Abstract
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory condition that is mediated by very complex mechanisms controlled by genetic, immune, and environmental factors. More than 74 kinds of genetically engineered mouse strains have been established since 1993 for studying IBD. Although mouse models cannot fully reflect human IBD, they have provided significant contributions for not only understanding the mechanism, but also developing new therapeutic means for IBD. Indeed, 20 kinds of genetically engineered mouse models carry the susceptibility genes identified in human IBD, and the functions of some other IBD susceptibility genes have also been dissected out using mouse models. Cutting-edge technologies such as cell-specific and inducible knockout systems, which were recently employed to mouse IBD models, have further enhanced the ability of investigators to provide important and unexpected rationales for developing new therapeutic strategies for IBD. In this review article, we briefly introduce 74 kinds of genetically engineered mouse models that spontaneously develop intestinal inflammation.
Keywords: epithelial barrier, ER stress, knockout mice, IBD susceptibility gene, immunoregulation, mouse models
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory condition that is classified into two major forms, Crohn’s disease (CD) and ulcerative colitis (UC) [1,2]. Dr. Kirsner first introduced an experimental colitis model in 1957, which was induced in rabbits by sensitization to egg albumin together with rectal instillation of formalin [3], and different types of colitis models such as chemically induced colitis models, cotton-top tamarin model, spontaneous mutation models, and adoptive T cell transfer model have since been established [4,5,6]. Development of genetic engineering technology in animals made an important turning point in IBD research [7]. In 1993, three kinds of knockout (KO) mice deficient for interleukin (IL)-2 [8], IL-10 [9], or T cell receptor α chain (TCRα) [10] were reported as the first murine models of spontaneous colitis. IL-10 and its receptors have since been identified as susceptibility genes for IBD [11], and IL-2 and its receptor also represent IBD susceptibility genes [12,13]. In contrast, TCRα per se is not an IBD susceptibility gene, but several IBD susceptibility genes such as mucin 1 (Muc1) serve as protective factors in the colitis of TCRα KO mice [14]. So far, more than 74 kinds of genetically engineered mouse strains have been reported to develop colitis and/or ileitis spontaneously. In addition, more than 790 kinds of genetically engineered mice have been demonstrated to increase or decrease the susceptibility to chemically induced colitis and/or epithelial barrier dysfunction [7]. The alteration of inflammatory processes in so many kinds of genetically engineered mice suggests that IBD is mediated by much more complicated mechanisms than previously predicted [15]. Indeed, human genome-wide association studies have identified more than 160 susceptibility genes in IBD [12,13,15], and 20 kinds of genetically engineered mouse models carry the susceptibility genes identified in human IBD. Since there are so many kinds of genetically engineered mice associated with IBD, this review focuses on only 74 genetically engineered mouse models that develop intestinal inflammation spontaneously.
Genetically engineered murine IBD models
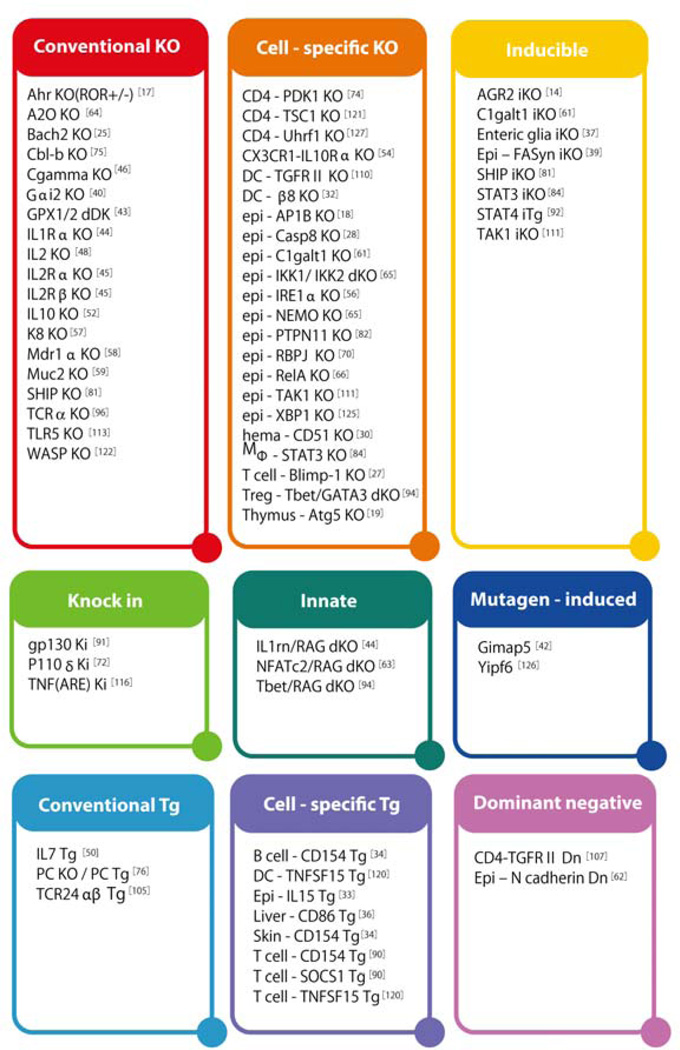
Genetically engineered mice for studying IBD are classified into 9 groups based on the gene construction strategies (Figure 1). Conventional transgenic (Tg) or knockout (KO) mice are genetically engineered to overexpress or lack a gene of interest in all cell types. Dominant negative transgenic (Dn) model is genetically engineered to overexpress a nonfunctional protein that interferes with the normal function of target protein by competing their ligand or receptor. Cell-specific Tg or KO model overexpresses or lacks a gene of interest in a specific cell type, respectively. Inducible knockout (iKO) models allow investigators to delete a gene of interest anytime during the adult life. Knock-in (KI) models carry a mutation in gene of interest for mimicking the polymorphism within the IBD susceptibility gene. Innate models lack a gene of interest in an immune deficient background. In this model, the single KO mice do not develop intestinal inflammation under immune sufficient conditions, but the development can be induced when they are further crossed with immune deficient mice such as RAG2 KO mice lacking both T and B cells. Mutagen-induced models carry a functional gene mutation that is randomly induced by N-ethyl-N-nitrosourea treatment.
Figure 1. Gene targeting strategies.
Genetically engineered mouse models of IBD are here classified into 9 groups based on the strategy used for the gene targeting, including conventional transgenic (TG), conventional knockout (KO), inducible KO (iKO), cell-specific TG, cell-specific KO, dominant negative (Dn); knock in (KI); Mutagen-induced; and innate models. For cell-specific genetic engineering, epithelial cells (epi), T cells (T), B cells (B), CD4+ T cells (CD4), dendritic cells (DC), macrophages (Mφ), Treg cells (Foxp3) are targeted.
In addition, mouse IBD models can be further classified into 4 groups depending on the organ that is affected by inflammation (Figure 2). Colitis models develop inflammation restricted to the colon, ileitis models develop inflammation restricted to small intestine, enterocolitis models develop inflammation in both colon and small intestine, and systemic models develop inflammation in multiple organs including the intestine.
Figure 2. Classification of IBD models depending on the organ affected by inflammation.
74 genetically engineered mouse models of IBD are further classified into four groups; colitis models, ileitis models, enterocolitis models developing colitis and ileitis, and systemic model developing inflammation in multiple organs including intestine. Mice carrying human IBD susceptibility genes are indicated by Italic Bold.
AGR2
Anterior gradient 2 (AGR2) is a potential IBD risk gene [16], encoding an Endoplasmic Reticulum (ER)-resident disulphide isomerase involved in protein folding. AGR2 KO mice spontaneously developed intestinal inflammation that was most severe in the terminal ileum, and to a lesser extent in the colon [16]. By utilizing inducible AGR2 KO mice, defects in Paneth cells were shown as the primary causal factor of this ileitis.
Ahr
Aryl hydrocarbon receptor (Ahr) plays an important role for the function of group 3 innate lymphoid cells (ILC3) that produce IL-22 to preserve epithelial barrier integrity. Ahr KO mice did not develop colitis, although spontaneous development of colitis became recognizable at 12–20 wk of age when haplodeficiency of RORγt (a transcription factor for Th17 differentiation) was genetically introduced in these mice [17]; their colitis was characterized by fewer ILC3 cells and markedly more IL-17+ IFNγ+ CD4+ T cells.
AP1B
Epithelial cell polarity is established by an epithelium-specific polarized sorting factor adaptor protein AP1B. Mice with epithelial cell-specific deletion of ab1b (AQ; Please check mouse gene abbreviation) spontaneously developed a Th17-dominant colitis at 8 wk of age [18]; the barrier function of epithelial cells was intact, but production of antibacterial peptides such as cathelicidin and defensin was significantly reduced. This colitis was treatable by antibiotics.
Atg5
Autophagy exerts diverse physiological functions ranging from microbial infections to antigen presentation [19]. The autophagy molecule Atg16L1 is produced by a major susceptibility gene of CD [12,13,20–22]. Atg16L1 KO mice were highly susceptible to a chemically-induced acute colitis, although they failed to develop colitis spontaneously [23]. In contrast, implantation of Atg5 KO thymi into nu/nu mice lacking T cells induced the development of multi-organ inflammation in the colon, liver, lung, and uterus [24].
Bach2
Bach2, which represents a susceptibility gene of CD, serves as a transcriptional repressor of Blimp-1 (an inducer of plasma cell differentiation) and of myeloid program to induce B cell development [25]. Bach2 KO mice developed a progressive wasting disease from 3 mo of age due to severe pneumonia. Some of these mice developed a mild and incompletely penetrating inflammation in the small intestine and stomach [26] that was associated with lack of efficient formation of regulatory T cells (Treg).
Blimp-1
B lymphocyte induced maturation protein-1 (Blimp-1) encoded by Prdm1 was initially identified as a B cell-specific transcription factor for plasma cell differentiation. However, subsequent studies have identified the expression of Blimp-1 in different cell types such as memory T cells. Prdm1 has been identified as a susceptibility gene for both CD and UC [12,13]. 20% of T cell-specific Blimp-1 KO mice developed a Th1-mediated colitis at 4 wk of age, and the penetration of colitis reached to 83% by 21 wk of age [27]. Treg cells were able to expand normally, but their regulatory function was impaired. In addition, T cells were more prone to differentiate into IL-17+ IFNγ+ CD4+ T cell subset that is seen in the inflamed intestine of CD patients.
Caspase-8 (Caspase8, cFLIP)
Caspase-8 is a cysteine protease involved in regulating apoptosis. More than 80% of mice with epithelial cell-specific deletion of caspase-8 developed ileitis but not colitis [28]. This ileitis was characterized by a marked destruction of ileal architecture and by the induction of apoptosis-independent type of programmed necrosis so called necroptosis.
Cellular FADD-like interleukin 1β-converting enzyme inhibitory protein (cFLIP) is an antiapoptotic protein through inhibition of caspase-8. Mice with epithelial cell-specific deletion of cflip died within one day after birth with massive intestinal bleeding due to abnormal epithelial homeostasis characterized by enhanced apoptosis that starts in the uterus (AQ: do you mean starts before birth?) [29].
CD51/integrin β8
CD51 is the most unselective α subunit, associating with β1, β3, β5, β6, and β8 subunits and participating in many cellular processes such as cell adhesion and cell survival. CD51 KO mice died before or shortly after birth from defects in brain vascular development. In contrast, mice with haematopoietic cell-specific deletion of cd51 developed inflammation in multiple organs around 14 wk of age, including colon, caecum, peritoneum, liver, lungs, and nasal cavity [30]. This inflammation was characterized by aberrant Th1- and Th2-type responses due to the inability of phagocytic cells to remove apoptotic cells that serve as a source of self-antigens [31].
Integrin β8 is associated with integrin αV subunit to form αVβ8 that is capable of activating the TGFβ pathway. Mice with specific deletion of β8 in both CD4+ T cells and DCs developed a progressive wasting disease from 4–5 mo of age, and all mice then developed colitis characterized by enhancement of both Th1 and Th2 responses by 10 mo of age [32].
CD86
CD86 is a costimulatory molecule expressed by antigen presenting cells (APCs) to interact with CD28 for T cell activation and with CTLA4 for immune regulation. Mice with liver-specific overexpression of soluble CD86 Ig Fc fusion protein developed transmural colitis by 8–10 wk of age [33]. This colitis was mediated by IFN-γ-producing T cells activated via CD28.
CD154
CD154 is expressed on T cells to interact with CD40 on APCs to provide bidirectional co-stimulatory signals between T cells and APCs for activation of both humoral and cellular immune responses. Mice with T cell-specific overexpression of Cd154 developed inflammation in multiple organs, including colon, stomach, kidney and lung at 3–6 weeks of age [34]. The colitis was characterized by lethal, transmural, granulomatous inflammation. Although B cells do not express CD154, except in some patients with systemic lupus erythematosus (SLE), mice with ectopic overexpression of Cd154 on B cells developed colitis, ileitis, and glomerulonephritis at 8–15 weeks of age [35]. In addition, mice with skin epidermis-specific overexpression of Cd154 developed dermatitis and systemic autoimmune diseases [36].
Enteric glia
Mice that were genetically engineered to express herpes simplex virus thymidine kinase under the control of glial fibrillary acidic protein (GFAP), lost the GFAP-positive glial cells of the small intestine after administration of Ganciclovir, an antiviral medication. This inducible ablation of enteric glial cells resulted in a fulminating ileitis with necrosis and haemorrhage through disrupting the intestinal epithelial barrier [37,38]. This necrotic ileitis was not improved by antibiotic treatment [37].
FASyn
Fatty acid synthase (FASyn) is an insulin-regulated enzyme that synthesizes saturated fatty acids for cell membranes, energy stores, and signalling molecules. Tamoxifen-induced epithelial cell-specific deletion of FASyn during adult life induced body weight loss from 5 d after tamoxifen treatment [39]. Spontaneous development of inflammation particularly in the caecum and to a lesser extent, colon was then observed. This colitis was treatable by antibiotics and characterized by a disruption of intestinal barrier due to impaired secretion of Muc2.
Gαi2
Gαi2 belongs to a family of GTP-binding protein involved in a wide variety of transmembrane signalling systems. All Gαi2 KO mice on a 129/sv background developed a lethal, pan-colitis at 16–20 wk of age and 30% developed adenocarcinoma [40]. In contrast, Gαi2 KO mice on a C57BL/6 background were relatively resistant to colitis development. Reduced production of IL-10 by DCs was seen in 129/sv, but not C57BL/6, Gαi2 KO mice [41].
Gimap5
GTPase of immunity-associated protein (Gimap), which shares a GTP-binding AIG homology domain, is expressed predominantly by lymphocytes to regulate their survival. Gimap5 is a susceptibility gene for SLE. Through screening of N-ethyl-N-nitrosourea-mutagenized mice, spontaneous development of colitis and hepatitis was identified in mice carrying a mutation in Gimap5 [42]. The colitis was seen from 4 wk of age, and severe colitis was established by 10 wk of age. This colitis was treatable by antibiotics and characterized by lymphopaenia with a functional defect of B cells.
GPX
Glutathione peroxidase (GPX) is a selenium-dependent hydroperoxidase-reducing enzyme to reduce H2O2 and fatty acid hydroperoxides. Mice deficient for both, but not either, Gpx1 and Gpx2 spontaneously developed colitis and ileitis with perianal ulceration by 24 d of age. Approximately 40% of these mice died before 36 d after birth [43]. Interestingly, a cholesterol diet exacerbated this colitis through reduction of ER stress responses.
IL-1Rn
IL-1 receptor antagonist (IL-1RA), encoded by the I1rn gene, serves as an endogenous IL-1 inhibitor. An association of Il1rn polymorphism with CD in a population in India has been reported. Mice with deletion of Il1rn on an immune deficient background (RAG2 KO mice lacking T and B cells) spontaneously developed colitis with high mortality [44]; 80% of mice developed rectal prolapse at 12 wk of age.
IL-2 pathway (IL-2, IL-2Rα, IL-2Rβ, and Common γ)
IL-2 binds to a heterotrimer receptor composed of IL-2Ra, IL-2Rβ, and Common γ chains to induce activation-induced cell death and elicit the function of Treg cells. Importantly, Il2ra encoding IL-2Ra is a CD susceptibility gene and the il2 gene is located within a susceptibility locus for UC [12,13]. IL-2 KO mice spontaneously developed a systemic autoimmune disease characterized by colitis, gastritis, hepatitis, pneumonia, pancreatitis, nephritis, and haemolytic anaemia [8]. In addition, IL-2Ra KO, IL-2Rβ KO, and Common γ chain KO mice all developed similar autoimmune phenotypes [45–47]. Furthermore, deficiency of Janus family tyrosine kinase (JAK) 3, which is an essential transducer of Common γ chain, caused spontaneous development of colitis in mice [46]. A unique feature of the IL-2 KO model was the dependence of colitis on an autoimmune mechanism, as indicated by the spontaneous development of colitis in germ-free condition [48]. The CD4+ TCRαβ+ T cells but not CD8+ TCRαβ+ T cells, TCRγδ+ T cells or B cells are required for the development of this colitis [49].
IL-7
IL-7 plays a major role in T cell homeostasis at various developmental stages, and its receptor encoded by Il7r gene has been identified as a candidate gene associated with UC [12,13]. IL-7Tg mice spontaneously developed colitis between 4 and 12 wk of age [50]. Systemic, but not intestinal, IL-7 contributed to the perpetuation of this colitis by generating long-lived colitogenic memory CD4+ T cells that reside in the bone marrow [51].
IL-10
The gene for IL-10, a regulatory cytokine, is a susceptibility gene not only for adult IBD but also for early onset of IBD in children [11–13]. IL-10 KO mice spontaneously developed a colitis sharing some features with CD after 3 mo of age [9]. A more severe colitis was observed when they were backcrossed to C3H or Balb/c backgrounds as compared to C57BL/6. Development of colitis was inhibited when IL-10 KO mice were maintained under germ-free conditions [52], and many studies focusing on enteric bacteria and probiotics have used this model [53].
CX3CR1+ macrophages have been proposed to represent a major source of IL-10 in the inflamed colon. However, CX3CR1-specific IL-10 KO mice failed to spontaneously develop colitis. In contrast, CX3CR1-specific IL-10Ra KO mice developed inflammation most prominently in the caecum and distal colon [54], suggesting that macrophages are a major responder to, rather than producer of, IL-10 in the inflamed colon.
IL-15
IL-2 and IL-15 bind to heterotrimeric receptors that have two receptor subunits in common, but these two cytokines have distinct roles in adaptive immune responses. Mice overexpressing Il15 in the thymus and intestinal epithelial cells under a control of T3b promoter spontaneously developed inflammation with an expansion of CD8αβ+ Natural Killer (NK) T cells in proximal small intestine [55]. This inflammation was recognized from 3 mo of age with 100% penetration at 6 mo of age.
IRE1α
Inositol Requiring Enzyme (IRE) 1α is an ER transmembrane protein that serves as a major sensor of ER stress. Mice with epithelial specific deletion of Ire1α spontaneously developed a colitis with goblet cell loss and epithelial barrier dysfunction [56]. Rectal bleeding was observed from 16–24 wk of age, and female mice were more susceptible.
K8
Keratin 8 (K8) serves as a structural protein for single layered epithelial cells. K8 KO mice on a C57BL/129 background suffered embryonic lethality, but K8 KO mice on an FVB/N background survived and developed colorectal, but not small intestinal, hyperplasia with rectal prolapse at 9 wk of age [57].
Mdr1a
Multiple drug resistance (Mdr) 1 protein pumps small amphiphilic and hydrophobic molecules across membranes for detoxification. MDR1 is a susceptibility gene for the late onset of UC in Japanese population [15]. 20–25 % of Mdr1a KO mice on an FVB background at one year of age developed a colitis that was improved by antibiotic treatment [58].
Mucus pathway (Muc2, C1galt1)
Muc2 is a component of the mucus layer, which serves as a lubricant and a physiological barrier between luminal contents and mucosal surfaces. Muc2 KO mice were initially established as a cancer model with spontaneous adenocarcinoma in the colon at 6 mo of age [59]. A subsequent study identified that colitis, with mild inflammatory cell infiltration, was present primarily in the distal colon of Muc2 KO mice from 5 wk of age [60].
Intestinal mucus carries large numbers of O-glycan ???chains, moieties? AQ: please clarify), which account for approximately 80% of the mucus molecule’s mass. O-glycan synthesis in epithelial cells is altered in approximately 30% of UC patients [61]. A glycosylation enzyme termed core 1β1,3-galactosyltransferase (C1galt1) is required for the initiation of O-glycan synthesis on mucus molecules. All mice with epithelial cell-specific deletion of c1galt1 spontaneously developed inflammation in the distal colon at 12 weeks of age [61]. The colitis still developed in the absence of T and B cells, and antibiotics treatment improved this colitis. In addition, inducible deletion of c1galt1 in epithelial cells during adult life rapidly elicited the development of colitis within 10 d after initiation [61].
N-cadherin
N-cadherin mediates homophilic adhesive interactions between epithelial cells. Chimeric mice that were generated from ES cells to carrying a dominant negative mutation of N-cadherin only in small intestinal epithelial cells developed transmural inflammation in the jejunum by 3 mo of age [62].
NFATc2/RAG
The nuclear factor of activated T cells, cytoplasmic 2 (NFATc 2) plays redundant functions in gene regulation for T cell differentiation. Although NFATc2 KO mice did not develop colitis, the offspring of crosses with RAG2 KO mice (lacking T and B cells) developed a spontaneous colitis around 15 wk of age [63]. Polyclonally-activated regulatory B cells participated in the suppression of this colitis. (AQ: please check that your original meaning has been retained)
NFκB1 pathway (NEMO, IKK1, IKK2, RelA, A20)
The nuclear factor (NF) κB1 pathway is activated via a trimer composed of IκB kinase (IKK)1, IKK2 and NFκB essential modulator (NEMO, also known as IKKγ). Although inhibition of NFκB1 in adaptive immune cells has been shown to improve colitis [64], mice with specific deletion of nemo in epithelial cells spontaneously developed colitis with epithelial apoptosis at 6 wk of age [65]. Absence of both, but not either, IKK1 and IKK2 in epithelial cells also led to spontaneous development of colitis [65]. In addition, 10–15% of epithelial cell-specific RelA (also known as NFκBp65) KO mice exhibited diarrhoea within 3 d after birth and died within 25 d [66]. Crypt structures in the small intestine were completely lost in these mice.
A20, also known as TNFα inducible protein 3 (TNFIP3), is an ubiquitin-editing enzyme involved in the termination of NFκB1 activation. A20 KO mice developed inflammation in multiple organs including the liver, kidney, intestine, and joints at 3–6 wk of age [67]. The colitis still developed in the absence of T and B cells. Myeloid lineage cells were primarily responsible for the development of this colitis [68]. Although epithelial cell-specific A20 KO mice failed to spontaneously develop colitis, both epithelial cell- and myeloid cell-specific A20 KO mice developed multi-organ inflammation [69].
Notch (RBP-J, Pofut1)
Notch receptors undergo proteolytic cleavage to release the Notch intracellular domain (NICD). These translocate into the nucleus to form a transcriptional activator complex with recombination signal binding protein for Igκ J region (RBP-J), and intestinal epithelial cells, this complex activates Notch target genes. A Th17-dominant colitis developed in 50% of mice with epithelial cell-specific deletion of rbpj. Interestingly, this colitis was characterized by an increased production of defensin and by goblet cell hyperplasia at 20–26 wk of age [70]. This colitis was improved by antibiotics treatment.
Protein O-fucosyltransferase 1 (Pofut1) is an enzyme that is required for Notch ligand binding. Mice with epithelial cell-specific deletion of Pofut1 exhibited goblet cell hyperplasia with enhanced expression of Muc2. Intestinal inflammation was evident from 4 wk of age, and all mice developed enterocolitis with transmural inflammation by 36 wk of age [71].
PI3K pathway (p110δ, PDK1, Cbl-b)
Phosphatidyl inositol 3-kinase (PI3K) is required for second messenger signalling from antigen receptors. A PI3K p100 subunit, p110δ is expressed predominantly in leukocytes. Knock-in (KI) mice carrying a point mutation in the P110δ gene locus at position 910 (D>A) spontaneously developed a focal inflammation restricted to the rectum and caecum [72]. The colitis of p110δ KI mice is mediated by impaired activation of Foxp3+ Treg [73].
Phosphoinositide-dependent kinase 1 (PDK1) is a key downstream effector of PI3K pathway. Mice with CD4-specific deletion of Pdk1 spontaneously developed colitis at 8 wk of age [74]. Interestingly, this colitis was characterized by an expansion of IL-17A expressing TCRγδ T cells in the intraepithelial compartment.
Casitas B-lineage lymphoma b (Cbl-b) proteins function as E3 ubiquitin ligases and molecular adaptors to promote ubiquitin conjugation to the p85 regulatory subunit of PI3K. Cbl-b acts as a negative regulator of T cell activation. Cbl-b KO mice spontaneously developed multi-organ inflammation (intestine, salivary glands, pancreas, liver, lung, heart, bladder, and connective tissues) with massive infiltration of activated T cells and B cells from 3 mo of age [75].
Protein C
Protein C (PC) pathway represents a key coagulation system. Since PC KO mice died soon after birth, PC KO mice were further crossed with PC transgenic mice (PCKO/PCtg) to allow them to express low levels of PC. Interestingly, the PCKO/PCtg mice spontaneously developed mild colitis, recognized by histological and endoscopic examinations at 6–8 wk of age [76].
PP4
Protein phosphatase 4 (PP4), encoded by Ppp4c, is a serine/threonine phosphatase. Approximately 60% of mice with CD4-specific deletion of ppp4c developed colitis with rectal prolapse by 15 wk of age [77]. Development of functional Tregs was impaired in this model.
Runx3
Runx3, which serves as a context-dependent transcription factor involved in thymopoiesis and dendritic cell maturation, has been reported as a susceptibility gene for UC in Dutch and Chinese populations [78]. All Runx3 KO mice spontaneously developed colitis, with an enhancement of both Th1 and Th2 responses by 4 weeks of age, and 20% of these mice featured extension of inflammation to the small intestine [79]. This intestinal inflammation was associated with impaired activation of Treg and CD8+ T cells [80].
SHIP
The Src homology 2 (SH2)-containing inositol-5 phosphatase (SHIP), which is activated in response to various growth factors as well as TCR and BCR ligations, is located within an IBD association locus at 2q37. Over 90% of SHIP KO mice developed transmural, segmental ileitis with granulomas (28%) at 6–8 wk of age [81]. Interestingly, inducible deletion of SHIP during adult life also led to the rapid development of ileitis within 5 wk after gene deletion. Granulocyte-monocyte lineage cells, but not T cells or NK cells, are primarily responsible for this ileitis.
SHP2 (PTPN11)
Src homology 2-containing protein tyrosine phosphatase 2 (SHP2), encoded by Ptpn11, is a ubiquitously expressed cytoplasmic protein. Ptpn11 was a susceptibility gene for UC in a Japanese population [82]. Mice with epithelial cell-specific deletion of Ptpn11 developed colitis at 3 wk of age, and some of them died by 10 wk of age [83].
STAT3 pathway (STAT3, SOCS3, gp130)
The signal transducer and activator of transcription (STAT) 3, which serves as a master transcriptional factor to control a broad spectrum of adaptive and innate immune responses such as Th17 differentiation and epithelial regeneration, represents a susceptibility gene for CD and UC [12,13]. Although activation of STAT3 in CD4+ T cells induced the development of colitis [84,85], macrophage/neutrophil-specific Stat3 KO mice spontaneously developed colitis at 20 wk of age [86]. Development of colitis in macrophage/neutrophil-specific Stat3 KO mice was abolished in the absence of T and B cells [87]. In contrast, specific deletion of Stat3 in epithelial cells failed to produce spontaneous colitis [88]. These findings suggest a pathogenic role for STAT-3-medicated activation of adaptive immunity and a protective role of STAT3-mediated activation of innate immunity in colitis. Interestingly, inducible deletion of Stat3 during adult life led to rapid development of colitis [89]. Since the deletion occurred in all cell types, the data from inducible Stat3 KO mice tend to suggest that the overall function of STAT3 in colitis depends more heavily on innate, rather than adaptive, immune cells.
Activation of STAT3 is regulated by suppressor of cytokine signaling (SOCS) protein. Approximately 40% of mice with T cell-specific overexpression of Socs1 developed colitis after 15 wk of age when they were kept in conventional, but not SPF, conditions [90].
IL-6 interacts with gp130 receptor to activate STAT3. Mice carrying a truncating mutation within Gp130 spontaneously developed ulcerations at the gastric pylorus and anorectal region at 4 mo of age when maintained under SPF conditions [91]. The distal ulceration region extended to the caecum when they were kept under conventional conditions. Arthritis was also recognized in some of these mice.
STAT4
STAT4, a transcription factor that promotes Th1 development, is a susceptibility gene for UC in Caucasians [92]. Mice that were genetically engineered to overexpress Stat4 when the cytomegalovirus (CMV) promoter is activated, developed transmural colitis within 7–14 d after immunization with DNP-KLH/CFA to activate the CMV promoter [93]; this colitis was mediated by CD4+ Th1 T cells.
T-bet/GATA3
Recent studies found the expression of T-bet (a transcription factor for Th1) and GATA3 (a transcription factor for Th2) in Treg. To test the role of these transcriptional factors in Treg function, mice with Treg-specific deletion (under control of Foxp3 promoter) of Tbet and/or Gata3 were generated. Interestingly, these mice started to display splenomegaly and lymphadenopathy from 6–8 wk of age and then developed inflammation in multiple organs, including colon, small intestine, liver, lung, and kidney [94].
T-bet/RAG
T-bet is a T-box transcription factor involved in the differentiation of Th1 T cells. T-bet KO mice did not develop colitis, although colitis did develop when they were crossed with RAG2 KO mice lacking T and B cells. A continuous colitis resembling that of UC was observed from 4 wk of age [95]. Interestingly, the colitis was transmissible to wild type mice both vertically (maternally from mother) and horizontally (between neighbours).
TCRα
The T cell receptor (TCR), which is composed of TCRα and TCRβ chains, is required for the recognition of antigens by T cells. Approximately 60% of TCRα KO mice spontaneously developed a Th2-mediated colitis (sharing some features with human UC) by 6 mo of age [10]. Mice with Balb/c and C3H/Hej backgrounds were relatively resistant to this colitis as compared with C57BL/6. As for the majority of IBD models, TCRα KO mice failed to develop colitis in a germ-free facility [96,97]. In contrast to other IBD models, the development of colitis in TCRα KO mice was abolished when exposed to wide spectrum of microorganisms in conventional facilities [98,99]. In addition, IL-10-producing regulatory B cells (termed Breg), which contribute to the suppression of colitis, were first identified in this model [100,101]. The existence of Breg in a subset of UC patients is supported by the exacerbation/induction of UC after B cell-depletion therapy [102–104].
TCR24αβ
24αβ transgenic mice carry TCR from a CD1d-reactive NKT cell line with expression of TCRVα3.2 and Vβ9. When 24αβ Tg mice were crossed with CD1d Tg mice to activate the NKT cells, 70% of them developed colitis at 6 mo of age [105].
TGFβ pathway (TGF-β1, TGFRII, TAK1, SMAD4)
Transforming growth factor (TGF)-β1 contributes to immune regulation and Treg differentiation. TGFβ1 KO mice developed necrotic inflammation in multiple organs and majority of them died before 3–4 weeks of age [106]. In contrast, CD4+ T cell-specific dominant negative TGFβ receptor type II transgenic mouse survived and spontaneously developed inflammation at 3–4 mo of age, with increased expression of both Th1 and Th2 cytokines in multiple organs, including the colon, liver, stomach, duodenum, pancreas, and kidney [107]. A protective role of B cells was demonstrated in this model [108]. Unlike CD4+ T cell-specific suppression of TGFβ signal, epithelial cell-specific suppression of TGFβ signalling failed to induce spontaneous development of colitis [109].
Mice with a DC-specific deletion of TGF-β receptor type II developed multi-organ inflammation from 4 wk of age, including hepatitis, pancreatitis, gastritis, and colitis. Only 30–40% of mice survived at 12 wk of age. Interestingly, abnormal expansion of CD25− Foxp3+ CD4+ T cells was seen in these mice [110].
TGFβ activated kinase 1 (TAK1) participates in the activation of innate immune pathways. Severe epithelial damage in the small intestine was seen in epithelial cell-specific TAK1 KO mice by 3 days after birth [111]. In addition, inducible epithelial specific deletion of Tak1 gene promptly led to intestinal epithelial damage within 3 d after deletion of TAK1.
Mothers against decapentaplegic homolog 4 (SMAD4) is a central mediator of TGFβ signalling. T cell-specific SMAD4 KO mice and CD4+ T cell-specific SMAD4 KO mice both developed inflammation throughout the gastrointestinal tract (gastritis, ileitis, colitis) at 3 mo of age [112]. Rectal prolapse (25%) and development of cancer throughout the gastrointestinal tract was also observed. In contrast, epithelial cell-specific SMAD4 KO mice did not develop inflammation or cancer.
TLR5
TLRs play important roles in innate immunity through recognizing different microbial products. TLR5 serves as a receptor for bacterial flagellin that has been shown to represent a dominant pathogenic antigen in CD [113]. In contrast, a polymorphism in tlr5 was proposed to be protective for CD [114]. Approximately 30% of TLR5 KO mice developed rectal prolapse and colitis restricted primarily to the cecum and proximal colon at 8–12 wk of age [115].
TNF
Neutralization of TNF-α activity has been widely used for the treatment of IBD, and CD-specific susceptibility variants have been identified within TNF in Japanese population [15]. The involvement of TNF-α in the pathogenesis of CD has been well supported by spontaneous development of ileitis in mice that exhibit an enhanced TNF-α activity due to deletion of adenosine-uracil multimers (AU-rich elements, ARE) responsible for the Tnf mRNA destabilization [116]. The inflammation in TNF(ARE) mice was localized primarily to the terminal ileum and occasionally to the proximal colon. This ileitis was mediated by CD8+ T cells, IL-12p40, TNF-α, and IFN-γ [117,118].
TNFSF14
TNF superfamily 14 (TNFSF14), which interacts with two receptors, herpes virus entry mediator and lymphotixin β receptor to control the response of T cells, is encoded by TNFSF14, another susceptibility gene for UC [12,13]. Mice with T cell-specific overexpression of human TNFSF14 developed hepatitis and an ileitis characterized by villus atrophy [119].
TNFSF15
TNFSF15, which binds to a death domain receptor 3 (TNFRSF25), is encoded by TNFSF15, a gene associated with CD and UC [12,13]. Mice with T cell-specific overexpression of Tnfsf15 spontaneously developed transmural inflammation in the small, but not large, intestine with 100% penetration after 6 weeks of age [120]. CD4+ T cells and IL-13 were primarily responsible for the development of this ileitis. In addition, mice with CD11c+ cell-specific overexpression of tnfsf15 developed ileitis, but the severity was milder as compared to T cell-specific TNFSF15 Tg mice.
TSC1
Tuberous sclerosis-1 (TSC1) is an upstream negative regulator of mammalian target of rapamycin (mTOR). Mice with CD4-specific deletion of Tsc1 spontaneously developed inflammation characterized by large lymphoid aggregates in the colon and liver around 6 mo of age [121]. The absence of Tsc1 reduced the expression of Foxp3 and induced the expression of IL-17 in Treg cells.
WASP
Wiskott-Aldrich syndrome protein (WASP) is an organizer involved in the remodelling of actin cytoskeleton to induce cell movement, cell signalling, and cell division. All WASP KO mice developed a UC-like colitis spontaneously at 6 mo of age [122]. The colitis was mediated by IL-4-expressing CD4+ T cells [123] and by impaired development of functional Treg [124].
XBP1
X-box-binding protein (XBP1) is required for unfolded protein response induced by ER stress. An association of XBP1 variants with CD and UC has been identified [125]. Although XBP1 plays an important role in the terminal differentiation of B cells into plasma cells, mice with specific deletion of Xbp1 in intestinal epithelial cells spontaneously developed ileitis with absence of Paneth cells that secrete defensins [125].
Yipf6
Through an environmentally sensitized genetic screen of approximately 6,000 mice that were treated with N-ethyl-N-nitrosourea to randomly induce genetic mutations, Yipf6 mutant mice were found to spontaneously develop ileitis and colitis at 16 mo of age [126]. Yipf6 is a Yip gene family member that regulates Rab-protein-mediated ER-to-Golgi membrane transport.
Uhrf1
Epigenetic regulation plays an important role in controlling the expression of many genes. Uhrf1 (ubiquitin-like, with pleckstrin-homology and RING-finger domains 1), which is encoded by Np95 in mice and ICBP90 in humans, serves as an epigenetic regulator. Mice with CD4-specific deletion of Np95 spontaneously developed colitis by 10 wk of age, and almost all mice succumbed to death before 24 wk of age [127]. The colitis was characterized by a defect in proliferation of colonic Treg.
Conclusion
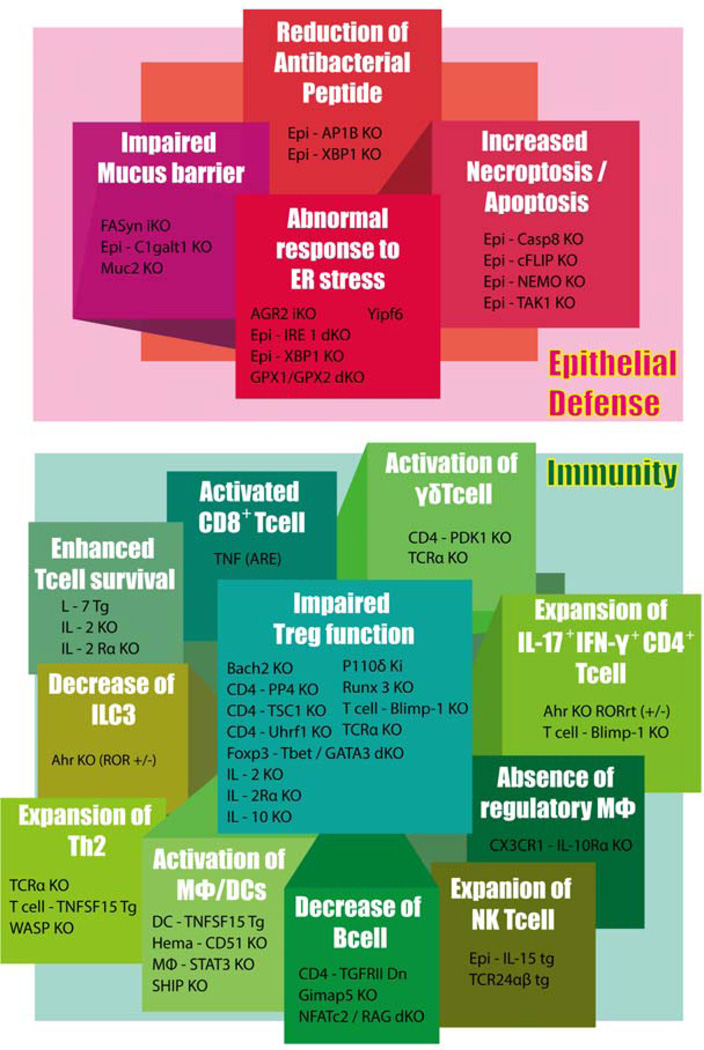
Spontaneous development of intestinal inflammation in more than 74 kinds of genetically engineered mouse strains, as well as presence of more than 160 IBD susceptibility genes in humans certainly indicate a much more complex mechanism of IBD than previously predicted. This raises a possibility that IBD includes more diverse disease conditions than the currently recognised two forms, CD and UC. In addition, data from these mouse models highlight the critical involvement of dysregulated immune responses and impaired epithelial defence in the pathogenesis of IBD (Figure 3).
Figure 3. Major pathways involved in the pathogenesis of IBD.
Genetically engineered mouse IBD models highlight immune response and epithelial defence for the pathogenesis of IBD. The pathways, which are primarily responsible for the development of intestinal inflammation in genetically engineered models, are indicated.
Acknowledgements
We greatly thank Arianna DeGruttola for her excellent editorial help. This work was supported by NIH RO1DK091247, DK080070, AI081807 and JSPS KAKENHI 26893315, 15H04813.
Footnotes
All authors declare no conflict of interest.
Author contributions:
AM and EM interpreted the data and organized the paper. TT, HH, and TO performed literature search and generated figures. All authors were involved in writing the paper and had final approval of the submitted and published versions
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50:495–507. doi: 10.1007/s00535-015-1064-1. [DOI] [PubMed] [Google Scholar]
- 3.Kirsner JB, Elchelepp J. The production of an experimental ulcerative colitis in rabbits. Tr A Am Physicians. 1957;70:102–119. [PubMed] [Google Scholar]
- 4.Podolsky DK, Lobb R, King N, Benjamin CD, et al. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993;92:372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22 low subset. J Exp Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 7.Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci. 2012;105:263–320. doi: 10.1016/B978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
- 8.Sadlack B, Merz H, Schorle H, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 9.Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 10.Mombaerts P, Mizoguchi E, Grusby MJ, et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 11.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lees CW, Barrett JC, Parkes M. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 13.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizoguchi A, Mizoguchi E. Animal models of IBD: linkage to human disease. Curr Opin Pharmacol. 2010;10:578–587. doi: 10.1016/j.coph.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arimura Y, Isshiki H, Onodera K, et al. Characteristics of Japanese inflammatory bowel disease susceptibility loci. J Gastroenterol. 2014;49:1217–1230. doi: 10.1007/s00535-013-0866-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao F, Edwards R, Dizon D, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu J, Guo X, Chen ZM, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi D, Hase K, Kimura S, et al. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology. 2011;141:621–632. doi: 10.1053/j.gastro.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 19.Stappenbeck TS, Rioux JD, Mizoguchi A, et al. Crohn disease: A current perspective on genetics, autophagy and immunity. Autophagy. 2010;7:335–374. doi: 10.4161/auto.7.4.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitoh T, Fujita N, Jang MH, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 24.Nedjic J, Aichinger M, Emmerich J, et al. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 25.Itoh-Nakadai A, Hikota R, Muto A, et al. The transcription repressors Bach2 and Bach1 promote B cell development by repressing the myeloid program. Nat Immunol. 2014;15:1171–1180. doi: 10.1038/ni.3024. [DOI] [PubMed] [Google Scholar]
- 26.Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 2013;498:506–510. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins GA, Cimmino L, Shapiro-Shelef M, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 28.Günther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piao X, Komazawa-Sakon S, Nishina T, et al. c-FLIP maintains tissue homeostasis by preventing apoptosis and programmed necrosis. Sci Signal. 2012;5:ra93. doi: 10.1126/scisignal.2003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacy-Hulbert A, Smith AM, Tissire H, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizoguchi A, Mizoguchi E, Smith RN, et al. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis MA, Reizis B, Melton AC, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim G, Turovskaya O, Levin M, et al. Spontaneous colitis occurrence in transgenic mice with altered B7-mediated costimulation. J Immunol. 2008;181:5278–5288. doi: 10.4049/jimmunol.181.8.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clegg CH, Rulffes JT, Haugen HS, et al. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111–1122. doi: 10.1093/intimm/9.8.1111. [DOI] [PubMed] [Google Scholar]
- 35.Higuchi T, Aiba Y, Nomura T, et al. Ectopic expression of CD40 ligand on B cells induces lupus-like autoimmune disease. J Immunol. 2002;168:9–12. doi: 10.4049/jimmunol.168.1.9. [DOI] [PubMed] [Google Scholar]
- 36.Mehling A, Loser K, Varga G, et al. Overexpression of CD40 ligand in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J Exp Med. 2001;194:615–628. doi: 10.1084/jem.194.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bush TG, Savidge TC, Freeman TC, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 38.Savidge TC, Newman P, Pothoulakis C, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 39.Wei X, Yang Z, Rey FE, et al. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe. 2012;11:140–152. doi: 10.1016/j.chom.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudolph U, Finegold MJ, Rich SS, et al. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 41.Peña JA, Thompson-Snipes L, Calkins PR, et al. Alterations in myeloid dendritic cell innate immune responses in the Galphai2-deficient mouse model of colitis. Inflamm Bowel Dis. 2009;15:248–260. doi: 10.1002/ibd.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes MJ, Aksoylar H, Krebs P, et al. Loss of T cell and B cell quiescence precedes the onset of microbial flora-dependent wasting disease and intestinal inflammation in Gimap5-deficient mice. J Immunol. 2010;184:3743–3754. doi: 10.4049/jimmunol.0903164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esworthy RS, Aranda R, Martín MG, et al. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–G855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 44.Akitsu A, Kakuta S, Saijo S, et al. Rag2-deficient IL-1 Receptor Antagonist-deficient Mice Are a Novel Colitis Model in Which Innate Lymphoid Cell-derived IL-17 Is Involved in the Pathogenesis. Exp. Animals. 2014;63:235–246. doi: 10.1538/expanim.63.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willerford DM, Chen J, Ferry JA, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 46.Murata Y, Yamashita A, Saito T, et al. The conversion of redox status of peritoneal macrophages during pathological progression of spontaneous inflammatory bowel disease in Janus family tyrosine kinase 3(−/−) and IL-2 receptor gamma(−/−) mice. Int Immunol. 2002;14:627–636. doi: 10.1093/intimm/dxf031. [DOI] [PubMed] [Google Scholar]
- 47.Poussier P, Ning T, Chen J, et al. Intestinal inflammation observed in IL-2R/IL-2 mutant mice is associated with impaired intestinal T lymphopoiesis. Gastroenterology. 2000;118:880–891. doi: 10.1016/s0016-5085(00)70174-0. [DOI] [PubMed] [Google Scholar]
- 48.Contractor NV, Bassiri H, Reya T, et al. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1998;160:385–394. [PubMed] [Google Scholar]
- 49.Ma A, Datta M, Margosian E, et al. T cells, but not B cells, are required for bowel inflammation in interleukin 2-deficient mice. J Exp Med. 1995;182:1567–1572. doi: 10.1084/jem.182.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe M, Ueno Y, Yajima T, et al. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998;187:389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomita T, Kanai T, Nemoto Y, et al. Systemic, but not intestinal, IL-7 is essential for the persistence of chronic colitis. J Immunol. 2008;180:383–390. doi: 10.4049/jimmunol.180.1.383. [DOI] [PubMed] [Google Scholar]
- 52.Matharu KS, Mizoguchi E, Cotoner CA, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology. 2009;137:1380–1390. doi: 10.1053/j.gastro.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoentjen F, Harmsen HJ, Braat H, et al. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut. 2003;52:1721–1727. doi: 10.1136/gut.52.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zigmond E, Bernshtein B, Friedlander G, et al. Macrophage-Restricted Interleukin-10 Receptor Deficiency, but Not IL-10 Deficiency, Causes Severe Spontaneous Colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Ohta N, Hiroi T, Kweon MN, et al. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta+NK1.1+ T cells for the development of small intestinal inflammation. J Immunol. 2002;169:460–468. doi: 10.4049/jimmunol.169.1.460. [DOI] [PubMed] [Google Scholar]
- 56.Zhang HS, Chen Y, Fan L, et al. The Endoplasmic Reticulum Stress Sensor IRE1α in Intestinal Epithelial Cells Is Essential for Protecting against Colitis. J Biol Chem. 2015;290:15327–15336. doi: 10.1074/jbc.M114.633560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baribault H, Penner J, Iozzo RV, et al. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- 58.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. [PubMed] [Google Scholar]
- 59.Velcich A, Yang W, Heyer J, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 60.Van der Sluis M, De Koning BA, De Bruijn AC, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Fu J, Wei B, Wen T, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 63.Gerth AJ, Lin L, Neurath MF, et al. An innate cell-mediated, murine ulcerative colitis-like syndrome in the absence of nuclear factor of activated T cells. Gastroenterology. 2004;126:1115–1121. doi: 10.1053/j.gastro.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Neurath MF, Pettersson S, Meyer zum Büschenfelde KH, et al. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 65.Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 66.Steinbrecher KA, Harmel-Laws E, Sitcheran R, et al. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180:2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 67.Lee EG, Boone DL, Chai S, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vereecke L, Sze M, Mc Guire C, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vereecke L, Vieira-Silva S, Billiet T, et al. A20 controls intestinal homeostasis through cell-specific activities. Nat Commun. 2014;5:5103. doi: 10.1038/ncomms6103. [DOI] [PubMed] [Google Scholar]
- 70.Y Takahashi D, Ebisawa M, Kakiguchi K, et al. Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J Immunol. 2012;188:2427–2436. doi: 10.4049/jimmunol.1101128. [DOI] [PubMed] [Google Scholar]
- 71.Guilmeau S, Flandez M, Bancroft L, et al. Intestinal deletion of Pofut1 in the mouse inactivates notch signaling and causes enterocolitis. Gastroenterology. 2008;135:849–860. doi: 10.1053/j.gastro.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 73.Patton DT, Garden OA, Pearce WP, et al. The phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 74.Park SG, Mathur R, Long M, et al. T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity. 2010;33:791–803. doi: 10.1016/j.immuni.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bachmaier K, Krawczyk C, Kozieradzki I, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 76.Vetrano S, Ploplis VA, Sala E, et al. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc Natl Acad Sci U S A. 2011;108:19830–19835. doi: 10.1073/pnas.1107140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao FH, Shui JW, Hsing EW, et al. Protein phosphatase 4 is an essential positive regulator for Treg development, function, and protective gut immunity. Cell & Bioscience. 2014;4:25. doi: 10.1186/2045-3701-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weersma RK, Zhou L, Nolte IM, et al. Runt-related transcription factor 3 is associated with ulcerative colitis and shows epistasis with solute carrier family 22, members 4 and 5. Inflamm Bowel Dis. 2008;14:1615–1622. doi: 10.1002/ibd.20610. [DOI] [PubMed] [Google Scholar]
- 79.Brenner O, Levanon D, Negreanu V, et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc Natl Acad Sci U S A. 2004;101:16016–16021. doi: 10.1073/pnas.0407180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sugai M, Aoki K, Osato M, et al. Runx3 is required for full activation of regulatory T cells to prevent colitis-associated tumor formation. J Immunol. 2011;186:6515–6520. doi: 10.4049/jimmunol.1001671. [DOI] [PubMed] [Google Scholar]
- 81.Kerr WG, Park MY, Maubert M, et al. (2011) SHIP deficiency causes Crohn’s disease-like ileitis. Gut. 2011;60:177–188. doi: 10.1136/gut.2009.202283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narumi Y, Isomoto H, Shiota M, et al. Polymorphisms of PTPN11 coding SHP-2 as biomarkers for ulcerative colitis susceptibility in the Japanese population. J Clin Immunol. 2009;29:303–310. doi: 10.1007/s10875-008-9272-6. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita H, Kotani T, Park JH, et al. Role of the Protein Tyrosine Phosphatase Shp2 in Homeostasis of the Intestinal Epithelium. PLOS ONE. 2014;9:e92904. doi: 10.1371/journal.pone.0092904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Durant L, Watford WT, Ramos HL, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 87.Reindl W, Weiss S, Lehr HA, et al. Essential crosstalk between myeloid and lymphoid cells for development of chronic colitis in myeloid-specific signal transducer and activator of transcription 3-deficient mice. Immunology. 2007;120:19–27. doi: 10.1111/j.1365-2567.2006.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alonzi T, Newton IP, Bryce PJ, et al. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine. 2004;26:45–56. doi: 10.1016/j.cyto.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Inagaki-Ohara K, Sasaki A, Matsuzaki G, et al. Suppressor of cytokine signalling 1 in lymphocytes regulates the development of intestinal inflammation in mice. Gut. 2006;55:212–219. doi: 10.1136/gut.2004.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ernst M, Inglese M, Waring P, et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194:189–203. doi: 10.1084/jem.194.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu QF, Li Y, Zhao QH, et al. Association of STAT4 rs7574865 polymorphism with susceptibility to inflammatory bowel disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2015:S2210–S7401. doi: 10.1016/j.clinre.2015.04.002. 00085-6. [DOI] [PubMed] [Google Scholar]
- 93.Wirtz S, Finotto S, Kanzler S, et al. Chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF- plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–1888. [PubMed] [Google Scholar]
- 94.Yu F, Sharma S, Edwards J, et al. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol. 2015;16:197–206. doi: 10.1038/ni.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mizoguchi A, Mizoguchi E, Saubermann LJ, et al. Limited CD4 T-cell diversity associated with colitis in T-cell receptor alpha mutant mice requires a T helper 2 environment. Gastroenterology. 2000;119:983–995. doi: 10.1053/gast.2000.18153. [DOI] [PubMed] [Google Scholar]
- 97.Dianda L, Hanby AM, Wright NA, et al. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 98.Gaskins HR, Vondrak-Juergens GL, McCracken BA, et al. Specific-pathogen-free conditions enhance inflammatory bowel disease in T-cell receptor knockout, but not C3H/HeJBir mice. Lab Anim Sci. 1997;47:650–5. [PubMed] [Google Scholar]
- 99.Shimomura Y, Mizoguchi E, Sugimoto K, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–737. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- 100.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 101.Mizoguchi A, Mizoguchi E, Takedatsu H, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 102.Goetz M, Atreya R, Ghalibafian M, et al. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007;13:1365–1368. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]
- 103.Ardelean DS, Gonska T, Wires S, et al. Severe ulcerative colitis after rituximab therapy. Pediatrics. 2010;126:e243–e246. doi: 10.1542/peds.2009-3395. [DOI] [PubMed] [Google Scholar]
- 104.El Fassi D, Nielsen CH, Junker P, et al. Systemic adverse events following rituximab therapy in patients with Graves’ disease. J Endocrinol Invest. 2010;34:e163–e167. doi: 10.3275/7411. [DOI] [PubMed] [Google Scholar]
- 105.Liao CM, Zimmer MI, Shanmuganad S, et al. dysregulation of CD1d–restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology. 2012;142:326–334. doi: 10.1053/j.gastro.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 108.Moritoki Y, Lian ZX, Lindor K, et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893–1903. doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beck PL, Rosenberg IM, Xavier RJ, et al. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramalingam R, Larmonier CB, Thurston RD, et al. Dendritic cell-specific disruption of TGF-β Receptor II leads to altered Regulatory T Cell phenotype and Spontaneous Multiorgan Autoimmunity. J Immunol. 2012;189:3878–3893. doi: 10.4049/jimmunol.1201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kajino-Sakamoto R, Inagaki M, Lippert E, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim BG, Li C, Qiao W, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 113.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gewirtz AT, Vijay-Kumar M, Brant SR, et al. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1157–G1163. doi: 10.1152/ajpgi.00544.2005. [DOI] [PubMed] [Google Scholar]
- 115.Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 117.Kontoyiannis D, Boulougouris G, Manoloukos M, et al. Genetic dissection of the cellular pathways and signaling mechanisms in modeled tumor necrosis factor-induced Crohn’s-like inflammatory bowel disease. J Exp Med. 2002;196:1563–1574. doi: 10.1084/jem.20020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Apostolaki M, Manoloukos M, Roulis M, et al. Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn’s disease. Gastroenterology. 2008;134:2025–2035. doi: 10.1053/j.gastro.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 119.Shaikh RB, Santee S, Granger SW, et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167:6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 120.Meylan F, Song YJ, Fuss I, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4:172–185. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoon Park, Hyung-Seung Jin, Justine Lopez, et al. TSC1 regulates the balance between effector and regulatory T cells. J Clin Invest. 2014;123:5165–5178. doi: 10.1172/JCI69751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 123.Nguyen DD, Maillard MH, Cotta-de-Almeida V, et al. Lymphocyte-dependent and Th2 cytokine-associated colitis in mice deficient in Wiskott-Aldrich syndrome protein. Gastroenterology. 2007;133:1188–1197. doi: 10.1053/j.gastro.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maillard MH, Cotta-de-Almeida V, Takeshima F, et al. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204:381–391. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brandle K, Tomisato W, Li X, et al. Yip1 domain family, member 6 (Yipf6) mutation induces spontaneous intestinal inflammation in mice. Proc Natl Acad Sci U S A. 2012;109:12650–12655. doi: 10.1073/pnas.1210366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Obata Y, Furusawa Y, Endo TA, et al. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat Immunol. 2014;15:571–579. doi: 10.1038/ni.2886. [DOI] [PubMed] [Google Scholar]