Abstract
Developmental dyslexia is commonly thought to arise from specific phonological impairments. However, recent evidence is consistent with the possibility that phonological impairments arise as symptoms of an underlying dysfunction of procedural learning. The nature of the link between impaired procedural learning and phonological dysfunction is unresolved. Motivated by the observation that speech processing involves the acquisition of procedural category knowledge, the present study investigates the possibility that procedural learning impairment may affect phonological processing by interfering with the typical course of phonetic category learning. The present study tests this hypothesis while controlling for linguistic experience and possible speech-specific deficits by comparing auditory category learning across artificial, nonlinguistic sounds among dyslexic adults and matched controls in a specialized first-person shooter videogame that has been shown to engage procedural learning. Nonspeech auditory category learning was assessed online via within-game measures and also with a post-training task involving overt categorization of familiar and novel sound exemplars. Each measure reveals that dyslexic participants do not acquire procedural category knowledge as effectively as age- and cognitive-ability matched controls. This difference cannot be explained by differences in perceptual acuity for the sounds. Moreover, poor nonspeech category learning is associated with slower phonological processing. Whereas phonological processing impairments have been emphasized as the cause of dyslexia, the current results suggest that impaired auditory category learning, general in nature and not specific to speech signals, could contribute to phonological deficits in dyslexia with subsequent negative effects on language acquisition and reading. Implications for the neuro-cognitive mechanisms of developmental dyslexia are discussed.
Keywords: Developmental dyslexia, category acquisition, procedural learning, speech, videogame training
1. Introduction
Developmental dyslexia is a neurological disorder characterized by a persistent inability to achieve typical reading levels that is not a result of disorders of general intelligence, emotional disturbances, gross neurological deficits or inadequate schooling. A central hypothesis is that dyslexia involves a core deficit in the direct access to, and manipulation of, phonemic language units retrieved from long-term declarative memory (Snowling, 2000). Consistent with this phonological hypothesis, impaired phonological awareness, poor verbal short term memory and slow lexical retrieval are among the most frequent symptoms associated with dyslexia (Vellutino, Fletcher, Snowling, & Scanlon, 2004). However, individuals with dyslexia have a wide range of non-linguistic deficits that are difficult to reconcile with a strictly phonological etiology (Facoetti et al., 2003; Howard, Howard, Japikse, & Eden, 2006; Tallal, 1980).
1.2 The Procedural Learning Deficit Account
One central theory that addresses these non-phonological deficits claims that difficulty in phonology, reading, writing and spelling skills in dyslexia may be related to selective impairment in procedural learning associated with the learning and control of established sensorimotor and cognitive habits, skills, and procedures (Nicolson & Fawcett, 2011). Individuals with dyslexia are impaired at a variety of tasks believed to be sub-served by procedural learning including motor adaptation (Brookes, Nicolson, & Fawcett, 2007), implicit sequence learning (Howard et al., 2006; Vicari, Marotta, Menghini, Molinari, & Petrosini, 2003), probabilistic category learning (Gabay, Vakil, Schiff, & Holt, in press), and artificial grammar learning (Pavlidou, Williams, & Kelly, 2009). Procedural learning among individuals with dyslexia is less stable, more prone to interference (Gabay, Schiff, & Vakil, 2012b), and less effectively consolidated (Gabay, Schiff, & Vakil, 2012a). Neuroimaging studies have revealed impairments in brain regions associated with procedural learning tasks among individuals with dyslexia (Nicolson et al., 1999; Pernet, Poline, Demonet, & Rousselet, 2009; Rae et al., 1998). However, the nature of the link between impaired procedural learning and phonological deficits is unresolved.
1.3 A possible relationship between procedural learning and the formation of speech categories
In the present study we examine a route by which impaired procedural learning may affect phonological processing in dyslexia. We begin with the observation that speech categories are inherently multidimensional, with no single acoustic dimension necessary or sufficient to signaling phonetic category membership. Acoustic information along these dimensions is highly variable as a result of talker differences, coarticulation, and other factors. Adding to the complexity, critical information is rapidly conveyed across tens of milliseconds. These factors converge to make explicit attempts to discover and integrate acoustic cues that are diagnostic to speech category identity extremely difficult. Speech learning is therefore a true procedural knowledge learning challenge in that listeners must discover diagnostic dimensions across highly variable sensory input and perceptually weight these dimensions according to how well the dimensions signal category membership (Chandrasekaran, Koslov, & Maddox, 2014; Chandrasekaran, Yi, & Maddox, 2014; Lim, Fiez, & Holt, 2014).
Furthermore, speech category acquisition ‘in the wild’ occurs under incidental conditions, without instructions to search for category-diagnostic dimensions, overt category decisions, or explicitly-provided feedback. Beyond ecological validity, this is an important issue because there is growing evidence that overt and incidental learning paradigms draw upon neural substrates with distinctive computational specialties (e.g. Doya, 1999; Lim, Fiez, Wheeler, & Holt, 2013; Tricomi, Delgado, McCandliss, McClelland, & Fiez, 2006). Other studies have demonstrated the existence of two distinct category learning systems that appear to operate for both visual and speech category learning (Ashby & Alfonso-Reese, 1998; Yi, Maddox, Mumford, & Chandrasekaran, 2014).
We propose that a general impairment in acquisition of procedural category knowledge in the auditory domain would be expected to impact phonetic category learning across speech signals, with the potential for cascading effects for language acquisition and learning to read. Although recent research with neurotypical individuals implicates procedural learning in phonetic (Chandrasekaran, Yi, et al., 2014) and nonspeech auditory category learning (Lim et al., 2013), acquisition of procedural category knowledge in the auditory domain has not been investigated in dyslexia. Previous studies examining speech perception among individuals with dyslexia have mostly used classic categorical perception tasks in which participants must identify speech sounds that morph between two phonemes in incremental steps along a continuum (Godfrey, Syrdal-Lasky, Millay, & Knox, 1981; Manis et al., 1997; Vandermosten et al., 2010). This approach examines the end product of learning by having participants access established categories. It remains an open question whether auditory category learning is impaired in dyslexia.
We hypothesize that procedural learning impairment in dyslexia may interfere with learning procedural knowledge characterizing speech categories. We further hypothesize that this impairment may be general in nature, affecting acquisition of auditory categories characterized by procedural knowledge, whether they are comprised of speech or nonspeech signals.
1.4 The present study
To test these hypotheses, we employed a first-person shooter videogame paradigm that has been used in previous research to incidentally train listeners to learn complex, artificial, nonlinguistic nonspeech categories (Leech, Holt, Devlin, & Dick, 2009; Lim et al., 2013; Lim, Lacerda, & Holt; S. Lim & Holt, 2011; Liu & Holt, 2011; Wade & Holt, 2005); see Figure 1. Participants’ task is to navigate through a space-themed virtual world, shooting and capturing alien creatures as they appear. There is no overt sound categorization task and no explicit categorization-related feedback. However, acoustically-variable sounds drawn from a category are consistently associated with the appearance of a particular alien creature. Therefore, learning the functional equivalence of within-category sounds can support success at the primary space navigation task. The virtual world is characterized by task demands that mimic some aspects of natural environments. Participants make goal-directed actions for which there is a positive or negative outcome contingent on behavior, the actions are performed in the context of expectations about outcomes, and there is an incentive to succeed. These task characteristics are known to robustly engage the striatal learning system of the basal ganglia (Delgado, Stenger, & Fiez, 2004; Tricomi et al., 2006), implicated as a contributor to learning procedural knowledge (Seger, 2006). Indeed, neuroimaging research reveals that successful auditory category learning within the videogame recruits striatal activation (Lim et al., 2013), engages putatively speech-selective left posterior superior temporal cortex for processing exemplars drawn from the newly-acquired nonspeech categories (Leech et al., 2009), and warps perceptual space and early auditory evoked responses in a manner like that observed in speech category acquisition (Liu & Holt, 2011). This incidental training is also effective in promoting phonetic category learning. Adult native-Japanese second-language learners of English significantly improve in categorizing English [r]-[l] (a notoriously difficult second-language phonetic learning challenge; Bradlow, Pisoni, Akahane-Yamada, & Tohkura, 1997; Ingvalson, Holt, & McClelland, 2012; Ingvalson, McClelland, & Holt, 2011) with just 2.5 hours of incidental training within the videogame (Lim & Holt, 2011). Moreover, the incidental training in the videogame is effective even when non-native speech categories are presented in continuous foreign-language speech, thus requiring listeners to simultaneously solve both speech segmentation and categorization learning challenges (Lim et al., in press)
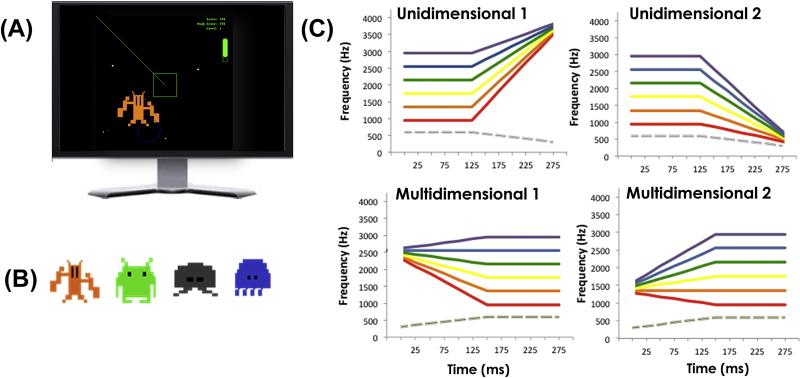
Figure 1.
(A) A screenshot of the Wade and Holt (2005) videogame. As an alien approaches, a sound exemplar from an associated sound category is presented. Participants make shooting or capturing actions according to whether the alien is a friend or foe, as indicated by shape of the central aiming mechanism (green square). (B) The four aliens have different colors, shapes, and characteristic movement patterns. (C) Each of the aliens is associated with one of the four sound categories depicted here. Each of the higher-frequency components indicated by different colors is paired with the grey lower-frequency component to create six exemplars per category. Unidimensional categories are characterized by a rising (top left) or falling (top right) frequency sweep whereas no single acoustic dimension defines the multidimensional categories (bottom).
2. Methods
2.1 Participants
Participants included adults with dyslexia (N=14, 6M, 8F) and controls (N=14, 6M, 8F) matched very closely in age, sex and nonverbal intelligence (See Table I). Ten of the participants with dyslexia and 8 control participants also completed an additional sound discrimination task (the remainder of the participants were unavailable to complete the task). All were university students in the area of Pittsburgh, PA. All participants were native English speakers with no reported signs of sensory or neurological deficits and came from families with middle to high socioeconomic status. Diagnosis of a comorbid learning disability such as ADHD was an exclusion criterion; two participants with dyslexia who had severe symptoms and a diagnosis of ADHD were excluded from the sample. A well-documented history of dyslexia was the inclusion criterion for the dyslexia group: 1) each individual received a formal diagnosis of dyslexia by a qualified psychologist; 2) each individual's diagnosis was verified by the diagnostic and therapeutic center at their university; and 3) each individual was receiving accommodations in educational settings. The Control group was age matched with the Dyslexia group, with no reading problems and the same level of cognitive ability (as measured by the Raven's Standard Progressive Matrices (SPM) test; Raven, 1992). Inclusion criteria for the Control group were no prior history of learning disabilities and performance at or above average on standardized measures of reading. Written informed consent was obtained from all participants.
Table I.
Demographic, cognitive and language-related data for Dyslexia and Control groups
| Group |
|||
|---|---|---|---|
| Measure | Dyslexia | Controls | p |
| Age (years) | 21.28 (3.75) | 21.57 (2.4) | n.s. |
| Raven | 56.71 (2.94) | 57.85 (1.56) | n.s. |
| Digit spana (combined) | 10.71 (2.67) | 15 (2.57) | <.01** |
| RAN objectsa | 99.85 (18.13) | 117.35 (12.07) | <.01* |
| RAN colorsa | 95.64 (12.21) | 111.92 (11.18) | <01** |
| RAN numbersa | 105.71 (5.51) | 113.35 (4.41) | <.01** |
| RAN lettersa | 101.78 (5.76) | 112 (4.16) | <.01** |
| WRMT-R WIa | 98.14 (4.22) | 109.42 (4.76) | <.01** |
| WRMT-R WAa | 96 (7.71) | 112.64 (10.87) | <.01** |
| Towre SW (A+B)a | 97.42 (7.31) | 119.28 (8.44) | <.01** |
| Towre PD (A+B)a | 89.42 (7.38) | 115.28 (9.44) | <.01** |
| Spoonerism accuracy | 8.28 (3.38) | 11.14 (2.1) | <.05* |
| Spoonerism Time | 138 (41.91) | 86 (21.57) | <.01** |
Indicates standardized scores; other scores presented as raw scores
All participants underwent a series of cognitive tests to evaluate general intelligence (Raven's SPM; Raven, 1992), verbal working memory (as measured by the forward and backward Digit Span from the Wechsler Adult Intelligence Scale-III; Wechsler, 1997), rapid naming (Wolf & Denckla, 2005), and phonological awareness (Spoonerism; Brunswick et al., 1999). In addition, all participants performed both un-timed and timed (fluency) tests of word reading and decoding skills and Word Identification (WI) and Word Attack (WA) subtests form the Woodcock Reading Mastery Test-Revised (WRMT-R; Woodcock, 1987). In addition, participants performed the Sight Word Efficiency, Forms A+B (i.e., rate of word identification) and Phonemic Decoding Efficiency, Forms A+B (i.e., rate of decoding pseudo words) subtests form the Test of Word Reading Efficiency (TOWRE-II; Torgesen, Wagner, & Rashotte, 1999). Results are presented in Table I.
The groups did not differ according to age or intelligence. However, the Dyslexia group differed significantly from the Control group on word reading and decoding skills across both rate and accuracy measures. In addition, the Dyslexia group was impaired compared with the Control group in three major phonological domains: phonological awareness (Spoonerisms), verbal short-term memory (digit span) and rapid naming (rapid automatized naming).
It is noted that all participants in the Dyslexia group were high functioning university students with dyslexia. Prior studies of dyslexia reveal that such participants exhibit average performance on standardized reading tests (including performance on low frequency words, as in the Woodcock Reading Mastery Test-Revised). Nevertheless, they differ significantly from matched control groups and continue to present phonological problems that can be assessed by phonological tests such as the Spoonerism test (Wilson & Lesaux, 2001). Our dyslexic participants fit this profile. Each individual had received a formal diagnosis of developmental dyslexia by a qualified psychologist and was receiving testing accommodations. The Dyslexia group differed significantly from the Control group on all literacy measures and exhibited phonological processing impairments (as indicated by the Spoonerism test), despite average performance on standardized tests. This profile is clearly indicative of a sample of dyslexic adults.
2.2 Stimuli
There were four auditory categories (identical to those used by Wade and Holt, 2005) (see also Emberson, Liu, & Zevin, 2013; Leech et al., 2009; Liu & Holt, 2011). Figure 1c shows schematized versions of the six exemplars defining each category. Two of the categories (unidimensional categories) were differentiated by a single, perceptually-salient acoustic dimension. The other two categories (multidimensional categories) were defined such that no single acoustic dimension determined category membership. Across all categories, each sound exemplar was 250 ms in duration and had a lower-frequency (P1, grey line, Fig 1c) and a single higher-frequency (P2, colored lines, Fig 1c) spectral peak. Exemplars were differentiated by the dynamics of the higher spectral peak, P2. The sounds were constructed with saw-tooth wave, square wave and noise carriers, rendering them unambiguously nonlinguistic.
In addition to the six exemplars defining each of the categories during training, five additional exemplars per category were created and reserved for testing generalization of category learning to novel exemplars. These stimuli had steady-state frequencies intermediate to those of the training stimuli. In other respects their acoustic characteristics matched those of their category. See Wade and Holt (2005) for further details.
2.3 Experimental Design
2.3.1 Videogame training
Participants experienced the sound category exemplars in a first-person shooter-style videogame that more closely models incidental sound category learning than traditional stimulus-response-feedback category learning paradigms, while providing tight control over the history of listening experience (see Wade & Holt, 2005 for full details on the game). Participants navigate through a pseudo-three-dimensional space environment while executing keystrokes to shoot and capture animated alien characters. Each of four visually distinct aliens is associated with a different sound category (Figure 1a, b). When a character is present on the screen one of the sound category exemplars associated with it is selected randomly and played repeatedly. Thus, across appearances, a character is correlated with a distinctive sound category defined by acoustically-variable exemplars. It is possible to play the game at lower levels without reliance on auditory categorization. However, the fast-paced nature of higher game levels increasingly demands auditory categorization for success. As game play speeds at higher levels (determined on the basis of participants’ success at the capture/shoot task, see Wade and Holt, 2005) characters originate further eccentric relative to the center of the screen. Thus, at higher levels it is possible to hear characters before seeing them. This allows participants to anticipate and plan the appropriate shooting/capturing response via incidental sound categorization. Nonetheless, the task demands of the videogame are predominantly visually-guided.
Participants were given no instructions or hints to use or attend to auditory information and a music soundtrack, stylistically similar to commercial space-themed videogames, contributed to a complex soundscape that minimized the prominence of sound category exemplars. Participants made no overt categorization judgments and received no feedback about sound categorization. They were not informed of the relationship between alien characters and sound categories. However, the acoustically-variable sound exemplars within a category were functionally equivalent in signaling an appropriate behavior. As a result, there were naturalistic task demands that encouraged the discovery of sound categories.
Participants played the game while seated in sound-attenuated chambers, wearing headphones and positioned directly in front of a computer monitor. Game navigation involved a sequence of keystrokes on a standard keyboard. During the first 5-10 minutes the experimenter explained the aim of the game (to shoot/capture the characters) and how to accomplish actions with keystrokes. Participants then practiced to assure understanding. Training commenced when the experimenter was satisfied that the participant understood the game mechanics and continued, self-paced, for 50 minutes. Full details regarding the videogame paradigm can be found in Wade and Holt (2005).
2.3.2 Overt categorization task
An explicit sound categorization test immediately followed videogame training. On each trial, participants heard a sound exemplar presented five times concurrent with visual presentation of all four visual alien characters, arranged across the four screen quadrants. Participants guessed which alien matched the sound. Sound-category exemplars in the test were 24 stimuli presented during game play and 20 novel sounds created to match the defining characteristics of each sound category (5 per category). These latter sounds were not experienced in training and thus tested generalization of category learning to novel exemplars, a hallmark of categorization. There was no feedback.
2.3.3 Sound discrimination task
Individuals with dyslexia may have difficulty with rapidly-varying acoustic information at the time scale that often differentiates phonetic categories (Tallal, 1980). The auditory categories of the present study are differentiated, at least in part, by temporal information within this range (P2 transition, Fig 1c). Therefore, we examined the possibility that the complex nonspeech sound category exemplars present auditory processing difficulties for listeners with dyslexia, separate from category learning. Participants discriminated stimulus pairs drawn from the multidimensional sound categories. These nonspeech sounds are spectrally complex and perceptually confusable (see Emberson et al., 2013; Wade & Holt, 2005), presenting the most challenging auditory discriminations among the present stimuli. The four 250-ms sounds were not experienced in the videogame, and were approximately equidistant in perceptual space (see Liu & Holt, 2011). The four sounds were presented in every pairwise combination (275 ms silent interval), with 48 trials/block across 10 blocks (1:1 ratio of same/different trials). Participants indicated same or different with a key press. There was no feedback.
3. Results
3.1 Videogame task
Participants with dyslexia were significantly poorer at acquiring the sound categories than their control-group counterparts, as evident in both overt category judgments and measures of category acquisition observed indirectly through participants’ pattern of videogame play.
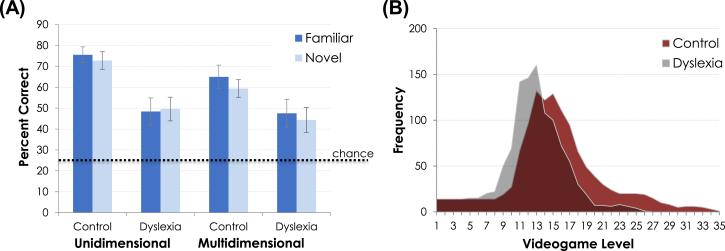
Immediately following game play, participants overtly labeled familiar sound exemplars from the game and also novel sounds drawn from the four categories by guessing which alien would be most likely to appear on screen based on each sound. A mixed model analysis of variance (ANOVA) was conducted with group (Dyslexia vs. Controls) as a between-subjects factor and exemplar (familiar vs. novel category exemplars to test generalization of learning) and category type (unidimensional vs. multidimensional, see Figure 1c) as within-subject factors and accuracy in the overt categorization test as the dependent measure. Results are presented in Figure 2a. Individuals with dyslexia (t(13)=3.93, p<.01) and matched control participants (t(13)=10.99, p<.01) were able to learn the sound categories at above-chance (25%) levels. However, participants with dyslexia were significantly less accurate (M=47.4%, S.E.=.05) in categorizing nonspeech sound exemplars than matched controls (M=68.1%, S.E.=.03), F (1, 26) =8.97, p = .006 ηp2= .266. Across all participants, familiar exemplars heard during the videogame were somewhat better categorized (M=59.1%, S.E.=.04) than novel, generalization exemplars (M=56.5%, S.E.=.04), F(1,26)=3.77, p=.062, ηp2= .056. Note that this pattern is consistent with robust generalization to novel, unfamiliar exemplars because categorization of novel exemplars was above-chance, t(27)=8.36, p<.01. There was also a main effect of category such that unidimensional category exemplars were more accurately categorized (M=61.5%, S.E. =.04) than multidimensional category exemplars (M=54.05%, S.E.=.04), F(1,26) = 6.91, p = .014, ηp2= .202, as has been found in previous studies with these stimuli (e.g., Wade & Holt, 2005). There were no significant interactions (p>.131).
Figure 2.
(A) Accuracy in the overt post-training categorization task for Control and Dyslexia groups across Unidimensional and Multidimensional categories. Both groups performed above chance, but performance among participants with dyslexia was significantly poorer. (B) Frequency of game play across videogame levels for Control and Dyslexia groups. The groups differed significantly, with Control listeners reaching higher levels. Since auditory category learning is increasingly necessary to succeed at higher levels, this is consistent with better auditory category learning among Control group participants.
A measure of category acquisition observed indirectly through participants’ pattern of videogame play also reveals poorer auditory category learning among participants with dyslexia. The videogame is structured so that successful action increasingly depends on auditory categorization as the game progresses to higher levels. As a result, the highest game level achieved is a useful index of incidental category learning that does not rely on overt category decisions. Control group participants advanced to significantly higher levels of game play (M=21.78, S.E.=1.55) than participants with dyslexia (M=17.14, S.E.=.95), t(26)=−2.54, p<.05 (Figure 2b). This is consistent with the group difference observed in the overt categorization task; poorer category learning among participants with dyslexia likely had a negative impact on category-dependent game performance at higher levels. Finally, there was a significant positive correlation between mean highest game level achieved and categorization accuracy in the overt categorization task, for both Dyslexia (r=.82, p<.05) and Control (r=.69, p<.05) groups. Thus, poorer performance in the overt categorization task among participants with dyslexia is unlikely to be a consequence of failing to transfer learning from incidental training to more explicit decision-making about sound categories.
Each group of participants experienced the same length of videogame training (50 minutes). However, since the pace of play is faster at higher game levels and Control group participants reached higher levels of game play, it is possible that the Control group's category learning and generalization in the overt categorization task was supported by greater overall experience with sound exemplars within the 50-min session. To investigate this possibility, we conducted an ANCOVA analysis with posttest categorization as the dependent variable, group as a between-subjects factor, and the number of trials presented during the game (as defined by the time from an aliens’ appearance to the participant's response) as a covariate. The main effect of group remained significant, F(1, 25) = 5.041, p<.05. Thus, experiencing a greater number of trials within the videogame was not the driving force behind the observed group differences.
3.2 Sound discrimination task
A subset of participants also completed a control task in which they discriminated novel exemplars drawn from the sound categories. Discrimination accuracy was very high and statistically indistinguishable in the Dyslexia (M=96.9%, S.E.=.006) and Control (M=98.2%, S.E.=.006) groups, t(16)=−1.46, p=.29. This suggests that the observed category-learning deficit did not arise from poorer discrimination of the sound exemplars by dyslexic participants. There was no correlation of auditory category learning assessed by overt categorization accuracy (r=.357, p = .346 for the Control group; r= − .164, p = .673 for the Dyslexia group) or highest game level (r=.527, p = .145 for the Control group; r = .298, p = .436 for the Dyslexia group) with discrimination performance for either group.
3.3 Data Analysis Related to the Language Battery
A battery of cognitive and language measures characterized impairments among dyslexic participants relative to control participants (Table I). A forward stepwise regression analysis assessed the relationship of categorization performance (across novel and familiar exemplars) in the overt post-training test and measures related to reading efficiency including naming latency (RAN), phonological awareness (Spoonerism) and verbal working memory (digit span) as well as a measure of general intelligence (Raven's score), which did not differ significantly across groups. In all, the following independent variables were included in the analyses: a) Word Identification score, WI; b) Word Attack score, WA; c) Sight Word efficiency score, SW; d) Phonemic Decoding efficiency score, PD; e) Digit span, DS; f) Spoonerism accuracy and time scores; g) Rapid automatized naming of letters/colors/objects and numbers scores; h) Raven's score; i) Group (coded as a dummy variable; 0 for dyslexia, 1 for Controls).
Only the time to accomplish the Spoonerism task (Brunswick, McCrory, Price, Frith, & Frith, 1999), which requires phonemic-level manipulation and is considered an especially good measure of adult phonological awareness (Walton & Brooks, 1995), was significantly correlated with nonspeech auditory category learning (R=.454, adjustedR=.433, see Table II). This measure of phonological awareness was associated with nonspeech auditory category learning across all participants, β= −.674, t= −4.654, p<.01. No other variables were significantly related to nonspeech auditory category learning. The time to accomplish the Spoonerism task was negatively correlated with auditory category learning, even with all variables entered into the regression model.
Table II.
Forward stepwise regression with accuracy on overt posttest categorization as the dependent variable
| Variable | B | S.E. | β | t | p | R2 | R2 adjusted |
|---|---|---|---|---|---|---|---|
| Spoonerism Time | −.003 | .157 | −.674 | −4.654 | .00 | .454 | .433 |
We conducted two additional regression analyses (across the same variables indicated above) with two within-videogame measures: highest level game attained and game score. Of these measures, the highest game level achieved is most closely associated with auditory category learning because the game is structured to require auditory categorization at its highest levels (categorization facilitates the quick action needed at the highest levels of game play because you can hear the aliens and begin to plan a response in the appropriate direction before seeing them). Game score is a measure weighted by the current level and the proximity of the alien character to the player at the time of shooting (see Wade and Holt, 2005 for details). More points are awarded for faster shooting and at higher levels of game play. This measure is less closely related with the auditory category learning demands present in the task.
For the highest game level attained, two variables emerge as significant: 1) Word attack measure 2) Spoonerism RT (R=.625, adjusted R= .342, see Table III). Only the ability to read pseudo words accurately and the time to accomplish the Spoonerism task were correlated significantly with the highest videogame level attained, an indirect measure of incidental category learning (β=.382, t= 2.217, p<.05; β=−.385, t=−2.077, p<.05 respectively). The fact that Spoonerism RT predicted both this indirect measure of category learning and the direct measure of generalization of category learning in the overt labeling task supports the possibility of a relationship between incidental category learning and phonological processing.
Table III.
Forward stepwise regression with highest level game attained as the dependent variable
| Variable | B | S.E. | β | t | p | R2 | R2 adjusted |
|---|---|---|---|---|---|---|---|
| Word Attack | .161 | .073 | .382 | 2.217 | .036 | .625 | .390 |
| Spoonerism Time | −.045 | .022 | −.358 | −2.077 | .048 | .625 | .390 |
For the game score (the indirect game measure less closely associated with task demands on category learning), two variables emerge as significant: 1) RAN colors test 2) Raven scores (R=.647, adjusted R= .372, see Table V). The ability to rapidly name colors and intelligence (as measured by the Raven's Progressive Matrices test) were correlated significantly with game score (β=.548, t= 3.594, p<.01; β=.333, t=−2.183, p<.05 respectively). We do not have a direct explanation of why RAN of colors and intelligence predicted game scores. However it should be noted that the relation between RAN and game score was found only for rapid naming of a specific category (colors) and not for other measures of RAN (rapid naming of letters, numbers and objects). Our confidence in the importance of this relationship is diminished by the fact that it does not hold up across categories that purportedly measure the same ability. Although intelligence and game score were correlated, these measures did not differ significantly across groups and so intelligence cannot account for the observed auditory category learning differences across groups.
Table V.
Forward stepwise regression with game score as the dependent variable
| Variable | B | S.E. | β | t | p | R2 | R2 adjusted |
|---|---|---|---|---|---|---|---|
| Ran colors | .161 | .073 | .382 | 2.217 | .036 | .625 | .390 |
| Raven | −.045 | .022 | −.358 | −2.077 | .048 | .625 | .390 |
It should be noted that RAN, Spoonerism and digit span tests all require phonological processing. Thus, to the extent that dysfunction in incidental auditory category learning is associated with phonological processing, each of these measures might be expected to be correlated with category learning outcomes. However, within the context of this study, we found that only the Spoonerism test was correlated with incidental auditory category learning. We speculate that this may be a reflection of the nature of our sample of dyslexics. The Spoonerism task is considered an especially good test with which to detect phonological processing impairments among high functioning adults with dyslexia (Snowling, Nation, Moxham, Gallagher, & Frith, 1997).
4. Discussion
The underlying biological and cognitive causes of dyslexia remain under extensive debate despite decades of intensive research (for a review, see Démonet, Taylor, & Chaix, 2004). Although the emphasis has been on whether dyslexia is characterized by a core phonological deficit, a growing literature documents procedural learning impairments among individuals with dyslexia (Gabay, Schiff, & Vakil, 2012c; Howard et al., 2006; Pavlidou & Williams, 2014; Sperling, Lu, & Manis, 2004; Stoodley, Harrison, & Stein, 2006; Stoodley, Ray, Jack, & Stein, 2008; Vicari et al., 2005; Vicari et al., 2003). However, it is not yet understood how procedural learning impairments relate to the phonological deficits so prominent in dyslexia. Some have hypothesized that impaired procedural learning may interfere with skill automation or articulation, in turn leading to impoverished phonological representations (Nicolson & Fawcett, 2011). This conceptualization emphasizes the motor and skill-acquisition functions associated with procedural learning systems (Doyon & Benali, 2005; Doyon, Penhune, & Ungerleider, 2003; Doyon, Ungerleider, Squire, & Schacter, 2002). However, the neural systems thought to contribute to procedural learning have diverse nonmotor roles (Middleton & Strick, 2000; Strick, Dum, & Fiez, 2009) including support for perceptual category learning (for a review see, Seger, 2008).
Perceptual category learning is highly significant in spoken language processing. The acoustic signature of phonemes varies dramatically across utterances. As a result, a simple match-to-sample approach to acquiring phonemes is not sufficient. Instead, phonetic learning can be conceived of as an example of complex sound categorization whereby acoustically distinct utterances sampling a highly multidimensional space come to be treated as functionally equivalent (Holt & Lotto, 2010). Listeners must discover the dimensions of linguistically-relevant acoustic variability that signal different phonemes while, at the same time, disregarding variability that does not differentiate phonemes (e.g., talker differences). Said another way, phonetic learning is an example of learning functional equivalence classes, or categories (Holt & Lotto, 2010). The nature of speech signals makes it difficult to acquire explicit knowledge about the dimensions that define speech categories. Therefore, learning speech categories involves acquiring procedural knowledge that cannot be explicitly verbalized. Although this learning begins in infancy (e.g., Kuhl, Williams, Lacerda, Stevens, & Lindblom, 1992), there is a long developmental tail extending into late childhood (Zevin, 2012). Even 8- to 12-year-olds are not adult-like in the details of speech categorization (Hazan & Barrett, 2000; Idemaru & Holt, 2013; Nittrouer, 1996).
In the present study, we investigated the possibility that a procedural learning impairment in dyslexia might interfere with acquiring procedural auditory category knowledge. We hypothesized that this impairment may be general, affecting procedural acquisition of even nonspeech auditory categories. Indeed, relative to age- and cognitive-ability matched control participants, dyslexic adults exhibited impaired acquisition of complex, artificial nonspeech categories in the context of an immersive virtual environment with task demands that mimic some elements of natural learning. The impairment was evident in auditory-category-driven behavior within the game and also in generalization of incidental category learning to an overt labeling task. This pattern of impairment is difficult to reconcile with a strictly phonological account of dyslexia. Although other research has suggested domain-general impairments in dyslexia, the present results are the first to demonstrate that individuals with dyslexia do not learn procedural auditory category knowledge as effectively as controls. Since phonetic category learning is highly dependent on acquisition of procedural category knowledge, this provides a link between procedural learning impairments and phonological deficits.
An advantage of the present approach is that the videogame training paradigm presents task demands considerably closer to learning in natural environments than traditional laboratory tasks. However, in light of this more complex, demanding environment, it is possible that participants with dyslexia encountered difficulty in navigating the videogame environment, or in staying attentive to the training task. If so, this might account for the poorer auditory category learning among individuals with dyslexia. However, the Dyslexia group (M=47186, S.E.=6141.2) did not differ from the Control group (M=59142, S.E.=5455.5) in the overall score attained in the game, t(26) = −1.48, p=.14. This may seem counterintuitive since control participants successfully completed significantly higher game levels than dyslexic participants (Figure 2b). However, it is possible to accumulate points at lower game levels within which auditory categorization is not critical to successful game navigation. The equivalence of groups’ high scores confirms that both groups were actively engaged and succeeding in the mechanics of the videogame. Individuals with dyslexia did not have greater difficulty in playing the videogame due to attentional impairments (which have been associated with dyslexia; Facoetti et al., 2010; Franceschini, Gori, Ruffino, Pedrolli, & Facoetti, 2012; Hari & Renvall, 2001), coordination of actions, or other factors. However, the dyslexic participants’ route through the game did not involve as much auditory category learning as their control group counterparts, as confirmed by significantly lower high-level achieved and poorer post-test categorization accuracy.
Note, as well, that the videogame does not require overt attention or response to the auditory stimulus dimensions that define categories. Although counterintuitive, studies of perceptual learning demonstrate that learning can suffer when attention is directed toward the learning-relevant stimulus dimensions as compared to situations in which attention is directed instead toward another task (for a comprehensive review see, Seitz & Watanabe, 2009). Indeed, a previous study using the same nonspeech sounds as the present study demonstrated poorer auditory category learning when attention was directed toward the stimulus dimensions in an unsupervised learning task, compared to the incidental learning in the videogame (Wade & Holt, 2005). Thus, it is difficult to account for the present results in terms of impaired attentional processing.
It is also possible that poorer auditory category learning among participants with dyslexia may have arisen due to difficulty in sensory processing of the sound exemplars, and not from impairment in category learning per se. This is an especially important alternative to consider because the sounds defining the present categories were characterized by spectrotemporal characteristics similar to those thought to present perceptual difficulties for listeners with dyslexia (Tallal, 1980; Vandermosten et al., 2011). However, mitigating this possibility, perceptual discrimination of category exemplars was excellent among the subset of dyslexic participants available to be tested. Dyslexia and Control groups’ accuracy in discriminating perceptually similar sound category exemplars (Emberson et al., 2013) was equivalent, and very near ceiling in accuracy. Goswami (2015) has argued that sensory deficits in dyslexia may arise as a consequence of reduced reading experience. The claim is that sensory processing deficits are not causally related to the neurocognitive basis of dyslexia, but rather arise as a consequence of reading less. Since our study examined adults with dyslexia, this is an important point of consideration. If reading less were to affect auditory processing, it may lead to difficulties in auditory category learning in adulthood unrelated to the ultimate neural basis of dyslexia. However, here we present the alternative hypothesis that it is the learning through which auditory (including phonetic) categories are acquired that is impaired in dyslexia, not auditory processing per se. In accordance with this perspective, we observe poor incidental auditory learning in the absence of auditory discrimination impairments for the to-be-learned stimuli among our dyslexic sample. Ultimately, as Goswami (2015) advocates, longitudinal studies will be needed to make progress in establishing causality. However, the present results do not appear to be well-accounted for by auditory processing deficits, whether primary or secondary from less reading experience. Rather, we propose that these results suggest a general impairment in acquisition of procedural category knowledge.
Accounts that posit procedural learning deficits in dyslexia are sometimes held to relate closely with another account, the anchoring-deficit hypothesis of dyslexia (Ahissar, 2007). The anchoring-deficit hypothesis suggests that dyslexics’ difficulties arise from poor perceptual anchoring across recently presented stimuli (Ahissar, 2007). Whereas control listeners’ perceptual acuity typically benefits from experimental paradigms in which there is a stable, repeating perceptual reference, individuals with dyslexia experience less of a performance boost from the presence of such “anchors.” Ahissar (2007) proposes that anchoring, the ability to construct and use an internal perceptual anchor, resembles short-term priming effects and is impaired in dyslexia. The specific proposal is that anchoring deficits would arise from poor utilization of stimuli experienced in the immediate perceptual environment, and not from impairments in longer-term modifications as a result of experience (Ahissar, 2007). This is the crux of the distinction from what we propose here. Specifically, we propose that individuals with dyslexia are impaired at forming long-term modifications supporting categorization via procedural learning.
Further relevant to the anchoring-deficit hypothesis, in the present study individuals with dyslexia were indistinguishable from control listeners on perceptual discrimination of the nonspeech auditory category exemplars. Within the present study, this task arguably would make the greatest demands on anchoring to immediate perceptual experience, yet the participants with dyslexia show no impairment in discrimination performance and ceiling-level performance. Ahissar, Lubin, Putter-Katz, and Banai (2006) make the case that dyslexics’ difficulties are evident only when a limited stimulus set is presented repetitively. Under this task demand, typical listeners benefit from forming perceptual anchors whereas individuals with dyslexia do not. The present discrimination task involves a small set of repetitive stimulus pairs and thereby would be expected to be a good assay of anchoring deficit in dyslexia. However, we observed no group differences. In a similar manner the results of the overt categorization test are of particular interest with regard to the anchoring-deficit hypothesis. A subset of the sounds tested in the categorization task was not previously experienced in the videogame. Success in labeling in labeling these exemplars thus required generalization of learning from within the videogame task, and not short-term priming. Moreover, since individual exemplars were presented on each trial the possibility that anchoring could either help or hinder performance is minimized. The present results thus are more consistent with longer-term modification and not ad hoc utilization of recently presented stimuli, as postulated by the anchoring-deficit hypothesis of dyslexia (Ahissar, 2007). The generalization results, in particular, lead us to believe that the anchoring-deficit hypothesis cannot entirely account for the present findings.
In the present study, we targeted nonspeech auditory categories to specifically address the question of whether there are general impairments in learning auditory procedural category knowledge because a general impairment would be expected also to impact phonetic category acquisition. However, to the extent that procedural learning impairments are present in dyslexia deficits in category learning would be predicted across modalities. In fact, previous study shows impaired visual category learning among individuals with dyslexia (Sperling et al., 2004). Moreover, adults with dyslexia are impaired at probabilistic visual category learning in the weather prediction task, which is thought to rely upon the procedural learning system (Gabay, Vakil, Schiff, & Holt, 2015). The parity in finding procedural learning deficits across both auditory and visual category learning is consistent with emerging research among control participants demonstrating commonalities across visual and auditory category learning (Ashby & Maddox, 2005; Chandrasekaran, Yi, et al., 2014).
Within this literature, some have argued that the acquisition of multidimensional categories is more likely to draw on procedural learning than unidimensional categories, for which the diagnostic dimension is more easily verbalized and may draw upon more explicit learning systems (Maddox et al., 2014; Yi et al., 2014). However, although the multidimensional categories present a different learning challenge than the unidimensional categories, recent neuroimaging research indicates that both types of categories draw on procedural learning in the videogame training paradigm (Lim et al., 2013). This is consistent with an emerging consensus that stimulus factors and task demands interact to affect the learning system engaged (Chandrasekaran, Yi, et al., 2014; Gabay, Dick, Zevin, & Holt, 2015; Lim, Fiez, & Holt, 2014). Further research that directly examines the interaction of stimulus factors and task demands on perceptual category learning among individuals with dyslexia may help to reveal the nature of learning deficits and possible routes for remediation through training.
In this regard, the present data suggest that training interventions that target non-procedural learning may be especially effective rehabilitation options. Auditory training has factored prominently in rehabilitation strategies for dyslexia (Kujala et al., 2001) and there is a growing appreciation of the multiple learning systems present in the nervous system (Doya, 1999). By manipulating the approach taken to structuring category exemplars, task feedback, stimulus repetition rate, and other factors known to affect the degree to which procedural learning is engaged (Ashby & Maddox, 2005) it may be possible to aid learners with dyslexia in acquiring robust phonetic categories.
With regard to captivating videogame training approaches, it is important to note that inasmuch as videogames can create complex, multimodal immersive environments the cognitive and perceptual skills they train can be very distinct. Our videogame training task was developed specifically to engage auditory category learning via procedural learning. It was designed to mimic some of the challenges of phonetic category acquisition in real-world environments, within which there typically is no explicit instruction or explicitly-provided feedback to support sound categorization. Since we see impairments among adults with dyslexia within this task, it suggests that there may be consequences for real-world acquisition of phonetic categories.
Other action video games have been found to improve reading among dyslexics by improving individuals’ attentional abilities (Franceschini et al., 2013). Since action videogames typically involve rapid, transient events and moving objects, a high perceptual and motor load, and an emphasis on visual processing in peripheral space, it has been proposed that action videogame training might improve the efficiency of the magnocellular-dorsal pathway or “action” stream. Although we postulate a rather different neural basis for dyslexia, the approaches are compatible in the attempts to understand the neurobiological bases of dyslexia and to seek new approaches for remediation.
4.1 Hypotheses regarding the neurocognitive basis of dyslexia
The present results also inform the neurocognitive basis of dyslexia. The non-phonological deficits observed in individuals with dyslexia have been suggested to relate to selective impairment in procedural learning associated with the learning and control of established sensorimotor and cognitive habits, skills and procedures (Nicolson & Fawcett, 2011). However, despite evidence for procedural learning impairments among individuals with dyslexia (Gabay et al., 2012a, 2012b, 2012c; Howard et al., 2006; Lum, Ullman, & Conti-Ramsden, 2013; Pavlidou & Williams, 2014; Stoodley, Harrison, & Stein, 2006; Stoodley et al., 2008; Vicari et al., 2005; Vicari et al., 2003), the nature of the link between impaired procedural learning and phonological deficits remains uncertain. The current version of the procedural learning hypothesis (Nicolson & Fawcett, 2011) suggests language-based, as opposed to motor-based, aspects of the procedural learning system should be the most impaired among those with dyslexia (although some individuals may present with motor deficits, as well). In support of this, Gabay et al. (2012) observe impaired language sequence learning along with intact motor sequence learning in adults with dyslexia. The present results to not speak strongly to the language/motor distinction. However, in concert with other recent results (for additional evidence see, Gabay, Thiessen, & Holt, 2015), the present findings argue that it will be important to refine accounts of procedural learning beyond language versus motor distinctions.
In this regard, the present results sharpen neurobiological models of impaired procedural learning in dyslexia. Previous examinations have mostly focused on the impact of cerebellar dysfunction as it relates to procedural learning impairments in dyslexia (Menghini, Hagberg, Caltagirone, Petrosini, & Vicari, 2006; Nicolson et al., 1999; Pernet et al., 2009; Rae et al., 1998). In contrast, recent research suggests that the basal ganglia play a role in learning within the videogame task and its recruitment appears to be significant in supporting changes in cortical representations of the to-be-learned sound categories. Using the same videogame training environment and sound categories used in the present study, Lim et al. (2013) observe bilateral activation of the posterior caudate of the basal ganglia related to nonspeech auditory category learning within the videogame. The basal ganglia are associated with reinforcement learning emerging as one builds and updates predictions about future rewards (Daw, Niv, & Dayan, 2005; Schultz, Dayan, & Montague, 1997). Since actions in the videogame are not directed at sound categorization, per se, training may elicit internally-generated reward prediction error feedback signals from the basal ganglia that indirectly induce changes in sound category representations that correlate with task success (see Lim et al., 2014). Based on these neuroimaging findings, we speculate that impairments in indirect reward prediction error driven learning via the basal ganglia may contribute to disrupting the typical course of category acquisition in dyslexia, with cascading effects on phonological processing. Specifically, it is possible that less robust learning signals through the striatum ultimately contribute to less robust cortical representations for auditory categories. Although advocates of the phonological deficit hypothesis have suggested that dyslexia can be viewed as a cortical disconnection syndrome originating from problems in cortical-cortical connectivity (Paulesu et al., 1996), the present results (along with the procedural learning account (Nicolson & Fawcett, 2010)) recommend that it will be fruitful to more fully examine cortical-subcortical interactions in dyslexia. Although there is evidence to suggest dysfunction in cortico-cerebellar interactions in dyslexia (Menghini et al., 2006; Nicolson et al., 1999; Pernet et al., 2009; Rae et al., 1998), little attention has been directed to understanding corticostriatal interactions. The present results, along with neuroimaging in the same paradigm with control participants (Leech et al., 2009; Lim et al., 2013), strongly argue that it will be important to carefully examine corticostriatal loops among individuals with dyslexia in future research.
4.2 The relation between procedural learning impairment and the formation of speech categories
The contribution of procedural learning to phonological impairments via auditory category learning provokes a reconsideration of current theories of dyslexia and broadens understanding of the underlying neurocognitive mechanisms involved. On the one hand, the phonological deficit hypothesis has been criticized for focusing on symptoms (phonological impairments) rather than on the cause of these symptoms (Nicolson & Fawcett, 2010). It may be possible that the focus on documenting the phonological deficits typical of dyslexia has led to too little consideration of the mechanisms involved in the formation of these phonological representations, which of course must be learned through experience with the native language. On the other hand, the procedural learning deficit hypothesis has been criticized because the link postulated between procedural learning impairment and phonological deficits relies heavily on speech articulation (Ramus et al., 2003). This conceptualization emphasizes the motor and skill-acquisition functions associated with procedural learning systems (Doyon & Benali, 2005; Doyon et al., 2003; Doyon et al., 2002) and posits that phonological development is impacted via dysfunction in articulatory development (Nicolson & Fawcett, 2011). Nonetheless, there is as yet no definitive connection to make clear how a procedural learning deficit results in phonological impairments typical of dyslexia.
The present results suggest a possible link between phonological deficits and procedural learning impairments. Although prior hypotheses have emphasized the possibility that impairment to the motor and skill-acquisition functions associated with procedural learning systems may be important in understanding dyslexia, the neural systems thought to contribute to procedural learning have diverse nonmotor roles (Middleton & Strick, 2000; Strick et al., 2009) including involvement in perceptual category learning (Seger, 2008). This is particularly relevant in making a link from procedural learning impairment to the phonological deficits typical of dyslexia because contemporary accounts of speech perception emphasize the significance of general auditory category learning mechanisms in acquiring speech categories (Holt & Lotto, 2008) and made a case for the involvement of the procedural learning system in this learning (Guediche, Holt, Laurent, Lim, & Fiez, 2014; S. Lim et al., 2014; Yi et al., 2014). We hypothesize that procedural learning impairment may lead to impaired perceptual category learning that results in impoverished representations of the phonological characteristics of speech and concomitant difficulties in grapheme-phenome conversion and in learning to read.
4.2 Conclusions
Phonological impairments have been emphasized as the cause of dyslexia (Snowling, 2000). However, the current data suggest that impaired auditory categorylearning, general in nature and not specific to speech, could contribute to phonological deficits in dyslexia with subsequent negative effects on reading and language acquisition. The present data are consistent with a general impairment in mapping probabilistic perceptual input to behaviorally-relevant category representations, not specific to speech. Nevertheless, the consequences of this general impairment might be quite prominently on display for speech because language learning and processing place such considerable demands on mapping probabilistic perceptual information to linguistically-significant representations. The present results provide a conceptual link between observations of procedural learning deficits on the one hand, and phonological impairments, on the other.
Acknowledgements
This work was supported by a grant to L.L.H. from the National Institutes of Health (R01DC004674).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahissar M. Dyslexia and the anchoring-deficit hypothesis. Trends in cognitive sciences. 2007;11(11):458–465. doi: 10.1016/j.tics.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Lubin Y, Putter-Katz H, Banai K. Dyslexia and the failure to form a perceptual anchor. Nature neuroscience. 2006;9(12):1558–1564. doi: 10.1038/nn1800. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Alfonso-Reese LA. A neuropsychological theory of multiple systems in category learning. Psychological review. 1998;105(3):442. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annu. Rev. Psychol. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Bradlow AR, Pisoni DB, Akahane-Yamada R, Tohkura Y. Training Japanese listeners to identify English/r/and/l: IV. Some effects of perceptual learning on speech production. The Journal of the Acoustical Society of America. 1997;101(4):2299. doi: 10.1121/1.418276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes RL, Nicolson RI, Fawcett AJ. Prisms throw light on developmental disorders. Neuropsychologia. 2007;45(8):1921–1930. doi: 10.1016/j.neuropsychologia.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price C, Frith C, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics A search for Wernicke's Wortschatz? Brain. 1999;122(10):1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Koslov SR, Maddox WT. Toward a dual-learning systems model of speech category learning. Frontiers in psychology. 2014;5 doi: 10.3389/fpsyg.2014.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Yi H-G, Maddox WT. Dual-learning systems during speech category learning. Psychonomic bulletin & review. 2014;21(2):488–495. doi: 10.3758/s13423-013-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature neuroscience. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Delgado M, Stenger V, Fiez J. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14(9):1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Démonet J-F, Taylor MJ, Chaix Y. Developmental dyslexia. The Lancet. 2004;363(9419):1451–1460. doi: 10.1016/S0140-6736(04)16106-0. [DOI] [PubMed] [Google Scholar]
- Doya K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural networks. 1999;12(7):961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Current opinion in neurobiology. 2005;15(2):161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Doyon J, Ungerleider LG, Squire L, Schacter D. Functional anatomy of motor skill learning. Neuropsychology of memory. 2002;3:225–238. [Google Scholar]
- Emberson LL, Liu R, Zevin JD. Is statistical learning constrained by lower level perceptual organization? Cognition. 2013;128(1):82–102. doi: 10.1016/j.cognition.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A, Lorusso ML, Paganoni P, Cattaneo C, Galli R, Umiltà C, Mascetti GG. Auditory and visual automatic attention deficits in developmental dyslexia. Cognitive Brain Research. 2003;16(2):185–191. doi: 10.1016/s0926-6410(02)00270-7. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Trussardi AN, Ruffino M, Lorusso ML, Cattaneo C, Galli R, Zorzi M. Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. Journal of Cognitive Neuroscience. 2010;22(5):1011–1025. doi: 10.1162/jocn.2009.21232. [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Pedrolli K, Facoetti A. A causal link between visual spatial attention and reading acquisition. Current Biology. 2012;22(9):814–819. doi: 10.1016/j.cub.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Viola S, Molteni M, Facoetti A. Action video games make dyslexic children read better. Current Biology. 2013;23(6):462–466. doi: 10.1016/j.cub.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Gabay Y, Dick FK, Zevin JD, Holt LL. Incidental Auditory Category Learning. Journal of Experimental Psychology: Human Perception & Performance. doi: 10.1037/xhp0000073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay Y, Schiff R, Vakil E. Attentional requirements during acquisition and consolidation of a skill in normal readers and developmental dyslexics. Neuropsychology. 2012a;26(6):744. doi: 10.1037/a0030235. [DOI] [PubMed] [Google Scholar]
- Gabay Y, Schiff R, Vakil E. Dissociation between online and offline learning in developmental dyslexia. Journal of clinical and experimental neuropsychology. 2012b;34(3):279–288. doi: 10.1080/13803395.2011.633499. [DOI] [PubMed] [Google Scholar]
- Gabay Y, Schiff R, Vakil E. Dissociation between the Procedural Learning of Letter Names and Motor Sequences in Developmental Dyslexia. Neuropsychologia. 2012c;50(10):2435–2441. doi: 10.1016/j.neuropsychologia.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Gabay Y, Thiessen ED, Holt LL. Impaired Statistical Learning in Developmental Dyslexia. Journal of Speech, Language, and Hearing Research. 2015 doi: 10.1044/2015_JSLHR-L-14-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay Y, Vakil E, Schiff R, Holt LL. Probabilistic Category Learning in Developmental Dyslexia: Evidence From Feedback and Paired-Associate Weather Prediction Tasks. 2015 doi: 10.1037/neu0000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey JJ, Syrdal-Lasky K, Millay KK, Knox CM. Performance of dyslexic children on speech perception tests. Journal of experimental child psychology. 1981;32(3):401–424. doi: 10.1016/0022-0965(81)90105-3. [DOI] [PubMed] [Google Scholar]
- Guediche S, Holt LL, Laurent P, Lim S-J, Fiez JA. Evidence for cerebellar contributions to adaptive plasticity in speech perception. Cerebral Cortex. 2014:bht428. doi: 10.1093/cercor/bht428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends in cognitive sciences. 2001;5(12):525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Hazan V, Barrett S. The development of phonemic categorization in children aged 6–12. Journal of phonetics. 2000;28(4):377–396. [Google Scholar]
- Holt LL, Lotto AJ. Speech perception within an auditory cognitive science framework. Current Directions in Psychological Science. 2008;17(1):42–46. doi: 10.1111/j.1467-8721.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LL, Lotto AJ. Speech perception as categorization. Attention, Perception, & Psychophysics. 2010;72(5):1218–1227. doi: 10.3758/APP.72.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44(7):1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Idemaru K, Holt LL. The developmental trajectory of children's perception and production of English/r/-/l. The Journal of the Acoustical Society of America. 2013;133:4232. doi: 10.1121/1.4802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvalson EM, Holt LL, McCLELLAND JL. Can native Japanese listeners learn to differentiate/r–l/on the basis of F3 onset frequency? Bilingualism: Language and Cognition. 2012;15(02):255–274. doi: 10.1017/S1366728912000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvalson EM, McClelland JL, Holt LL. Predicting native English-like performance by native Japanese speakers. Journal of phonetics. 2011;39(4):571–584. doi: 10.1016/j.wocn.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255(5044):606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Kujala T, Karma K, Ceponiene R, Belitz S, Turkkila P, Tervaniemi M, Näätänen R. Plastic neural changes and reading improvement caused by audiovisual training in reading-impaired children. Proceedings of the National Academy of Sciences. 2001;98(18):10509–10514. doi: 10.1073/pnas.181589198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Holt LL, Devlin JT, Dick F. Expertise with artificial nonspeech sounds recruits speech-sensitive cortical regions. The Journal of Neuroscience. 2009;29(16):5234–5239. doi: 10.1523/JNEUROSCI.5758-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-J, Fiez JA, Holt LL. How may the basal ganglia contribute to auditory categorization and speech perception? Frontiers in neuroscience. 2014;8 doi: 10.3389/fnins.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-J, Fiez JA, Wheeler ME, Holt LL. Investigating the Neural Basis of Video-game-based Category Learning. Paper presented at the Journal of Cognitive Neuroscience. 2013 [Google Scholar]
- Lim S-J, Lacerda F, Holt LL. Discovering functional units in continuous speech. Journal of Experimental Psychology: Human Perception & Performance. doi: 10.1037/xhp0000067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Fiez JA, Holt LL. How may the basal ganglia contribute to auditory categorization and speech perception? Auditory Cognitive Neuroscience. 2014;8:230. doi: 10.3389/fnins.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Holt LL. Learning Foreign Sounds in an Alien World: Videogame Training Improves Non-Native Speech Categorization. Cognitive science. 2011;35(7):1390–1405. doi: 10.1111/j.1551-6709.2011.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Holt LL. Neural changes associated with nonspeech auditory category learning parallel those of speech category acquisition. Journal of Cognitive Neuroscience. 2011;23(3):683–698. doi: 10.1162/jocn.2009.21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JA, Ullman MT, Conti-Ramsden G. Procedural learning is impaired in dyslexia: Evidence from a meta-analysis of serial reaction time studies. Research in developmental disabilities. 2013;34(10):3460–3476. doi: 10.1016/j.ridd.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox WT, Chandrasekaran B, Smayda K, Yi H-G, Koslov S, Beevers CG. Elevated depressive symptoms enhance reflexive but not reflective auditory category learning. Cortex. 2014;58:186–198. doi: 10.1016/j.cortex.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis FR, McBride-Chang C, Seidenberg MS, Keating P, Doi LM, Munson B, Petersen A. Are speech perception deficits associated with developmental dyslexia? Journal of experimental child psychology. 1997;66(2):211–235. doi: 10.1006/jecp.1997.2383. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: An fMRI study. Neuroimage. 2006;33(4):1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31(2):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Fawcett A. Dyslexia, learning, and the brain. The MIT Press; 2010. [Google Scholar]
- Nicolson RI, Fawcett AJ. Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex. 2011;47(1):117–127. doi: 10.1016/j.cortex.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. The Lancet. 1999;353(9165):1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nittrouer S. Discriminability and perceptual weighting of some acoustic cues to speech perception by 3-year-olds. Journal of Speech and Hearing Research. 1996;39(2):278. doi: 10.1044/jshr.3902.278. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome? Brain. 1996;119(1):143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Pavlidou EV, Williams JM. Implicit learning and reading: Insights from typical children and children with developmental dyslexia using the artificial grammar learning (AGL) paradigm. Research in developmental disabilities. 2014;35(7):1457–1472. doi: 10.1016/j.ridd.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Pavlidou EV, Williams JM, Kelly LM. Artificial grammar learning in primary school children with and without developmental dyslexia. Annals of Dyslexia. 2009;59(1):55–77. doi: 10.1007/s11881-009-0023-z. [DOI] [PubMed] [Google Scholar]
- Pernet C, Poline J, Demonet J, Rousselet G. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC neuroscience. 2009;10(1):67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Lee MA, Dixon RM, Blamire AM, Thompson CH, Styles P, Stein JF. Metabolic abnormalities in developmental dyslexia detected by< sup> 1</sup> H magnetic resonance spectroscopy. The Lancet. 1998;351(9119):1849–1852. doi: 10.1016/S0140-6736(97)99001-2. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126(4):841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Raven J, Court JH, Raven J. Standard progressive matrices. Manual for Raven's Progressive Matrices and Vocabulary Scales. 1992 [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seger CA. The basal ganglia in human learning. The Neuroscientist. 2006;12(4):285–290. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- Seger CA. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neuroscience & Biobehavioral Reviews. 2008;32(2):265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. The phenomenon of task-irrelevant perceptual learning. Vision research. 2009;49(21):2604–2610. doi: 10.1016/j.visres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling M, Nation K, Moxham P, Gallagher A, Frith U. Phonological processing skills of dyslexic students in higher education: A preliminary report. Journal of Research in Reading. 1997;20(1):31–41. [Google Scholar]
- Snowling MJ. Dyslexia. Blackwell Publishing; 2000. [Google Scholar]
- Sperling AJ, Lu Z-L, Manis FR. Slower implicit categorical learning in adult poor readers. Annals of dyslexia. 2004;54(2):281–303. doi: 10.1007/s11881-004-0014-z. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Harrison EP, Stein JF. Implicit motor learning deficits in dyslexic adults. Neuropsychologia. 2006;44(5):795–798. doi: 10.1016/j.neuropsychologia.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Harrison EPD, Stein JF. Implicit motor learning deficits in dyslexic adults. Neuropsychologia. 2006;44(5):795–798. doi: 10.1016/j.neuropsychologia.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Ray NJ, Jack A, Stein JF. Implicit Learning in Control, Dyslexic, and Garden-Variety Poor Readers. Annals of the New York Academy of Sciences. 2008;1145(1):173–183. doi: 10.1196/annals.1416.003. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual review of neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain and Language. 1980;9(2):182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner R, Rashotte C. TOWRE–2 Test of Word Reading Efffciency. 1999 [Google Scholar]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. Journal of cognitive neuroscience. 2006;18(6):1029–1043. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Luts H, Poelmans H, Golestani N, Wouters J, Ghesquière P. Adults with dyslexia are impaired in categorizing speech and nonspeech sounds on the basis of temporal cues. Proceedings of the National Academy of Sciences. 2010;107(23):10389–10394. doi: 10.1073/pnas.0912858107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Luts H, Poelmans H, Wouters J, Ghesquière P. Impairments in speech and nonspeech sound categorization in children with dyslexia are driven by temporal processing difficulties. Research in developmental disabilities. 2011;32(2):593–603. doi: 10.1016/j.ridd.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): What have we learned in the past four decades? Journal of Child Psychology and Psychiatry. 2004;45(1):2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Vicari S, Finzi A, Menghini D, Marotta L, Baldi S, Petrosini L. Do children with developmental dyslexia have an implicit learning deficit? Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(10):1392–1397. doi: 10.1136/jnnp.2004.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Marotta L, Menghini D, Molinari M, Petrosini L. Implicit learning deficit in children with developmental dyslexia. Neuropsychologia. 2003;41(1):108–114. doi: 10.1016/s0028-3932(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Wade T, Holt LL. Incidental categorization of spectrally complex non-invariant auditory stimuli in a computer game task. The Journal of the Acoustical Society of America. 2005;118:2618. doi: 10.1121/1.2011156. [DOI] [PubMed] [Google Scholar]
- Walton D, Brooks P. The Spoonerism Test. Educational and Child Psychology. 1995 [Google Scholar]
- Wechsler D. WAIS-III: Wechsler adult intelligence scale. Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wilson AM, Lesaux NK. Persistence of phonological processing deficits in college students with dyslexia who have age-appropriate reading skills. Journal of Learning Disabilities. 2001;34(5):394–400. doi: 10.1177/002221940103400501. [DOI] [PubMed] [Google Scholar]
- Wolf M, Denckla MB. RAN/RAS: Rapid automatized naming and rapid alternating stimulus tests. Pro-ed; 2005. [Google Scholar]
- Woodcock RW. Woodcock reading mastery tests, revised. American Guidance Service Circle Pines; MN.: 1987. [Google Scholar]
- Yi H-G, Maddox WT, Mumford JA, Chandrasekaran B. The Role of Corticostriatal Systems in Speech Category Learning. Cerebral Cortex. 2014:bhu236. doi: 10.1093/cercor/bhu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin JD. A sensitive period for shibboleths: The long tail and changing goals of speech perception over the course of development. Developmental Psychobiology. 2012;54(6):632–642. doi: 10.1002/dev.20611. [DOI] [PubMed] [Google Scholar]