Abstract
Airborne silica dust (quartz) is common in coal mines and represents a respiratory hazard that can lead to silicosis, a potentially fatal lung disease. With an eye toward developing a portable monitoring device for rapid analysis of silica dust, laser-induced breakdown spectroscopy (LIBS) was used to quantify quartz in coal dust samples collected on filter media. Pure silica (Min-U-Sil™ 5), Georgia kaolin, and Pittsburgh-4 and Illinois-6 coal dusts were deposited separately and at multiple mass loadings onto 37-mm polyvinylchloride (PVC) filters. LIBS-generated silicon emission was monitored at 288.16 nm, and non-silica contributions to that signal from kaolinite were removed by simultaneously detecting aluminum. Measurements of the four samples were used to calculate limits of detection (LOD) for silicon and aluminum of approximately 0.08 µg/cm2 and 0.05 µg/cm2, respectively (corresponding to 0.16 µg/cm2 and 0.20 µg/cm2 for silica and kaolinite, respectively). Relative errors of prediction are around 10%. Results demonstrate that LIBS can dependably quantify silica on filter samples of coal dust and confirm that accurate quantification can be achieved for very lightly loaded samples, which supports the potential application of LIBS for rapid, in-field monitoring.
Index Headings: Laser-induced breakdown spectroscopy, LIBS, Coal dust, Silicon, Silica, Silicosis, Filter, Aerosols
INTRODUCTION
Silicosis is an incurable and potentially fatal lung disease caused by the inhalation of airborne particles containing crystalline silica (further referred to as “silica”), leading to inflammation and scarring in the upper lobes of the lung.1,2 Other health effects linked to silicosis include pulmonary tuberculosis, autoimmune disease, lung cancer, and chronic obstructive pulmonary disease (COPD).2,3 The National Institute for Occupational Safety and Health (NIOSH) estimates the current silicosis death rate in the U.S. at nearly 200 people per year.4 Approximately two million Americans are regularly exposed to silica, one hundred thousand of which are employed in settings where there are large amounts of silica dust in the air, liberated by activities such as rock drilling, mining, and sandblasting.3 There are currently no known therapies that reduce the mortality rate of people affected with silicosis, but a simple line of defense against the disease is to monitor the amount of silica dust in the air and limit worker exposure.5 While silicosis is easily preventable in principle, it still persists,6,7 motivating NIOSH to investigate potential new monitoring approaches including Fourier transform infrared (FT-IR) spectroscopy,8,9 and in the current study, LIBS.
Silica is found in the matrix of most coal deposits, both in the form of aluminosilicate minerals and in its elemental form (pure quartz), which is commonly called “free silica.”10 Silica-bearing minerals may also occur within lenses or partings visible within the coal seam, such as sandstone lenses deposited by meandering streams or ash bands resulting from volcanic eruptions. While there are many trace minerals and compounds in coal deposits, the most prevalent minerals are quartz and kaolin.11 The amount of quartz in typical coal mine dusts varies from about 2 to 14% and is often even higher in the host rock.11 Fragmentation of the coal matrix and other silica-bearing minerals during drilling and mining operations liberates particles of silica, causing exposure to airborne silica dust and potential health problems in coal mines.
Policy limits exposure to coal mine dust to 2 mg/m3 if the concentration of silica is 5% or less. For coal dusts with a higher mass fraction of silica, the regulated limit in units of mg/m3 is calculated as 10 divided by the percentage of silica.8,12 To measure exposure to coal dust, NIOSH developed and certified a direct reading monitor.13,14 This personal dust monitor (PDM) successfully aided miners in reducing their exposure to coal dust by making changes to their work activities and implementing dust control practices based on the continuous reading provided by the device.15 However, while the PDM measures miners’ exposure to total dust, it does not directly measure silica. For mine conditions where the silica content is known to be relatively constant, a rough estimate of silica exposure can be calculated by using the PDM data combined with the percent silica.
The amount of silica in coal dust samples is currently determined in the U.S. by collecting a filter sample and submitting it to the Mine Safety and Health Administration (MSHA). The filter is then pre-treated via ashing and analyzed by the P7 analytical method,16 using FT-IR spectroscopy. The P7 method, when conducted on samples of respirable-sized dust, has a detection limit of 4 µg/filter, a linear dynamic range of 20 to 700 µg/filter, and a precision of 7–10%. The lag time from sample collection to the return of silica exposure data is on the order of weeks, so the information cannot be used in a timely way to improve work conditions, especially in environments where the dust conditions vary rapidly. Therefore, NIOSH is evaluating field-portable technologies capable of end-of-shift (EOS) or near-real-time quantification of silica in coal dust samples.8 This paper evaluates the use of laser-induced breakdown spectroscopy (LIBS) for that purpose.
LIBS uses a high powered, pulsed laser beam to generate a plasma on the surface of a targeted sample.17 As the plasma cools, electronically excited neutral and ionized atomic species relax and emit fluorescence at various wavelengths. Elemental abundances are determined through analysis of resulting spectra. The technique has been applied to measurements of solids, liquids, and gases, and here we focus on measurements of particulate matter collected onto filters. Alternatively, individual aerosols can also be measured directly by LIBS as demonstrated previously.18–21
For filter-collected particles, detection limits are strongly dependent on chemical composition and measurement conditions.22–25 Neuhauser et al. measured 12 toxic metals collected on glass microfiber filters by LIBS with a 532 nm laser.22 Each filter was measured 125 times with a spot size and spacing of 220 and 240 µm, respectively. Detection limits ranged from 0.01 to 0.44 µg/cm2, but other figures of merit were not reported, including shot-to-shot variation, dynamic range, or sensitivity.
Cremers and Radziemski detected beryllium on filters using a long-spark technique with a photo-multiplier tube and monochromator arrangement.23 After loading, filter samples were spray coated with a clear acrylic material to reduce the loss of particles in areas surrounding the laser spot that results from the plasma-induced pressure wave. Even with the coating applied, the laser spark generally influenced a total area equal to three widths of the laser pulse. Detection limits ranged from 0.012 to 0.450 µg/cm2.
Panne et al. measured heavy metal particles collected on quartz fiber filters.24 Self-absorption was observed above mass loadings of 50 to 100 µg/cm2. Scanning electron microscopy (SEM) images showed that for a 532 nm laser pulse with energy of approximately 30 mJ and a typical spot size of 220 µm, the laser pulse and resulting plasma did not fully penetrate through the filter. The authors report that for mass loadings below 10 µg/cm2 a majority of the aerosol mass is ablated in a single pulse. Detection limits ranged from 0.01 to 0.91 µg/cm2.
The aim of our study was to evaluate the efficacy of LIBS to quantify silica in coal dust samples with an eye toward developing an EOS or rapid, in-field silica monitoring method. The first goal of the study was to quantify the precision and accuracy of the correlation between the LIBS Si signal and the mass of silica on filter samples of coal dust. The second goal was to determine if the sensitivity of LIBS for measuring silica lends itself to the development of an EOS silica monitor. Parallel sets of filter samples consisting of pure silica (Min-USil™ 5), Georgia kaolin, and two types of coal dusts on 37 mm polyvinylchloride (PVC) filters were generated for analysis by LIBS and the MSHA P7 method. The Min-U-Sil™ 5, Georgia kaolin, Illinois-6, and Pittsburgh-4 coal dusts were used to determine system sensitivity and to develop a correction scheme to remove the interference of silicon derived from kaolin within the coal dusts. The silica content, measured by P7, was then correlated to the LIBS Si signal.
EXPERIMENTAL
Laser-Induced Breakdown Spectroscopy
LIBS experiments were conducted using a commercial Insight™ bench top system (TSI, Inc.); a general description of the optical layout can be found in a previous publication by Stipe et al.26 A 7 ns, 80 mJ pulse of 1064 nm light is focused by a 3× microscope objective to a 300 µm spot on the surface of the particle-laden filter, generating a plasma. As the plasma cools, the resulting fluorescence is collected by a fiber-optic cable to a 0.3 m Czerny–Turner style spectrometer. The light is dispersed with a 600 grooves/mm grating onto an intensified charge-coupled device (iCCD) camera. The delay and gate are each 1 µs. To achieve a consistent spot size across all samples by maintaining a constant lens-to-sample distance, each filter is brought into the focal plane of the video camera that images the sample through the same 3× objective that focuses the pulsed laser.
Filter Sample Preparation
Filter samples with known mass of Min-U-Sil™ 5, Georgia kaolin, and coal dust were generated by aerosolizing each of these materials at ambient conditions (25 °C, 50% relative humidity (RH)) in a Marple calm air chamber,27 using a fluidized bed aerosol generator (TSI, Inc., Model 3400A). The sample loading and P7 results are shown in Table I. Samples were collected onto low-ash, 37 mm, 5.0 µm pore size, PVC filters (SKC® Corp., Inc.) that were pre-weighed using a gravimetric balance and housed in plastic cassettes. The cassettes reduced the diameter of the loaded material to 34 mm. A Dorr-Oliver cyclone with a 4 µm 50% cut-point removed large particles from the sampling flow, resulting in respirable particles. The samples were mounted in groups of six, with each sampler connected to a critical orifice calibrated to 1.7 liters per minute (lpm) and each group connected to a flow manifold. Five manifolds were used per run. The chamber concentration was monitored using two tapered element oscillating microbalances (TEOM) (Thermo Scientific, Model TEOM 1400a) connected to Dorr-Oliver cyclones and operated at a flow rate of 1.7 lpm. After collection, all filter samples were post-weighed to determine the mass of loaded dust. The six samples from each loading were divided into two sets of triplicates—one for MSHA P7 analysis and the other for LIBS analysis.
TABLE I.
Filter sample gravimetric and MSHA P7 results.
| MSHA P7 | MSHA P7 | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Mass (µg) |
Kaolin (µg) |
Quartz (µg) |
Sample | Mass (µg) |
Kaolin (µg) |
Quartz (µg) |
|
| Minusil-5 | Georgia kaolin | |||||||
| M-1 | 14 | 0 | 16 | G-1 | 9 | 12 | 2 | |
| M-2 | 13 | 0 | 17 | G-2 | 12 | 12 | 2 | |
| M-3 | 17 | 0 | 13 | G-3 | 14 | 12 | 2 | |
| M-4 | 28 | 0 | 29 | G-4 | 22 | 23 | 3 | |
| M-5 | 26 | 0 | 32 | G-5 | 23 | 23 | 3 | |
| M-6 | 31 | 0 | 31 | G-6 | 20 | 23 | 3 | |
| M-7 | 62 | 0 | 60 | G-7 | 50 | 45 | 5 | |
| M-8 | 61 | 0 | 60 | G-8 | 51 | 47 | 5 | |
| M-9 | 64 | 0 | 50 | G-9 | 52 | 45 | 5 | |
| M-10 | 111 | 0 | 113 | G-10 | 90 | 84 | 10 | |
| M-11 | 109 | 0 | 110 | G-11 | 100 | 84 | 9 | |
| M-12 | 116 | 0 | 106 | G-12 | 109 | 86 | 9 | |
| M-13 | 218 | 0 | 204 | G-13 | 217 | 181 | 19 | |
| M-14 | 215 | 0 | 211 | G-14 | 208 | 178 | 18 | |
| M-15 | 217 | 0 | 209 | G-15 | 231 | 179 | 18 | |
| Pittsburgh-4 | Illinois-6 | |||||||
| P-1 | 9 | 3 | 1 | I-1 | 10 | 1 | 1 | |
| P-2 | 11 | 3 | 1 | I-2 | 13 | 0 | 0 | |
| P-3 | 8 | 3 | 1 | I-3 | 11 | 1 | 1 | |
| P-4 | 42 | 8 | 3 | I-4 | 32 | 3 | 1 | |
| P-5 | 31 | 8 | 3 | I-5 | 31 | 3 | 2 | |
| P-6 | 35 | 8 | 3 | I-6 | 32 | 3 | 2 | |
| P-7 | 65 | 14 | 5 | I-7 | 61 | 6 | 2 | |
| P-8 | 59 | 12 | 4 | I-8 | 57 | 6 | 3 | |
| P-9 | 62 | 14 | 5 | I-9 | 55 | 6 | 3 | |
| P-10 | 123 | 22 | 8 | I-10 | 108 | 11 | 5 | |
| P-11 | 128 | 24 | 9 | I-11 | 115 | 11 | 5 | |
| P-12 | 126 | 23 | 8 | I-12 | 120 | 11 | 5 | |
| P-13 | 167 | 30 | 10 | I-13 | 172 | 16 | 6 | |
| P-14 | 179 | 33 | 12 | I-14 | 182 | 17 | 6 | |
| P-15 | 171 | 34 | 11 | I-15 | 184 | 17 | 7 | |
In a previous study, Min-U-Sil™ 5 was shown to be 92.4% silica.28 In the current study, by comparing the P7 data with gravimetric data for many samples, the Min-U-Sil™ 5 was measured to have approximately 95% silica. The material chosen for developing the kaolin correction scheme was a well-characterized source of Georgia kaolin used in previous research studies observing IR signals for various types of kaolin.29 The two types of coal dust used in the current study were Pittsburgh-4 and Illinois-6. Pittsburgh-4 is derived from the Pittsburgh coal seam and was ground to a nominal mean diameter of 4 µm, while the Illinois-6 coal dust was ground slightly finer, with a target mean diameter of 3 µm.
MSHA P7 Method
Currently, airborne silica in coal dust is quantified by the U.S. Mine Safety and Health Administration (MSHA) P7 method.16 The method involves ashing the PVC filters in a low-temperature radio-frequency asher to destroy organic constituents including coal dust and the PVC filter itself. Ashed samples are redeposited in a 9 mm diameter circular area on a vinyl acrylic copolymer filter (VAC-DM450). The redeposited ashed samples are then scanned by FT-IR spectrometry to determine both the quartz and kaolinite content. Since the main doublet peak used to quantify silica has an overlapping kaolinite peak, a correction scheme is used to remove that contribution and the value of quartz in the sample is estimated.30
For this study, parallel sets of samples were generated and analyzed by the MSHA P7 method, to be used for comparison with the LIBS-analyzed samples. Each of the parallel sets of filters consisted of triplicate samples at each mass loading condition. The relationships between mass loading and silica concentration for the P7-analyzed samples are shown in Figs. 1a through 1d. The relationship between mass loading and kaolinite is shown in Figs. 2a through 2c. Prediction errors in Figs. 1 and 2 are two standard deviations.
Fig. 1.
Relationships between deposited mass and silica content determined by MSHA P7 analysis for (a) Min-U-Sil™ 5, (b) Georgia kaolin, and (c) Pittsburgh-4 and (d) Illinois-6 coal dusts. Error bars are two standard errors of prediction.
Fig. 2.
Relationship between deposited mass and kaolinite content determined by MSHA P7 analysis for (a) Georgia kaolin and (b) Pittsburgh-4 and (c) Illinois-6 coal dusts. No kaolinite was detected in Min-U-Sil™ 5 samples. Error bars are two standard errors of prediction.
RESULTS AND DISCUSSION
Method Development
In initial experiments, single lasershot measurements were performed at multiple locations across a filter surface loaded with Min-U-Sil™ 5 dust. Analysis of the spectral results showed that material vaporized by each laser pulse redeposited on the filter surface, thereby influencing measurements made by subsequent laser shots on the same filter. To eliminate this problem, each filter was cut into approximately 20 pieces, and each piece was measured individually. The 34 mm diameter circle of deposited particulate matter on each filter was cut into approximately 5 mm × 5 mm squares. The 300 µm laser spot was centered in each square. The relative standard deviation (RSD) of the 20 shots ranged from approximately 10 to 15%, due to both variations in plasma formation and non-uniform particle deposition across the filter surface. A future portable system should therefore perform the LIBS analysis either in a sample chamber that isolates the laser shot to a confined area or deposits the particles in separate locations on a filter tape.
Initial experiments also helped to determine the chosen laser energy and spot size of 80 mJ and 300 µm, respectively. At lower fluences, the laser-ablated hole did not fully penetrate the PVC filter. At higher fluences, the filter sample fractured excessively and shot-to-shot variations in emission signals increased. Representative spectra of Min-U-Sil™ 5, Georgia kaolin, the coal dusts, and a blank filter are shown in Fig. 3.
Fig. 3.
Representative LIBS spectra of blank filter and filters loaded separately with Min-U-Sil™ 5, Georgia kaolin, and Pittsburgh-4 and Illinois-6 coal dusts. The blank filter shows a carbon peak at 247.9 nm and no interference at the silicon peak locations (251.6 and 288.2 nm) or at the aluminum doublet (308.2 and 309.2 nm). Min-U-Sil™ 5 exhibits the carbon peak from the filter and silicon emission, while Georgia Kaolin and the coal dusts contain carbon, silicon, and aluminum.
Previous studies using LIBS to measure individual aerosolized particles show that the LIBS signal is mass dependent as long as the particles are fully vaporized by the laser-induced plasma. An early study by Radziemski et al.21 estimated the upper size limit for full particle vaporization of beryllium particles around 10 µm, while two later studies found the size limit to be 2.1 µm for silica particles and 5 µm for glucose particles.31,32 These studies were of individual particles in air, and the results, likely dependent on the laser wavelength and fluence, may not be representative of measurements of particles on a filter matrix. For particles collected on a filter, Cremers and Radziemski23 measured beryllium particles with sizes of 0.05, 0.5–5.0, 15, 45, and 65 µm. The beryllium signal was an order of magnitude larger for the 0.05 to 5.0 µm range of particles than for the 15 µm particles, and the authors attribute the difference to incomplete vaporization. In our study, all particles were sampled through a 4 µm cut-point cyclone to restrict the deposited mass to only the respirable fraction.
Silicon light emission was quantified by integrating the peak area at 288.16 nm (Table II). As is evident in the spectra, LIBS detects atomic silicon and not silica (SiO2) directly. Using the P7 data for the Min-U-Sil™ 5 samples, the LIBS silicon signal was correlated to silica mass using the ratio of molar masses MSi/MSiO2 of approximately 0.467. To determine the silica content in other samples, all contributions to silicon fluorescence not from silica must be removed.
TABLE II.
Peak locations and energy levels for carbon, silicon, and aluminum.
| Element | Wavelength (nm) | E1 (eV) | E2 (eV) |
|---|---|---|---|
| Carbon | 247.86 | 2.6840 | 7.6848 |
| Silicon | 288.16 | 0.7810 | 5.0823 |
| Aluminum | 308.22 | 0.0000 | 4.0215 |
| 309.27 | 0.0138 | 4.0216 | |
| 309.28 | 0.0138 | 4.0214 |
The only sources of silicon in coal are silicate minerals, which exist as either quartz or kaolinite (Al2Si2O5(OH)4).30 While other silicon-containing minerals exist33 in some settings, they are not present in most coal dusts. Kaolinite contains aluminum, so to quantify the contribution of kaolinite to the total silicon signal, aluminum fluorescence at 308.22 nm, 309.27 nm, and 309.28 nm (Table II) was monitored. A calibration curve was generated relating aluminum fluorescence to aluminum mass loading. The kaolinite content was determined by the MSHA P7 method, as described earlier and shown in Fig. 2. The mass of aluminum in the samples was found using the ratio of molar masses MAl/Mkaolinite = 0.209, and the silicon content in kaolinite was found using MSi/Mkaolinite = 0.218. Using this approach, the silicon from silica is found by subtracting the silicon from kaolinite from the total silicon determined from the LIBS signal. For the Min-U-Sil™ 5 samples, MSHA P7 analysis did not detect any kaolinite; therefore, it was not necessary to correct these samples.
Calibrations
Min-U-Sil™ 5, Georgia kaolin, Illinois-6, and Pittsburgh-4 were analyzed by LIBS, and the silicon, aluminum, and carbon emissions were monitored. For each material, three filters at each loading condition were measured. Initial measurements of silicon in the four sample types showed that the sensitivity of the LIBS silicon signal depends on the material measured. These differences in sensitivity, or chemical matrix effects, are shown in Fig. 4. To correct for these matrix effects, carbon emission, predominantly from the PVC filter material, was monitored at 247.86 nm (Table II) and used as an internal standard. Note that since the average mass of a PVC filter is roughly 13 grams, and the mass of the most heavily loaded coal filter was approximately 250 µg, the carbon content of the coal contributes less than 0.01% to the total carbon content and has only a minor effect on the normalization approach.
Fig. 4.
LIBS silicon emission for Min-U-Sil™ 5, Georgia kaolin, Illinois-6, and Pittsburgh-4 at different mass loadings. Each plotted data point is an average of 20 measurements of three similarly loaded filters. The silicon content was calculated using the MSHA P7 analysis of silica and kaolinite.
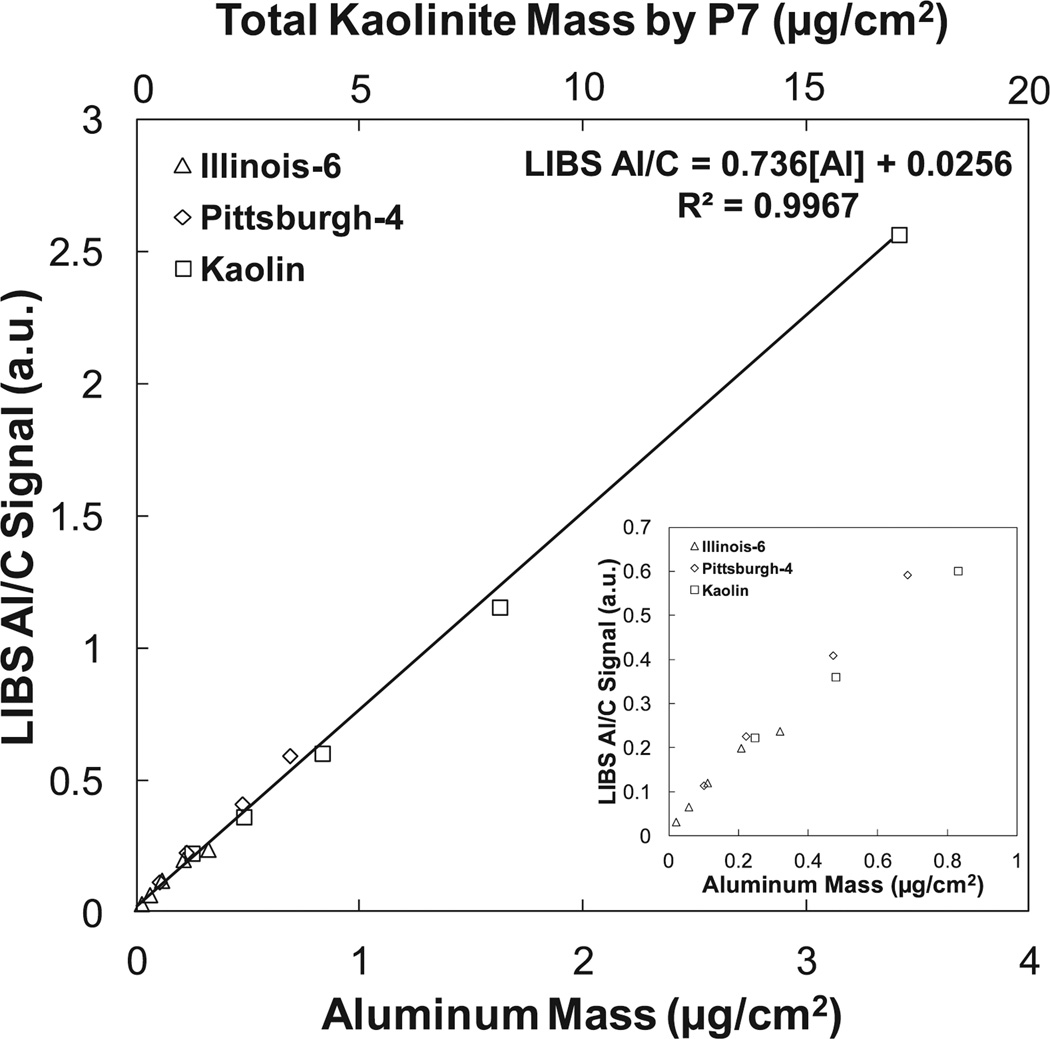
The calibration curve for LIBS-generated aluminum emission normalized by carbon emission as a function of deposited aluminum mass is shown in Fig. 5. The aluminum content is inferred from the kaolinite mass determined by MSHA P7 on the parallel set of samples (Figs. 2a through 2c). The calibration curve is linear with an R2 of 0.997. Only data from Georgia kaolin, Illinois-6, and Pittsburgh-4 are shown since Min-U-Sil™ 5 contains no kaolinite. The limit of detection (LOD) of aluminum in kaolinite, calculated as three times the standard deviation of the background divided by the slope of the calibration curve, is 0.48 µg/filter (0.05 µg/cm2) or roughly 37 picograms of material per laser shot. The limit of quantification (LOQ), usually 3.3 times the LOD, is approximately 0.17 µg/cm2. For kaolinite, the LOD and LOQ are 0.20 and 0.66 µg/cm2, respectively.
Fig. 5.
LIBS silicon emission at 288.16 nm normalized by carbon emission at 247.86 nm for Minusil-5, Georgia kaolin, Illinois-6, and Pittsburgh-4. Each plotted data point is an average of measurements of three similarly loaded samples. The silicon content was calculated using the MSHA P7 analysis of silica and kaolinite.
The calibration curve of the LIBS-generated silicon emission normalized by carbon as a function of silicon loading is given in Fig. 6. The silicon content for the LIBS-measured samples was calculated using the gravimetric and MSHA P7 relationships shown previously in Figs. 1a through 1d. The calibration data were fit to a second-order polynomial regression to capture the nonlinear behavior at loading conditions above 5 µg/cm2 of silicon. This nonlinearity is likely due to self-absorption effects caused by the high population of Si atoms generated in the plasma at the higher silica concentrations.17 The LOD is 0.72 µg/filter (0.08 µg/cm2) or roughly 56 picograms of material per laser shot, and the LOQ is 0.26 µg/cm2. This corresponds to a silica LOD and LOQ of 0.16 and 0.53 µg/cm2, respectively.
Fig. 6.
LIBS silicon emission at 288.16 nm normalized by carbon emission at 247.86 nm for Min-U-Sil™ 5, Georgia kaolin, Illinois-6, and Pittsburgh-4. Each plotted data point is an average of measurements of three similarly loaded samples. The silicon content was calculated using the MSHA P7 analysis of silica and kaolinite. To determine the silica content, the kaolinite contribution to the total silicon signal would be subtracted using Fig. 5.
Application of the Method to Coal Mine Dust
Respirable coal dust samples from mines are required to be below 5% quartz in a 2.0 mg/m3 sample,12 which on average limits the respirable quartz concentration to 100 µg/m3. Since the results of this study indicate that the LOQ for silica using LIBS is on the order of 0.5 µg/cm2, or over 25 times more sensitive than the MSHA P7 method, it suggests that LIBS may be useful as a near-real-time technique that can quantify silica in airborne dust at the levels expected in a mine. Assuming a respirable quartz concentration of 100 µg/m3 and a sampling rate of 2 lpm across a diameter of 34 mm, the required time to reach the LOQ would be approximately 20 minutes. The measurement time resolution may be reduced by increasing the sampling flow rate or reducing the collection area of the filter, each of which would increase the velocity through the filter face. In a study by Lee et al.,9 a high volumetric flow rate sample collection method was successfully demonstrated at a flow rate of 11.2 lpm, which would reduce the required sampling time to 4 minutes.
CONCLUSIONS
The efficacy of using LIBS to measure silica in filter samples of coal dust was demonstrated. The limit of detection and limit of quantification for silica were 0.16 and 0.53 µg/cm2, respectively, making it more than 25 times more sensitive than the MSHA P7 method. Since LIBS detects elemental silicon, a correction scheme was developed to remove the influence of silicon originating from kaolinite in the coal samples. The correlation of the LIBS Si signal with mass of silicon in samples was excellent (R2 = 0.995) over a relatively wide range of filter loadings (approximately 0.6–19 µg/cm2 of silica), suggesting that LIBS may be used as an EOS method for measuring silica.
Practical application of this method would require developing a miniaturized LIBS system for fast, in-field monitoring of silica in an underground mining environment. As currently envisioned, the system would entail deposition of coal dust onto a translating filter tape that moves the deposited particles into a measurement chamber for LIBS analysis. To reduce the size and weight of such an instrument, a single-channel spectrometer—covering a wavelength range that captures carbon, silicon, and aluminum (240–315 nm)—and a passively Q-switched pulsed laser could be used. Based on the demonstrated LOQ and minimum sampling rate of 2 lpm, the maximum time resolution is on the order of 20 minutes, or 4 minutes if high flow rate collection is employed.
ACKNOWLEDGMENTS
The authors would like to thank TSI, Inc. for use of its Insight™ LIBS system, and William J. Archer and Jeanne Zimmer for generating the filter samples used in this study. This work was partially funded by the Seattle University Summer Faculty Fellowship Program and a Welsch Scholarship for undergraduate research.
References
- 1.Ziskind M, Jones R, Weill H. Silicosis. Am. Rev. Respir. Dis. 1976;113(5):643–665. doi: 10.1164/arrd.1976.113.5.643. [DOI] [PubMed] [Google Scholar]
- 2.NIOSH. Publication No. 2002-129. Cincinnati, OH: National Institute for Occupational Safety and Health; 2002. NIOSH Hazard Review: Health Effects of Occupational Exposure to Respirable Crystalline Silica. [Google Scholar]
- 3.Yassin A, Yebesi F, Tingle R. Occupational Exposure to Crystalline Silica Dust in the United States, 1988–2003. Environ. Hlth. Perspect. 2005;113(3):255–260. doi: 10.1289/ehp.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIOSH. “ http://www2.cdc.gov/drds/WorldReportData/”. eWoRLD Report online. 2012
- 5.Wagner GR. The Inexcusable Persistence of Silicosis. Am. J. Pub. Hlth. 1995;85(10):1346–1347. doi: 10.2105/ajph.85.10.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez C, Prieto A, Garcia L, Quero A, González S, Casan P. Silicosis: a Disease with an Active Present. Arch. Bronconeumol. 2010;46(2):97–100. doi: 10.1016/j.arbres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Laney SA, Petsonk EL, Atfield MD. Pneumoconiosis Among Underground Bituminous Coal Miners in the United States: Is Silicosis Becoming More Frequent? Occup. Environ. Med. 2010;67:5. doi: 10.1136/oem.2009.047126. [DOI] [PubMed] [Google Scholar]
- 8.Miller AL, Drake PL, Murphy NC, Noll JD, Volkwein JC. Evaluating Portable Infrared Spectrometers for Measuring the Silica Content of Coal Dust. J. Environ. Monit. 2012 doi: 10.1039/c1em10678c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee T, Kim SW, Chisholm WP, Slaven J, Harper M. Performance of High Flow Rate Samplers for Respirable Particle Collection. Ann. Occup. Hyg. 2010;54(6):697–709. doi: 10.1093/annhyg/meq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyers RA. Coal Handbook. New York and Basel: Marcel Dekker, Inc.; 1981. pp. 1–17. [Google Scholar]
- 11.Schatzel SJ. Identifying Sources of Respirable Quartz and Silica Dust in Underground Coal Mines in Southern West Virginia, Western Virginia, and Eastern Kentucky. Intnl. J. Coal Geo. 2009;78:110–118. [Google Scholar]
- 12. [accessed 17th October 2011];U.S. Code of Federal Regulations 30CFR70 Sub-part B 70.101. 2010 Jul 1; http://www.gpoaccess.gov/nara/index.html.
- 13.Page S, Volkwein J, Vinson R, Joy G, Mischler S, Tuchman D, McWilliams L. Equivalency of a Personal Dust Monitor to the Current United States Coal Mine Respirable Dust Sampler. J. Environ. Monit. 2008;10(1):96–101. doi: 10.1039/b714381h. [DOI] [PubMed] [Google Scholar]
- 14.Tuchman DP, Volkwein JC, Vinson RP. Implementing Infrared Determination of Quartz Particulates on Novel Filters for a Prototype Dust Monitor. J. Environ. Monit. 2008;10(5):671–678. doi: 10.1039/b803804j. [DOI] [PubMed] [Google Scholar]
- 15.Peters RH, Vaught C, Hall EE, Volkwein JC. DHHS (NIOSH), Publ. No. 2008-110, IC 9501. 2008. IC 9501(47 pp) [Google Scholar]
- 16.MSHA. Infrared Determination of Quartz in Respirable Coal Mine Dust - Method No. MSHA P7. US Dept of Labor-MSHA-Pittsburgh Safety and Health Technology Center; 2008. [Google Scholar]
- 17.Cremers DA, Radziemski LJ. Handbook of Laser-Induced Breakdown Spectroscopy. West Sussex, UK: John Wiley and Sons; 2006. p. 283. [Google Scholar]
- 18.Hahn DW. Laser-induced Breakdown Spectroscopy for Sizing and Elemental Analysis of Discrete Aerosol Particles. Appl. Phys. Lett. 1998;72:2960–2963. [Google Scholar]
- 19.Park K, Cho G, Kwak J. Development of an Aerosol Focusing-Laser Induced Breakdown Spectroscopy (Aerosol Focusing-LIBS) for Determination of Fine and Ultrafine Metal Aerosols. Aero. Sci. Technol. 2008;43:375–386. [Google Scholar]
- 20.Lithgow GA, Robinson AL, Buckley SG. Ambient Measurements of Metal-containing PM2.5 in an Urban Environment Using Laser-induced Breakdown Spectroscopy. Atmos. Environ. 2004;38:3319–3328. [Google Scholar]
- 21.Radziemski LJ, Loree TR, Cremers DA, Hoffman NM. Time-Resolved Laser-Induced Breakdown Spectrometry of Aerosols. Anal. Chem. 1983;55(8):1246–1252. [Google Scholar]
- 22.Neuhauser RE, Panne U, Niessner R. Laser-Induced Plasma Spectroscopy (LIPS): a Versatile Tool for Monitoring Heavy Metal Aerosols. Anal. Chim. Acta. 1999;392:47–54. [Google Scholar]
- 23.Cremers DA, Radziemski LJ. Direct Detection of Beryllium on Filters Using the Laser Spark. Appl. Spectrosc. 1985;39(1):57–63. [Google Scholar]
- 24.Panne U, Neuhauser RE, Theisen M, Fink H, Niessner R. Analysis of Heavy Metal Aerosols on Filters by Laser-Induced Plasma Spectroscopy. Spectrochim. Acta, Part B. 2001;56:839–850. [Google Scholar]
- 25.Kuhlen T, Fricke-Begemann C, Strauss N, Noll R. Analysis of Size-Classified Fine and Ultrafine Particulate Matter on Substrates with Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta, Part B. 2008;63:1171–1176. [Google Scholar]
- 26.Stipe CB, Hensley BD, Boersema JL, Buckley SG. Laser-Induced Breakdown Spectroscopy of Steel: A Comparison of Univariate and Multivariate Calibration Methods. Appl. Spectrosc. 2010;64(2):154–160. doi: 10.1366/000370210790619500. [DOI] [PubMed] [Google Scholar]
- 27.Marple VA, Rubow KL. An Aerosol Chamber for Instrument Evaluation and Calibration. Am. Ind. Hyg. Assoc. J. 1983;44:361–367. [Google Scholar]
- 28.Stacy P, Kauffer E, Moulut J-C, Dion C, Beauparlant M, Fernandez P, Key-Schwartz R, Friede B, Wake D. An International Comparison of the Crystallinity of Calibration Materials for the Analysis of Respirable Alpha Quartz Using X-ray Diffraction and a Comparison with Results from the Infrared KBr Disc Method. Ann. Occup. Hyg. 2009;53(6):639–649. doi: 10.1093/annhyg/mep038. [DOI] [PubMed] [Google Scholar]
- 29.Anderson CC. Collaborative Tests of Two Methods for Determining Free Silica in Airborne Dust. DHHS (NIOSH) Publ. No. 83-124. 1983:157. [Google Scholar]
- 30.Ainsworth SM. Infrared Analysis of Respirable Coal Mine Dust for Quartz: Thirty Five Years. J. ASTM Intl. 2005;2:1–13. [Google Scholar]
- 31.Carranza JE, Hahn DW. Assessment of the Upper Particle Size Limit for Quantitative Analysis of Aerosols Using Laser-Induced Breakdown Spectroscopy. Anal. Chem. 2002;74(21):5450–5454. doi: 10.1021/ac020261m. [DOI] [PubMed] [Google Scholar]
- 32.Vors E, Salmon L. Laser-induced Breakdown Spectroscopy (LIBS) for Carbon Single Shot Analysis of Micrometer-sized Particles. Anal. Bioanal. Chem. Special Issue. 2006;385:281–287. doi: 10.1007/s00216-006-0320-x. [DOI] [PubMed] [Google Scholar]
- 33.Ojima J. Determining of Crystalline Silica in Respirable Dust Samples by Infrared Spectrophotometry in the Presence of Interferences. J. Occup. Health. 2003;45:94–103. doi: 10.1539/joh.45.94. [DOI] [PubMed] [Google Scholar]