Abstract
The G protein-coupled receptor kinase-2 (GRK2) is upregulated in the injured heart and contributes to heart failure pathogenesis. GRK2 was recently shown to associate with mitochondria but its functional impact in myocytes due to this localization is unclear. This study was undertaken to determine the effect of elevated GRK2 on mitochondrial respiration in cardiomyocytes. Sub-fractionation of purified cardiac mitochondria revealed that basally GRK2 is found in multiple compartments. Overexpression of GRK2 in mouse cardiomyocytes resulted in an increased amount of mitochondrial-based superoxide. Inhibition of GRK2 increased oxygen consumption rates and ATP production. Moreover, fatty acid oxidation was found to be significantly impaired when GRK2 was elevated and was dependent on the catalytic activity and mitochondrial localization of this kinase. Our study shows that independent of cardiac injury, GRK2 is localized in the mitochondria and its kinase activity negatively impacts the function of this organelle by increasing superoxide levels and altering substrate utilization for energy production.
Keywords: GRK2, Cardiomyocytes, Mitochondria, Respiration
Introduction
Activation of G protein-coupled receptors (GPCRs) and downstream signaling are paramount for a myriad of vital cellular responses. GPCR kinases (GRKs) are essential for fine-tuning these signals as phosphorylation induces the loss of signaling [1]. GRK2 is a major GRK expressed in the myocardium and is elevated after injury and/or stress [1]. Elevated GRK2 in the heart can induce the loss of β-adrenergic receptor (βAR) mediated inotropic reserve, a condition associated with heart failure (HF) [2]. Targeted inhibition of GRK2 improves cardiac function and βAR signaling in the heart and expression of a peptide inhibitor of GRK2, known as the βARKct, has rescued several animal models of HF [1, 2]. Recently, additional non-canonical mechanisms of GRK2 action in the heart have been shown to contribute to the pathological nature of GRK2 in its promotion of cardiac disease. GRK2 has been shown to be a pro-death kinase due to its negative regulation of nitric oxide signaling and its stress-induced localization to mitochondria [3]. GRK2 is localized to mitochondria [3-5] and its presence under basal conditions suggests a potential importance in energy production, which is particularly relevant for tissues that depend heavily on energy expenditure such as the heart. The adult heart primarily uses fatty acids for ATP production, however substrate preference can change in various cardiac pathologies[6]. Overall, the functional impact of mitochondrial-localized GRK2 on mitochondrial energy dynamics has not been examined. Herein, we elucidate the impact of GRK2 in cardiomyocyte mitochondrial function including its regulation of respiration, ATP production and superoxide levels. We found that inhibition of GRK2 increased ATP production whereas elevated GRK2 increased superoxide levels and negatively regulated FA oxidation, findings that are important during pathogenesis of cardiac disease.
Methods
Detailed methods are available in the online supplement.
Results and Discussion
Immuno-gold EM studies in hearts of transgenic mice overexpressing GRK2 (TgGRK2) and controls (NLC) suggested that GRK2 localized to and was distributed throughout mitochondria [3]. In this study we sub fractioned rat heart mitochondria and found GRK2 in multiple mitochondrial compartments (Figure 1A). This result was consistent with our previous EM result and suggests that GRK2 could be involved in regulating basal metabolic mitochondrial functions, such as energy production.
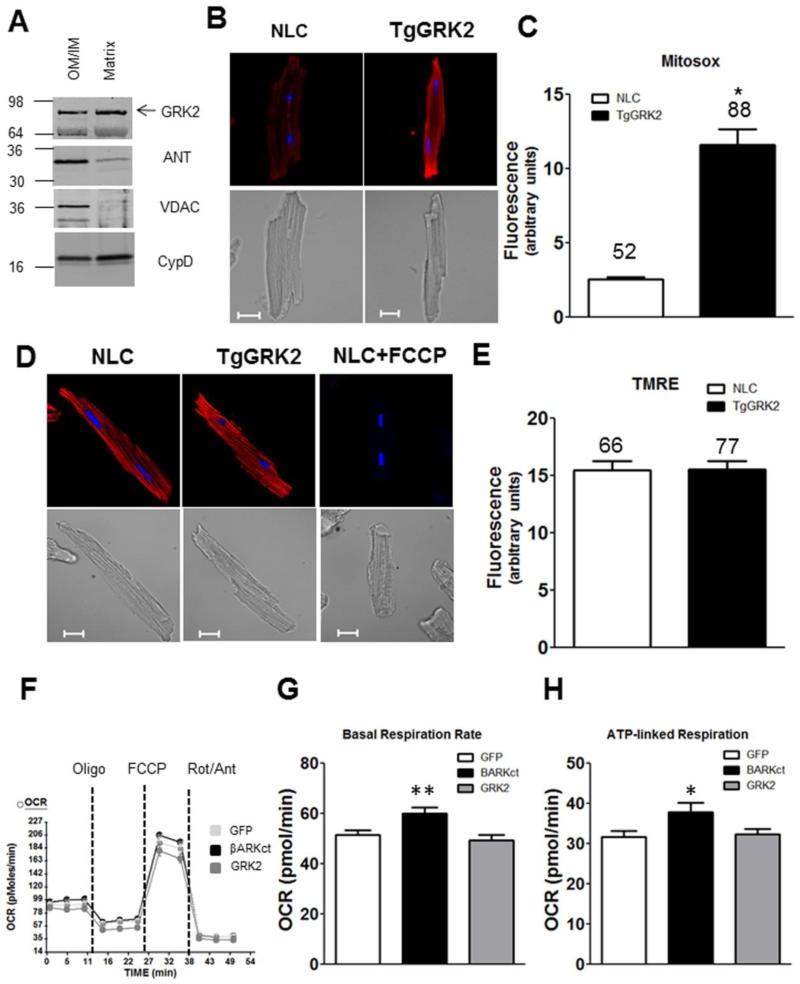
Figure 1. GRK2 localization, effect on superoxide formation and respiration under stress-free conditions.
A: GRK2 localized to both OM/IM compartment and the matrix (VDAC: OM marker, ANT: IM marker, Cyclophilin D: matrix marker). Equal amount of total protein was loaded in each lane. Shown is a representative blot from 3 experiments. B: Top, representative confocal images of MitoSox red (superoxide) in AMCs from TgGRK2 or NLC, Hoechst (nuclear marker). Bottom, corresponding DIC images of each representative cell. Scale bar is 20μm. C: Quantification of superoxide levels in TgGRK2 myocytes. Total number of cells quantified is noted above each bar (p<0.0001 TgGRK2 vs NLC). D: Top, representative confocal images of AMCs from TgGRK2 or NLC mice labeled with TMRE (mitochondrial membrane potential, ΔΨ) and Hoechst (nuclear marker). Bottom, respective DIC images of each representative cell. Scale bar is 20μm. FCCP (20μM) induced complete mitochondrial depolarization. E: Quantification of three independent experiments show no difference in ΔΨ. Total number of cells quantified is noted above each bar. F: Representative OCR recording of one Seahorse® Flux Analyzer plate (n=12-14 wells/group) in NRVMs expressing the indicated proteins. G-H: βARKct treatment increased basal respiratory rate (**p<0.001 βARKct vs GFP) and increased ATP production (* p<0.05 βARKct vs GFP) N=4 independent plates, 46-52 wells/per group.
Research over the last two decades indicates that elevation of GRK2 in the hearts of animals or humans, occurring after cardiac stress/injury, is detrimental to myocardial function [1, 2, 8, 9]. Moreover, lowering GRK2 levels or activity in myocytes improves cardiac function [1, 2, 10]. Studies have shown that increased levels of GRK2 can lead to decreased contractility and impairment in βAR-mediated responses. In addition to these canonical GPCR actions, GRK2 has novel receptor-independent actions that contribute to its pathological effects [1]. As an example, the oxidative stress-induced translocation of GRK2 to mitochondria results in GRK2 acting as a pro-death kinase [3]. In this report, we add the novel finding that raising cellular levels of GRK2 to those seen in human HF results in significantly increased superoxide levels (Figure 1B&C). These studies were conducted using adult mouse cardiomyocytes (AMCs) isolated from TgGRK2 or NLC mice loaded with the mitochondrial superoxide reporter, MitoSOX Red, and imaged via confocal microscopy. The level of GRK2 in the hearts of TgGRK2 mice were similar to those observed in HF (3-4 fold elevation) [2]. This suggests that elevation of myocyte GRK2 can make cells more prone to injury and also participate in HF pathogenesis. This result is opposite to that reported in macrophages where GRK2 knockdown increased reactive oxygen species (ROS) [11]. Next, we measured mitochondrial membrane potential (ΔΨ) in the adult myocytes from these transgenic mouse hearts but found no difference with GRK2 overexpression (Figure 1D&E).
To explore functional consequences of mitochondrial GRK2 localization when it is elevated as in cardiac stress/injury, we measured oxygen consumption rate (OCR) using the Seahorse flux analyzer in neonatal rat ventricular myocytes (NRVMs) treated with adenoviruses encoding for the expression of GFP, GRK2, or the βARKct, which can inhibit GRK2 and block its mitochondrial accumulation after oxidative stress[3]. A representative tracing and the quantified results are shown in Figure 1F-H. Interestingly, we found that both basal respiratory rate and ATP-linked respiration, a parameter to measure ATP production was significantly increased when GRK2 was inhibited with the βARKct (Figure 1H). We also measured maximal respiration rates, spare capacity, and coupling efficiency but found no significant changes (data not shown). Finally, extracellular acidification rates (ECAR), which is an indirect measurement of glycolytic activity, was not altered (data not shown). The beneficial effect of BARKct on respiratory rate and ATP production in this assay is consistent with our previous report demonstrating that this peptide limited mitochondrial accumulation of GRK2 after oxidative stress[3]. Again, our findings differ from a previous study that reported decreased GRK2 led to an almost total depletion of ATP in HEK cells[5]. This discrepancy could be due to cellular differences, however, at least in the myocyte, our result with βARKct is consistent with over 20 years of cardiac physiological data demonstrating beneficial outcomes when GRK2 is lower or inhibited[1].
Substrate utilization varies throughout development and cardiac pathologies [6]. It is known that NRVMs prefer glucose whereas the adult heart heavily depends on fatty acid oxidation (FAO) for energy production [7]; therefore, we tested NRVMs using the energy substrate preferred by adult cells. NRVMs expressing GFP, βARKct, or GRK2 were exposed to substrate limiting conditions which allowed cells to use the exogenous fatty acid, palmitate, for energy production (Figure 2). Under these conditions OCR measurements revealed that elevated GRK2 negatively affect myocyte FAO and FA-mediated oxygen consumption. Specifically, GFP and βARKct expressing NRVMs showed the expected increase in OCR in the presence of palmitate while myocytes with increased GRK2 were not capable of utilizing palmitate as effectively (Figure 2A). In addition, basal respiration due to utilization of exogenous FAs (Figure 2B) as well as palmitate-induced ATP-linked respiration (Figure 2C) was significantly decreased compared to GFP when GRK2 was higher. No change was observed in maximal respiration due to utilization of exogenous FAs (Figure 2D).
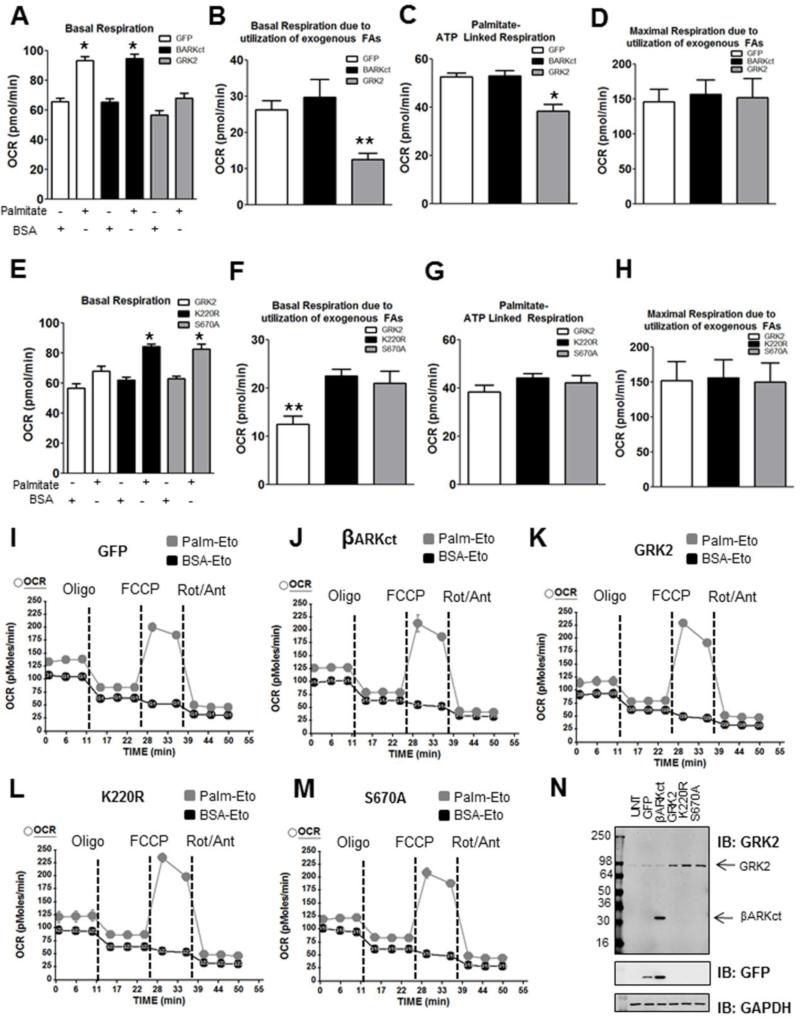
Figure 2. GRK2 negatively regulates fatty acid-mediated respiration in NRVM.
A: Basal OCR profile of cells expressing GFP, βARKct, or GRK2 and exposed to Palmitate or BSA (* p<0.001 GFP Palm vs BSA and βARKct Palm vs BSA). B: Basal respiration due to utilization of exogenous palmitate is decreased by GRK2 overexpression (** p<0.02 GRK2 vs GFP). C: GRK2 overexpression decreases palmitate-linked ATP production (*p<0.001 GRK2 vs GFP). D: No significant change was observed in maximal respiration rates due to utilization of exogenous FAs. E: GRK2 mutants K220R and S670A restore basal OCR in presence of palmitate. (*p<0.0001 K220R palm vs BSA and S670A palm vs BSA). F: Basal OCR due to utilization of exogenous FAs is restored when K220R or S670A is overexpressed compared to wild-type GRK2 elevation (**p<0.05 GRK2 vs K220R and GRK2 vs S670A). G: A trend for increased Palmitate-ATP production in myocytes when K220R or S670A is overexpressed. H: No significant change was observed in maximal respiration rates due to utilization of exogenous FAs in any myocyte group. A-H: N=4 independent experiments, 12-16 wells/per group I-M: Representative traces of an individual plate showing GFP, βARKct, GRK2, K220R, S670A in the presence of Palm or BSA (n=3-4 wells per group for this individual plate). N: Western blot analysis of cells from Seahorse® wells confirmed adenoviral expression of βARKct, GRK2, and the GRK2 mutants. GRK2 infections were designed to overexpress GRK2 to an extent similar to that in TgGRK2 mice and in human HF.
To specifically address whether these negative changes in FAO were due to increased accumulation of GRK2 to mitochondria, we overexpressed a mutant GRK2 (S670A) in myocytes that has been previously shown to have limited ability to translocate to mitochondria during oxidative stress [3]. Interestingly, when this GRK2 mutant is overexpressed in myocytes palmitate was effectively utilized and basal respiration was normal (Figure 2E). In addition, in order to determine kinase-dependence we also overexpressed a kinase-dead GRK2 (K220R) in myocytes and these cells also had normal palmitate utilization (Figure 2E). Indeed, basal respiration due to utilization of exogenous FAs that was impaired with elevated GRK2 was significantly increased with expression of K220R or S670A (Figure 2F). These two mutants of GRK2 also produced a trend towards increased palmitate-induced ATP production compared to wild-type GRK2 (Figure 2G). No change in maximal respiration due to utilization of FAs was observed with any GRK2 (Figure D&H). A representative trace of one plate for GFP, βARKct, GRK2, K220R, S670A treated NRVMs (exposed to BSA or palmitate) is shown in Figure 2I-M. These data are important as they indicate that GRK2-mediated FAO defects in myocytes depend on its mitochondrial localization and kinase activity. Overall, these data suggest that elevation of GRK2 levels in myocytes, which occurs after stress or injury can in itself be damaging to the myocyte and consistent with it being a key molecule in HF pathogenesis[9, 12].
Our results may also be significant to other diseases such as diabetes where energy production depends heavily on FAO [6], and where increased levels of GRK2 have been linked to insulin resistance[13]. In line with results obtained here, a recent study showed that in obese mice GRK2 ablation was able to prevent further weight gain during high fat diet[14], potentially by decreasing FA synthesis or increasing FA oxidation. Collectively, data obtained from many studies over decades indicate that an elevated level of GRK2 in the heart is detrimental to cardiac function, and this study provides further evidence that GRK2 has a negative impact on cellular homeostasis through a non-canonical action on mitochondrial function. Finally, it was reported that in human HF there is an excessive uptake and accumulation of myocardial FFA [15], which is consistent with our finding that increased GRK2 leads to decreased utilization of exogenous fatty acids. Further studies are needed to address the specific mechanisms of how GRK2 impacts mitochondrial FAO and substrate utilization.
Supplementary Material
Highlights.
Mitochondrial GRK2 is localized to multiple sub-mitochondrial locations.
Overexpression of GRK2 in cardiomyocytes increases superoxide levels and impairs fatty acid-driven oxygen consumption rate
GRK2 kinase activity is necessary for the deleterious effect on mitochondrial energy dynamics
βARKct, a peptide inhibitor of GRK2, enhances basal oxygen consumption rates and ATP production
Acknowledgements
We thank Zuping Qu and Samantha Baxter for their expert maintenance and genotyping of our transgenic mouse colony, and Dr. Kevin Bittman of Seahorse® Biosciences for technical guidance with our experiments.
Sources of Funding:
P.Y.S was supported by an AHA Post-Doctoral Fellowship. W.J.K is the W.W. Smith Chair in Cardiovascular Medicine at Temple University School of Medicine. This project was supported by National Institute of Health (NIH) grant P01 HL091799. W.J.K. is also supported by NIH grants R37 HL061690, R01 HL085503, P01 HL075443 (Project 2) and P01 HL108806 (Project 3). K.D. was supported by a NIH pathway to independence grant R00 HL112853.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
None
REFERENCES
- [1].Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol Rev. 2015;95:377–404. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–3. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- [3].Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, et al. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ Res. 2013;112:1121–34. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Obrenovich ME, Palacios HH, Gasimov E, Leszek J, Aliev G. The GRK2 Overexpression Is a Primary Hallmark of Mitochondrial Lesions during Early Alzheimer Disease. Cardiovasc Psychiatry Neurol. 2009;2009:327360. doi: 10.1155/2009/327360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fusco A, Santulli G, Sorriento D, Cipolletta E, Garbi C, Dorn GW, 2nd, et al. Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. Cell Signal. 2012;24:468–75. doi: 10.1016/j.cellsig.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kolwicz SC, Jr., Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–16. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- [8].White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–33. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, et al. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–8. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- [10].Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–22. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sorriento D, Fusco A, Ciccarelli M, Rungi A, Anastasio A, Carillo A, et al. Mitochondrial G protein coupled receptor kinase 2 regulates proinflammatory responses in macrophages. FEBS Lett. 2013;587:3487–94. doi: 10.1016/j.febslet.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, et al. Lymphocyte levels of GRK2 (betaARK1) mirror changes in the LVAD-supported failing human heart: lower GRK2 associated with improved beta-adrenergic signaling after mechanical unloading. J Card Fail. 2006;12:360–8. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- [13].Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, et al. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–17. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [14].Vila-Bedmar R, Cruces-Sande M, Lucas E, Willemen HL, Heijnen CJ, Kavelaars A, et al. Reversal of diet-induced obesity and insulin resistance by inducible genetic ablation of GRK2. Sci Signal. 2015;8:ra73. doi: 10.1126/scisignal.aaa4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lommi J, Kupari M, Yki-Jarvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol. 1998;81:45–50. doi: 10.1016/s0002-9149(97)00804-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.