Abstract
Objective
To examine the effect of modafinil on depression via a secondary data analysis of a RCT of modafinil for fatigue in cancer patients. The primary aim is to elucidate factors that contributed to the effectiveness of modafinil in the parent trial.
Methods
541 cancer patients receiving chemotherapy and experiencing fatigue (Brief Fatigue Inventory (BFI) Item 3≥3) were randomized to receive 200mg modafinil (N=260) or placebo (N=281) daily from baseline (Cycle 2) to post-test (Cycle 4). Patients completed the Center for Epidemiologic Studies Depression Scale (CES-D) and POMS Depression-Dejection subscale (POMS-DD) at baseline and post-test. We used linear regression to address the hypothesis that modafinil would be associated with reduced depression, particularly in those experiencing severe fatigue (BFI≥7).
Results
Modafinil did not have a significant effect on depression, even for those patients with severe fatigue. However, for subjects with severe fatigue (BFI ≥ 7), those receiving modafinil had lower depression scores than controls. Modafinil significantly moderated the relationship between baseline fatigue and CES-D total scores (p = 0.04), and was marginally significant as a moderator for the relationship between baseline fatigue and POMS-DD scores (p = 0.07). Modafinil also significantly moderated the relationship between baseline fatigue and CES-D Positive Affect subscale scores (p = 0.003), but not CES-D Somatic, Negative Affect, or Interpersonal subscale scores.
Conclusion
Modafinil differentially impacts depression based on a patient’s level of fatigue, and reduced depressive symptoms only in those with extreme fatigue. This effect may be driven by increases in positive affective symptoms. These results have significant implications for intervention; in patients with high levels of fatigue, modafinil might also reduce depression. Future RCTs are needed to confirm these results.
Keywords: cancer, depression, cancer-related fatigue, modafinil, psychostimulant
Introduction
Symptoms of depression and fatigue are common among cancer patients: nearly 25% meet criteria for major depression, with an additional 30% reporting depressive symptoms.1 Similarly, cancer-related fatigue (CRF) among cancer patients exceeds 60% in many studies.2 Depression is associated with increased non-compliance with cancer treatments,3 increased inflammation,4 disease progression,5 and increased risk of mortality.6 CRF is associated with decreased treatment adherence, physical and psychosocial functioning, and quality of life.7 Taken together, the prevalence and negative outcomes of depression and CRF underscore the necessity of developing effective interventions to control these symptoms.
In populations of cancer patients, depression and fatigue frequently co-occur. In fact, the correlation between the two has been reported to range from r = 0.35 to 0.58, accounting for more than one third of the variance in several studies.8–10 Thus, it is not surprising that psychostimulants used for the treatment of CRF are often used to augment antidepressant treatment in psychiatry, leading to parallel improvements in both fatigue and depressive symptoms.11 Modafinil, a stimulant medication that has been approved for treatment of narcolepsy, shift work sleep disorder, and sleep apnea,12 has received attention in the psycho-oncology literature as a potential pharmacological intervention for CRF. Although there is mixed evidence in the literature for the efficacy of modafinil for the treatment of CRF,13–15 modafinil has received substantial attention in psychiatry for management of depression. A meta-analysis by Gross and colleagues16 found modafinil to be an effective augmentation strategy that reduced both depression and fatigue. Randomized controlled studies have also suggested that modafinil may provide benefit as an adjunctive therapy for patients with partial response or non-response to typical antidepressants.17–18 A review by Chang, Sato, and Han19 found that modafinil may be an affective adjunctive treatment for depression, but is most appropriate for targeting somatic symptoms of depression such as fatigue and sleepiness.
Taken together, these findings have led researchers to hypothesize that the improvements in fatigue observed in conjunction with psychostimulant medication are secondary to improvements in mood.20 For example, a recent editorial by Ruddy and colleagues suggests that modafinil might benefit a subset of cancer patients who suffer from depression or narcotics-induced fatigue.21 Thus, the aim of the present research was to examine the moderating effect of modafinil on the relationship between fatigue and depression in a sample of cancer patients undergoing chemotherapy. We hypothesized that modafinil would be associated with reduced levels of depressive symptoms, particularly in those who were experiencing high levels of fatigue. To test this hypothesis, we used data from a multicenter, double-blind randomized clinical trial (RCT) examining the effects of modafinil versus placebo on fatigue in cancer patients undergoing chemotherapy.15
Materials and Methods
Design
This is a secondary data analysis of prospectively collected data for an RCT.15 A multicenter, double-blind, placebo-controlled, phase 3 RCT examined the effect of modafinil on CRF in cancer patients undergoing chemotherapy. The primary outcomes of the RCT and a full description of all participant (N=541) characteristics have been previously published.15
Procedures
Data were obtained from a total of 877 fatigued cancer patients beginning chemotherapy at 23 geographically separate Community Clinical Oncology Program (CCOP) affiliates. Eligibility and exclusion criteria for the present study are the same as those for the original study.15 Details of the RCT, including procedures for informed consent, accrual, and randomization have been published elsewhere.15 Patients were randomized to receive 200mg modafinil or placebo daily, and were instructed not to initiate any new medications during this time. Cephalon, Inc. supplied modafinil and placebo tablets for the present study. Patients completed self-report measures of fatigue and depressive symptoms at home for one week after their second chemotherapy cycle (baseline) and fourth chemotherapy cycle (post-test).
The original report of the primary outcomes from the RCT15 provides information about study flow. A sample of 877 cancer patients beginning chemotherapy was assessed for fatigue. Those who reported fatigue (N=867) were enrolled in the study and randomized to the receive modafinil (N=431) or placebo (N=436). One hundred and seventy one (40%) patients in the modafinil group are not included in this secondary data analysis due to study dropout (n=112), failure to complete depression measures at follow-up (n= 49), expiration (n=5), or ineligibility (n=5). One hundred and fifty five (36%) patients in the placebo group are not included in this secondary data analysis due to study dropout (n=109), failure to complete depression measures at follow-up (n= 39), expiration (n=5), or ineligibility (n=2). Thus, a total of 541 had complete data on depression and were available for the current analyses. As indicated in the original report of the primary outcomes from the RCT, the rate of missing values for the drug arm was numerically larger than that of the placebo arm but was not statistically significant.15 Across groups, the most common reasons for study dropout included: changes or delays in treatments (n=46, 21%), other medical complications (n=40, 18%), and possible related side effects (n=20, 9%). Compared to patients who completed all follow-up measures, patients who dropped out demonstrated no statistically significant differences in fatigue or depressive symptoms (all ps > 0.05).
Participants
The sample was primarily female (70%), Caucasian (95%), non-Hispanic (99%), married (70%), with a mean age of 59 years (range= 18–87, SD = 11.5 years), and had at least a high school degree (92%). The three most frequent primary tumor locations were breast (39%), alimentary (24%), and lung (14%). Forty three percent of the sample had received prior chemotherapy at baseline, and most (93%) had received or were continuing with some type of cancer treatment beyond chemotherapy (radiation therapy=76%, hormonal therapy=90%).
Measures
Fatigue
CRF was assessed by using the Brief Fatigue Inventory (BFI).22 Patients rate 9 items on a scale from 0 (no fatigue or does not interfere) to 10 (worst possible fatigue or completely interferes). The mean of the nine BFI items can be used as a global BFI score. The reliability, validity, and internal consistency (up to Cronbach’s α = 0.96) of the BFI have been demonstrated in previous samples of cancer patients.22
Depressive symptoms
Two measures were used. (1) The Center for Epidemiological Studies Depression scale (CES-D)23 measures depressive symptoms over the past week. Twenty items are rated on a 4-point scale from 0 (hardly ever or never) to 3 (much or most of the time). Scores range from 0 to 60; higher scores reflect greater depressive symptoms. Scores greater than 16 are clinically significant. The CES-D has 4 subscales: Depressed Affect, Positive Affect, Somatic Symptoms, and Interpersonal Relations. Because the somatic symptoms subscale is easily parsed out, the CES-D is commonly used in research with cancer patients.24 (2) The Depression-Dejection subscale of the Profile of Mood States (POMS-DD)25 assesses depressive symptoms over the past week. Fifteen items are rated on a 5-point scale from 0 (not at all) to 4 (extremely). Scores range from 0 to 32; higher scores indicate greater depressive symptoms.
Analytic Strategy
Descriptive statistics were calculated for demographic and clinical characteristics at baseline. Baseline (Cycle 2) summary statistics for predictors are reported in Table 1. No sociodemographic, disease, and treatment variables were significantly correlated with the outcome variable; thus, none of these variables were included as controls in subsequent analyses.
Table 1.
Summary statistics for participants (N = 541) on measures of fatigue and depressive symptoms.
| Baseline (Cycle 2) | Post-Test (Cycle 4) | ||||
|---|---|---|---|---|---|
| Measure | Number of Items |
Mean (SD) | Observed Range |
Mean (SD) | Observed Range |
| Brief Fatigue Inventory (BFI) | 9 | 5.47 (2.01) | 0.29–10.00 | 5.09 (2.32) | 0.00–9.78 |
| Profile of Mood States Depression-Dejection Subscale (POMS-DD) | 15 | 3.98 (3.97) | 0–c18 | 3.29 (3.62) | 0–20 |
| Center for Epidemiological Studies Depression Scale (CES-D) Total Score | 20 | 16.94 (9.75) | 0–49 | 15.55 (9.84) | 0–49 |
| Negative Affect Subscale | 5 | 3.19 (3.27) | 0–15 | 2.85 (3.15) | 0–15 |
| Positive Affect Subscale | 4 | 3.54 (3.09) | 0–12 | 3.25 (2.95) | 0–12 |
| Somatic Symptoms Subscale | 5 | 7.13 (3.10) | 0–14 | 6.63 (3.47) | 0–15 |
| Interpersonal Subscale | 2 | 0.20 (0.69) | 0–4 | 0.16 (0.58) | 0–6 |
For the tests of modafinil as a moderator, six linear models were used to address the hypotheses. Models specified the following variables at post-test as outcomes: CES-D, POMS-DD, and CES-D subscale scores (Positive Affect, Negative Affect, Somatic Symptoms, and Interpersonal Symptoms). All models specified BFI baseline, Arm, and BFI*Arm interaction term as independent variables. Thus, the regression models addressed the hypothesis that baseline (Cycle 2) fatigue would predict depression at post-test (Cycle 4), and that intervention arm would moderate this relationship. All analyses were based on intention to treat. The data were screened for outliers and other anomalies, and the assumptions underlying each of the analyses were thoroughly checked. P-values less than 0.05 indicated statistical significance (two-tailed). The final sample size of 541 participants provided a more than acceptable power level for these analyses (1-β > 0.95; post-hoc power analyses conducted using the program G*Power26). All further statistical analyses were completed using SPSS Version 19.0 and R Version 2.15.1.
Results
Summary statistics of measures at baseline (Cycle 2) and post-test (Cycle 4) are reported in Table 1. An independent samples t-test confirmed that study arms were balanced on baseline levels of depression and fatigue. There were no differences between arms in BFI (p=0.63), CES-D (p=0.13), or POMS-DD scores (p=0.48). The correlation between CES-D and BFI scores at baseline was statistically significant (r=0.53, p < 0.01), as was the correlation between baseline BFI and post-test CES-D scores (r=0.29, p < 0.01). The baseline correlation between the POMS-DD and BFI was not statistically significant (r=−0.02, p=0.73). However, the correlation between baseline BFI and post-test POMS-DD scores was statistically significant (r=0.17, p < 0.01)
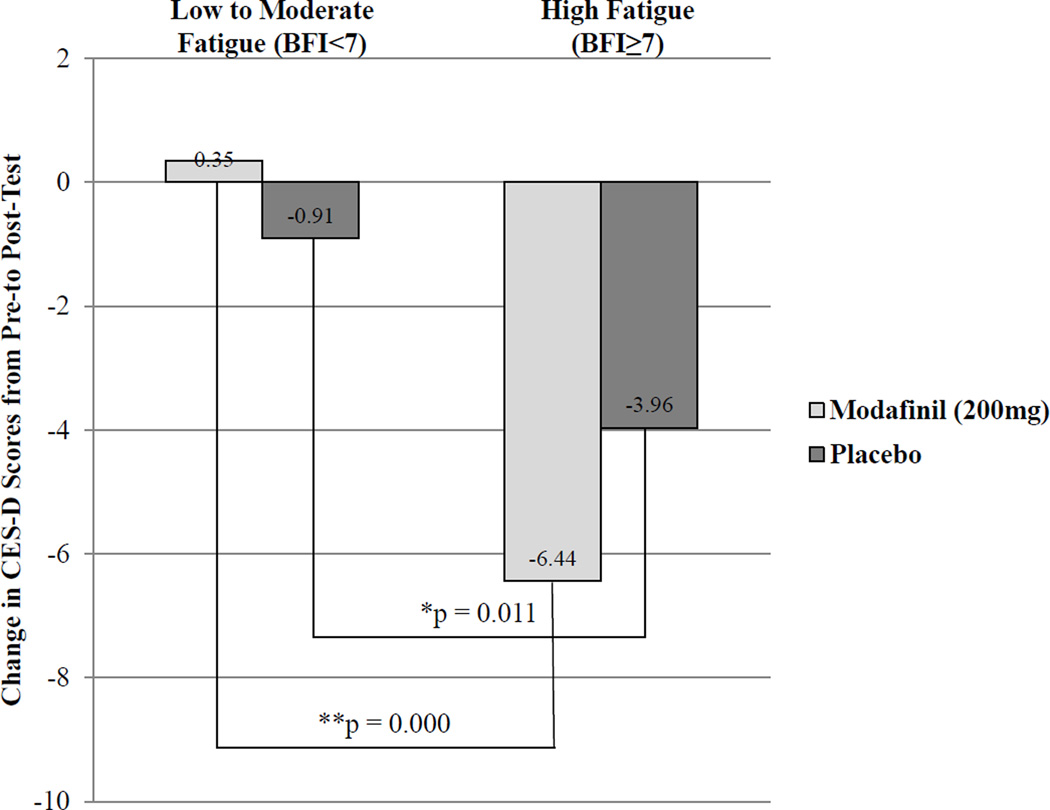
We found no overall effect of modafinil on depression, even in the high fatigue group; however, there was a significant overall association between baseline fatigue and post-test CES-D total scores (p = 0.0001). In addition, the linear model demonstrated a significant moderating effect of modafinil on the relationship between baseline fatigue and CES-D total scores (p = 0.04). For subjects with severe fatigue (BFI≥7), those in active treatment had greater reductions in depression scores than controls (see Figure 1). High fatigue subjects in active treatment had a significant 6.44 point reduction in CES-D scores from pre-test to post-test (t=4.79, df=54, p=0.000); high fatigue subjects in the control arm had a significant 3.96 point reduction in CES-D scores (t=3.56, df=73, p=0.001). Changes in CES-D scores were small (active treatment: 0.35, control: 0.91) and non-significant for the low-to-moderate fatigue group.
Figure 1.
Change in CES-D total scores by baseline level of fatigue.
We also found a significant overall association between baseline fatigue and post-test POMS-DD scores (p=0.0002). However, the moderating effect of modafinil on the relationship between baseline fatigue and POMS-DD total scores was only marginally significant (p=0.07).
Finally, we examined the moderating effect of modafinil on the relationships between baseline levels of fatigue and the four CES-D subscales. Modafinil significantly moderated the relationship between baseline fatigue and Positive Affect subscale scores (p=0.007), but did not significantly moderate the relationship between baseline fatigue and the Somatic, Negative Affect, or Interpersonal subscales (all p > 0.05).
Discussion
To our knowledge, this is one of the first studies to evaluate the moderating effect of a commonly used psychostimulant on the relationship between fatigue and depression in cancer patients. Our results indicate that modafinil differentially impacts depression based on a patient’s level of fatigue: modafinil produced improvements in depression for those individuals who were experiencing high levels of fatigue. This finding is consistent with other studies of modafinil throughout the psycho-oncology literature, which have repeatedly found that modafinil is most efficacious for patients who were experiencing high levels of fatigue at baseline.13, 15
The results of this study improve our understanding of fatigue and depression management in cancer patients who are undergoing chemotherapy. Because fatigue is a symptom of depression, one would expect that improvements in depressive symptoms would be driven by changes in the CES-D somatic symptoms subscale. Unexpectedly, in this sample it was positive affective symptoms, rather than somatic symptoms, that were linked to fatigue and to improvement following modafinil. This not only speaks to the antidepressant effects of psychostimulants, it also indicates a population for whom modafinil might be particularly effective. Rather than considering psychostimulants as a treatment option for all fatigued cancer patients, perhaps we should be attempting to identify a symptom cluster of fatigue and depression.20 These results have significant implications for intervention; in highly fatigued patients, modafinil might also reduce depression by increasing positive affect.
There are several limitations to the present study which should be noted. First, our sample was homogenous. Most subjects were Caucasian, female, and partnered. Thus, the generalizability of our results to other racial and ethnic groups is limited. Second, the eligibility criteria for the original study were extremely specific. All patients were required to be at least mildly fatigued, and it is possible that this eligibility criterion introduced ceiling effects for improvement in depressive symptoms. Third, no data was collected regarding subjects’ prior psychiatric history or receipt of treatment for depression. Third, the present study was a secondary analysis of data collected in a RCT;15 the primary outcome measure of this trial was fatigue, and depression was secondary. Thus, this study was not specifically designed to assess the effects of modafinil on the relationship between fatigue and depressive symptoms. Future research would be needed to confirm these results. Specifically, RCTs of modafinil for depression in cancer patients are needed. In addition, a “pragmatic” trial, where eligibility criteria are widened, would provide more insight into the generalizability of these results.
Despite these limitations, the results of the current study fill a gap in the cancer care literature and indicate the need for mechanism-driven research on the effects of psychostimulants on cancer-related fatigue. This research works towards disentangling a complicated relationship between depression and fatigue and the management of both symptoms. The longitudinal nature of the data allows for a strong statistical examination of the hypothesized moderating relationship.27 In prior research involving modafinil there has been a substantial placebo effect;28 however, the inclusion of the control group in the present research allows us to draw conclusions beyond the potentially significant placebo effect. In addition, this study has a very large sample, which a single institution alone may not have been able to recruit. Finally, there is a dearth of hypothesis-driven, mechanism-focused research on the effects of psychostimulants. The present study provides support for future research specifically designed to assess the effects of modafinil on depressive symptoms in those with severe fatigue and additionally assess psychological and biological mechanisms that may help further understand these relationships and provide targeted interventions.
Acknowledgments
Source of Funding: This research was supported by grants from the National Cancer Institute (CA189961, CA102618, CA168911, CA168886, CA181659, CA185678). Cephalon, Inc. supplied modafinil and placebo tablets for the present study.
Footnotes
Conflicts of Interest: All authors declare that there are no conflicts of interest.
References
- 1.Spiegel D, Giese-Davis J. Depression and cancer: Mechanisms and disease progression. Biol Psychiat. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 2.Morrow GR, Shelke AR, Roscoe JA, et al. Management of cancer-related fatigue. Cancer Invest. 2005;23:229–239. doi: 10.1081/cnv-200055960. [DOI] [PubMed] [Google Scholar]
- 3.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 4.Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 5.Chida Y, Hamer M, Wardle J, et al. Do stress-related psychological factors contribute to cancer incidence and survival: A systematic review and meta-analysis. Nat Rev Clin Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 6.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 7.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong TS, Cohen MZ, Eriksen LR, et al. Symptom clusters in oncology patients and implications for symptom research in people with primary brain tumors. J Nurs Scholarship. 2004;36:197–206. doi: 10.1111/j.1547-5069.2004.04038.x. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–743. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier G, Verhoef MJ, Khatri N, et al. Quality of life in brain tumor patients: The relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57:41–49. doi: 10.1023/a:1015728825642. [DOI] [PubMed] [Google Scholar]
- 11.Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61:378–381. doi: 10.4088/jcp.v61n0510. [DOI] [PubMed] [Google Scholar]
- 12.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 13.Shaw EG, Case D, Bryant D, et al. Phase II double-blind placebo-controlled study of armodafinil for brain radiation induced fatigue. J of Clin Oncol 2013 ASCO Annual Meeting Abstracts. 2013;31:9505. [Google Scholar]
- 14.Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: Results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol. 2014;32:1882–1888. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 15.Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy. Cancer. 2010;116:3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross AJ, Kaser M, Costafreda SG, et al. Modafinil augmentation therapy in unipolar and bipolar depression: A systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2013;74:1101–1107. doi: 10.4088/JCP.13r08560. [DOI] [PubMed] [Google Scholar]
- 17.DeBattista C, Lembke A, Solvason HB, et al. A prospective trial of modafinil as an adjunctive treatment of major depression. J Clin Psychopharmacol. 2004;24:87–90. doi: 10.1097/01.jcp.0000104910.75206.b9. [DOI] [PubMed] [Google Scholar]
- 18.Taneja I, Haman K, Shelton RC, et al. A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol. 2007;27:76–78. doi: 10.1097/jcp.0b013e31802eb7ea. [DOI] [PubMed] [Google Scholar]
- 19.Chang CM, Sato S, Han C. Evidence for the benefits of nonantipsychotic pharmacological augmentation in the treatment of depression. CNS Drugs. 2013;27(Suppl 1):S21–S27. doi: 10.1007/s40263-012-0030-1. [DOI] [PubMed] [Google Scholar]
- 20.Barton DL. New Insights in the Treatment and Effects of Fatigue and Insomnia. Chicago, IL: Paper presented June 2, 2013 at the 2013 American Society of Clinical Oncology Annual Meeting; [Google Scholar]
- 21.Ruddy KJ, Barton D, Loprinzi CL. Laying to rest psychostimulants for cancer-related fatigue? J Clin Oncol. 2014;32:1865–1867. doi: 10.1200/JCO.2014.55.8353. [DOI] [PubMed] [Google Scholar]
- 22.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 25.Norcross JC, Guadagnoli E, Prochaska JO. Factor structure of the Profile of Mood States (POMS): two partial replications. J Clin Psych. 1984;40:1270–1277. doi: 10.1002/1097-4679(198409)40:5<1270::aid-jclp2270400526>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Lang AG, et al. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 27.Kraemer HC, Stice E, Kazdin A, et al. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J of Psychiat. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R. Approved and investigational uses of modafinil. Drugs. 2008;68:1803–1839. doi: 10.2165/00003495-200868130-00003. [DOI] [PubMed] [Google Scholar]