed] [Google Scholar]
- 32. Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001;Appendix 3:Appendix 3B 10.1002/0471142735.ima03bs21 . [DOI] [PubMed] [Google Scholar]
- 33. Santos DB, Schiar VP, Ribeiro MC, Schwab RS, Meinerz DF, Allebrandt J, et al. Genotoxicity of organoselenium compounds in human leukocytes in vitro. Mutat Res. 2009;676:21–6. 10.1016/j.mrgentox.2009.03.006 . [DOI] [PubMed] [Google Scholar]
- 34. Konca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Gozdz S, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 2003;534:15–20. . [DOI] [PubMed] [Google Scholar]
- 35. Dong Y, Zhang J. Testing the genotoxicity of coking wastewater using Vicia faba and Hordeum vulgare bioassays. Ecotoxicol Environ Saf. 2010;73:944–8. 10.1016/j.ecoenv.2009.12.026 . [DOI] [PubMed] [Google Scholar]
- 36. Liu JR, Dong HW, Tang XL, Sun XR, Han XH, Chen BQ, et al. Genotoxicity of water from the Songhua River, China, in 1994–1995 and 2002–2003: Potential risks for human health. Environ Pollut. 2009;157:357–64. 10.1016/j.envpol.2008.10.004 . [DOI] [PubMed] [Google Scholar]
- 37. Fracasso ME, Leone R, Brunello F, Monastra C, Tezza F, Storti PV. Mutagenic activity in wastewater concentrates from dye plants. Mutat Res. 1992;298:91–5. . [DOI] [PubMed] [Google Scholar]
- 38. Gauthier L, Van der Gaag MA, L'Haridon J, Ferrier V, Fernandez M. In vivo detection of waste water and industrial effluent genotoxicity: use of the Newt Micronucleus Test (Jaylet Test). Sci Total Environ. 1993;138:249–69. . [DOI] [PubMed] [Google Scholar]
- 39. Durgo K, Orescanin V, Lulic S, Kopjar N, Eljezic DZ, Colic JF. The assessment of genotoxic effects of wastewater from a fertilizer factory. J Appl Toxicol. 2009;29:42–51. 10.1002/jat.1381 . [DOI] [PubMed] [Google Scholar]
- 40. Ferk F, Misik M, Grummt T, Majer B, Fuerhacker M, Buchmann C, et al. Genotoxic effects of wastewater from an oncological ward. Mutat Res. 2009;672:69–75. 10.1016/j.mrgentox.2008.08.022 . [DOI] [PubMed] [Google Scholar]
- 41. De Flora S, Bagnasco M, Serra D, Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat Res. 1990;238:99–172. . [DOI] [PubMed] [Google Scholar]
- 42. Gambelunghe A, Piccinini R, Ambrogi M, Villarini M, Moretti M, Marchetti C, et al. Primary DNA damage in chrome-plating workers. Toxicology. 2003;188:187–95. . [DOI] [PubMed] [Google Scholar]
- 43. Gibb HJ, Lees PS, Pinsky PF, Rooney BC. Lung cancer among workers in chromium chemical production. Am J Ind Med. 2000;38:115–26. . [DOI] [PubMed] [Google Scholar]
- 44. Celik A, Ogenler O, Comelekoglu U. The evaluation of micronucleus frequency by acridine orange fluorescent staining in peripheral blood of rats treated with lead acetate. Mutagenesis. 2005;20:411–5. . [DOI] [PubMed] [Google Scholar]
- 45. Piao F, Cheng F, Chen H, Li G, Sun X, Liu S, et al. Effects of zinc coadministration on lead toxicities in rats. Industrial health. 2007;45:546–51. . [DOI] [PubMed] [Google Scholar]
- 46. Robbiano L, Carrozzino R, Puglia CP, Corbu C, Brambilla G. Correlation between induction of DNA fragmentation and micronuclei formation in kidney cells from rats and humans and tissue-specific carcinogenic activity. Toxicology and applied pharmacology. 1999;161:153–9. 10.1006/taap.1999.8796 . [DOI] [PubMed] [Google Scholar]
- 47. Tapisso JT, Marques CC, Mathias Mda L, Ramalhinho Mda G. Induction of micronuclei and sister chromatid exchange in bone-marrow cells and abnormalities in sperm of Algerian mice (Mus spretus) exposed to cadmium, lead and zinc. Mutat Res. 2009;678:59–64. 10.1016/j.mrgentox.2009.07.001 . [DOI] [PubMed] [Google Scholar]
- 48. Agarwal DK, Lawrence WH, Nunez LJ, Autian J. Mutagenicity evaluation of phthalic acid esters and metabolites in Salmonella typhimurium cultures. J Toxicol Environ Health. 1985;16:61–9. 10.1080/15287398509530719 . [DOI] [PubMed] [Google Scholar]
- 49. Kleinsasser NH, Kastenbauer ER, Wallner BC, Weissacher H, Harreus UA. [Genotoxicity of phthalates. On the discussion of plasticizers in children's toys]. Hno. 2001;49:378–81. . [DOI] [PubMed] [Google Scholar]
- 50. Ginsberg G, Toal B, Kurland T. Benzothiazole toxicity assessment in support of synthetic turf field human health risk assessment. J Toxicol Environ Health A. 2011;74:1175–83. 10.1080/15287394.2011.586943 . [DOI] [PubMed] [Google Scholar]
- 51. Phillips BJ, James TE, Gangolli SD. Genotoxicity studies of di(2-ethylhexyl)phthalate and its metabolites in CHO cells. Mutat Res. 1982;102:297–304. . [DOI] [PubMed] [Google Scholar]
- 52. Schmid P, Schlatter C. Excretion and metabolism of di(2-ethylhexyl)phthalate in man. Xenobiotica. 1985;15:251–6. . [DOI] [PubMed] [Google Scholar]
- 53. Single versus multiple dosing in the micronucleus test: the summary of the fourth collaborative study by CSGMT/JEMS.MMS. Collaborative Study Group for the Micronucleus Test, the Mammalian Mutagenesis Study Group of the Environmental Mutagen Society, Japan (CSGMT/JEMS.MMS). Mutat Res. 1990;234:205–22. . [PubMed] [Google Scholar]
- 54. Llorente MT, Parra JM, Sanchez-Fortun S, Castano A. Cytotoxicity and genotoxicity of sewage treatment plant effluents in rainbow trout cells (RTG-2). Water Res. 2012;46:6351–8. 10.1016/j.watres.2012.08.039 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)


