Abstract
This was a randomized controlled pilot study of Lactobacillus rhamnosus GG versus standard of care to prevent gastrointestinal multidrug-resistant organism (MDRO) colonization in ICU patients. Seventy subjects were included in analyses. There were no significant differences in acquisition or loss of any MDROs (p>0.05). There were no probiotic-associated adverse events.
INTRODUCTION
Infections with multidrug-resistant organisms (MDRO) are a serious threat to critically ill patients, leading to increased morbidity and mortality.1,2 Gastrointestinal colonization with MDROs increases patients’ risk of infection, and colonized patients are the major reservoir for MDRO transmission to other hospitalized patients.3 One potential strategy to prevent MDRO colonization is to use probiotics to promote healthy intestinal flora, but data on probiotics in ICU patients are limited. We conducted a prospective, randomized controlled pilot study to determine if Lactobacillus rhamnosus GG could safely prevent intestinal colonization with MDROs in a critically ill population.
METHODS
This study was conducted at Barnes-Jewish Hospital (BJH) in St. Louis, MO, a 1,250 bed university-affiliated hospital, from February 2012- October 2013, and was approved by the Washington University Human Research Protection Office. The primary outcome was the acquisition of gastrointestinal MRDO colonization. The secondary endpoints were safety and loss of MDRO colonization.
Inpatients age ≥18 admitted to the medical or coronary ICUs with anticipated length of stay >48 hours were eligible. Exclusion criteria included: pregnancy, immunosuppression, HIV with CD4 <200 cells/mcl, absolute neutrophil count <500 K/cumm, transplant recipients, ongoing chemotherapy, prosthetic valve or valvuloplasty, vascular graft, left ventricular assist device (LVAD), balloon pump, cardiac arrest, cardiac trauma, pancreatitis, endocarditis, history of rheumatic fever, congenital cardiac abnormality, tracheostomy, gastrointestinal bleeding or injury, esophageal varices, oropharyngeal mucosal injury, diarrhea, and unwillingness or inability to consent.
Subjects were randomly assigned to the probiotic or standard of care group (SOC) in a 1:1 ratio using permutation blocks (n=4 per block) by APACHE II scores. Study assignment was unblinded. Subjects randomized to the probiotic group received 1 capsule containing 1010 cells of L. rhamnosus GG (Culturelle, i-Health, Inc., Cromwell, CT) twice a day. If subjects were unable to swallow due to intubation or presence of a nasogastric tube, the probiotic was administered in a saline slurry via syringe through the tube after removal of the gelatin capsule. Subjects in the probiotic group received probiotic for 14 days or until study exit (death or hospital discharge), whichever came first.
Stool samples or rectal swabs were obtained at study enrollment (prior to the first dose of probiotic), study day 3, and every 3 days until study exit. Study exit was defined as death or day 14 post-enrollment, whichever came first. Patients were included in outcomes analyses if they had ≥3 samples. Acquisition of MDRO was defined as negative cultures on enrollment and positive cultures on day 3 and/or at study exit. Loss of MDRO colonization was defined as positive cultures on enrollment and negative cultures on day 3 and study exit.
Selective media were used to isolate MDROs as follows: HardyCHROM™ CRE Agar (Hardy Diagnostics, Santa Maria, CA) for CRE, ChromID® VRE agar (bioMerieux, Durham, NC) for VRE, HardyCHROM™ ESBL Agar (Hardy Diagnostics, Santa Maria, CA) for ESBL and HardyCHROM™ ChromID® Pseudomonas (Biomerieux, Durham, NC) agar for Pseudomonas. Cycloserine-cefoxitin mannitol broth with taurocholate lysozyme cysteine (Anaerobe Systems, Morgan Hill, CA) was used for C. difficile culture as previously published.4 Organisms recovered from selective media were identified using VITEK MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system, IVD v2.0 (bioMerieux).
Data collected included demographics, medical history, APACHE II scores, length of stay, type of intensive care unit, inpatient medication exposures, ventilation status, hospital mortality, and diagnosis of infections due to L. rhamnosus GG. Chi-square tests, univariate logistic regression, and Mann-Whitney U tests were performed as appropriate. A p ≤ 0.05 was considered significant. SPSS version 21 (IBM, Armonk, NY) was used.
RESULTS
One hundred three patients were enrolled and randomized to probiotics or standard of care (SOC). 70 patients had at ≥3 specimens available for analyses: 30 (43%) in the probiotic group and 40 (57%) in the SOC group. There were no significant differences between groups in demographics, pre-enrollment length of stay, or severity of illness (Table 1). There was a trend towards older age in the probiotic group (median age=65 years vs. 59, p=0.06). Patients in the probiotic group were more likely to have received aztreonam prior to enrollment (17% vs. 0; p=0.01); (Table 1).
TABLE 1.
Pre-enrollment Patient Characteristics
| Variable | Probiotic (n = 30) | Standard of care (n = 40) | P value |
|---|---|---|---|
| Age, median (range), y | 65 (29–82) | 59 (32–82) | .06 |
| Female sex | 18 (60) | 20 (50) | .41 |
| Nonwhite race | 11 (37) | 16 (40) | .78 |
| Pre-enrollment hospital LOS, median (range), d | 6.0 (1–17) | 4.5 (1–23) | .31 |
| Pre-enrollment ICU LOS, median (range), d | 4.5 (1–16) | 3.53 (1–22) | .18 |
| Patient location | |||
| Cardiac ICU | 13 (43) | 14 (35) | Reference |
| Medical ICU | 17 (57) | 26 (65) | .48 |
| Pre-enrollment mechanical ventilation | 20 (67) | 20 (50) | .16 |
| APACHE II | |||
| 1–17 | 12 (40) | 17 (43) | Reference |
| 18–24 | 12 (40) | 15 (38) | .82 |
| ≥25 | 6 (20) | 8 (20) | .93 |
| Pre-enrollment medication exposures ≥ 12 hours | |||
| Aztreonam | 5 (17) | 0 (0) | .01 |
| Carbapenems | 4 (13) | 6 (15) | >.99 |
| Cephalosporins | 12 (40) | 20 (50) | .41 |
| Metronidazole PO/IV | 5 (17) | 2 (5) | .13 |
| Penicillins | 2 (7) | 3 (8) | >.99 |
| Vancomycin IV | 13 (43) | 18 (45) | .89 |
| Any antibiotic | 22 (73) | 25 (63) | .34 |
| PPI/H2 blocker | 20 (67) | 25 (63) | .72 |
NOTE. Data are no. (%) of subjects unless otherwise indicated. APACHE II, Acute Physiology and Chronic Health Evaluation II; H2 blocker, H2 receptor antagonists; ICU, intensive care unit; IV, intravenous; LOS, length of stay; PO, by mouth; PPI, proton pump inhibitor.
Colonization status throughout enrollment is summarized in Table 2. There was no significant difference in colonization with any MDROs on enrollment (43% of probiotic group vs. 33% of SOC group; p=0.35). Only one subject was colonized with an ESBL and one with P. aeruginosa at enrollment. More patients were colonized with VRE and C. difficile, and rates were similar between groups (p=0.34 and p=0.80, respectively).
TABLE 2.
Colonization Status Throughout Enrollment
| Organism | Probiotic (n = 30) | Standard of Care (n =40) | P value |
|---|---|---|---|
| Colonization at enrollment | |||
| ESBL/CRE | 0 (0) | 1 (3)a | >.99 |
| VRE | 7 (23) | 5 (13) | .34 |
| Pseudomonas aeruginosa | 1 (3) | 0 (0) | .43 |
| Clostridium difficile | 6 (20) | 9 (23) | .80 |
| Acquisition of colonizationb | |||
| ESBL/CRE | 2/30 (7) | 0/39 (0) | .19 |
| VRE | 4/23 (17) | 3/35 (9) | .42 |
| P. aeruginosa | 2/29 (7) | 3/40 (8) | >.99 |
| C. difficile | 0/24 (0) | 2/31 (6) | .50 |
| Loss of colonizationc | |||
| ESBL/CRE | N/A | 0/1 (0) | |
| VRE | 0/7 (0) | 0/5 (0) | |
| P. aeruginosa | 0/1 (0) | N/A | |
| C. difficile | 0/6 (0) | 1/9 (11) | >.99 |
NOTE. CRE, carbapenem-resistant Enterobactcriaceae; ESBL, extended-spectrum beta-lactamase; N/A, not applicable; VRE, vancomycin-resistant Enterococcus.
This was ESBL.
Negative culture results on enrollment and positive culture results on day 3 and/or study exit, excluding those colonized at day 0.
Positive culture results on enrollment and negative culture results on day 3 and/or study exit.
There was no significant difference in overall acquisition of any MDROs between the two groups (10% of probiotic group vs. 15% of SOC group; p=0.72). Two (7%) patients in the probiotic group acquired ESBL colonization (p=0.19). 17% of the probiotic group vs. 9% in the SOC group acquired VRE (p=0.42). 7% patients in the probiotic group and 8% SOC acquired P. aeruginosa (p=>0.99). No patients in the probiotic group and 7% in the SOC group acquired C. difficile (p=0.50).
The single patient colonized with an ESBL-producing Enterobacteriaceae on enrollment (SOC group) remained colonized throughout hospitalization. No patients in any group lost colonization with VRE or P. aeruginosa. One SOC patient lost C. difficile colonization (p>.99).
All 103 patients were included in the safety assessment. There were no significant differences between probiotic and standard of care patients in the number of patients who died (22% probiotic group vs. 21% SOC; p=0.88). There were no infections due to probiotic or clinical cultures positive for L. rhamnosus GG in either group. No adverse events associated with the probiotic occurred.
DISCUSSION
No differences in acquisition or loss of MDRO colonization between the probiotic and SOC group were identified in this study. These results may indicate that either our sample size was not large enough to detect a difference between groups, our study duration was too short, or that L. rhamnosus GG at the dose used did not affect MDRO colonization. There were no infections related to probiotics, suggesting that probiotics may be safe in a select cohort of critically ill patients, with care to minimize probiotic contamination when administered by tube.
Previous studies evaluating probiotics have had conflicting results.5–8 A meta-analysis found that probiotics in critically ill adults did not significantly reduce mortality but did reduce ICU-acquired pneumonia and ICU length of stay.9 Another meta-analysis indicated probiotics were associated with reductions in infectious complications but had no effect on mortality or length of stay.10 These differences may be due to varying sample size, rates of MDRO carriage, types and doses of probiotic used, or the underlying complexity of the microbiome.
This study has limitations, including small sample size, duration of follow-up, and inclusion of a single type and dose of probiotic. We did not survey for gastric or upper airway colonization, which may be an important site for MDRO colonization. Finally, our extensive exclusion criteria may limit the generalizability of this study.
There are unresolved controversies regarding probiotics, including the type of patients who may benefit most from probiotics, the ideal probiotic organism(s) and dose. The effect of prolonged probiotic administration on the gut microbiome is an area for further investigation. Future, larger, studies are needed to evaluate the effectiveness of probiotics in preventing intestinal colonization due to MDROs in critically ill patients.
FIGURE 1.
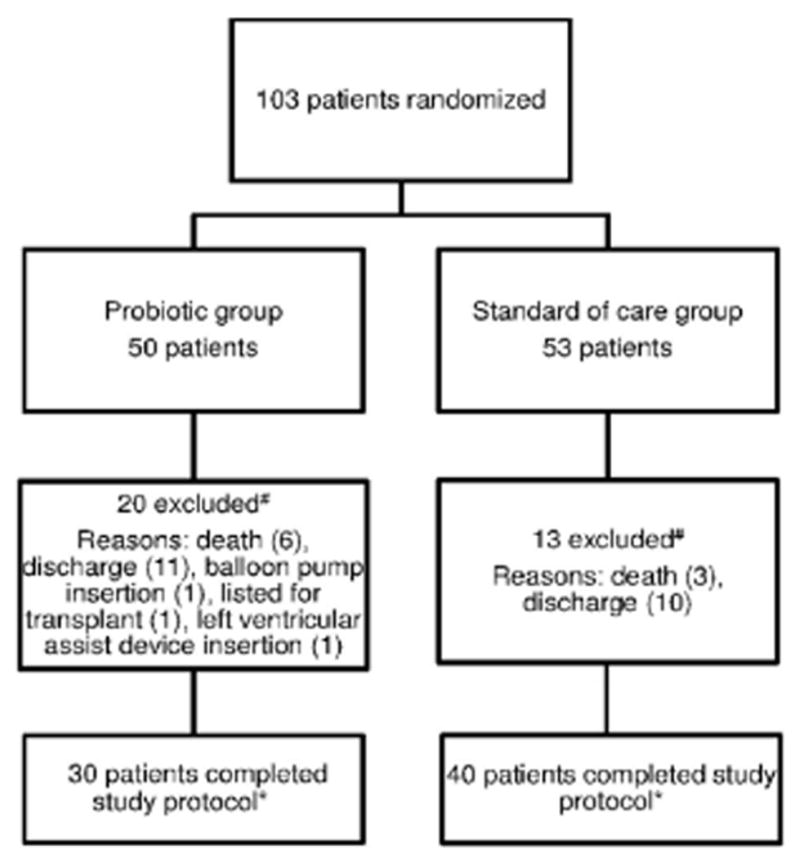
Flowchart of study protocol. #Submitted < 3 samples. *Submitted ≥ 3 samples.
Acknowledgments
Financial Support: This study was supported by the CDC Prevention Epi-Center Grant: U54CK000162. The probiotic Lactobacillus rhamnosus GG (Culturelle) was provided free of charge by iHealth. iHealth took no part in study design, data collection, management, analysis, interpretation, presentation of data, description of findings or final manuscript.
Footnotes
Conflict of Interest: J.H.K., K.M.B., K.A.R., S.M.S., T.H., H.M.B., M.H.K., V.J.F., E.R.D. report no conflicts of interest. C.D.B. reports grants from bioMerieux, personal fees from bioMerieux, personal fees from Thermofisher, grants from Cepheid, grants from Accelerate Diagnostics, personal fees from Nanosphere, outside the submitted work. V.J.F. reports grants from the CDC and non-financial support from iHealth during the conduct of the study.
Reference List
- 1.Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis. 2011;11:30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 2.Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;23:47. doi: 10.1186/2110-5820-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39:219–26. doi: 10.1086/422002. [DOI] [PubMed] [Google Scholar]
- 4.Hink T, Burnham CA, Dubberke ER. A systematic evaluation of methods to optimize culture-based recovery of Clostridium difficile from stool specimens. Anaerobe. 2013;19:39–43. doi: 10.1016/j.anaerobe.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Regt MJ, Willems RJ, Hene RJ, et al. Effects of probiotics on acquisition and spread of multiresistant enterococci. Antimicrob Agents Chemother. 2010;54:2801–05. doi: 10.1128/AAC.01765-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249–57. doi: 10.1016/S0140-6736(13)61218-0. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. 2013;5:CD006095. doi: 10.1002/14651858.CD006095.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Plummer S, Weaver MA, Harris JC, et al. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7:59–62. [PubMed] [Google Scholar]
- 9.Barraud E, Bollaert PE, Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients: a meta-analysis of randomized controlled trials. Chest. 2013;143:646–55. doi: 10.1378/chest.12-1745. [DOI] [PubMed] [Google Scholar]
- 10.Petrof EO, Dhaliwal R, Manzanares W, et al. Probiotics in the critically ill: a systematic review of the randomized trial evidence. Crit Care Med. 2012;40:3290–302. doi: 10.1097/CCM.0b013e318260cc33. [DOI] [PubMed] [Google Scholar]


