Abstract
Objective
To utilize a number of methods to control for confounding and selection bias to examine the association between lymphadenectomy and survival in a large cohort of women with endometrial cancer.
Methods
A retrospective cohort study using the National Cancer Database was performed to identify women with endometrioid adenocarcinoma of the endometrium who underwent hysterectomy with or without lymphadenectomy from 1998–2011. Traditional regression analysis, propensity score and an instrumental variable using regional variation in the rate of lymphadenectomy as an instrument were used to examine the association between lymphadenectomy and survival.
Results
A total of 151,089 women treated at 1336 hospitals were identified; 99,052 (65.6%) patients underwent lymphadenectomy while 52,037 (34.4%) did not. In a multivariable regression model, lymphadenectomy was associated with a 16% reduction in mortality (HR=0.84; 95% CI, 0.81–0.87). The results were similar after adjustment for adjuvant therapy (HR=0.85; 95% CI, 0.82–0.87). The results were largely unchanged and suggested that lymphadenectomy was associated with improved survival after application of a propensity score analysis. In contrast, in the instrumental variable analysis there was not a statistically significant association between lymphadenectomy and survival (HR=0.75; 95% CI, 0.53–1.06), even after adjustment for adjuvant treatment (HR=0.76; 95% CI, 0.54–1.06). The results were unchanged for women with T1A and T1B tumors.
Conclusion
Lymphadenectomy is associated with a modest, if any, effect on survival for women with endometrial cancer.
Introduction
The treatment of endometrial cancer has evolved over the last three decades. In the 1980’s, the disease was predominantly treated with intracavitary radiation followed by hysterectomy. With the greater understanding of the patterns of spread, treatment shifted to primary surgery with lymph node sampling in higher risk patients. Nodal status is used to tailor adjuvant therapy; women with nodal disease were treated with pelvic radiation, while those with negative nodes received brachytherapy or observation.1,2 Data from observational studies emerged suggesting a therapeutic benefit for lymphadenectomy, even in women without nodal metastasis, and nodal evaluation became more widespread and shifted from sampling to a full lymphadenectomy of the pelvic and para-aortic nodes.3–10
The benefits of lymph node dissection for endometrial cancer were challenged by the publication of two randomized trials.11,12 These European trials both reported no association between lymphadenectomy and survival.11,12 However, concern has been raised that these trials were underpowered to detect a benefit for lymphadenectomy, that the quality of lymphadenectomy dissection performed was suboptimal, and the ability of the status of the nodes to guide therapy unclear.1,13,14 In the U.S., lymphadenectomy remains a component of therapy for many women with endometrial cancer.
An important limitation of observational studies is the inability to control for confounding factors that influence outcome.15–17 A variety of statistical techniques are now available to help overcome this limitation. Propensity score analysis estimates the probability of treatment, the propensity score, and then uses this score to assess outcomes while controlling for measured confounders15. An instrumental variable analysis is a technique that leverages variation in treatment, referred to as an instrument, to control for both measured and unmeasured confounding factors.15,16
Given the conflicting data surrounding the benefits of lymph node dissection for endometrial cancer, we performed a population-based analysis to examine the association between lymphadenectomy and survival. We analyzed a large cohort of women using a variety of statistical methodologies to control for measured and unmeasured confounders.
Materials and Methods
A retrospective cohort study using the National Cancer Data Base was performed18,19 Data on incident cancer cases from over 1500 Commission on Cancer affiliated hospitals encompassing approximately 70% of all newly diagnosed cancers is captured in the dataset. Data on patient demographics, clinical data, tumor characteristics, staging, treatment, and overall survival is collected.18,19 The database contains deidentified data and was deemed exempt by the Columbia University Institutional Review Board.
Women with endometrioid adenocarcinoma of the endometrium diagnosed from 1998–2011 who underwent hysterectomy were selected. Patient who received preoperative radiation and those who had another primary tumor prior to the diagnosis of uterine cancer were excluded. As the study spanned the time frame including the American Joint Commission on Cancer staging systems 5–7, we converted the T stage of all patients to uniform nomenclature and included the following T stages: T1A (tumor limited to the endometrium or <50% of the myometrium), T1B (tumor with >50% myometrial invasion), or T2 (cervical stromal involvement). As the goal of the analysis was to explore the influence of lymphadenectomy on outcome, we included women regardless of their nodal status (positive lymph nodes, negative lymph nodes, lymph nodes unknown). Women with primary tumor spread beyond the uterus (>T2) or metastatic disease were excluded.
Lymph node dissection was considered the removal of any lymph nodes. We performed sensitivity analyses in which the extent of lymphadenectomy was assessed. In these analyses, the cohort was stratified as those women who had <10 lymph nodes removed and those who had ≥10 nodes removed (extensive lymphadenectomy).
Clinical variables analyzed included age (<40, 40–49, 50–59, 60–6, ≥70 years), race (white, black, other), insurance (commercial, Medicare, Medicaid, uninsured, other), region of residence, and area level education (percentage of residents who did not complete high school: <14%, 14–19.9%, 20–28.9%, ≥29%). Tumor grade was classified as well, moderately, or poorly differentiated. Use of adjuvant radiotherapy was grouped as: none, brachytherapy, and external beam radiation (with or without brachytherapy). Use of chemotherapy during the first course of treatment was recorded.
Hospital location was classified as metropolitan, urban, rural and hospitals are also classified as academic–research cancer centers or community cancer centers.19 Annualized hospital volume was recorded as the mean annual number of cases cared for in years in which a given hospital recorded at least one patient and included as a continuous variable in all analyses.
Frequency distributions between groups were compared using a standardized difference with a value of ≤0.10, considered to indicate good balance.20 Multivariable generalized estimating equations (GEE) with a Poisson distribution and log link function were developed to examine predictors of lymphadenectomy while controlling for other clinical, demographic, and hospital characteristics.
The association between performance of lymphadenectomy and overall survival was assessed using multivariable Cox proportional hazards analysis, through use of a propensity score (matching, inverse probability of treatment weights, and stratification/deciles) and an instrumental variable analysis. For each methodology, we developed a model which included only lymphadenectomy, a clinical model that included lymphadenectomy and all patient (clinical and tumor) and hospital characteristics, and a clinical and treatment model that included all of the variables in the clinical model as well as adjuvant therapy (chemotherapy and radiation) administered.
The propensity score (PS) is the predicted probability of treatment.15,16,21 To calculate the PS, we fit a logistic regression model that included all of the clinical, oncologic and hospital characteristics and two way interaction terms (only those interaction terms with a P-value of <0.05 for stage T1B and T2 to allow model convergence) to determine to the probability of undergoing lymphadenectomy. The predicted probability (the propensity score) was estimated for each patient and ranged from 0 to 1.
The propensity score matching relied on a Greedy 5 to 1 digit matching algorithm. Women who underwent lymphadenectomy were matched to controls to 5 digits of the PS. For those subjects for whom a match was not identified, a 4-digit match was applied. This process was continued down to a 1-digit match for those who remained unmatched (Appendix 1, available online at http://links.lww.com/xxx). We also applied an inverse probability of treatment weighting approach (IPTW) for PS analysis.16,22 Using an IPTW approach, each patient was assigned a differential weight based on their calculated PS. Using this approach allows inclusion of all subjects and does not require a match. The weighting assumptions of the IPTW approach assigned patients who underwent a lymphadenectomy a weight of 1/propensity score and those who did not undergo a lymphadenectomy a weight of 1/(1-propensity score).16,22 Marginal Cox proportional hazards regression models were used as the final model for PS analysis to estimate hazard ratio for mortality with receipt of lymphadenectomy, accounting for hospital clustering.
An instrumental variable analysis (IVA) is an analytic methodology that attempts to adjust for measured and unmeasured confounders through application of an exogenous instrument.15,16,23 The instrument, or instrumental variable (IV), is a characteristic associated with treatment but not outcome. Variation in the instrument approximates randomization and results in groups of patients with similar characteristics, including unmeasured factors.16
The IV for our analysis was geographic variation in performance of lymphadenectomy. Within the dataset, hospitals are classified into 9 unique geographic regions. We calculated the predicted probability of performance of lymphadenectomy for each patient while controlling for all of the clinical, demographic, and hospital characteristics. Within each region, we then calculated the difference between the observed and expected rate of lymphadenectomy. The difference in the observed to expected rate served as the instrumental variable. Regions with a positive value of the IV had more patients who underwent lymphadenectomy than predicted, while regions with a negative value for the IV had fewer patients than predicted undergo lymphadenectomy. We used the 1-year lagged rate of lymphadenectomy (performance of lymphadenectomy in the regions in the year prior) to allow greater independence from current patients’ medical conditions as previously described.16 A sensitivity analysis using the current year rate of lymphadenectomy was also performed.
The primary IVA relied on the two-stage residual inclusion methodology.16,24 In the first-stage, a logistic regression model was used to generate the residual (difference between observed and predicted probability of lymphadenectomy). We noted that the lagged lymphadenectomy had a statistically significant effect on patient’s receipt of lymphadenectomy, and there was substantial geographic variation (F=758.08) (P<0.001). The residual obtained from first-stage was then included in the second-stage of a marginal Cox proportional hazards regression model to estimate the hazard ratio for mortality with performance of lymphadenectomy. We performed further sensitivity analyses for the IVA using the two-stage predictor substitution (2SPS) methodology to explore the effects of model specification on the estimates of the treatment effect.25–27
The effect of lymphadenectomy on survival outcome was also estimated in absolute scale (survival difference and standard error) through adjusted survival curves in clinical and adjuvant treatment adjusted models for traditional regression, propensity score analysis and IV analysis.28,29 All analyses were performed with SAS version 9.3 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
A total of 151,089 women treated at 1336 hospitals were identified. Within the cohort, 99,052 (65.6%) patients underwent lymphadenectomy while 52,037 (34.4%) did not have lymph node sampling (Table 1). The rate of lymphadenectomy increased over time, from 51.8% in 1998 to a peak of 70.6% in 2007, and then declined slightly through 2011 (Figure 1). The overall rate of lymphadenectomy was 60.7% for T1A tumors, 78.7% for T1B tumors, and 77.9% for T2 tumors. Within the cohort the median follow-up time was 54.5 months in women who underwent lymphadenectomy and 60.9 months in those women who did not (Appendix 2, available online at http://links.lww.com/xxx).
Table 1.
Clinical and demographic characteristics of the cohort.
| Original cohort (n=151,089) | Propensity score inverse probability of treatment weighted cohort (n=302,664)2 | Instrumental variable analysis cohort (n=146,764)3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No nodal dissection (n=52,037) |
Nodal dissection (n=99,052) | No nodal dissection (n=151,725) |
Nodal dissection (n=150,939) |
Below median (n=72,838) |
Equal or above median (n=73,926) |
||||||||||
| N | (%) | N | (%) | SD1 | N | (%) | N | (%) | SD1 | N | (%) | N | (%) | SD1 | |
|
Instrumental variable (mean) |
−0.0358 | 0.0359 | |||||||||||||
|
Actual treatment, % lymph node dissection |
0 | (0.0) | 99,052 | (100.0) | 45,999 | (63.2) | 50,812 | (68.7) | |||||||
| Region | 0.18 | 0.01 | - | ||||||||||||
| New England | 4,708 | (9.1) | 5,995 | (6.1) | 10,758 | (7.1) | 10,664 | (7.1) | 10,260 | (14.1) | 0.0 | (0) | |||
| Middle Atlantic | 9,533 | (18.3) | 16,907 | (17.1) | 26,647 | (17.6) | 26,492 | (17.6) | 25,680 | (35.3) | 0.0 | (0) | |||
| South Atlantic | 8,394 | (16.1) | 20,307 | (20.5) | 29,093 | (19.2) | 28,706 | (19) | 0 | (0) | 280,66 | (38) | |||
| East north central | 10,162 | (19.5) | 20,743 | (20.9) | 31,000 | (20.4) | 30,858 | (20.4) | 3,075 | (4.2) | 269,13 | (36.4) | |||
| East south central | 2,706 | (5.2) | 5,413 | (5.5) | 8,169 | (5.4) | 8,116 | (5.4) | 4,193 | (5.8) | 3,711 | (5) | |||
| West north central | 4,124 | (7.9) | 8,118 | (8.2) | 12,243 | (8.1) | 12,173 | (8.1) | 9,017 | (12.4) | 2,889 | (3.9) | |||
| West south central | 3,842 | (7.4) | 6,020 | (6.1) | 9,831 | (6.5) | 9,819 | (6.5) | 9,310 | (12.8) | 296 | (0.4) | |||
| Mountain | 2,231 | (4.3) | 4,435 | (4.5) | 6,642 | (4.4) | 6,656 | (4.4) | 3,207 | (4.4) | 3,310 | (4.5) | |||
| Pacific | 6,337 | (12.2) | 11,114 | (11.2) | 17,344 | (11.4) | 17,454 | (11.6) | 8,096 | (11.1) | 8,741 | (11.8) | |||
| Age | 0.18 | 0.00 | 0.02 | ||||||||||||
| <40 | 2,213 | (4.3) | 2,897 | (2.9) | 5,152 | (3.4) | 5,124 | (3.4) | 2,401 | (3.3) | 2,550 | (3.5) | |||
| 40–49 | 6,852 | (13.2) | 9,501 | (9.6) | 16,444 | (10.8) | 16,388 | (10.9) | 7,971 | (10.9) | 7,904 | (10.7) | |||
| 50–59 | 16,841 | (32.4) | 30,743 | (31) | 47,601 | (31.4) | 47,495 | (31.5) | 23,113 | (31.7) | 23,348 | (31.6) | |||
| 60–69 | 14,576 | (28) | 32,065 | (32.4) | 46,857 | (30.9) | 46,545 | (30.8) | 22,460 | (30.8) | 22,963 | (31.1) | |||
| 70–79 | 7,534 | (14.5) | 17,468 | (17.6) | 25,208 | (16.6) | 24,988 | (16.6) | 12,044 | (16.5) | 12,014 | (16.3) | |||
| ≥80 | 4,021 | (7.7) | 6,378 | (6.4) | 10,464 | (6.9) | 10,399 | (6.9) | 4,849 | (6.7) | 5,147 | (7.0) | |||
| Race | 0.06 | 0.00 | 0.09 | ||||||||||||
| White | 46,645 | (89.6) | 87,432 | (88.3) | 134,585 | (88.7) | 134,983 | (88.8) | 64,995 | (89.2) | 65,086 | (88) | |||
| Black | 2,993 | (5.8) | 6,419 | (6.5) | 9,504 | (6.3) | 9,396 | (6.2) | 3,821 | (5.3) | 5,395 | (7.3) | |||
| Other | 1,480 | (2.8) | 3,599 | (3.6) | 5,069 | (3.4) | 5,078 | (3.4) | 2,671 | (3.7) | 2,311 | (3.1) | |||
| Unknown | 919 | (1.8) | 1,602 | (1.6) | 2,567 | (1.7) | 2,481 | (1.6) | 1,351 | (1.9) | 1,134 | (1.5) | |||
| Year of diagnosis | 0.24 | 0.01 | - | ||||||||||||
| 1998 | 2,084 | (4) | 2,241 | (2.3) | 4,334 | (2.9) | 4,303 | (2.9) | - | -- | - | -- | |||
| 1999 | 2,366 | (4.6) | 2,840 | (2.9) | 5,233 | (3.5) | 5,210 | (3.5) | 2,347 | (3.2) | 2,859 | (3.9) | |||
| 2000 | 2,818 | (5.4) | 3,334 | (3.4) | 6,175 | (4.1) | 6,096 | (4) | 3,487 | (4.8) | 2,665 | (3.6) | |||
| 2001 | 3,398 | (6.5) | 4,700 | (4.7) | 8,038 | (5.3) | 8,078 | (5.4) | 4,446 | (6.1) | 3,652 | (4.9) | |||
| 2002 | 3,510 | (6.8) | 5,082 | (5.1) | 8,674 | (5.7) | 8,604 | (5.7) | 5,173 | (7.1) | 3,419 | (4.6) | |||
| 2003 | 3,462 | (6.7) | 5,704 | (5.8) | 9,118 | (6) | 9,157 | (6.1) | 4,891 | (6.7) | 4,275 | (5.8) | |||
| 2004 | 3,781 | (7.3) | 6,510 | (6.6) | 10,343 | (6.8) | 10,300 | (6.8) | 3,684 | (5.1) | 6,607 | (8.9) | |||
| 2005 | 3,831 | (7.4) | 7,540 | (7.6) | 11,486 | (7.6) | 11,365 | (7.5) | 4,092 | (5.6) | 7,279 | (9.9) | |||
| 2006 | 3,863 | (7.4) | 8,696 | (8.8) | 12,536 | (8.3) | 12,554 | (8.3) | 5,554 | (7.6) | 7,005 | (9.5) | |||
| 2007 | 3,889 | (7.5) | 9,351 | (9.4) | 13,325 | (8.8) | 13,255 | (8.8) | 5,986 | (8.2) | 7,254 | (9.8) | |||
| 2008 | 4,184 | (8) | 10,014 | (10.1) | 14,348 | (9.5) | 14,196 | (9.4) | 7,779 | (10.7) | 6,419 | (8.7) | |||
| 2009 | 4,866 | (9.4) | 10,272 | (10.4) | 15,131 | (10) | 15,135 | (10) | 9,637 | (13.2) | 5,501 | (7.4) | |||
| 2010 | 4,762 | (9.2) | 10,988 | (11.1) | 15,900 | (10.5) | 15,715 | (10.4) | 7,101 | (9.8) | 8,649 | (11.7) | |||
| 2011 | 5,223 | (10) | 11,780 | (11.9) | 17,085 | (11.3) | 16,971 | (11.2) | 8,661 | (11.9) | 8,342 | (11.3) | |||
| Insurance | 0.05 | 0.00 | 0.07 | ||||||||||||
| Not insured | 1,711 | (3.3) | 3,313 | (3.3) | 5,049 | (3.3) | 5,013 | (3.3) | 2,242 | (3.1) | 2,635 | (3.6) | |||
| Commercial | 29,050 | (55.8) | 53,894 | (54.4) | 83,182 | (54.8) | 82,841 | (54.9) | 41,044 | (56.4) | 39,767 | (53.8) | |||
| Medicaid | 2,267 | (4.4) | 3,881 | (3.9) | 6,204 | (4.1) | 6,123 | (4.1) | 3,118 | (4.3) | 2,901 | (3.9) | |||
| Medicare | 17,581 | (33.8) | 35,332 | (35.7) | 53,234 | (35.1) | 52,901 | (35.1) | 24,629 | (33.8) | 26,533 | (35.9) | |||
| Other | 363 | (0.7) | 845 | (0.9) | 1,211 | (0.8) | 1,197 | (0.8) | 448 | (0.6) | 739 | (1) | |||
| Unknown | 1,065 | (2.1) | 1,787 | (1.8) | 2,844 | (1.9) | 2,863 | (1.9) | 1,357 | (1.9) | 1,351 | (1.8) | |||
| Education (area residents who did not complete high school) | 0.03 | 0.00 | 0.10 | ||||||||||||
| ≥29% | 7,280 | (14) | 13,570 | (13.7) | 21,039 | (13.9) | 20,827 | (13.8) | 9,830 | (13.5) | 10,481 | (14.2) | |||
| 20–28.9% | 11,181 | (21.5) | 21,242 | (21.5) | 32,705 | (21.6) | 32,356 | (21.4) | 14,801 | (20.3) | 16,699 | (22.6) | |||
| 14–19.9% | 12,815 | (24.6) | 23,429 | (23.7) | 36,352 | (24) | 36,187 | (24) | 17,745 | (24.4) | 17,393 | (23.5) | |||
| < 14% | 18,719 | (36) | 36,756 | (37.1) | 55,527 | (36.6) | 55,460 | (36.7) | 27,984 | (38.4) | 25,878 | (35) | |||
| Not Available | 2,042 | (3.9) | 4,055 | (4.1) | 6,102 | (4) | 6,109 | (4.1) | |||||||
| T Stage | 0.37 | 0.01 | 0.03 | ||||||||||||
| T1A | 43,092 | (82.8) | 66,498 | (67.1) | 109,609 | (72.2) | 109,402 | (72.5) | 53,219 | (73.1) | 53,204 | (72) | |||
| T1B | 5,747 | (11) | 21,281 | (21.5) | 27,455 | (18.1) | 27,038 | (17.9) | 12,802 | (17.6) | 13,520 | (18.3) | |||
| T2 | 3,198 | (6.2) | 11,273 | (11.4) | 14,661 | (9.7) | 14,499 | (9.6) | 6,817 | (9.4) | 7,202 | (9.7) | |||
| Grade | 0.51 | 0.00 | 0.06 | ||||||||||||
| Well | 33,585 | (64.5) | 41,244 | (41.6) | 74,946 | (49.4) | 74,649 | (49.5) | 35,238 | (48.4) | 37,537 | (50.8) | |||
| Moderate | 12,872 | (24.7) | 36,935 | (37.3) | 50,170 | (33.1) | 49,829 | (33) | 24,408 | (33.5) | 23,816 | (32.2) | |||
| Poorly | 2,665 | (5.1) | 14,851 | (15) | 17,705 | (11.7) | 17,522 | (11.6) | 8,497 | (11.7) | 8,452 | (11.4) | |||
| Unknown | 2,915 | (5.6) | 6,022 | (6.1) | 8,905 | (5.9) | 8,940 | (5.9) | 4,695 | (6.5) | 4,121 | (5.6) | |||
| Facility location | 0.05 | 0.00 | 0.07 | ||||||||||||
| Metropolitan | 40,901 | (78.6) | 78,077 | (78.8) | 119,516 | (78.8) | 118,977 | (78.8) | 58,262 | (80) | 57,320 | (77.5) | |||
| Urban | 7,662 | (14.7) | 15,102 | (15.3) | 22,922 | (15.1) | 22,687 | (15) | 10,198 | (14) | 11,930 | (16.1) | |||
| Rural | 953 | (1.8) | 1,958 | (2.0) | 2,885 | (1.9) | 2,903 | (1.9) | 1,308 | (1.8) | 1,530 | (2.1) | |||
| Unknown | 2,521 | (4.8) | 3,915 | (4.0) | 6,402 | (4.2) | 6,372 | (4.2) | 3,070 | (4.2) | 3,146 | (4.3) | |||
| Facility type | 0.15 | 0.00 | −0.17 | ||||||||||||
| Academic/research program | 19,106 | (36.7) | 43,554 | (44.0) | 62,698 | (41.3) | 62,606 | (41.5) | 33,364 | (45.8) | 27,553 | (37.3) | |||
| Non-academic program | 32,931 | (63.3) | 55,498 | (56.0) | 89,027 | (58.7) | 88,333 | (58.5) | 39,474 | (54.2) | 46,373 | (62.7) | |||
| Hospital volume | 0.29 | −0.00 | −0.00 | ||||||||||||
| Median (IQR) | 15.9 (6.7–29.3) |
21.6 (11.4–36.1) |
20.5 (8.8–34.7) |
19.9 (9.6–34.1) |
20.8 (9.7–36.5) |
20.7 (9.4–32.8) |
|||||||||
| Radiation Treatment | 0.33 | 0.15 | 0.09 | ||||||||||||
| None | 43,305 | (83.2) | 70,425 | (71.1) | 116,517 | (76.8) | 112,097 | (74.3) | 53,673 | (73.7) | 57,016 | (77.1) | |||
| External beam | 4,859 | (9.3) | 13,532 | (13.7) | 21,058 | (13.9) | 18,151 | (12) | 8,977 | (12.3) | 8,544 | (11.6) | |||
| Brachytherapy | 2,778 | (5.3) | 13,117 | (13.2) | 11,279 | (7.4) | 17,541 | (11.6) | 8,499 | (11.7) | 7,104 | (9.6) | |||
| Unknown | 1,095 | (2.1) | 1,978 | (2.0) | 2,872 | (1.9) | 3,149 | (2.1) | 1,689 | (2.3) | 1,262 | (1.7) | |||
| Chemotherapy | 0.25 | 0.15 | 0.03 | ||||||||||||
| No | 50,016 | (96.1) | 90,975 | (91.9) | 144,721 | (95.4) | 139,956 | (92.7) | 68,478 | (92.9) | 68,403 | (93.7) | |||
| Yes | 395 | (0.8) | 4,862 | (4.9) | 2,735 | (1.8) | 6,587 | (4.4) | 2,821 | (3.8) | 2,397 | (3.3) | |||
| Unknown | 1,626 | (3.1) | 3,215 | (3.2) | 4,269 | (2.8) | 4,395 | (2.9) | 2,420 | (3.3) | 2,245 | (3.1) | |||
Standardized difference (SD)=difference in means or proportions divided by standard error. SD of ≤0.10 is considered to indicate good balance between groups.
Frequency numbers are rounded to integers based on weight. The conventional SD for weighted cohort is consistent with the analysis in weighted regression model using SURVEYLOGISTIC procedure.
4325 patients treated in 1998 excluded since data from 1997 to calculate the previous year lymphadenectomy rate was unavailable.
Figure 1.
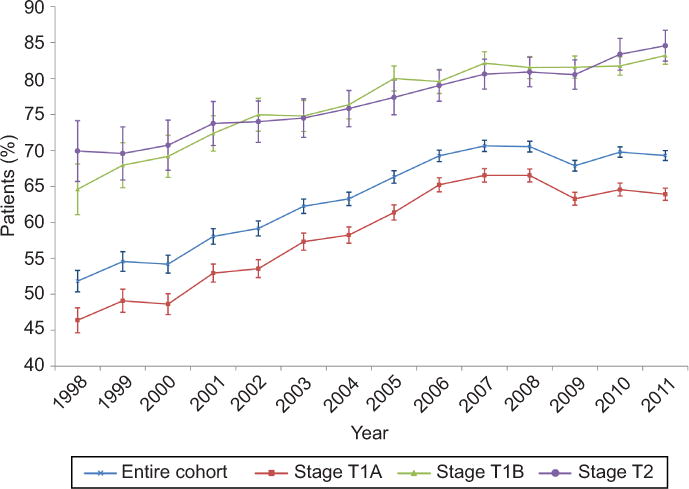
Trends in performance of lymphadenectomy over time. P<.05 for the overall cohort and each subset.
In the unadjusted analysis, there were significant differences in the clinical and demographic characteristics of patients who underwent lymphadenectomy (Table 1). In a multivariable model, more recent year of diagnosis, non-white, non-black race, older age, commercial insurance, residence outside of New England, higher area-level education, higher tumor grade and T stage, and treatment at an academic center were all associated with performance of lymphadenectomy (Appendix 3, available online at http://links.lww.com/xxx.)
After calculation of the propensity score and matching or application of an inverse probability of treatment weighting algorithm, the patient and hospital characteristics were well balanced across the cohorts (Table 1 and Appendix 4 [Appendix 4 is available online at http://links.lww.com/xxx]). The primary instrument, the regional rate of lymphadenectomy varied from 56.0–61.0% in the lowest quintile region to 70.7% in the highest quintile region (Appendix 5, available online at http://links.lww.com/xxx). When the cohort was divided at the median value of the instrument (the lagged difference between observed and predicted lymphadenectomy rate), there was a 7.2% difference in the instrument’s value between the two groups (Table 1). Patients in the group below the median IV value were 3.6% less likely to undergo lymphadenectomy, while those above the median were, on average, 3.6% more likely to undergo lymphadenectomy. Grouping patients by the instrument resulted in a similar distribution of the characteristics in the two groups except for facility type.
In a regression model adjusted for clinical characteristics, performance of lymphadenectomy was associated with a 16% reduction in mortality (HR=0.84; 95% CI, 0.81–0.87) (Table 2). The results were similar after adjustment for clinical characteristics and adjuvant therapy (HR=0.85; 95% CI, 0.82–0.87). The results were largely unchanged and suggested that lymphadenectomy was associated with improved survival after application of propensity score stratification, matching, or inverse probability treatment weighting. In contrast, in the instrumental variable analysis, there was not a statistically significant association between lymphadenectomy and survival (HR=0.75; 95% CI, 0.53–1.06); the results were similar after adjustment for adjuvant treatment (HR=0.76; 95% CI, 0.54–1.06).
Table 2.
Hazard ratio for mortality associated with performance of lymphadenectomy stratified by stage and modeling strategy.
| Hazard ratio for mortality with performance of lymphadenectomy Adjusted HR (95% CI) |
||||
|---|---|---|---|---|
| Entire cohort (n=151,089) |
Stage T1A (n=109,590) |
Stage T1B (n=27,028) |
Stage T2 (n=14,471) |
|
| Unadjusted survival model | 1.10 (1.06, 1.14)** | 1.03 (0.98, 1.07) | 0.73 (0.68, 0.77)** | 0.84 (0.78, 0.90)** |
| Multivariable survival model | ||||
| Clinical characteristics1 | 0.84 (0.81, 0.87)** | 0.87 (0.83, 0.91)** | 0.77 (0.72, 0.81)** | 0.85 (0.79, 0.91)** |
| Clinical and treatment characteristics2 | 0.85 (0.82, 0.87)** | 0.87 (0.83, 0.91)** | 0.77 (0.73, 0.82)** | 0.85 (0.79, 0.92)** |
| Propensity score analysis | ||||
| Stratification by deciles | ||||
| Propensity score alone | 0.86 (0.83, 0.89)** | 0.88 (0.84, 0.93)** | 0.77 (0.72, 0.82)** | 0.83 (0.77, 0.90)** |
| Propensity score plus clinical characteristics1 | 0.83 (0.80, 0.86)** | 0.86 (0.82, 0.90)** | 0.76 (0.72, 0.81)** | 0.84 (0.78, 0.91)** |
| Propensity score plus clinical and treatment characteristics2 | 0.84 (0.81, 0.86)** | 0.86 (0.82, 0.90)** | 0.77 (0.72, 0.81)** | 0.85 (0.79, 0.92)** |
| Propensity score matched cohort | ||||
| Propensity score alone | 0.84 (0.81, 0.87)** | 0.90 (0.85, 0.95)** | 0.72 (0.66, 0.77)** | 0.83 (0.77, 0.90)** |
| Propensity score plus clinical characteristics1 | 0.84 (0.80, 0.88)** | 0.92(0.86, 0.98)** | 0.69(0.63, 0.76)** | 0.82(0.74, 0.91)** |
| Propensity score plus clinical and treatment characteristics2 | 0.84(0.80, 0.88)** | 0.92(0.86, 0.98)** | 0.69 (0.63, 0.76)** | 0.84 (0.75, 0.94)* |
| Inverse probability of treatment weighting | ||||
| Propensity score alone | 0.83 (0.80, 0.86)** | 0.87 (0.83, 0.92)** | 0.74 (0.67, 0.80)** | 0.81 (0.75, 0.88)** |
| Propensity score plus clinical characteristics1 | 0.80 (0.78, 0.83)** | 0.85 (0.80, 0.89)** | 0.71 (0.65, 0.78)** | 0.80 (0.74, 0.87)** |
| Propensity score plus clinical and treatment characteristics2 | 0.80 (0.77, 0.83)** | 0.84 (0.80, 0.89)** | 0.71 (0.65, 0.78)** | 0.80 (0.74, 0.87)** |
| Instrumental variable analysis | ||||
| Instrumental variable plus clinical characteristics1 | 0.75 (0.53, 1.06) | 1.26 (0.83, 1.92) | 0.79 (0.38, 1.66) | 0.15 (0.04, 0.61)** |
| Instrumental variable plus clinical and treatment characteristics2 | 0.76 (0.54, 1.06) | 1.38 (0.86, 2.19) | 0.77 (0.37, 1.64) | 0.20 (0.05, 0.81)* |
Clinical model adjusted for age, race, insurance status, area level education, year of diagnosis, grade, stage, region, annualized hospital volume, facility type, urbanity.
Clinical and treatment characteristics model adjusted for age, race, insurance status, area level education, year of diagnosis, grade, stage, region, annualized hospital volume, facility type, urbanity, radiation treatment and chemotherapy.
P<0.05
P<0.001
When stratified by T stage, the results for T1A and T1B tumors were similar; the multivariable survival models and propensity score models all suggested reduced mortality with lymphadenectomy. However, the lagged IV analysis found no statistically significant association between lymphadenectomy and survival. In contrast, for women with T2 tumors all of the analytic methodologies noted reduced mortality in women who underwent lymphadenectomy (Table 2). Among women with T1A tumors, 5-year survival was 93.2% (95% CI, 93.0–93.4%) in women who underwent lymphadenectomy versus 92.4% (95% CI, 92.2–92.7%) in those without lymphadenectomy (Table 3). For those with T1B tumors, 5-year survival was 82.4% (95% CI, 81.8–83.0%) after lymphadenectomy compared to 78.4% (95% CI, 77.4–79.5%) without lymphadenectomy.
Table 3.
One, 3, and 5-year adjusted survival probabilities based on multivariable adjustment, inverse probability of treatment weighted propensity score analysis and instrumental variable analysis
| Stage T1A | Stage T1B | Stage T2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted Survival probability (95% CI) |
Survival Difference (95%CI)1 |
Adjusted Survival probability (95% CI) |
Survival Difference (95%CI) 1 |
Adjusted Survival probability (95% CI) |
Survival Difference (95%CI) 1 |
||||
| No lymph node dissection |
Lymph node dissection |
No lymph node dissection |
Lymph node dissection |
No lymph node dissection |
Lymph node dissection |
||||
| 1-year | |||||||||
| Multivariate Regression model | 98.9% (98.8,99.0) |
99.0% (99.0, 99.1) |
−0.13 (−1.67, −0.09)* |
96.5% (96.2, 96.8) |
97.2% (97.0, 97.4) |
−0.73 (−0.93, −0.53)* |
96.1% (95.7, 96.5) |
96.6% (96.3, 96.9) |
−0. 52 (−0.79, −0.25)* |
| Propensity Score (IPTW) | 98.8% (98.8, 98.9) |
99.0% (99.0, 99.0) |
−0.17 (−0.21, −0.13)* |
96.1% (95.9, 96.3) |
97.1% (97.0, 97.2) |
−0.99 (−1.11, −0.87)* |
95.4% (95.1, 95.7) |
96.2% (96.0, 96.4) |
−0. 78 (−0.98, −0.98)* |
| Instrumental variable analysis | 99.1% (99.0, 99.3) |
98.8% (98.6, 99.1) |
0.29 (−0.06, 0.64) |
96.2% (94.1, 98.3) |
97.3% (96.9, 97.8) |
−1.16 (−3.67, 1.35) |
84.8% (69.7, 100.0) |
97.2% (96.9, 97.6) |
−12.50 (−29.3, 4.28)* |
| 3-year | |||||||||
| Multivariate Regression model | 95.9% (95.7, 96.1) |
96.3% (96.2, 96.5) |
−0.45 (−0.61, −0.29,)* |
86.8% (86.1, 87.5) |
89.4% (89.0, 89.8) |
−2.57 (−3.26, −1.88)* |
84.9% (84.0, 85.9) |
86.8% (86.2, 87.4) |
−1.82 (−2.78, −0.86)* |
| Propensity Score (IPTW) | 95.7% (95.6, 95.8) |
96.3% (96.2, 96.4) |
−0.60 (−0.70, −0.50)* |
85.1% (84.7, 85.5) |
88.7% (88.4, 89.1) |
−3.60 (−4.03, −3.17)* |
83.8% (83.3, 84.4) |
86.4% (85.9, 86.9) |
−2.58 (−3.19, −1.97)* |
| Instrumental variable analysis | 96.8% (96.1, 97.4) |
95.7% (95.0, 96.4) |
1.07 (−0.24, 2.38) |
85.7% (78.7, 93.2) |
89.7% (88.3, 91.2) |
−4.07 (−12.69, 4.55) |
57.0% (33.8, 96.1) |
89.2% (88.3, 90.2) |
−32.2 (−62.78, −1.62)* |
| 5-year | |||||||||
| Multivariate Regression model | 92.4% (92.2, 92.7) |
93.2% (93.0, 93.4) |
−0.80 (−1.07, −0.53)* |
78.4% (77.4, 79.5) |
82.4% (81.8, 83.0) |
−3.93 (−4.97, −2.89)* |
76.1% (74.8, 77.4) |
78.7% (77.9, 79.5) |
−2.65 (−4.04, −1.25)* |
| Propensity Score (IPTW) | 92.1% (92.0, 92.3) |
93.2% (93.0, 93.4) |
−1.06 (−1.23, −0.88)* |
76.1% (75.5, 76.6) |
81.6% (81.1, 82.1) |
−5.53 (−6.20, −4.86)* |
74.7% (74.0, 75.5) |
78.6% (77.9, 79.2) |
−3.83 (−4.73, −2.93)* |
| Instrumental variable analysis | 94.0% (92.9, 95.2) |
92.2% (91.0, 93.4) |
1.88 (−0.41, 4.17) |
76.6% (66.7, 88.2) |
82.9% (80.7, 85.2) |
−6.17 (−19.05, 6.71) |
42.8% (21.7, 84.2) |
82.4% (80.9, 84.0) |
−39.68 (−70.02, −9.34)* |
Survival Difference= adjusted survival probability of NO LND – Adjusted survival probability of LND.
Multivariable Cox proportional hazard regression model adjusted for clinical characteristics and adjuvant treatment.
Statistically significant survival difference (P<0.05).
We performed a series of sensitivity analyses to estimate the robustness of our findings. When the same year rate of lymphadenectomy (as opposed to lagged lymphadenectomy rate) was assessed as the instrument, the results were similar except that the findings for T2 tumors were attenuated somewhat (Appendix 6, available online at http://links.lww.com/xxx). When the analytic methodology used to calculate the IV was altered through use of a two-stage predictor substitution, the results were very similar. Finally, when the study was limited to only those women who underwent lymphadenectomy and outcomes were compared based on the number of nodes removed (<10 vs. ≥10), the results of the IV analysis suggested no association between extensive lymphadenectomy and survival for patients with T1A and T1B tumors (Appendixes 7 and 8, available online at http://links.lww.com/xxx).
Discussion
These findings suggest that lymphadenectomy is associated with a modest, if any, effect on survival for early-stage endometrial cancer. Although our regression analysis demonstrated improved survival with lymphadenectomy, the instrumental variable analysis did not identify a statistically significant association with survival, suggesting that unmeasured confounding factors may underlie some of the previously reported association with survival.
Prior observational studies have suggested that lymphadenectomy is associated with survival for women with higher risk, early-stage endometrial cancer.3–10 A report of over 12,000 women noted improved survival in women with deep myometrial invasion or high grade, superficially invasive endometrial cancer who underwent lymphadenectomy.3 In our cohort, we also noted improved survival in the observational analysis for all substages and the magnitude of the findings were largely unchanged even after application of propensity score matching. In contrast, the IV analysis found no association between lymphadenectomy and survival for either T1A or T1B tumors.
Our data are in accord with two prior randomized trials that both demonstrated no association between lymphadenectomy and survival.11,12 A trial of over 1400 women from the United Kingdom found no difference in survival with lymphadenectomy for apparent uterine-confined disease.11 An Italian trial of 514 patients reported similar findings.12 Despite the consistent findings of these trials, methodologic concerns, including the quality of lymphadenectomy (low number of nodes, lack of para-aortic nodes removed), enrollment of few patients with positive nodes, as well as concerns regarding the power of the studies to detect differences in survival, have led to continued controversy about the utility of lymphadenectomy.1,13,14
The goal of an instrumental variable analysis is to provide a pseudo-randomization to help control for unmeasured confounding.16,30 The results of our instrumental variable analysis are in line with the randomized data for lymphadenectomy and also suggest minimal association between the procedure on survival. Further, given the large sample size included, we could specifically analyze higher risk women and yet we still found no association between lymphadenectomy and survival. Finally, to address concerns regarding the quality of the lymphadenectomy performed, we performed sensitivity analyses limiting the cohort to removal of 10 or more nodes and still noted no association with survival.
Even if lymphadenectomy is not directly associated with survival, the procedure provides data to tailor adjuvant therapy, potentially avoiding treatment in lower risk patients. One report noted that when matched by grade and stage, women with high-risk disease who underwent lymphadenectomy were less likely to receive adjuvant whole pelvic radiotherapy.31 However, the lack of standardized adjuvant therapy recommendations for endometrial cancer further complicates the interpretation of trials of lymphadenectomy. Women with nodal metastases are now commonly treated with chemotherapy, often in combination with radiation.32 However, women with early-stage disease with high-risk features are increasingly also receiving chemotherapy, thus potentially negating some of the benefit of lymphadenectomy.33
We recognize a number of limitations. While an instrumental variable analysis compensates for unmeasured confounders, the methodology is sensitive to a number of underlying assumptions. First, the instrument should be associated with variation in treatment. An F statistic of >10 has been used as a surrogate to fulfill this assumption. In our analysis, the F statistic for the lagged lymphadenectomy rate was 758. Second, and more difficult to assess statistically, the instrument should not be directly associated with the outcome.34 While this assumption is difficult to verify, geographic variation has been widely used as an IV.15,16,30,34 Third, the dataset does not capture complete data on some factors that may have affected decision-making, including lymphvascular space invasion, intraoperative findings, and comorbidity. Similarly, using administrative data, it is impossible to distinguish patients with grossly enlarged nodes who underwent resection versus diagnostic sampling. In our analysis, by definition, these women were included in the lymphadenectomy cohort. Lastly, there is no standard definition for what constitutes an adequate lymphadenectomy. We performed a wide range of sensitivity analyses examining removal of different numbers of lymph nodes.
For gynecologists, these data highlight the difficulty in the decision to perform lymphadnectomy A recent decision analysis for clinical stage I tumors, 3 year survival rates ranged from 88–93% across various scenarios, suggesting that outcomes are good regardless of the therapeutic approach chosen.1,13 Similarly, our data suggest that at the population-level any survival benefit from lymphadenectomy is likely very small. Whether the small potential benefit of lymphadenectomy justifies the costs and potential complications of the procedure and whether further prospective study is warranted or even feasible remains a question of active debate.1
Supplementary Material
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants and Dr. Tergas is the recipient of a fellowship (NCI R25 CA094061-11) from the National Cancer Institute.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.McMeekin DS. Designing the next lymphadenectomy trial: what should we learn for our prior experiences. Gynecol Oncol. 2012;126:1–2. doi: 10.1016/j.ygyno.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 3.Chan JK, Cheung MK, Huh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006;107:1823–30. doi: 10.1002/cncr.22185. [DOI] [PubMed] [Google Scholar]
- 4.Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–75. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 5.Lutman CV, Havrilesky LJ, Cragun JM, et al. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol Oncol. 2006;102:92–7. doi: 10.1016/j.ygyno.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Kilgore LC, Partridge EE, Alvarez RD, et al. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 7.Mohan DS, Samuels MA, Selim MA, et al. Long-term outcomes of therapeutic pelvic lymphadenectomy for stage I endometrial adenocarcinoma. Gynecol Oncol. 1998;70:165–71. doi: 10.1006/gyno.1998.5098. [DOI] [PubMed] [Google Scholar]
- 8.Lowery WJ, Gehrig PA, Ko E, Secord AA, Chino J, Havrilesky LJ. Surgical staging for endometrial cancer in the elderly – is there a role for lymphadenectomy? Gynecol Oncol. 2012;126:12–5. doi: 10.1016/j.ygyno.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Bendifallah S, Koskas M, Ballester M, Genin AS, Darai E, Rouzier R. The survival impact of systematic lymphadenectomy in endometrial cancer with the use of propensity score matching analysis. Am J Obstet Gynecol. 2012;206:500 e1–11. doi: 10.1016/j.ajog.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 10.Chino JP, Jones E, Berchuck A, Secord AA, Havrilesky LJ. The influence of radiation modality and lymph node dissection on survival in early-stage endometrial cancer. Int J Radiat Oncol Biol Phys. 2012;82:1872–9. doi: 10.1016/j.ijrobp.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 13.Naumann RW. The role of lymphadenectomy in endometrial cancer: was the ASTEC trial doomed by design and are we destined to repeat that mistake? Gynecol Oncol. 2012;126:5–11. doi: 10.1016/j.ygyno.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 14.Creasman WT, Mutch DE, Herzog TJ. ASTEC lymphadenectomy and radiation therapy studies: are conclusions valid? Gynecol Oncol. 2010;116:293–4. doi: 10.1016/j.ygyno.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 15.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. Jama. 2007;297:278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–93. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012;30:4215–22. doi: 10.1200/JCO.2012.41.6701. [DOI] [PubMed] [Google Scholar]
- 18.The National Cancer Data Base. at https://www.facs.org/qualityprograms/cancer/ncdb.)
- 19.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmila MR, Birkmeyer NJ, Arbabi S, Osborne NH, Wahl WL, Dimick JB. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145:939–45. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 22.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. Jama. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo YF, Montie JE, Shahinian VB. Reducing bias in the assessment of treatment effectiveness: androgen deprivation therapy for prostate cancer. Med Care. 2012;50:374–80. doi: 10.1097/MLR.0b013e318245a086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27:531–43. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrido MM, Deb P, Burgess JF, Jr, Penrod JD. Choosing models for health care cost analyses: issues of nonlinearity and endogeneity. Health Serv Res. 2012;47:2377–97. doi: 10.1111/j.1475-6773.2012.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Malley AJ, Frank RG, Normand SL. Estimating cost-offsets of new medications: use of new antipsychotics and mental health costs for schizophrenia. Stat Med. 2011;30:1971–88. doi: 10.1002/sim.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan F, Small D, Bekelman JE, Mitra N. Bias in estimating the causal hazard ratio when using two-stage instrumental variable methods. Stat Med. 2015 doi: 10.1002/sim.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–58. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–9. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Wright JD, Ananth CV, Tsui J, et al. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer. 2014;120:1246–54. doi: 10.1002/cncr.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma C, Deutsch I, Lewin SN, et al. Lymphadenectomy influences the utilization of adjuvant radiation treatment for endometrial cancer. Am J Obstet Gynecol. 2011;205:562 e1–9. doi: 10.1016/j.ajog.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 33.Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226–33. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Garabedian LF, Chu P, Toh S, Zaslavsky AM, Soumerai SB. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann Intern Med. 2014;161:131–8. doi: 10.7326/M13-1887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


