Abstract
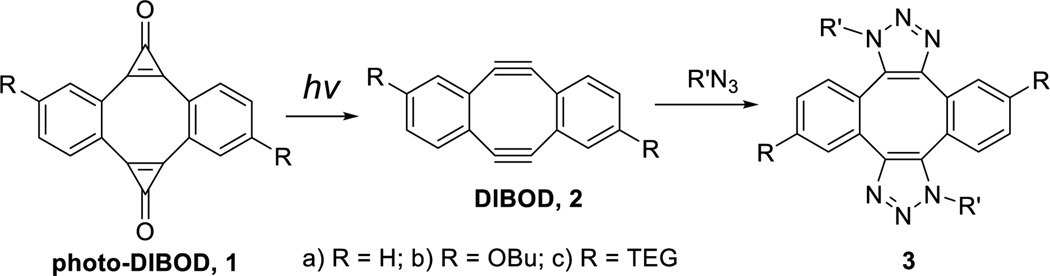
The first fully conjugated bis-cyclopropenone (photo-DIBOD), a derivative of dibenzo[a,e][8]annulene, has been synthesized. 350–420 nm irradiation of this robust compounds results in the efficient formation of dibenzo [a, e] cyclooctadiyne, an unstable, but useful SPAAC cross-linking reagent. Since photo-DIBO doesn’t react with organic azides, this method allows for the spatiotemporal control of the ligation of two azide-tagged substrates.
In recent years the copper (I) - catalyzed 1,3-dipolar cycloaddition of azides to terminal acetylenes (CuAAC),1 as well as the strain-promoted, catalyst – free version of this reaction (SPAAC),2 have become very popular tools for the functionalization, cross-linking, and immobilization of various substrates.3 These quintessential “click-chemistries” have found numerous applications in molecular biology,3b–c,4 drug development,5 biotechnology,6 and materials science.3a,7 The SPAAC ligation is better suited for applications in living systems as it avoids the use of cytotoxic copper catalysts.8 In addition, terminal acetylenes were found to inhibit cysteine proteases by forming thioethers with the catalytically active thiol.9 The utility of the SPAAC technique, on the other hand, is somewhat restricted by the requirement for the derivatization of the coupling partners with two different functionalities. While introduction of an azide group is well developed for a large variety of substrates, the incorporation of cyclooctyne moieties often requires multiple synthetic steps. A limited number of pre-functionalized SPAAC reagents are available commercially, but are rather expensive. Alternatively, two azide-derivatized substrates can be ligated using a bifunctional linker.10,11
Dibenzo[a,e]cyclooctadiyne (DIBOD 2a, a.k.a. Sondheimer diyne), represents a unique cross-linking platform in this sense, as it contains two azide-reactive strained triple bonds within the same cyclic structure and allows for double-SPAAC click ligation of azide-tagged substrates.12 Diyne 2a has been employed for the cross-coupling of various azide-functionalized substrates from biomolecules to metal – organic frameworks.12,13 The utility of this method, however, is limited by the low stability of the Sondheimer diyne. In the neat form 2a completely decomposes within two days and exhibits half lifetime of only 10 min in a neutral aqueous solution (1mM in PBS, pH 7.4, r.t.).14
To address this deficiency of the DIBOD cross - linker, we envisaged protecting (or “caging”) triple bonds in the molecule with a photolabile functionality. Photochemical decarbonylation of cyclopropenones is one of the most efficient methods for the generation of reactive acetylenes, as it is characterized by very fast rate, quantitative chemical and high quantum yields.15 This reaction can be induced by NIR light under two-photon excitation conditions16 and proceeds smoothly in the solid state.17 We have already employed this reaction for the generation of reactive triple bonds in ynols,18 enediynes,19 and dibenzocyclooctynes.20 Irradiation of bis-cyclopropenone 1 (photo-DIBOD) results in the formation of the Sondheimer diyne 2, which, in turn reacts with two azide-functionalized substrates to create a stable covalent cross-link (Scheme 1). It is important to note that the addition of second equivalent of azide to the diyne 2a proceeds at much higher rate than the first cycloaddition.12a
Scheme 1.
Photochemical generation of cross-linker 2 from azide-inert precursor 1 is not only reagent-free, but also allows for the spatiotemporal control of the ligation process. This reaction expands the family of recently developed “photo-click” ligation methods,21 including cycloaddition of alkenes to photochemically generated nitrile imines,22 photo-initiated thiol-ene,23 and thiol-yne24 reactions, light-induced Diels-Alder,25 as well as photo-Michael reaction.26 Additionally, photo-DIBOD (1) is an interesting molecule in its own right, representing the first known fully conjugated bis-cyclopropenone. Despite the presence of the cyclooctatetraene core and the obvious angle strain, this compound is surprisingly stable in the solid state and in solutions (vide infra).
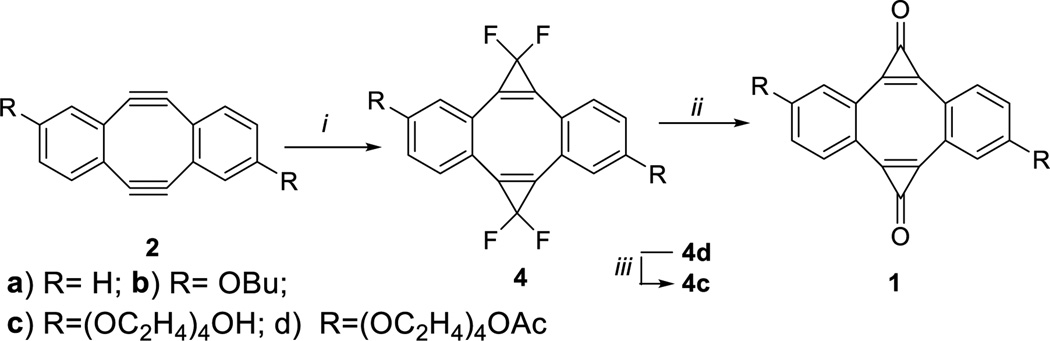
We envisioned the preparation of dibenzo[a,e]dicyclopropa[c,g] [8]annulene-1,6-dione (1a) and its derivatives by the double addition of dihalocarbene across the triple bonds in dibenzocyclooctyne 2 followed by the controlled hydrolysis. To address the limited solubility of the parent diyne 2a, we have also synthesized 2,8-dibutoxy- (2b) and 2,8-bis-[tetra(ethylene glycol)]- (2c) substituted analogs. Diynes 2a–c were prepared using the modified method of Otera and coworkers.14,27 Standard protocols for the generation of difluorocarbene require elevated temperature and harsh conditions28 that are not compatible with the highly sensitive diynes 2a–c. The milder conditions of the recently developed Hu-Prakash reaction29 allow for the efficient formation of difluorocarbene without significant decomposition of the substrate. Treatment of diynes 2a,b,d with TMSCF3 resulted in the formation of the bis-difluorocyclopropenes 4a,b,d, which were subsequently hydrolyzed on wet silica gel to afford the target bis-cyclopropenones 1a–c (Scheme 2). The tetra(ethylene glycol) substituents in 2c were capped with acetyl groups prior to cyclopropenation. The ester protection was later removed by base-promoted hydrolysis (Scheme 2).14
Scheme 2.
Synthesis of photo-DIBOD Reagents and conditions: i) TMSCF3, NaI, 110°C, 4a: 60%, 4b: 80%, 4c: 25%; ii) wet silica gel, 1a: 81%, 1b: 73%; iii) K2CO3, MeOH (1c: 53% over 2 steps, ii and iii)
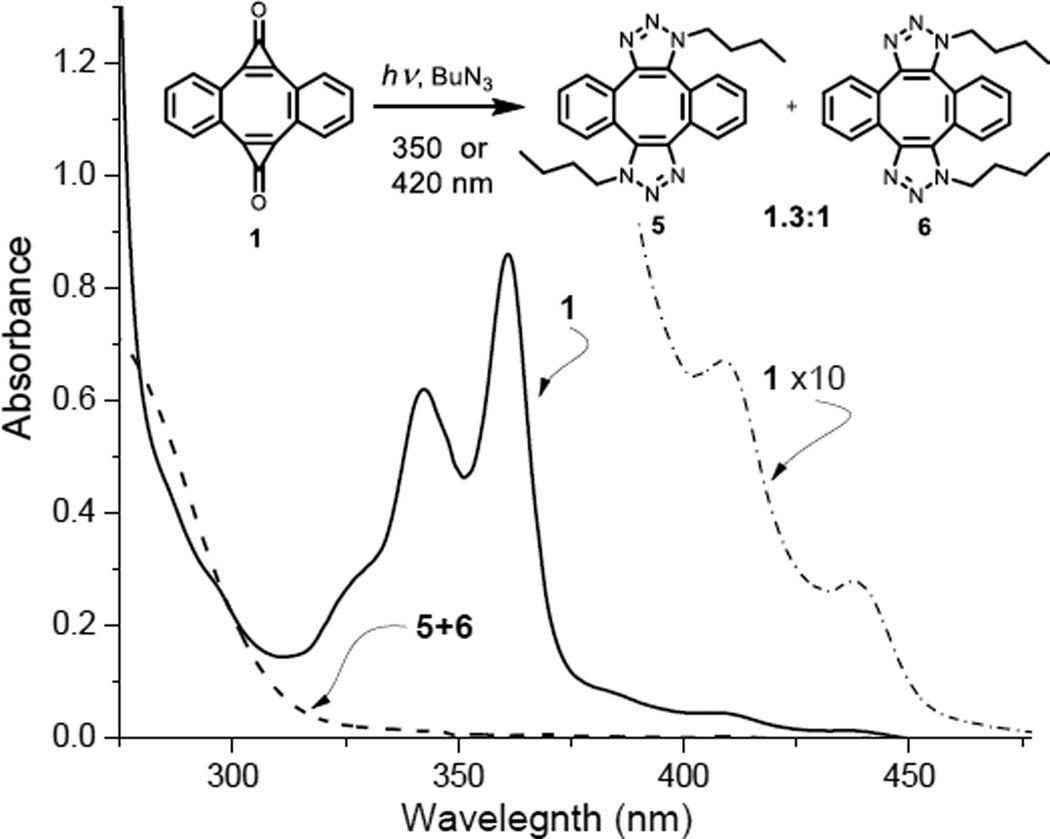
Bis-cyclopropenones 1a–c are bright yellow high-melting crystalline compounds14 that have a long shelf life if stored in the dark. The spectrum of photo-DIBOD 1a in the UVA region (Fig. 1) contains two close-lying bands at 342 (log ε=4.0) and 361 nm (log ε= 4.1), as well as two intense bands at shorter wavelengths (260 and 268 nm, log ε~ 4.8). Two weaker bands in the visible region (409 nm, log ε= 2.9; 438 nm, log ε= 2.6) are responsible for the color of these compounds (Fig. 1). Alkoxy-substitution in 1b and 1c results in the 17 nm red shift of the UVA bands, which is accompanied by a moderate hyperchromic effect (360 nm, log ε ~ 4.5, 378 nm, log ε~ 4.4). Photo-DIBODs 1a–c do not react with organic azides under ambient conditions. Irradiation of a methanol solution of photo-DIBO 1a with 350 nm light in the presence of butyl azide results in efficient decomposition of the starting material (Φ=0.05), which could be observed by the bleaching of the 342–361 nm bands. An isomeric mixture of head-to-tail (5) and head-to-head (6) bis-triazole adducts was isolated from the photolysate in 77% preparative yield (Fig. 1).
Figure 1.
UV spectra of ca. 80 µM solutions of photo-DIBOD (1a, solid line) and a mixture of bis-triazoles (5 and 6, dashed line), as well as 0.78 mM solution photo-DIBOD (1a, dash-dotted line) in MeOH-CH2Cl2 (9:1).
Interestingly, while the absorbance bands at 409 and 438 nm have relatively low intensity, irradiation of photo-DIBOD 1a with 420 nm fluorescent tubes in the presences of butyl azide produced the same mixture of triazoles 5 and 6. Complete conversion, however, requires longer irradiation times than with 350 nm lamps. Sensitivity to the violet light makes the photo-DIBOD cross-linking procedure compatible with the majority of modern confocal fluorescent microscopes.
Since photo-DIBOD derivatives 1a–c have very low aqueous solubility, we decided to explore the feasibility of conducting photo-crosslinking experiments employing nanocrystalline suspension of photo-DIBOD. Garcia-Garibay has previously reported that irradiation of the nanocrystalline suspension of diphenylcyclopropenone results in the efficient decarbonylation of the substrate.17 In fact, the quantum yield of the process in the solid state is about 4 times higher than in solution due to “quantum chain reaction.”17,30 The nanocrystalline suspension of photo-DIBOD 1a was prepared using reprecipitation techniques.17 A methanol solution of the substrate was slowly added to an aqueous PBS buffer containing 15 mM of SDS under sonication. Dynamic light scattering (DLS) studies performed on a photo-DIBOD suspension revealed a mean distribution of particles at 572±130 nm.14 The aqueous suspension of photo-DIBOD is chemically stable in the dark. While the intensity of the absorbance bands of 1a slowly decreases with time, this observation can be attributed to the precipitation of the substrate. Even after 48 h, full absorbance value was restored by sonication of the mixture for 15 min. In accordance with previously reported observations,17,30 the efficiency of photodecarbonylation of 1a shows dramatic enhancement in crystalline state (Φ = 0.41±0.8).
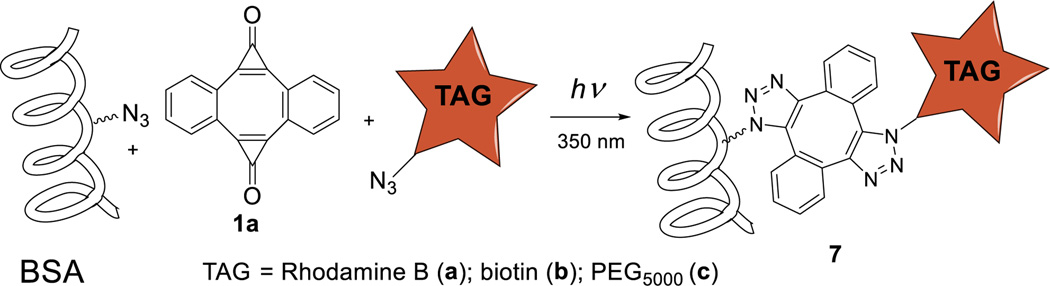
To demonstrate the utility of the photo-DIBOD crosslinker for biochemical ligations, we have derivatized a model protein, azide-tagged bovine serum albumin (azido-BSA), with a variety of functional moieties. The latter was prepared by treating the commercially available BSA with 1-azido-3-iodopropane in TRIS buffer.14 Fluorescent labeling of azido-BSA was achieved by irradiation of 0.4 µM PBS solution of the protein containing 4 µM of the nanocrystalline suspension of 1a and 4 µM of Rhodamine B azide for 8 min using 350 nm fluorescent tubes (Scheme 3).
Scheme 3.
Photo-labeling of azido-BSA
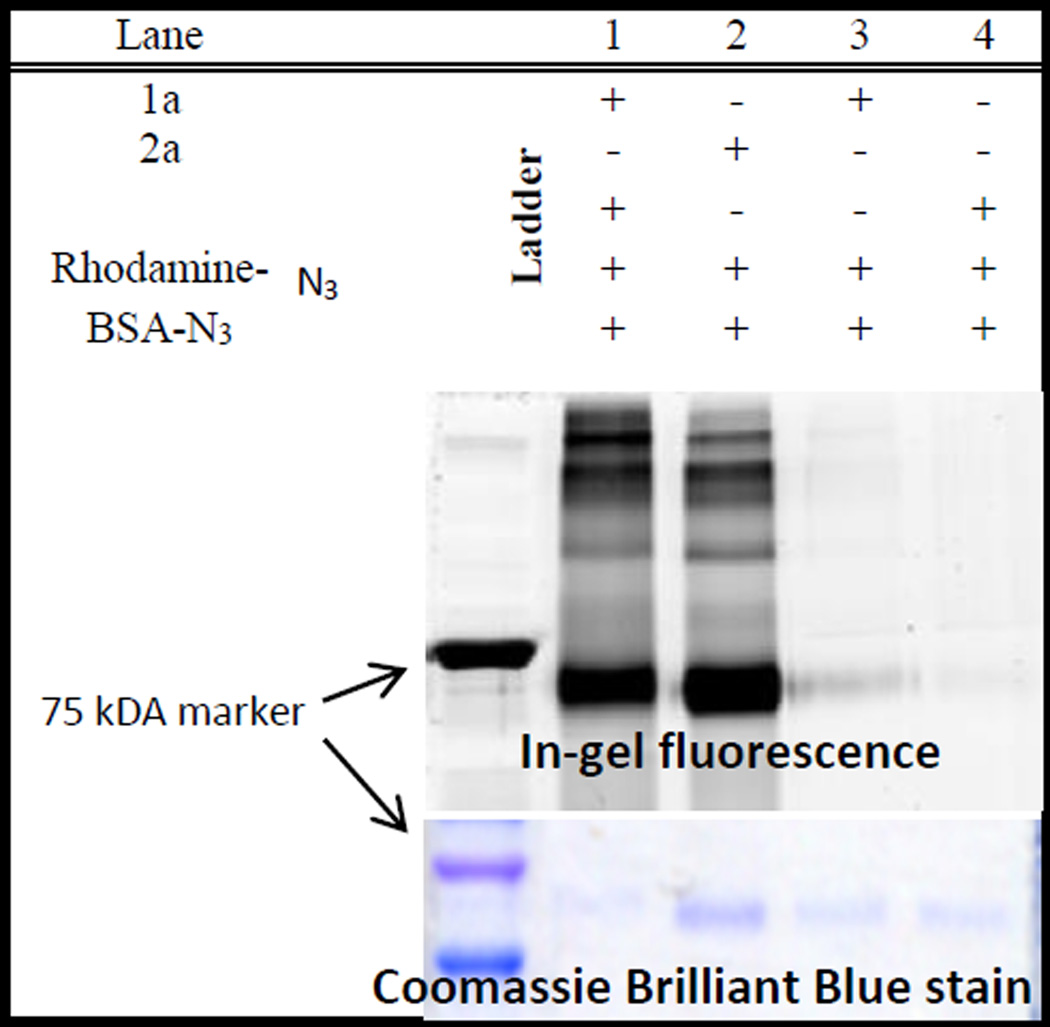
The SDS-PAGE analysis of the photolysate, conducted after overnight incubation in the dark, clearly shows the efficient cross-conjugation of azido-BSA with fluorescent dye (Fig. 2). Incubation of the same mixture in the dark overnight, as well as irradiation of azido-BSA – Rhodamine-azide mixture, does not produce labelled BSA 7a. As a positive control experiment, we have incubated azido-BSA – Rhodamine-azide mixture with freshly prepared Sondheimer diyne 2a. As the gel image illustrates, the photo-DIBOD conjugation is at least as efficient as the use of dibenzocyclooctadiyne 2a (Fig. 2).
Figure 2.
SDS-PAGE analysis of BSA - Rhodamine B conjugation. Lane 1: photo-DIBOD 1a, azido-BSA and Rhodamine B - azide irradiated; Lane 2: 2a, azido-BSA, and Rhodamine B - azide incubated overnight; Lane 3: photo-DIBOD 1a, azido-BSA, and Rhodamine B - azide incubated overnight in the dark. Lane 4: azido-BSA and Rhodamine B - azide incubated overnight.
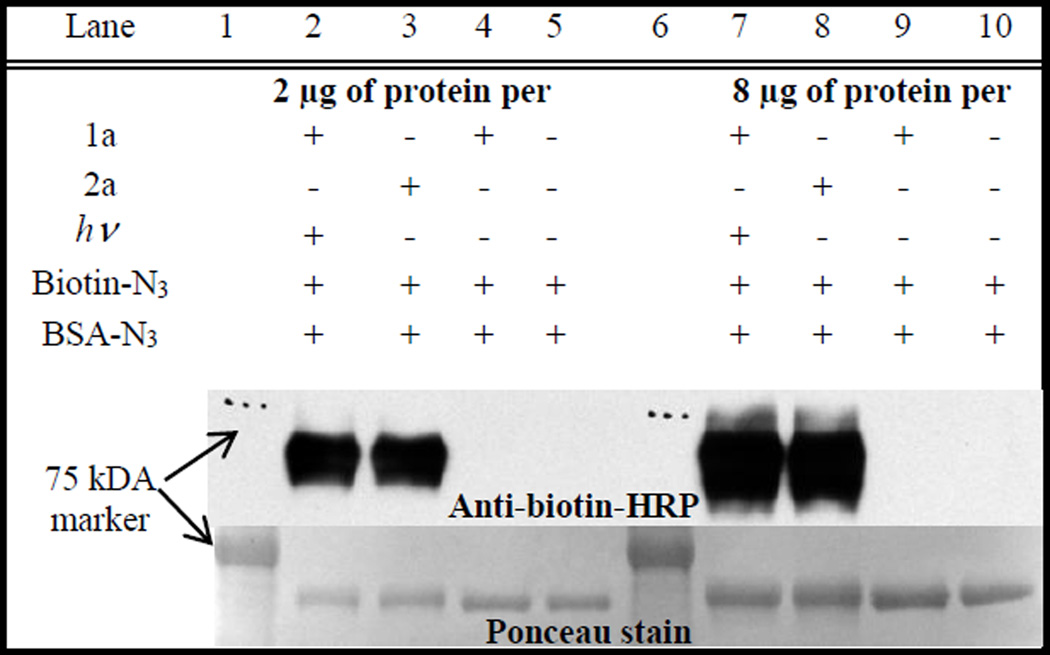
Photo-biotinylation of azido-BSA has been conducted following a similar procedure. A PBS solution of azido-BSA (0.4 µM), biotin-azide (4 µM), and nanocrystalline suspension of 1a (4 µM) was irradiated with 350 nm light for 5 min and incubated overnight. The protein was separated from low-molecular weight compounds by spin-filtration and further purified by size-exclusion chromatography. Western blot - anti-biotin-HRP analysis demonstrates the efficient photo-biotinylation of azido-BSA (Fig. 3).14 As in the previous experiment, azido-BSA – biotin-azide cross-linking using Sondheimer diyne 2a in the dark has been employed as a positive control.
Figure 3.
Western blot analysis of azido-BSA – Biotin-azide conjugation using different protein loading: lanes 1–5, 2 µg per lane and lanes 6–7, 2 µg per lane. Lanes 2,7: photo-DIBOD 1a, azido-BSA and Biotin-azide irradiated; Lanes 3,8: 2a, azido-BSA, and Biotin-azide incubated overnight; Lanes 4,9: photo-DIBOD 1a, azido-BSA, and Biotin-azide incubated overnight in the dark; Lane 5,10: azido-BSA and Biotin-azide incubated overnight.
The labeling of BSA with Rhodamine B and biotin demonstrated the utility of photo-DIBOD for protein functionalization with relatively small moieties. To test the capability of this platform for the conjugation of larger substrates, we conducted the cross-linking of azido-BSA with PEG5000-azide31 (Scheme 3). A PBS solution of azido-BSA (0.4 µM), PEG5000-azide (4 µM), and nanocrystalline suspension of 1a (4 µM) was irradiated with 350 nm light for 8 min and incubated overnight. The functionalized protein (7c) was concentrated via spin filtration (MWCO 10,000), purified on a Sephadex column, and lyophilized. Surprisingly, the MALDI-TOF mass spectrum revealed a mixture of BSA derivatives with various degrees of functionalization (Fig. S1).14 To rule out the possibility of non-specific reaction of photo-DIBOD 1a or of photo-generated 2a with the protein, we have conducted copper-catalyzed “click” conjugation of azido-BSA with PEG5000-acetylene.32 Gratifyingly, the MALDI-TOF spectrum of the resulting product shows the same distribution of PEGylated BSA derivatives as in the photo-conjugation experiment (Fig. S2).14 These experiments demonstrate that the photo-derivatization of proteins with 1a occurs only at azide moieties and is as efficient as CuAAC ligations. Incubation of azido-BSA, photo-DIBOD, and PEG5000-azide in the dark does not produce any BSA-PEG5000 conjugates.14
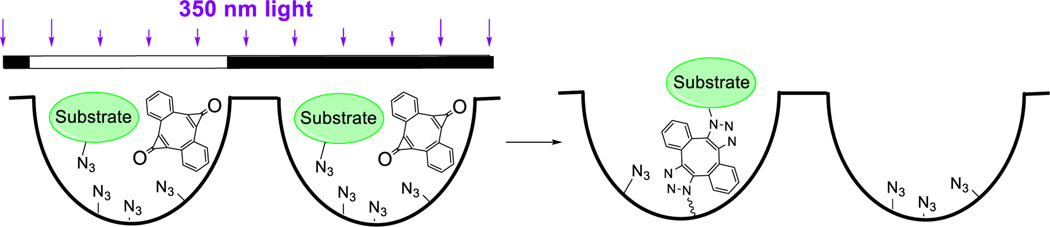
Finally, photo-DIBOD can be useful for the patterned immobilization of various substrates. To illustrate this capability of the new photo-crosslinking platform, we have immobilized fluorescent dye in selected wells of 96-well plates.14 Amine binding plates were functionalized with azide moieties by incubation with 3-azidopropan-1-amine. Methanol solution of Rhodamine B-azide and photo-DIBOD were added to each well and the loaded plate was irradiated with a 350 nm handheld fluorescent lamp via a shadow mask (Scheme 4). The plate was thoroughly washed with methanol and fluorescent intensity of individual wells was recorded using a plate reader (Table S1).14 Intense fluorescence of the irradiated wells demonstrates covalent immobilization of the dye.
Scheme 4.
Light-directed Immobilization of Rhodamine B on a 96-well plate
In conclusion, we have reported the first synthesis of a conjugated bis-cyclopropenone-fused dibenzo[a,e]cyclo-octatetraene (photo-DIBOD 1a) and its butoxy- (1b) and tetra-ethylene glycol (1c) derivatives. Irradiation of photo-DIBODs gives the corresponding bis-alkyne with a quantum yield of Φ =0.05, which acts as a linchpin in conjugating two azides. In aqueous solutions, nanocrystalline suspension of photo-DIBOD 1a serves as an efficient photo-crosslinker. The use of the nanocrystalline suspension has an additional benefit by enhancing the quantum yield of the process by almost an order of magnitude. We have demonstrated the general applicability of this platform by functionalizing a model protein, BSA, with a variety of functional fragments. In addition, photo-DIBOD was employed for the light-directed immobilization of azide-derivatized substrates.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. Y. G. Zheng and Mr. Z. Han’s help in the analysis of dye-labeled BSA. This project was supported by grants from the National Science Foundation (VP, CHEM-1213789) and the National Institutes of Health (RS, GM107012).
Footnotes
Electronic Supplementary Information (ESI) available: synthetic procedures and spectral data for the new compounds. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Kolb HC, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Tornøe CW, Christensen C, Meldal MJ. Org. Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 2.Baskin J, Prescher J, Laughlin S, Agard N, Chang P, Miller I, Lo A, Codelli J, Bertozzi CR. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Some recent reviews: Delaittre G, Guimard NK, Barner-Kowollik C. Acc. Chem. Res. 2015;48:1296. doi: 10.1021/acs.accounts.5b00075. Shieh P, Bertozzi CR. Org. Biomol. Chem. 2014;12:9307. doi: 10.1039/c4ob01632g. Tang W, Becker ML. Chem. Soc. Rev. 2014;43:7013. doi: 10.1039/c4cs00139g.
- 4.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS Chem. Biol. 2006;1:644. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]; Tanrikulu IC, Schmitt E, Mechulam Y, Goddard WA, Tirrell DA. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15285. doi: 10.1073/pnas.0905735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpless KB, Manetsch R. Exp. Opin. Drug Discov. 2006;1:525. doi: 10.1517/17460441.1.6.525. [DOI] [PubMed] [Google Scholar]
- 6.Smith MT, Hawes AK, Bundy BC. Curr. Op. Biotech. 2013;24:620. doi: 10.1016/j.copbio.2013.01.011. [DOI] [PubMed] [Google Scholar]; Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL. Chem. Rev. 2013;113:1904. doi: 10.1021/cr300143v. [DOI] [PubMed] [Google Scholar]
- 7.Lahann J. Click Chemistry for Biotechnology and Materials Science. West Sussex: Wiley; 2009. [Google Scholar]; Moses JE, Moorhouse AD. Chem. Soc. Rev. 2007;36:1242. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]; Arnold RM, Patton DL, Popik VV, Locklin J. J. Acc. Chem. Res. 2014;47:2999. doi: 10.1021/ar500191m. [DOI] [PubMed] [Google Scholar]
- 8.Gaetke LM, Chow CK. Toxicology. 2003;189:147. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]; Burrows CJ, Muller JG. Chem. Rev. 1998;98:1109. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- 9.Arkona C, Rademann J. Angew. Chem. Int. Ed. 2013;52:8210. doi: 10.1002/anie.201303544. [DOI] [PubMed] [Google Scholar]; Sommer S, Weikart ND, Linne U, Mootz HD. Bioorg. Med. Chem. 2013;21:2511. doi: 10.1016/j.bmc.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Beal DM, Albrow VE, Burslem G, Hitchen L, Fernandes C, Lapthorn C, Roberts LR, Selby MD, Jones LH. Org. Biomol. Chem. 2012;10:548. doi: 10.1039/c1ob06398g. [DOI] [PubMed] [Google Scholar]; Elamari H, Meganem F, Herscovici J, Girard C. Tetrahedron Lett. 2011;52:658. [Google Scholar]; Zong H, Goonewardena SN, Chang HN, Otis JB, Baker JR. Bioorg. Med. Chem. 2014;22:6288. doi: 10.1016/j.bmc.2014.07.015. [DOI] [PubMed] [Google Scholar]; Lai C-H, Chang TC, Chuang YJ, Tzou DL, Lin CC. Bioconjugate Chem. 2013;24:1698. doi: 10.1021/bc400219t. [DOI] [PubMed] [Google Scholar]
- 11.Arumugam S, Popik VV. J Org. Chem. 2014;79:2702. doi: 10.1021/jo500143v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Kii L, Shiraishi A, Hiramatsu T, Matsushita T, Uekusa H, Yoshida S, Yammamoto M, Kudo A, Hagiwara M, Hosoya T. Org. Biomol. Chem. 2010;8:405. doi: 10.1039/c0ob00003e. [DOI] [PubMed] [Google Scholar]; (b) Xu F, Peng L, Shinohara K, Morita T, Yoshida S, Hosoya T, Orita A, Otera JJ. Org. Chem. 2014;79:11592. doi: 10.1021/jo502248p. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S, Shiraishi A, Kanno K, Matsushita T, Johmoto K, Uekusa H, Hosoya T. Sci. Rep. 2011;1:8. doi: 10.1038/srep00082. [DOI] [PMC free article] [PubMed] [Google Scholar]; Horner A, Volz D, Hagendorn T, Furniss D, Greb L, Ronicke F, Nieger M, Schepers U, Brase S. RSC Adv. 2014;4:11528. [Google Scholar]
- 14.Supporting Information
- 15.(a) Poloukhtine A, Popik VV. J Org. Chem. 2003;68:7833. doi: 10.1021/jo034869m. [DOI] [PubMed] [Google Scholar]; (b) Poloukhtine A, Popik VV. J Phys. Chem. A. 2006;110:1749. doi: 10.1021/jp0563641. [DOI] [PubMed] [Google Scholar]
- 16.Urdabaev NK, Poloukhtine A, Popik VV. Chem. Comm. 2006:454. doi: 10.1039/b513248g. [DOI] [PubMed] [Google Scholar]
- 17.Kuzmanich G, Gard MN, Garcia-Garibay MA. J Am. Chem. Soc. 2009;131:11606. doi: 10.1021/ja9043449. [DOI] [PubMed] [Google Scholar]
- 18.Chiang Y, Kresge AJ, Paine SW, Popik VV. J Phys. Org. Chem. 1996;9:361. [Google Scholar]
- 19.Pandithavidana DR, Poloukhtine A, Popik VV. J Am. Chem. Soc. 2009;131:351. doi: 10.1021/ja8077076. [DOI] [PubMed] [Google Scholar]; Poloukhtine A, Popik VV. J Org. Chem. 2006;71:7417. doi: 10.1021/jo061285m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNitt CD, Popik VV. Org. Biomol. Chem. 2012;10:8200. doi: 10.1039/c2ob26581h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Tasdelen MA, Yagci Y. Angew. Chem. Int. Ed. 2013;52:5930. doi: 10.1002/anie.201208741. [DOI] [PubMed] [Google Scholar]; (b) Delaittre G, Goldmann AS, Mueller JO, Barner-Kowollik C. Angew. Chem. Int. Ed. 2015;54:11388. doi: 10.1002/anie.201504920. [DOI] [PubMed] [Google Scholar]
- 22.(a) Lim RK, Lin Q. Acc. Chem. Res. 2011;44:828. doi: 10.1021/ar200021p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu Z, Ohulchanskyy TY, An P, Prasad PN, Lin Q. J Am. Chem. Soc. 2013;135:16766. doi: 10.1021/ja407867a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Forest CA, Polizzotti BD, Anseth KS. Nature Materials. 2009;8:659. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fiore M, Marra A, Dondoni A. J Org. Chem. 2009;74:4422. doi: 10.1021/jo900514w. [DOI] [PubMed] [Google Scholar]; Killops KL, Campos LM, Hawker CJ. J Am. Chem. Soc. 2008;130:5062. doi: 10.1021/ja8006325. [DOI] [PubMed] [Google Scholar]; Chan JW, Yu B, Hoyle CE, Lowe AB. Chem. Commun. 2008:4959. doi: 10.1039/b813438c. [DOI] [PubMed] [Google Scholar]
- 24.Hensarling RM, Doughty VA, Chan JW, Patton DL. J Am. Chem. Soc. 2009;131:14673. doi: 10.1021/ja9071157. [DOI] [PubMed] [Google Scholar]
- 25.(a) Dillmore W, Yousaf M, Mrksich M. Langmuir. 2004;20:7223. doi: 10.1021/la049826v. [DOI] [PubMed] [Google Scholar]; (b) Rusmini F, Zhong Z, Feijen J. Biomacromolecules. 2007;3:1775. doi: 10.1021/bm061197b. [DOI] [PubMed] [Google Scholar]; (c) Arumugam S, Orski S, Locklin J, Popik VV. J Am. Chem. Soc. 2012;134:179. doi: 10.1021/ja210350d. [DOI] [PubMed] [Google Scholar]; (d) Bauer DM, Rogge A, Stolzer L, Barner-Kowollik C, Fruk L. Chem. Commun. 2013;49:8626. doi: 10.1039/c3cc43291b. [DOI] [PubMed] [Google Scholar]
- 26.(a) Arumugam S, Popik VV. J Am. Chem. Soc. 2012;134:8408. doi: 10.1021/ja302970x. [DOI] [PubMed] [Google Scholar]; (b) Bowman CN, Xi W, Kloxin C, Krieger M. Chem. Commun. 2013;49:4504. doi: 10.1039/c3cc41123k. [DOI] [PubMed] [Google Scholar]
- 27.Orita A, Hasegawa D, Nakano T, Otera J. Chem. Eur. J. 2002;8:2000. doi: 10.1002/1521-3765(20020503)8:9<2000::AID-CHEM2000>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Brahms D, Dailey W. Chem Rev. 1996;96:1585. doi: 10.1021/cr941141k. [DOI] [PubMed] [Google Scholar]; Tien F, Kruger V, Bautista O, Duan J-X, Li A-R, Dolbier W, Chen Q. Org. Lett. 2000;2:563. doi: 10.1021/ol0055622. [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Luo T, Hu J, Wang Y, Krishnan H, Jog P, Ganesh S, Prakash G, Olah G. Angew. Chem. Int. Ed. 2011;50:7153. doi: 10.1002/anie.201101691. [DOI] [PubMed] [Google Scholar]; Wang F, Zhang W, Zhu J, Li H, Huang K-W, Hu J. Chem. Comm. 2011;47:2411. doi: 10.1039/c0cc04548a. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen A, Kuzmanich G, Garcia-Garibay MA. J Phys. Chem. A. 2014;118:1858. doi: 10.1021/jp501216z. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier M, Klok H. Chem. Comm. 2008;23:2591. doi: 10.1039/b719689j. [DOI] [PubMed] [Google Scholar]
- 32.Friscourt F, Fahrni C, Boons G-J. J Am. Chem. Soc. 2012;134:18809. doi: 10.1021/ja309000s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.