Abstract
Traumatic spinal cord injury (SCI) is a devastating injury causing significant morbidity and mortality. Experimental studies have demonstrated that SCI induced cellular damage and disruption of the blood-spinal cord-barrier can initiate an autoimmune response. This response is thought to be pathogenic and contribute to poor outcome. The objective of this research was to investigate whether human SCI mounts an autoimmune response to self-antigens. Plasma samples were collected longitudinally from SCI patients (n=18) at acute (T1, <48 hr) and subacute (T2, 2-4 wk) time points to probe western blots of human brain homogenates in order to screen patients for the presence of putative autoantibodies. To identify the corresponding antigens, 2-dimensional (2D) gel electrophoresis, western blot and liquid chromatography coupled with mass spectrometry (LC-MS/MS) analyses were performed. We found that 4 of 18 patients (22%) had novel immunoreactive bands ranging in size from 36-42 kDa present in subacute, but not in acute, plasma samples suggesting post-injury production. To identify the cross-reacting antigens, we separated brain proteins by 2D gel electrophoresis and identified 9 immunoreactive spots. Amino acid sequence analysis of these spots identified peptides that mapped to glial fibrillary acidic protein (GFAP). Our results suggest that approximately 22% of SCI patients generated autoantibodies to GFAP. Future studies will be required to determine if these autoantibodies contribute to the pathogenic sequelae of SCI.
Keywords: GFAP, SCI, autoantibody
INTRODUCTION
Traumatic spinal cord injury (SCI) is a devastating injury that can profoundly alter quality of life. Deficits resulting from SCI can be permanent and, at present, there is no cure for SCI. Experimental studies have shown that SCI can initiate a B-cell-mediated autoimmune response leading to autoantibody production. For example, Popovich and colleagues reported that experimental SCI caused autoantibody production to DNA, RNA, and several CNS proteins, which contributed to exaggerated tissue damage and poor functional outcome.[1] Furthermore, injection of IgG purified from SCI mice into uninjured mice resulted in poor motor outcome and spinal cord pathology similar to that seen in SCI mice.[2] In a study of chronic SCI patients, the group mean autoantibody titer to GM1 ganglioside was significantly elevated relative to healthy controls, with 6 of 24 (25%) SCI patients’ titers exceeding the threshold of the control mean + 3 SD.[3] In an effort to identify other autoantibodies that may contribute to human SCI pathology, we used western blotting to screen plasma samples from SCI patients.
METHODS
The protocol for the use of human subjects was reviewed and approved by the University of Texas Health Science Center Committee for the Protection of Human Subjects. Blood samples were obtained with subjects’ informed consent. Adult subjects with non-penetrating SCI and no history of other neurological disease, injury, recent infection or cancer were enrolled in the study. Plasma was collected within the first 48 hours of SCI (T1) and again between 2-4 weeks after injury (T2) to perform within-patient comparisons to identify changes in antibody cross-reactivity that may indicate autoantibody production. These time points were chosen as IgG production typically peaks between 2-3 weeks after presentation of an antigen.[4] Patient demographic and clinical information are shown in Table 1.
Table 1.
Demographic and clinical information for subjects
| Value (%) | ||
|---|---|---|
| Gender | Males | 17(94) |
| Females | 1 (6) | |
| Age (average, years[range]) | 38.1±14.6 [18-69] | |
| Race | White | 15 (83) |
| Black/African American | 3 (17) | |
| Ethnicity | Hispanic | 3 (17) |
| Non-Hispanic | 15 (83) | |
| Injury – highest level | Cervical | 14 (78) |
| Thoracic | 4 (22) | |
| ASIA Impairment Grade* | A (complete) | 12 (67) |
| B (sensory incomplete) | 2 (11) | |
| C (motor incomplete) | 4 (22) |
* American Spinal Injury Association
A = Complete. No sensory or motor function is preserved in the sacral segments S4-S5.
B = Sensory Incomplete. Sensory but not motor function is preserved below the neurological level and includes the sacral segments S4-S5 and no motor function is preserved more than three levels below the motor level on either side of the body.
C = Motor Incomplete. Motor function is preserved below the neurological level, and more than half of key muscle functions below the single neurological level of injury have a muscle grade less than 3.
Human cadaver brain tissue was obtained from the UTHealth Willed Body Program and was frozen at −80°C. Western blots were performed on Immobilon-P membranes using diluted plasma (1:1000) as the primary antibody, a goat anti-human alkaline phosphatase-conjugated secondary antibody, and immunoreactive bands were detected using the chemilumenescent substrate CDP-Star. To identify cross-reacting self-antigens, large format (20 × 22 cm) 2D gels (Kendrick Labs) and western analysis were performed.[5,6] Gels were run in duplicate; one for western blotting and one for Coomassie staining. A section of the PVDF membrane surrounding molecular weight 42 kDa was used for western blotting with T1 and T2 plasma to minimize use of reagents. Immunoreactive spots with signal intensities that were higher when probed with the T2 sample as compared to the T1 sample from the same patient were selected. The corresponding spots from the Coomassie stained gels were carefully cut out for liquid chromatography coupled with mass spectrometry (LC-MS/MS) identification. Gel punches were washed, rehydrated and trypsin digested. The peptide mixture was analyzed by LC-MS/MS using a NanoAcuity UPLC coupled to a Q-TOF Ultima API MS (Micromass/Waters, Milford, MA), as previously described.[7] A Mascot and ProteinLynx Global Server (PLGS) database search provided a list of proteins for each gel spot. The MS/MS spectra of proteins identified by either one peptide or a Mascot score lower than 25 were verified to eliminate false positive results.
RESULTS
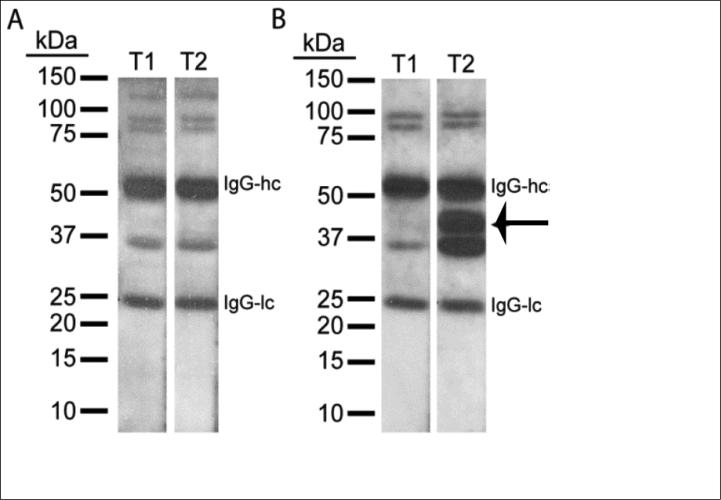
Pictures of representative western blots from two SCI patients are shown in Figure 1. Figure 1A shows a patient that had no substantial change to the immunoreactive band pattern detected when total brain proteins were probed with plasma collected at acute (T1) and subacute (T2) time points after injury. In contrast, Figure 1B shows the results from a patient in which there was a large increase in staining of the band at 36 kDa, and the appearance of a new immunoreactive band at 42 kDa, suggesting the post-injury generation of autoantibodies. Screening of T1 and T2 plasma from a total of 18 SCI patients identified 4 subjects (22%) that demonstrated increased staining of protein bands between 36 and 42 kDa.
Figure 1.
Western blots of human SCI plasma Time 1 and Time 2. Pictures of representative western blots probed with T1 (<48 hr) and T2 (2-4 weeks post-injury) plasma from two SCI patients. A. Although a few cross-reacting bands are detected for this patient, the intensity of immunoreactive bands was not specifically enhanced at T2. B. T2 plasma from a second patient cross-reacted with bands ranging from 36 to 42 kDa whose intensities were higher compared to T1 plasma from the same patient. (IgG-hc: Immunoglobulin G heavy chain, IgG-lc: Immunoglobulin G light chain.)
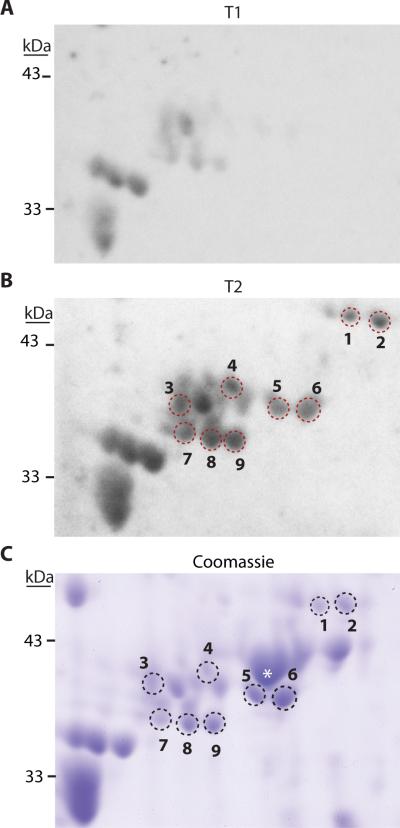
To identify the self-antigen(s), we probed blots of total brain extracts separated by 2D gel electrophoresis with T1 and T2 plasma. Pictures of representative western blots (Figure 2A and 2B) revealed several immunoreactive spots with higher signal intensities when probed with the T2 plasma as compared to the T1 plasma from the same patient. Selected immunoreactive spots (1-9 in Figure 2B) were matched to a corresponding Coomassie-stained 2D gel (Figure 2C), and carefully excised for LC-MS/MS analysis. Mass spectrometry results for spots 1-9 are presented in Table 2, showing peptides that mapped to GFAP were present in all spots. Searches of the Mascot and PLGS databases revealed that 81% of the identified unique peptides (131 of 161) mapped to human GFAP. The remaining peptides may have resulted from contamination of nearby proteins. For example, a minority of peptides that mapped to β-actin were present in spots 5 and 6. However, when we re-probed the blot with antibodies specific to β-actin, we determined the immunoreactive signal did not co-localize with the location of spots 5 and 6, but rather an abundant adjacent spot (Figure 2C, marked by an asterisk, data not shown).
Figure 2.
Western blots and Coomassie stained gel showing increased immunoreactivity after SCI. Picture of a representative western blot from membranes probed with A. T1 plasma, and B. T2 plasma from a patient positive for autoantibodies. C. Coomassie-stained 2D gel from the same area as shown in the membranes. Spots excised for LC-MS/MS are indicated by numbers; * indicates location of β-actin. (T1, (<48hrs) T2, (2-4 weeks) post-injury.)
Table 2.
LC-MS/MS results for individual spots. Spot numbers correspond to the spot indicated in Figure 2.
| Spot # | Protein Identified | Mascot Score | # GFAP Unique Peptides | % Sequence Coverage |
|---|---|---|---|---|
| 1 | glial fibrillary acidic protein [Homo sapiens] | 66 | 4 | 13 |
| 2 | glial fibrillary acidic protein, isoform 1 [Homo sapiens] | 1362 | 30 | 67 |
| 3 | glial fibrillary acidic protein [Homo sapiens] | 410 | 13 | 29 |
| 4 | glial fibrillary acidic protein [Homo sapiens] | PLGS | 3 | 7 |
| 5 | glial fibrillary acidic protein [Homo sapiens] | 171 | 5 | 15 |
| 6 | glial fibrillary acidic protein [Homo sapiens] | 483 | 16 | 38 |
| 7 | glial fibrillary acidic protein [Homo sapiens] | 139 | 8 | 20 |
| 8 | glial fibrillary acidic protein [Homo sapiens] | 780 | 19 | 45 |
| 9 | glial fibrillary acidic protein [Homo sapiens] | 1345 | 33 | 59 |
GFAP Geneinfo identifiers include the following: gi|4503979, gi|196115290, gi|334688844, gi|38566198, gi|16265836, gi|119571954, gi|62896925, gi|226236
DISCUSSION
Our 2D gel and LC-MS/MS results indicate that the examined protein spots contained GFAP, although their molecular weights slightly varied. The molecular weight and isoelectric point diversity of the GFAP-immunoreactive spots could have resulted from different post-translational modifications, breakdown of full-length GFAP in the post-mortem tissue, or from alternatively spliced variants of GFAP. At least 8 GFAP isoforms (α,γ,δ/ε,κ,Δ135,Δ164,Δexon6, Δexon7) have been described in the human brain.[8,9] Multiple GFAP isoforms and their post-translational modifications have previously been shown to occur in staircase-like patterns in brain tissue on 2D gels.[10,11] Post-translational changes, including glycosylation, phosphorylation and increased GFAP expression, are thought to be from astrogliosis, tissue damage and inflammation.[11] Alternatively, degradation of GFAP in post-mortem brain could give rise to a change in protein spot migration. Previous studies have shown that while most proteins are reasonably stable over time in the post-mortem brain, the abundance of different GFAP spots changed in relation to post-mortem interval, even at 4 degrees, indicating that GFAP is particularly susceptible to degradation.[12] Thus, some of the cross-reacting spots could be breakdown products, alternatively spliced GFAP or post-translationally modified forms of GFAP present in the tissue sample. Although autoantibodies to GFAP have been detected in patients following TBI and in persons with other neurological diseases, [13-16] this is the first study to demonstrate that autoantibodies to GFAP occur after SCI.
One limitation of this study is that the incidence of GFAP autoantibodies in our study population (22%) is limited by the small sample size and the time points chosen for analysis. In a traumatic brain injury (TBI) study evaluating 53 severely injured patients, a higher incidence of autoantibodies against GFAP has been reported.[16] Although we detected GFAP autoantibodies only in patients with an American Spinal Injury Association (ASIA) grade A (4/12; 33%), the incidence in milder injury grades could not be determined due to the limited number of patients in those grades (Table 1). At present, it is not clear if GFAP or a GFAP breakdown product (proposed biomarkers in CNS injury) released as a result of SCI initiated the autoantibody response.[16,17] Previous studies have reported that GFAP can be detected in the serum of SCI patients where it can elicit an immune response.[18] Alternatively, an immune response could have been initiated within the injured spinal cord itself where the blood-spinal cord barrier is disrupted and the concentration of released GFAP/GFAP breakdown products are likely to be substantially higher. Another limitation of the current study is that the consequences of autoantibody production on outcome have not been investigated. GFAP autoantibodies in SCI patients may interfere with the ability of GFAP to assemble into its functional filamentous structure, resulting in malfunctioning astrocytes. As astrocytes are intimately linked to blood-spinal cord barrier function, this could lead to prolonged disruption in barrier function. Additionally, impaired astrocyte function may cause inefficient removal of extracellular glutamate, which could exacerbate tissue damage. Alternately, GFAP autoantibodies could serve a protective role by limiting glial scar formation, a major hindrance to axonal regeneration post-SCI. Future studies will address these interesting possibilities.
Acknowledgement
The authors would like to thank Dr. Jon Johansen and Dr. Costel C. Darie for their expertise in 2D gel electrophoresis and mass spectrometry analysis, respectively.
Source of Funding: This work was supported in part by a grant from Mission Connect/TIRR Foundation and grants from NIH (NS087149 and NS088298).
Footnotes
Conflicts of Interest: no conflicts of interest are declared.
Reference List
- 1.Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 2.Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K, et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19:753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- 4.Skoda D, Kranda K, Bojar M, Glosova L, Baurle J, Kenney J, et al. Antibody formation against beta-tubulin class III in response to brain trauma. Brain Res Bull. 2006;68:213–216. doi: 10.1016/j.brainresbull.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Burgess-Cassler A, Johansen JJ, Santek DA, Ide JR, Kendrick NC. Computerized quantitative analysis of coomassie-blue-stained serum proteins separated by two-dimensional electrophoresis. Clin Chem. 1989;35:2297–2304. [PubMed] [Google Scholar]
- 6.O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 7.Darie CC, Deinhardt K, Zhang G, Cardasis HS, Chao MV, Neubert TA. Identifying transient protein-protein interactions in EphB2 signaling by blue native PAGE and mass spectrometry. Proteomics. 2011;11:4514–4528. doi: 10.1002/pmic.201000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–130. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Ciesielski-Treska J, Goetschy JF, Aunis D. Proteolytic degradation of vimentin and glial fibrillary acidic protein in rat astrocytes in primary culture. Eur J Biochem. 1984;138:465–471. doi: 10.1111/j.1432-1033.1984.tb07939.x. [DOI] [PubMed] [Google Scholar]
- 11.Korolainen MA, Auriola S, Nyman TA, Alafuzoff I, Pirttila T. Proteomic analysis of glial fibrillary acidic protein in Alzheimer's disease and aging brain. Neurobiol Dis. 2005;20:858–870. doi: 10.1016/j.nbd.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Crecelius A, Gotz A, Arzberger T, Frohlich T, Arnold GJ, Ferrer I, et al. Assessing quantitative post-mortem changes in the gray matter of the human frontal cortex proteome by 2-D DIGE. Proteomics. 2008;8:1276–1291. doi: 10.1002/pmic.200700728. [DOI] [PubMed] [Google Scholar]
- 13.Lotosh NG, Savel'eva EK, Selishcheva AA, Savel'ev SV. Autoantibodies to neuron-specific proteins S100, GFAP, MBP and NGF in the serum of rats with streptozotocin-induced diabetes. Bull Exp Biol Med. 2013;155:48–51. doi: 10.1007/s10517-013-2077-5. [DOI] [PubMed] [Google Scholar]
- 14.Mecocci P, Parnetti L, Romano G, Scarelli A, Chionne F, Cecchetti R, et al. Serum anti-GFAP and anti-S100 autoantibodies in brain aging, Alzheimer's disease and vascular dementia. J Neuroimmunol. 1995;57:165–170. doi: 10.1016/0165-5728(94)00180-v. [DOI] [PubMed] [Google Scholar]
- 15.Wei P, Zhang W, Yang LS, Zhang HS, Xu XE, Jiang YH, et al. Serum GFAP autoantibody as an ELISA-detectable glioma marker. Tumour Biol. 2013;34:2283–2292. doi: 10.1007/s13277-013-0770-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Zoltewicz JS, Mondello S, Newsom KJ, Yang Z, Yang B, et al. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS One. 2014;9:e92698. doi: 10.1371/journal.pone.0092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papa L, Lewis LM, Falk JL, Zhang Z, Silvestri S, Giordano P, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59:471–483. doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahadi R, Khodagholi F, Daneshi A, Vafaei A, Mafi AA, Jorjani M. Diagnostic Value of Serum Levels of GFAP, pNF-H, and NSE Compared With Clinical Findings in Severity Assessment of Human Traumatic Spinal Cord Injury. Spine. 2015;40:E823–E830. doi: 10.1097/BRS.0000000000000654. [DOI] [PubMed] [Google Scholar]