Abstract
This work emphasizes the detection of Candidatus “Rickettsia amblyommii” in questing Haemaphysalis juxtakochi and Amblyomma mixtum. From February 2009 to December 2012, questing ticks were collected from the vegetation and leaf-litter of four protected forests and two grassy areas around the Panama Canal basin. DNA was extracted from Amblyomma mixtum, Amblyomma naponense, Amblyomma oblongoguttatum, Amblyomma pecarium, Amblyomma tapirellum, Haemaphysalis juxtakochi, and unidentified immature Amblyomma. Specific primers of citrate synthase gene gltA were used to detect and identify the rickettsiae. Amplicons with the expected band size were purified and sequenced. DNA of C. “R. amblyommii” was found in A. mixtum, H. juxtakochi and Amblyomma immatures. To our knowledge, these finding represent the first report of C. “R. amblyommii” in free-living ticks in the wilderness of Central America.
Keywords: Candidatus “Rickettsia amblyommii”, Ixodidae, questing ticks, Panama Canal Basin
Introduction
The causative agents of tick-borne rickettsiosis (TBR) include 16 Rickettsia species with great relevance in worldwide public health, most notably because of their ability to cause disease in animals and humans [1, 2]. In Latin America, TBR caused by Rickettsia rickettsii is the most important zoonosis transmitted by ticks, with a high mortality in untreated cases [2, 3]. In Panama, TBR has been implicated in two outbreaks, separated by nearly 60 years. The first affected five people and caused two fatalities during 1950–1952 [4–6], and the second cluster of cases occurred from 2004 to 2014, when seven people were infected and six fatalities occurred [7–9]. In both outbreaks, R. rickettsii was the species involved. The most recent outbreak instigated new studies to determinate the distribution of TBR in rural areas and were identification of R. rickettsii and Candidatus “Rickettsia amblyommii” in ticks collected from domestic mammals [10, 11].
However, in Panama there are no studies that demonstrate the presence of rickettsiae in the wilderness environment, even though the greatest diversity of ticks occurs in forests [12]. The presence of TBR in the environment around the Panama Canal Basin (PCB) was recently reported by a seroprevalence survey [13] and included the description of one human case [9], but the identities of the species of ticks vectoring these infectious agents have not been resolved.
In this paper we present new data about the presence of Rickettsia in questing ixodid ticks from the natural environments around the PCB.
Methods
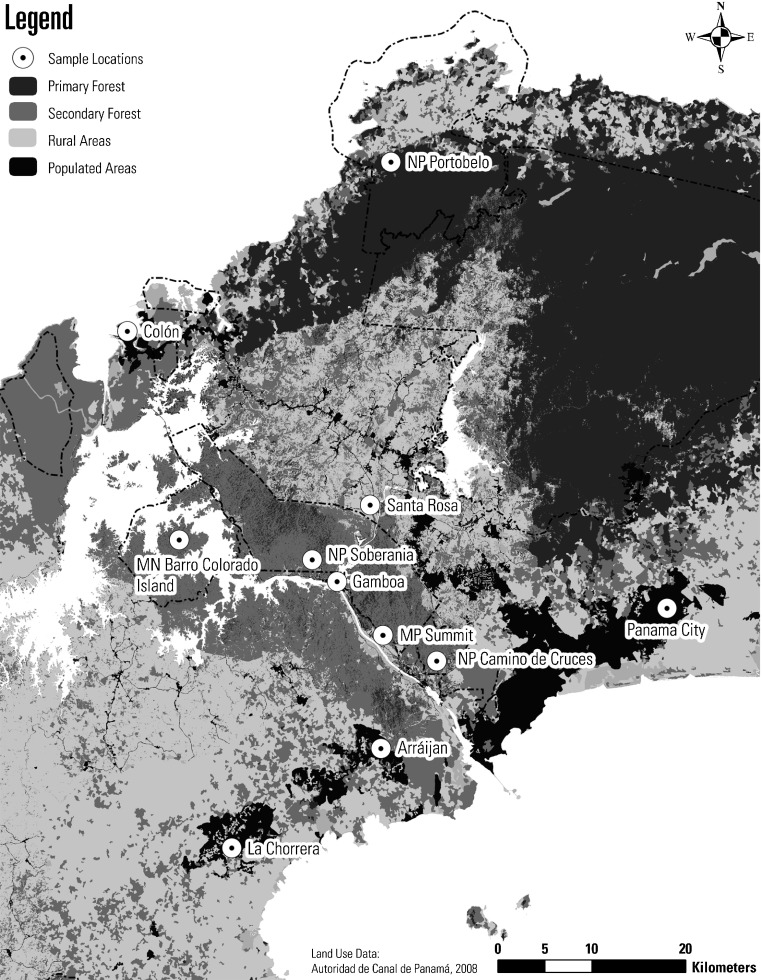
Study area. The PCB covers 1,474 km2 (Fig. 1), encompassing one of the most biodiverse areas in the country [14–16]. It includes conditions that support a wide diversity of tick-host interactions [17]. Annually, this area is visited by thousands of people undertaking eco-tourism activities, and it surrounds some of the most important urban areas of the Republic of Panama, including the cities of Panama and Colon [18]. Four sites were established in native forests, characterized by the presence of mature secondary tropical rain forests (Soberania and Portobelo National Parks, Metropolitan Natural Park and Summit Municipal Park). In addition, two riparian areas near to the towns of Clayton and Achiote were included. The sites were visited monthly from February 2009 to December 2012, during the morning hours, 0800 to 1200.
Fig. 1.
Map of the study area.
Tick sampling. A 100 m2 plot was established in each area and a white cloth (45 × 45 cm) was dragged along the leaf-litter and vegetation inside the plot. This method permits the capture of ticks that climb on vegetation (e.g. Haemaphysalis juxtakochi, Amblyomma tapirellum) or that actively seek hosts (e.g. Amblyomma immature); but it is inefficient in catching species showing other types of behavior (e.g. Ixodes spp, Amblyomma nodosum). Ticks that crawled onto the cloth were manually collected, deposited in 95% ethanol, and moved to the Department of Medical Entomology of the Gorgas Memorial Institute of Health Studies, for identification and counting. Taxonomic keys were used for identifying adults of Amblyomma, Ixodes and all stages of Haemaphysalis [12], and Amblyomma nymphs [19]. However, because the larvae and nymphs of some species of Amblyomma have not been described, some immature ticks were identified only at the genus level. Additionally, we followed the taxonomic criteria proposed by Nava et al. [20] for designation as Amblyomma mixtum within the Amblyomma cajennense species group.
Molecular analysis. Ticks were separated by species and site; adults were processed individually, while immature ticks were analyzed in pools of 10 larvae and 5–7 nymphs. We used Qiagen DNeasy Blood and Tissue extraction kit, following the manufacturer’s instructions. Extracted DNA was stored at −20°C for later use. We used 5 μl of DNA for PCR analysis, and used the primers CS-78 and CS-323 (GCAAGTATCGGTGAGGATGTAAT and GCTTCCTTAAAATTCAATAAATCAGGAT), which amplify a 401-bp fraction of a portion of the citrate synthase gene (gltA), as an initial test, following the suggestion of Labruna et al. [21]. Amplification was confirmed by gel electrophoresis on 1% agarose, followed by and staining with ethidium bromide using a 100 bp ladder. Amplicons with expected band size were purified using Agar Ace (Promega). Direct cycle sequencing was performed on each clean PCR reaction and then sequenced in an automatic sequencer (Applied Biosystens, model ABI Prism 3130xl Genetic Analyzer, California, US). Partial sequences were subjected to BLAST analysis to determine similarities to other Rickettsia species.
Results
We collected 7339 ticks (6981 immature ticks and 358 adults), corresponding to Amblyomma dissimile (seven specimens), Amblyomma mixtum (72), Amblyomma naponense (45), Amblyomma oblongoguttatum (84), Amblyomma pecarium (seven), Amblyomma sabanerae (two), Amblyomma tapirellum (36), Haemaphysalis juxtakochi (101), Ixodes affinis (four), and unidentified immature Amblyomma and Ixodes spp.
DNA was only extracted from 96 adults ticks (18 A. mixtum, 16 A. naponense, eight A. oblongoguttatum, two A. pecarium, 29 A. tapirellum and 23 H. juxtakochi) and 146 immature pools (47 larvae and 99 nymphs). Six adults were positive for rickettsiae DNA by PCR, corresponding to five A. mixtum and one H. juxtakochi. Three and nine pools of larvae and nymphs of Amblyomma were positive for rickettsiae DNA, in addition to two pools of H. juxtakochi nymphs.
Out of these 22 PCR-positive samples, 20 were successfully sequenced for the gltA gene (377 bp). All 20 sequences were 99.5–100% identical to corresponding sequences of C. “R. amblyommii” in GenBank (DQ517290.1, CP003334.1). Two sequenced data were submitted to GenBank under the accession numbers KM654281 and KM652482. The prevalence of C. “R. amblyommii” in ticks is shown in Table 1 as percentage and minimum infection rate (MIR) of ticks in a pool with detectable Rickettsia.
Table 1.
Candidatus “Rickettsia amblyommii” infection in free-living ticks from Panama. Values are given as infected ticks/individuals tested (%) for adults and positive pools/individuals tested (MIR in %) for immatures.
| Species | Locality | |||||
|---|---|---|---|---|---|---|
| Riparian Forest | Forest | PNP | ||||
| Clayton | Achiote | MNP | SMP | SNP | ||
| Adults1 | ||||||
| Amblyomma mixtum | 1/3 (33.3) | 2/6 (33.1) | 1/4 (25) | 0/3 (0) | — | 1/2 (50) |
| Amblyomma naponense | — | — | — | 0/15 (0) | — | 0/1 (0) |
| Amblyomma oblongoguttatum | — | — | 0/3 (0) | 0/3 (0) | — | 0/2 (0) |
| Amblyomma pecarium | — | — | — | 0/2 (0) | — | — |
| Amblyomma tapirellum | — | — | — | 0/13 (0) | 0/16 (0) | — |
| Haemaphysalis juxtakochi | 0/2 (0) | — | 0/2 (0) | 1/9 (11.1) | 0/6 (0) | 0/4 (0) |
| Immature2 | ||||||
| Amblyomma sp. (larvae) | 0/1 (0) | 0/1 (0) | 0/6 (0) | 0/9 (0) | 3/15 (2) | 0/1 (0) |
| Amblyomma sp. (nymph) | 1/2 (8.3) | 0/3 (0) | 2/14 (2.8) | 2/26 (1.3) | 4/20 (3.4) | 0/6 (0) |
| Haemaphysalis juxtakochi (larvae) | — | — | 0/3 (0) | 0/3 (0) | 0/7 | 0/1 (0) |
| Haemaphysalis juxtakochi (nymph) | 0/1 (0) | — | 0/1 (0) | 2/14 (2.4) | 0/9 (0) | 0/3 (0) |
1 Adults samples were analyzed for individuals. In parenthesis, the percentage of adults infected.
2 Immature ticks were analyzed in pools of 10 larvae and 5–7 nymphs. In parenthesis, the MIR corresponded to the number of positive pools/number of individuals examined.
No rickettsiae DNA were detected in A. naponense, A. pecarium, A. oblongoguttatum or A. tapirellum.
Discussion
The questing phases comprise almost 90% of the life cycle of ticks [22–23]. In these phases, rickettsiae infection must occur before the molt or when the infection is passed from the engorged female to offspring, implicating trans-stadial and trans-ovarial transmission. Thus, studies focusing on questing ticks may allow a better understanding of the relationship between ticks and rickettsiae, than those devoted to ticks parasitizing hosts. To our knowledge, this is the first report of C. “R. amblyommii” infesting free-living H. juxtakochi, it provides new data regarding A. mixtum, which may demonstrate trans-stadial transmission into these species. It is important to note that these species are among the most common tick species in the studied area [17] and also the most likely to bite humans [24].
Amblyomma mixtum was found mainly in riparian forest, with few ticks collected in secondary or primary forest. Before the reinstatement of A. mixtum in Panama [20], all records of this species were mentioned as A. cajennense. Thus, it is possible that A. mixtum correspond to the former report of A. cajennense infected with R. rickettsii [6] and C. “R. amblyommii” from rural areas of Panama [10, 11]. In the rural setting, horses and cattle have been considered the preferred hosts for A. mixtum in Panama [12]; however, during the present study no domestic animals were observed, suggesting that other vertebrate are likely involved in the ecology of C. “R. amblyommii” at PCB. In this area, ponchos [25] and white-tailed deer seem to be the main hosts of A. mixtum. Hence, further research is necessary to determine the role of these mammals in the maintenance of TBR ecology in PBC.
In contrast to A. mixtum, H. juxtakochi prefer secondary and primary forest [12, 26], environments with a more diverse community of potential hosts than riparian forests. In general, adults of both tick species are considered to be parasites of ungulates [12, 27]; however, again there is little information available about the immature hosts. The great diversity of vertebrates in PCB, the incomplete information about the hosts of H. juxtakochi, and the absence of serologic evidence of wild mammals infested with TBR make it difficult to determine the relationships among wildlife, ticks and C. “R. amblyommii”.
Candidatus “R. amblyommii” has a wide distribution in the Americas, reported in seven countries and detected in nine species of ticks [3, 28]. Hitherto, its pathogenicity has not been completely demonstrated in humans, although some authors have mentioned that it might cause mild fevers or rash at the site of the tick bite [29–32]. The effects in other mammals seem to demonstrate low infection levels. Laboratory rabbits and guinea pigs inoculated with C. “R. amblyommii” did not develop illness [28, 33, 34]. Other recent information has confirmed that previous infection of C. “R. amblyommii” is protective against infection with the more virulent R. rickettsii in laboratory guinea pigs [35]. Candidatus “R. amblyommii” can also an induce immunological reaction in dogs and horses, without the animal presenting signs of illness [11, 36, 37]. Moreover, experimental models showed a successful trans-ovarial transmission of C. “R. amblyommii” at least in A. americanum [38], which could explain the high rates of C. “R. amblyommii” in other tick species [39–41].
Because antigens of many species of TBR may exhibit cross-reaction in serological tests, some authors have remarked on the possibility of C. “R. amblyommii” interfering in clinical diagnosis of TBR [28]. This fact is important to consider with regard to the many reports of TBR seroprevalence in Summit Municipal Park personnel [13].
Finally, even though neither R. rickettsii nor any other pathogenic rickettsia was detected in this study, our findings present a first approximation on the presence of rickettsiae in wooded areas of Panama. In addition, more studies that include vertebrates are required to determine the potential risk of contracting TBR in these environments.
Acknowledgements
This work was partially sponsored by Secretaria Nacional de Ciencia y Tecnología (SENACYT) (grant COL-07-045) and the Red Iberoamericana de Investigación y Control de Enfermedades Rickettsiales (RIICER-CYTED). We sincerely thank to the National Authority of Environmental (ANAM), Panama Canal Authority (ACP), Nestor Correa (Summit Municipal Park) and Amelia Muñoz (Metropolitan Natural Park) for their permission to conduct this study and assistance and courtesy. Speciall thanks go to Robyn Nadolny (Old Dominion University, VA, USA) for her review and comments on the manuscript.
Conflict of Interest
All authors declare no conflict of interests.
References
- 1.Fournier PE, Raoult D. Bacteriology, taxonomy, and phylogeny of Rickettsia. In: Raoult D and Parola P, eds. Rickettsial diseases. D. Informa Healthcare; 2007. pp 1–14. [Google Scholar]
- 2.Labruna MB, Mattar S, Nava S, et al. . Rickettsioses in Latin America, Caribbean, Spain and Portugal. Rev MVZ Córdova 2011; 16: 2435–2457. [Google Scholar]
- 3.Parola C, Padock C, Socolovschi C, et al. . Update on tick-borne rickettsioses around the world, a geographic approach. Clin Microbiol Rev 2013; 26: 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodaniche E, Rodaniche A. Spotted fever in Panama; isolation of the etiologic agent from a fatal case. Am J Trop Med Hyg 1950; 30: 511–517. [DOI] [PubMed] [Google Scholar]
- 5.Calero C, Nuñez J, Silva-Goytía R. Rocky Mountain spotted fever in Panama. Report of two cases. Am J Trop Med Hyg 1952; 1: 631–636. [PubMed] [Google Scholar]
- 6.Rodaniche E. Natural infection of the tick, Amblyomma cajennense, with Rickettsia rickettsii in Panama. Am J Trop Med Hyg 1953; 2(4): 696–699. [DOI] [PubMed] [Google Scholar]
- 7.Estripeaut D, Aramburú MG, Saéz-Llórens X, et al. . Rocky Mountain Spotted Fever, Panama. Emerg Infect Dis 2007; 13: 1763–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tribaldos M, Zaldivar Y, Bermúdez SE, et al. . Rocky Mountain spotted fever in Panama, a cluster description. J Infect Dev Ctries 2011; 5: 737–741. [DOI] [PubMed] [Google Scholar]
- 9.De Lucas J, García E, García G, et al. . Nuevo caso de rickettsiosis humana en Panamá, primer reporte proveniente de un área silvestre. Rev Med Panamá 2013; 34: 40–43. [Google Scholar]
- 10.Eremeeva ME, Karpathy SE, Levin ML, et al. . Spotted fever Rickettsiae, Ehrlichia and Anaplasma, in ticks from peridomestic environments in Panama. J Clin Microbiol Infect 2009; 15: 1–3. [DOI] [PubMed] [Google Scholar]
- 11.Bermúdez SE, Zaldívar Y, Spolidorio MG, et al. . Rickettsial infection in domestic mammals and their ectoparasites in El Valle de Antón, Coclé, Panamá. Vet Parasitol 2011; 177: 134–138. [DOI] [PubMed] [Google Scholar]
- 12.Fairchild GB, Kohls GM, Tipton VJ. The ticks of Panama Acarina, Ixodoidea. Ectoparasites of Panama. In: Tipton, ed. Chicago, Illinois: Field Museum of Natural History; 1966. pp 167–207. [Google Scholar]
- 13.Bermúdez SE, Lyons C, García G, et al. . Serologic evidence of human Rickettsia infection found in three locations in Panama. Rev Biomédica 2013; 33(1): 31–37. doi, http://dx.doi.org/10.7705/biomedica.v33i0.831. [PubMed] [Google Scholar]
- 14.Moreno R, Bustamante-Ho A. Uso de cámaras trampas y observación de huellas de mamíferos y otras especies en el Camino del Oleoducto, Parque Nacional Soberanía, Panamá. InformeTécnico. Smithsonian Tropical Research Institute-Programa de Conservación de Felinos Península Osa y Panamá. 2008. p 13. [Google Scholar]
- 15.Anonymous. 2009. (Homepage on the internet). Autoridad Nacional del Ambiente, Panama. Available: http://www.anam.gob.pa
- 16.Moreno R, Meyer N. Distribution and conservation status of the white-lipped peccary (Tayassu pecari) in Panama. Suiform Soundings 2014; 13(1): 31–34. [Google Scholar]
- 17.Bermúdez SE, Miranda RJ, Smith DE. Ticks (Ixodida) species in the Summit Municipal Park and adjacent areas, Panama City, Panama. Exp Appl Acarol 2010; 52: 439–448. doi, http://dx.doi.org/10.1007/s10493-010-9374-8. [DOI] [PubMed] [Google Scholar]
- 18.Mapes K. Expanding ecotourism, embedding environmental sustainability in Panama’s Burgeoning Tourist Industry. Harvard Environmental Law Review 2009; 33: 225–261. [Google Scholar]
- 19.Martins T, Onofrio V, Barros-Battesti D, et al. . Nymphs of the genus Amblyomma (Acari, Ixodidae) of Brazil, descritions, redescriptions and identifications keys. Ticks and Ticks-Borne Dis 2010; 1: 75–99. [DOI] [PubMed] [Google Scholar]
- 20.Nava S, Beati L, Labruna M, et al. . Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp., and Amblyomma patinoi n. sp., and the reinstatement of Amblyomma mixtum Koch, 1844 and Amblyomma scultum Berlese, 1888 (Ixodida, Ixodidae). Ticks and Ticks-Borne Dis 2014; 5: 252–276. [DOI] [PubMed] [Google Scholar]
- 21.Labruna MB, Whitworth T, Horta MC, et al. . Rickettsia species infecting Amblyomma cooperi ticks from an area in the State of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J ClinMicrobiol 2004; 42: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Needham G, Teel P. Off-host physiological ecology of ixodid ticks. Ann Rev Entomol 1991; 36: 659–681. [DOI] [PubMed] [Google Scholar]
- 23.Bechara G, Szabó M, Duarte J, et al. . Ticks associated with wild animals in the Nhecolandia Pantanal, Brazil. Ann NY Acad Sci 2000; 289–297. [DOI] [PubMed] [Google Scholar]
- 24.Bermúdez SE, Castro A, Esser H, et al. . Ticks (Ixodida) on humans from central Panama, Panama (2010–2011). Exp Appl Acarol 2012; 58(1): 81–88. doi, http://dx.doi.org/10.1007/s10493-012-9564-7. [DOI] [PubMed] [Google Scholar]
- 25.Garcia G, Castro A, Rodríguez I, et al. . Ixodidae ticks in Hydrochoerus isthmius Goldman 1912 (Rodentia: Caviidae) in Panama. Syst Appl Acarol 2014; 19(4): 404–408. [Google Scholar]
- 26.García G, Castro A, Bermúdez S, et al. . Some ecological aspects of free-living Haemaphysalis juxtakochi Cooley, 1946 (Acari: Ixodidae) in Panama. Revista de Medicina Veterinaria y Zoonosis 2014; 19(1): 3984–3986. [Google Scholar]
- 27.Guglielmone AA, Estrada-Peña A, Keirans JE, et al. . Ticks (Acari, Ixodida) of the neotropical zoogeographic region. International Consortion on Tick and Tick-Borne Dis Argentina 2003; 173. [Google Scholar]
- 28.Saraiva D, Nieri-Bastos F, Horta M, et al. . Rickettsia amblyommii infecting Amblyomma auricularium ticks in Pernambuco, northeastern Brazil, isolation, transovarial transmission, and transstadial perpetuation. V Borne Zoonotic Dis 2013; 13: 615–618. [DOI] [PubMed] [Google Scholar]
- 29.Dasch G, Kelly O, Richards A, et al. . Western Blotting analysis of sera from military personnel exhibiting serological reactivity to Spotted Fever Group Rickettsiae. Am J Trop Med Hyg 1993; 49. [Google Scholar]
- 30.Billeter SA, Blanton HL, Little SE, et al. . Detection of Rickettsia amblyommii in association with a tick bite rash. Vector Borne Zoonotic Dis 2007; 7: 607–610. [DOI] [PubMed] [Google Scholar]
- 31.Apperson CS, Engber B, Nicholson WL, et al. . Tick-borne diseases in North Carolina, is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis 2008; 8: 597–606. [DOI] [PubMed] [Google Scholar]
- 32.Jiang J, Yarina T, Miller MK, et al. . Molecular detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. V Borne and Zoon Dis 2010; 10: 329–340. [DOI] [PubMed] [Google Scholar]
- 33.Burgdorfer W, Hayes S, Thomas L, et al. A new spotted fever group rickettsia from the lone star tick, Amblyomma americanum. In: Burgdorfer W and Anacker R, eds. Rickettsiae and rickettsial diseases. New York: Academic; 1981. pp 595–602. [Google Scholar]
- 34.Zanetti A, Pomwiroon W, Kearney M, et al. . Characterization of rickettsial infection in Amblyomma americanum (Acari, Ixodidae) by quantitative real-time polymerase chain reaction. J Med Entomol 2008; 45: 267–275. [DOI] [PubMed] [Google Scholar]
- 35.Blanton LS, Mendell NL, Walker DH, et al. . “Rickettsia amblyommii” induces cross protection against lethal rocky mountain spotted fever in a guinea pig. V Borne and Zoon Dis 2014; 14(8): 557–562. doi, http://dx.doi.org/10.1089/vbz.2014.1575. [DOI] [PubMed] [Google Scholar]
- 36.Labruna M, Horta M, Aguiar D, et al. . Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro Municipality, Western Amazon, Brazil. Vector-Borne Zoonotic Dis 2007; 7: 249–255. [DOI] [PubMed] [Google Scholar]
- 37.Melo A, Martins T, Horta M, et al. . Seroprevalence and risk factors to Ehrlichia spp. and Rickettsia spp. in dogs from the Pantanal Region of Mato Grosso State, Brazil. Ticks and Tick-Borne Dis 2011; 2: 213–218. [DOI] [PubMed] [Google Scholar]
- 38.Burgdorfer W, Hayes S, Thomas L, et al. A new Spotted Fever Group Rickettsia from the Lone Star Tick, Amblyomma americanum. In: Burgdorfer W and Anacker R, eds. Rickettsiae and rickettsial diseases. New York: Academic; 1981. pp 595–602. [Google Scholar]
- 39.Smith M, Ponnusamy L, Jiang J, et al. . Bacterial Pathogens in Ixodid Ticks from a Piedmont County in North Carolina: Prevalence of Rickettsial Organisms. V Borne and Zoon Dis 2010; 10(10): 939–952. [DOI] [PubMed] [Google Scholar]
- 40.Moncayo A, Cohen S, Fritzen C, et al. . Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg 2010; 83: 653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hun L, Troyo A, Taylor L, et al. . First report of the isolation and molecular characterization of Rickettsia amblyommii and Rickettsia felis in Central America. V Borne and Zoon Dis 2011; 11(10): 1395–1397. [DOI] [PubMed] [Google Scholar]