Abstract
Objective
Activation of PI3K/Akt signaling protects the myocardium from ischemia/reperfusion injury. MicroRNAs have been demonstrated to play an important role in the regulation of gene expression at the post-transcriptional level. In this study, we examined whether miR-130a will attenuate cardiac dysfunction and remodeling after myocardial infarction (MI) via PI3K/Akt dependent mechanism.
Approaches and Results
To determine the role of miR-130a in the proliferation and migration of endothelial cells, HUVECs were transfected with miR-130a mimics before the cells were subjected to scratch-induced wound injury. Transfection of miR-130a mimics stimulated the migration of endothelial cells into the wound area and increased phosphor-Akt levels. To examine the effect of miR-130a on cardiac dysfunction and remodeling after MI, Lentivirus expressing miR-130a (LmiR-130a) was delivered into mouse hearts seven days before the mice were subjected to MI. Cardiac function was assessed by echocardiography before and for up to 21 days after MI. Ejection fraction (EF%) and fractional shortening (FS%) in the LmiR-130a transfected MI hearts were significantly greater than in LmiR-control and untransfected control MI groups. LmiR-130a transfection increased capillary number and VEGF expression, and decreased collagen deposition in the infarcted myocardium. Importantly, LmiR-130a transfection significantly suppressed PTEN expression and increased the levels of phosphorylated Akt in the myocardium. However, treatment of LmiR-130a-transfected mice with LY294002, a PI3K inhibitor, completely abolished miR-130a-induced attenuation of cardiac dysfunction after MI.
Conclusions
miR-130a plays a critical role in attenuation of cardiac dysfunction and remodeling after MI. The mechanisms involve activation of PI3K/Akt signaling via suppression of PTEN expression.
Keywords: Myocardial infarction, microRNA-130a, angiogenesis, PTEN, PI3K/Akt signaling
Introduction
Cardiovascular disease is the number one killer in the United States. Each year, an estimated 785,000 Americans will have a new coronary attack, 470,000 will have a recurrent attack and 195,000 Americans will have “silent” myocardial infarctions [1].
Acute myocardial ischemia results in increases in the production of reactive oxygen and activation of deleterious cellular signaling that enhances the expression of proinflammatory cytokines and chemokines in endothelial cells, leading to the infiltration of inflammatory cells to the infarcted region [2]. The death of cardiac myocytes and loss of capillaries in and near the infarcted area promote progressive remodeling of the remaining active myocardium. Furthermore, left ventricular remodeling induces fibrosis, and causes left ventricular dilatation, resulting in heart failure. Importantly, insufficient myocardial capillary density after myocardial infarction (MI) has been identified as a critical event in the remodeling process.
Activation of phosphatidylinositol 3-kinase mediated Akt (PI3K/Akt) dependent signaling plays an important role in the induction of angiogenesis [3], cell proliferation and survival as well as anti-apoptosis [4]. We have previously reported that activation of PI3K/Akt signaling improved cardiac function, reduced infarct size, and decreased myocardial apoptosis following myocardial ischemia/reperfusion (I/R) injury [5-7]. Phosphatase and tensin homologue deleted on chromosome ten (PTEN) is a tumor suppressor gene that negatively regulates PI3K/Akt signaling [8]. PTEN suppression has been reported to attenuate myocardial ischemic injury via activation of PI3K/Akt signaling [9]. In addition, PTEN/PI3K/Akt signaling also plays a critical role in the induction of angiogenesis [3].
MicroRNAs (miRs) are 21 to 23 nucleotide non-protein-coding RNA molecules, which have been identified as novel regulators of gene expression at the post-transcriptional level by binding to target messenger RNAs (mRNAs) [10, 11]. Recently published data indicates that several miRs are involved in ischemic heart disease [12-16]. MiR-130a has been reported to down-regulate PTEN expression [17] and suppress the expression of anti-angiogenic genes GAX and HoxA5 in endothelial cells [18]. However, the role of miR-130a in MI-induced cardiac dysfunction has not been investigated.
In the present study, we demonstrated that increased expression of miR-130a by transfection of lentivirus expressing miR-130a (LmiR-130a) into the myocardium significantly attenuates cardiac dysfunction and improves remodeling after MI. The mechanisms involve activation of PI3K/Akt signaling via suppression of PTEN expression.
Materials and Methods
Animals
C57BL/6 male mice were purchased from The Jackson Laboratory (Indianapolis, IN). The mice were maintained in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU). The experiments described in this manuscript conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication, 8th Edition, 2011). All animal care and experimental protocols were approved by the ETSU Committee on Animal Care.
qPCR assay of microRNAs
MicroRNAs were isolated from heart tissues or cultured cells using the mirVanaTM miR isolation kit (Ambion) in accordance with the manufacturer's protocol and as described previously [19, 20]. Quantitative real-time PCR (qPCR) was conducted using a 4800 Real-time PCR machine (Bio-Rad). MicroRNA levels were quantified by qPCR using specific Taqman assays for miR (Applied Biosystems, USA) and Taqman Universal Master Mix (Applied Biosystems). Specific primers for miR-130a were obtained from Applied Biosystems [primer identification numbers: 000454 for hsa-miR-130a and 001973 for U6 small nucleolar RNA (snRU6)]. MicroRNA-130a levels were quantified with the 2(-ΔΔct) relative quantification method that was normalized to the snRU6.
Construction of lentiviral expressing miR-130a
MicroRNA-130a was constructed into the lentivirus expression vector using a lentivirus expressing system (Invitrogen corporation) as described previously [19, 20]. Briefly, the oligonucleotides for miR-130a were synthesized at Integrated DNA Technologies, annealed and ligated into pcDNATM6.2-GW/EmGFP-miR. The pcDNATM6.2-GW/EmGFP-miR cassette was subsequently transferred to pDONR221TM and final pLenti6/V5-DEST by two sequential Gateway BP and LR recombination. The lentiviral control vector contains a non-sense miR sequence that allows formation of a pre-miRNA hairpin predicted not to target any known vertebrate gene (Invitrogen Corporation). The viral particles were produced by third-generation packaging in 293FT cells and Lentiviral stocks were concentrated using ultracentrifugation [19, 20].
In vitro experiments
Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial basal medium-2 (EBM-2) supplemented with EGM-2 SingleQuots Kit (Lonza) and 20% fetal bovine serum (FBS). H9C2 cardiomyoblasts were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) and penicillin (Gibco) [19, 20]. When ECs and H9C2 cells reached 70-80% confluence, they were transfected with miR-130a mimics (40 nmol; invitrogen), scrambled-miR mimics (40 nmol; invitrogen), and anti-miR-130a mimics (80 nmol; invitrogen) using Lipofectamine RNAiMAX reagent (invitrogen). Scramble-miR mimics served as miR-control. Forty-eight hours after transfection, the HUVECS and H9C2 cells were harvested for isolation of microRNAs. The expression of miR-130a in HUVECs and H9C2 cells was examined by qPCR.
Twenty-four hours after transfection, the HUVECs and H9C2 cells were incubated with hypoxic medium at 37°C with 5% CO2 and 0.1% O2 in a hypoxia chamber (Pro-Ox Model C21, BioSpherix Ltd., Redfield NY) for 4 hours, and then the HUVECs and H9C2 cells were exposed to reoxygenation with normal medium for 24 hours in an incubator at 37°C with 5% CO2 [19, 20]. The cells (HUVECs and H9C2) that were not subjected to hypoxia/reoxygenation (H/R) were incubated at 37°C with 5% CO2 and served as control (normoxia). The cellular proteins were prepared for Western blot.
Endothelial cell migration capacity was determined by the scratch assay after 24 hours of transfection. HUVECs were scratched with 200 μl tips and cells were photographed at 0, 12, 24 hours after injury. The percent closure of the wound was analyzed by an image analyzer (Image J, NIH).
In vivo transfection of lentivirus expressing miR-130a into mouse hearts
Mice were intubated and anesthetized with mechanical ventilation using 5% isoflurane. Anesthesia was maintained by inhalation of 1.5-2% isoflurane in 100% oxygen. Body temperature was maintained at 37°C by heating pad. Following the skin incision, the right common carotid artery (CCA) and the right external carotid artery (ECA) were carefully exposed. Microvascular aneurysm clips were applied to the right CCA and right ECA. A micro-catheter was introduced into the right external carotid artery and positioned into the aortic root. One hundred microliters of LmiR-130a (1 × 108 PFU) or LmiR-control were injected through the micro-catheter [19, 20]. The micro-catheter was gently removed and the external carotid artery was tightened before the skin was closed. Seven days after transfection, the hearts were harvested for isolation of microRNAs. The expression of miR-130a in heart tissues was examined by qPCR.
Induction of myocardial infarction
Myocardial infarction was induced as previously described [21]. Briefly, mice (28-30g) were anesthetized by 5.0% isoflurane, intubated, and ventilated with room air using a rodent ventilator. Anesthesia was maintained by inhalation of 1.5-2% isoflurane driven by 100% oxygen flow. Body temperature was regulated at 37°C by heating pad. Following the skin incision, the hearts were exposed through a left thoracotomy in the fourth intercostal space. The left anterior descending (LAD) coronary artery was permanently ligated with an 8-0 silk ligature. The skin was closed, anesthesia was discontinued, and the animals were allowed to recover in pre-warmed cages.
In separate experiments, mice were treated with Pam3CSK4 (Catalog number: tlrl-pms, InvivoGen; 2mg/Kg body weight) or CpG-ODN (Catalog number: ODN 1826, InvivoGen; 0.4mg/Kg body weight) by i.p injection immediately after permanent ligation of LAD coronary. Twenty-four hours after ligation of LAD artery, hearts were harvested and ischemic areas were collected for isolation of microRNAs. MiR-130 levels were measured by qPCR.
Determination of myocardial infarct size induced by myocardial ischemia/reperfusion (I/R) injury
Myocardial infarct size was measured as described previously in a myocardial I/R model [5, 6, 22]. Briefly, mice were anesthetized by 1.5-2% isoflurane with 100% oxygen flow. The hearts were subjected to ligation of the left anterior descending (LAD) coronary artery with an 8-0 silk ligature. Forty-five min after ligation, the coronary artery was reperfused by pulling on the exteriorized suture to release the knot. The skin was closed, anesthesia was disconnected, and the animals were allowed to recover in the pre-warmed cages. Twenty-four hours after reperfusion, the hearts were harvested and infarct size was measured by TTC (triphenyltetrazolium chloride, Sigma-Aldrich) staining as described previously [5, 6, 22].
Echocardiography
Seven days after transfection of LmiR-130a or LmiR-control, the mice were subjected to permanent ligation of LAD coronary artery (MI). Cardiac function was examined by echocardiography before (Baseline) and after MI (1, 3, 7, 14, and 21 days). M-mode tracings were used to measure LV wall thickness, LV end-systolic diameter, and LV end-diastolic diameter. Percent fractional shortening (FS%) and ejection fraction (EF%) were calculated as described previously [5, 6].
Examination of cardiac fibrosis
Cardiac fibrosis was determined by Masson's trichrome stain. In brief, mice were transfected with LmiR-130a or LmiR-control 7 days before induction of MI. Three weeks after MI, hearts were harvested and sliced horizontally. One slice below the ligation site was immersion-fixed in 4% buffered paraformaldehyde, embedded in paraffin, and cut at a 5 mm thickness. The sections were stained by trichrome stain (Masson) kit (Sigma-Aldrich) according to the manufacturer's protocol, as described previously [23]. The stained-sections were examined using 12.5 × magnification Microscope and analyzed by an image analyzer (Image J, NIH).
In situ apoptosis assay
Myocardial apoptosis was examined as described previously [5, 6, 22] using the in situ cell death detection kit, fluorescein (Roche, USA). Briefly, hearts were harvested and sliced horizontally. One slice below the ligation site was immersion-fixed in 4% buffered paraformaldehyde, embedded in paraffin, and cut at a 5 mm thickness. The sections were incubated at 37°C for 1 hour with the commercially prepared labeling mixture supplied by the manufacturer. The nuclei of living and apoptotic cells were stained with ProLong Gold Antifade Reagent with DAPI (Invitrogen). Fields of the border areas were randomly evaluated for the percentage of apoptotic cells. The images were viewed on an EVOS-fl digital inverted fluorescent microscope (Advanced Microscopy Group, Bothell, WA). Total cells were counted in each field, and apoptotic cells are presented as the percentage of total cells counted.
Western blot
Western blot was performed as described previously [19, 20]. Briefly, the cellular proteins were extracted from ischemic hearts and cultured cells. The protein concentrations were determined by BCA protein assay kit (Thermo Scientific). The cellular proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, Piscataway, NJ, USA). The ECL membranes were incubated with the appropriate primary antibody, including anti-PTEN, anti-phospho-Akt, anti-Bax, (Cell Signaling Technology, Inc, Danvers, MA), anti-Akt, anti-Bcl-2,anti-VEGF, anti-HOXA5 (Santa Cruz Biotechnology), respectively, followed by incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Inc.) and analysis by the ECL system (Amersham Pharmacia, Piscataway). To control for lane loading, the same membranes were probed with anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase, Biodesign, Saco, Maine). The signals were quantified using the G: Box gel imaging system by Syngen (Syngene, USA, Fredrick, MD, USA).
Immunohistochemistry staining
Immunohistochemistry was performed as described previously [5, 24]. Briefly, one slice below the ligation site was immersion-fixed in 4% buffered paraformaldehyde, embedded in paraffin, and cut at 5 um sections. The sections were stained with specific anti-CD31 antibody (1:50 dilution, Abcam, ab28364) and treated with the ABC staining system (Santa Cruz Biotechnology). Three slides from each block were evaluated, counterstained with hematoxylin, and examined with bright-field microscopy. Four fields on each slide were examined at the infarct areas using a defined rectangular field area with 40 × magnification.
Caspase-3/7 and caspase-8 activities Assay
Caspase-3/7 and caspase-8 activities were measured using a Caspase-Glo assay kit (Promega) according to the manufacturer's protocol as described previously [5].
Statistical analysis
The data are expressed as mean ± SEM. Comparisons of data between groups were performed using either student t-test or analysis of variance (ANOVA), and the least significant difference procedure for multiple-range tests was performed. P< 0.05 was considered to be significant.
Results
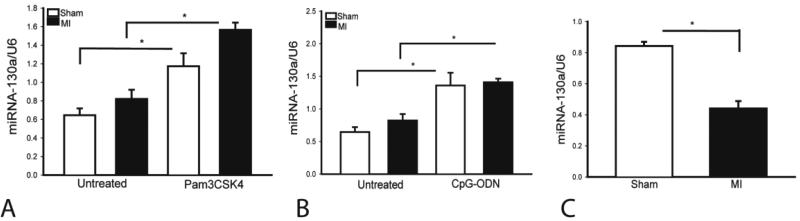
MiR-130a levels were decreased following Myocardial infarction
The role of miR-130a in myocardial infarction has not been investigated. We examined whether myocardial miR-130a expression would be altered following MI. As shown in Figure 1A and B, the levels of miR-130a were not significantly altered 24 hours after MI. However, miR-130a expression was significantly decreased (↓47.6%) three days after MI (Figure 1C), indicating that miR-130a may require protection against cardiac injury following myocardial infarction. We have previously reported that the TLR2 ligand (Pam3CSK4) [5] or a TLR9 ligand (CpG-ODN) [7] induces protection against myocardial ischemia/reperfusion (I/R) injury via activation of PI3K/Akt signaling. We then examined whether the TLR ligands increased miR-130a expression after MI. Mice were treated with either TLR2 ligand or TLR9 ligand before induction of MI. MiR-130a expression in the myocardium was assessed by qPCR. Figure 1A and B shows that administration of Pam3CSK4 significantly increased myocardial miR-130a levels in the absence (↑95%) or presence (↑91%) of MI. Similarly, CpG-ODN treatment also markedly increased miR-130a levels in the myocardium by 127% in sham control and by 72% in MI hearts. This data indicates that miR-130a may play a protective role in the myocardial ischemic injury through activation of PI3K/Akt signaling.
Figure 1.
TLR ligands increase the expression of miR-130a and MI-induced decreases in the levels of miR-130a in the myocardium. (A and B) Mice were treated with and without Pam3CSK or CpG-ODN immediately after permanent ligation of LAD coronary artery. Sham control mice were also treated with and without Pam3CSK4 or CpG-ODN. Twenty-four hours after LAD ligation, hearts were harvested for microRNA measurement. (C) MI decreased expression of miR-130a in the myocardium. Three days after MI, hearts were harvested for microRNA measurement. N=3 in sham group. N=4 in MI group. *p<0.05 compared with indicated group.
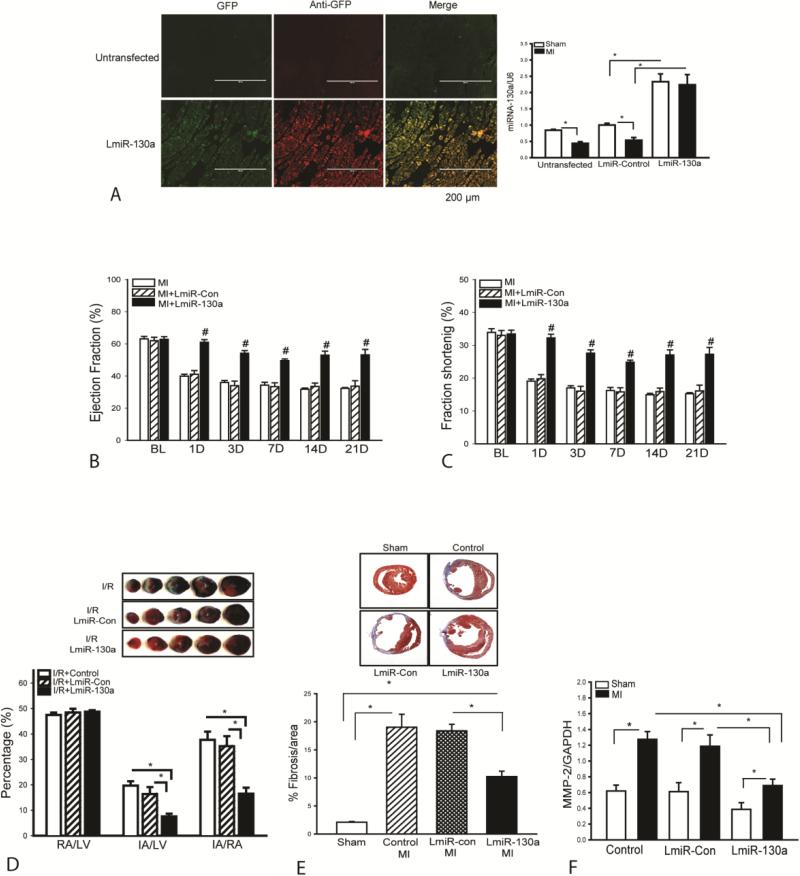
Transfection of lentivirus expressing miR-130a attenuates cardiac dysfunction after MI
To evaluate the role of miR-130a in cardiac function following MI, we constructed a lentivirus expressing miR-130a (LmiR-130a) and transfected it into the myocardium seven days before the hearts were subjected to MI. Lentivirus expressing scrambled miR served as control (LmiR-control). Figure 2A shows that MI resulted in a significant decrease in the expression of miR-130a (↓ 47.6%) compared with sham control. However, transfection of LmiR-130a significantly increased the levels of miR-130a in sham control (↑133%) and in infarcted hearts (↑315%), when compared with respective LmiR-Con controls. Figures 2B and C show that transfection of LmiR-130a into the myocardium markedly attenuated MI-induced cardiac dysfunction. MI markedly decreased ejection fraction (EF%) (B) and fractional shortening (FS%) values (C) in the untransfected hearts from day one up to day 21 after MI, when compared with the baseline. In contrast, the values of EF% and FS% in LmiR-130a transfected hearts were significantly greater than in the untransfected group at all the time points measured after MI. Transfection of LmiR-control did not prevent the MI-induced decrease in EF% and FS% values. There were no significant differences in baseline of cardiac function between untransfected, LmiR-control transfected, and LmiR-130a transfected hearts.
Figure 2.
Transfection of lentivirus expressing miR-130a (LmiR-130a) attenuates cardiac dysfunction and decreases collagen deposition after MI. Mice were transfected with LmiR-130a or LmiR-con for 7 days before induction of MI. (A) Increased expression of miR-130a in the myocardium following LmiR-130a transfection. Hearts were harvested at 3 days after MI, sectioned and viewed by fluorescent microscopy. Ischemic areas were collected for miR-130a measurement. (B and C) Cardiac function was examined by echocardiography before (Baseline) and at 1, 3, 7, 14, and 21 days after MI. Transfection of LmiR-130a maintained the levels of ejection fraction (EF%) and fractional shortening (FS%). N=24 in untransfected group. N=11 in LmiR-Control group. N=9 in LmiR-130a group. (D) LmiR-130a transfection decreases infarct size in myocardial ischemia/reperfusion (I/R) injury. Seven days after transfection with LmiR-130a, the mice were subjected to myocardial ischemia (45 minutes) and followed by reperfusion (24 hours). Hearts were harvested for measurement of infarct size by TTC staining. Ratios of risk area vs. left ventricle area (RA/LV) and infarct area vs. risk area (IA/RA) are presented in the graphs. Photographs of representative heart sections are shown above. N=7 in untransfected and LmiR-Control group. N=8 in LmiR-130a group. (E) Increased expression of miR-130a decreases collagen deposition in the myocardium. Three weeks after MI, hearts were harvested for analysis of cardiac fibrosis. Representative images of heart sections stained with Masson's trichrome. N=3 in untransfected and LmiR-Control groups. N=5 in LmiR-130a group. (F) Increased expression of miR-130a attenuates MI-induced increases in the levels of MMP-2 (n=5/group). #p<0.05 compared with LmiR-control and untransfected MI groups at the same time point. *p<0.05 compared with indicated group.
Increased expression of miR-130a decreases infarct size following myocardial ischemia/reperfusion (I/R) injury
We examined the effect of increased expression of miR-130a on myocardial infarct size in a model of myocardial I/R injury. LmiR-130a was transfected into the myocardium 7 days before the hearts were subjected to ischemia (45 min) followed by reperfusion (24 h). The infarct size was evaluated by Tetrazolium Chloride (TTC) staining. Figure 2D shows that I/R induced myocardial infarction in the untransfected mice. However, the infarct size in LmiR-130a transfected hearts was markedly reduced (↓53%) compared with the LmiR-control group. Transfection of LmiR-control did not affect I/R-induced infarct size. There was no significant difference in the ratio of risk area/left ventricle (RA/LV), which reflects the position of the coronary artery ligation, among the three groups.
Increased expression of miR-130a decreases collagen deposition in the myocardium following MI
Myocardial infarction induces collagen deposition, i.e. fibrosis in the myocardium which contributes to cardiac dysfunction23. Figure 2E shows that, 21 days after MI, fibrosis occurred in the infarcted area as evidenced by Masson's Trichrome staining of collagen in the heart tissue sections. In LmiR-130a transfected hearts, the percentage of fibrotic area was significantly reduced by 44.3% compared with the LmiR-control group. There was no significant difference in MI-induced fibrosis deposition between LmiR-control and untransfected MI groups.
Increased expression of miR-130a attenuates MI-induced increase in the expression of matrix metalloproteinase-2 (MMP-2) in the myocardium
Matrix metalloproteinase-2 (MMP-2) plays an important role in collagen degradation following MI [27, 28]. We examined the effect of miR-130a on the expression of MMP-2 in the myocardium following MI. Figure 2F shows that MMP-2 levels were significantly increased (↑105%) after MI, compared with sham control. In contrast, transfection of LmiR-130a into the myocardium markedly attenuated MI-induced increases in the expression of MMP-2 (↓41.5%), when compared with LmiR-control MI group. Transfection of LmiR-control did not alter MI-increased MMP-2 expression in the myocardium.
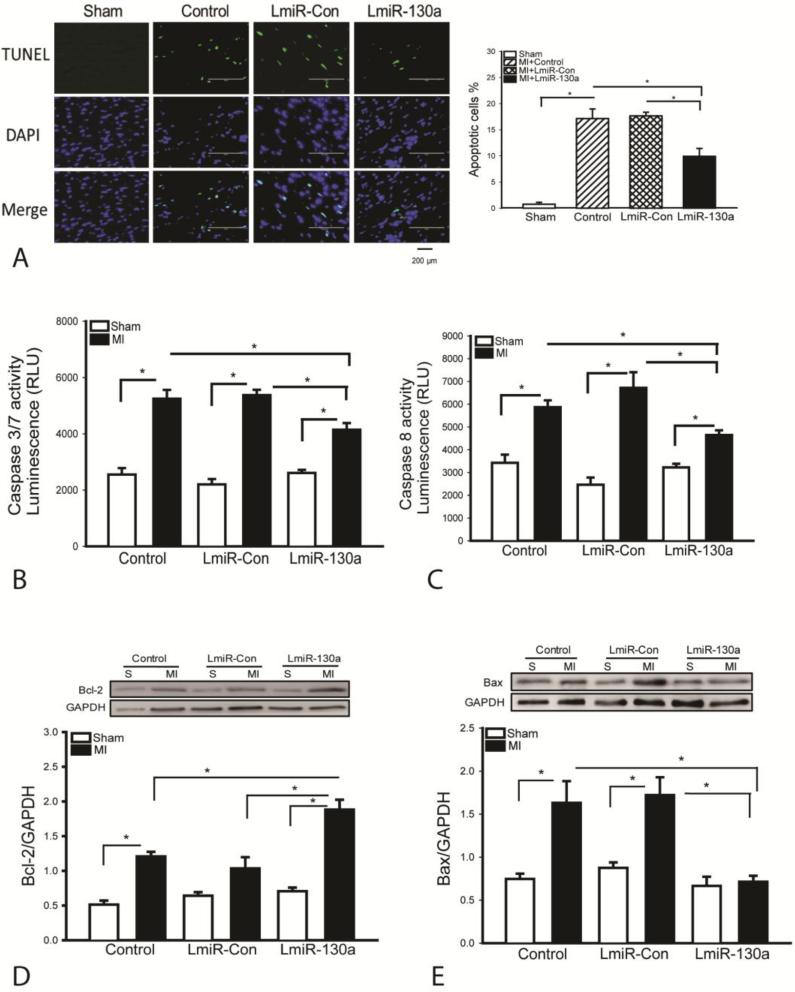
Transfection of LmiR-130a attenuates myocardial apoptosis following MI
Myocardial apoptosis plays a role in cardiac dysfunction following MI[29]. We examined the effect of miR-130a on MI-induced myocardial apoptosis. As shown in Figure 3A, MI induced myocardial apoptosis, as evidenced by TUNEL positive staining heart sections, when compared with sham control (17.1±1.8 vs 0.7±0.4). MI also induced increases in caspase-3/7 and caspase-8 activities by 105.6% and 71.3% respectively, compared with the sham control (Figure 3B and C). In LmiR-130a transfected hearts, the TUNEL positive apoptotic cells were markedly reduced by 44% (9.9±1.5 vs 17.7±2.3) and caspase-3/7 as well as caspase-8 activities were reduced by 22.9% and by 30.8% compared with LmiR-control group (Figures 3B and C). Bcl2 is an anti-apoptotic protein while Bax is a pro-apoptotic protein[30]. Figure 3D shows that MI induced an increase in the levels of Bcl2 in the myocardium compared with sham control. However, the Bcl2 levels in LmiR-130a transfected MI hearts were significantly greater by 82.5% than in LmiR-control MI group. MI markedly increased the levels of Bax by 118.6%, when compared with sham control (Figure 3E). In contrast, LmiR-130a transfection prevented MI-induced increases in Bax levels. Transfection of LmiR-control did not alter MI-induced apoptosis, caspase-3/7 and -8 activities, Bcl2 and Bax levels (Figures 3A-E).
Figure 3.
Increased expression of miR-130a attenuates myocardial apoptosis following MI. Three days after MI, hearts were harvested and sectioned for TUNEL assay. (A) TUNEL staining positive apoptotic cells (green fluorescence). DAPI staining nucleus (blue color). Qualitative data shows the percent apoptotic cells. (B and C) Increased expression of miR-130a attenuated MI-induced caspase-3/7 and -8 activities in the myocardium. (D and E) Transfection of LmiR-130a increased Bcl2 and decreased Bax levels in the myocardium after MI. N=4 in sham control group. N=6 in MI group. *p<0.05 compared with indicated group.
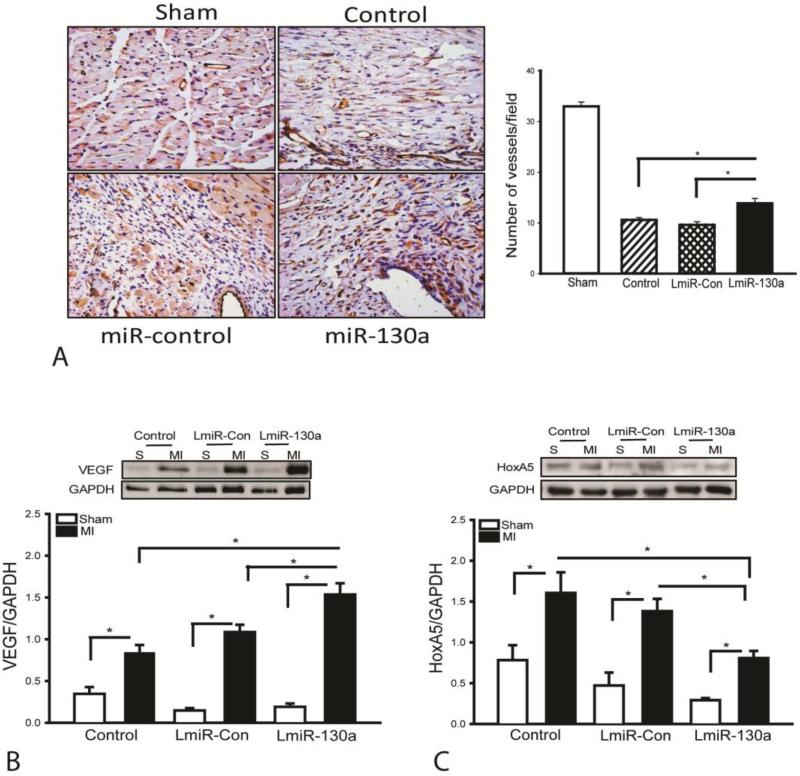
Transfection of LmiR-130a increases microvascular density in the myocardium following MI
In vitro data indicates that miR-130a promotes endothelial cell proliferation and migration. We examined whether increased expression of miR-130a would stimulate angiogenesis in the myocardium following MI. As shown in Figure 4A, the microvascular density in infarcted hearts was significantly reduced (↓67%) compared with sham control. However, LmiR-130a transfection markedly enhanced the microvascular density by 44.8% compared with the miR-control MI group at 21 days after MI. Vascular endothelial growth factor (VEGF) is an important pro-angiogenic growth factor[31] while homeobox protein HoxA5 is an antiangiogenic factor[32]. Figures 4B and C show that MI-induced increases in the levels of VEGF by 142.1% and HoxA5 by 105.2% respectively, compared with sham control. However, LmiR-130a transfection further enhanced VEGF levels by 41.5% and decreased HoxA5 levels by 41%, when compared with the LmiR-control MI groups (Figures 4B and C). There was no significant difference in the microvascular densities and the levels of VEGF as well as HoxA5 between LmiR-control and untransfected groups following MI.
Figure 4.
Transfection of LmiR-130a increases small vascular density following MI. After induction of MI for 21 days, hearts were harvested and sectioned for staining with anti-CD31 antibody. (A) Representative images of heart sections stained with anti-CD31 antibody. Quantification of immunohistochemistry staining with anti-CD31 antibody in the ischemic zone shows increased numbers of small vessel density following transfection with LmiR-130a. N=3-5/group. Transfection of LmiR-130a increased VEGF (B) and decreased HoxA5 (C) levels in the myocardium. N=4 in sham control group. N=6 in MI group. *p<0.05 compared with indicated group.
Increased miR-130a expression promotes endothelial cell migration
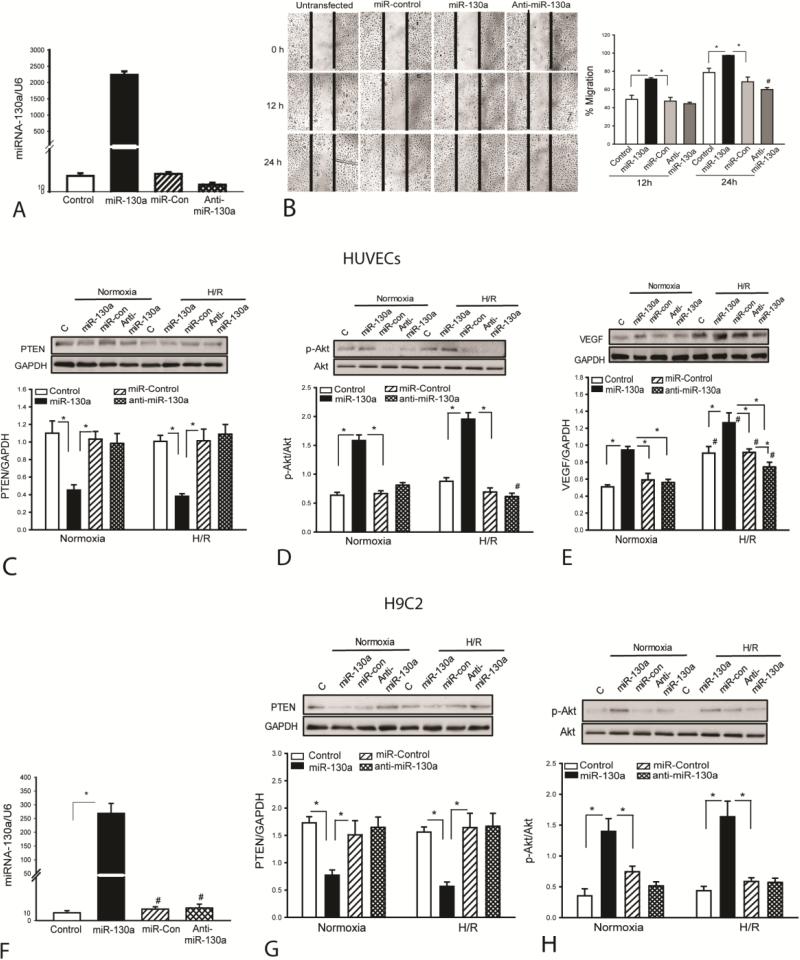
Our in vivo data shows that enhanced myocardial angiogenesis by increased expression of miR-130a is an important factor for improvement of cardiac function and attenuation of cardiac remodeling after MI [25]. To confirm the role of miR-130a in promoting endothelial cell proliferation and migration into the wound area, we performed in vitro experiments using human umbilical vein endothelial cells (HUVECs) that were subjected to scratch-induced wound area. HUVECs were transfected with miR-130a mimics, scrambled-miR mimics, and anti-miR-130a mimics respectively, before the cells were subjected to scratch-induced wound area. Figure 5A shows that transfection of HUVECs with miR-130a mimics significantly increased the levels of miR-130a (>1,000 fold) compared with the untransfected cells. Scratch stimulated the migration of endothelial cells into the wound area by 49.2% at 12 h and by 78.7% at 24 h (Figure 5B). In contrast, the endothelial cells that were transfected with miR-130a mimics migrated into the wound area by 71.4% at 12 h and 97.4% at 24 h following scratch (Figure 5B). However, treatment of the cells with anti-miR-130a markedly reduced endothelial cell migration into the wound area 24 h after scratch. Transfection of miR-control did not stimulate the migration of endothelial cells into the wound area.
Figure 5.
MiR-130a promotes endothelial cell migration, suppresses PTEN expression and increases Akt phosphorylation. HUVECs and H9C2 cardiomyoblasts were transfected with miR-130a mimics, scrambled miR-control mimics, or anti-miR-130a mimics, respectively. Twenty four hours after transfection, cells were subjected to hypoxia (4 h) followed by reoxygenation (24 h). The cells that were cultured at normal condition served as control (normoxia). (A) Increased miR-130a levels in HUVECs 48 h after transfection of miR-130a mimics. (B) Transfection of miR-130a mimics promotes HUVEC migration into the wound area. Representative images were taken before, 12 and 24 hours after injury (×4 magnification). Dashed line indicates the width of gap. N=3/group. #p<0.05 compared to all other groups. Transfection of miR-130a mimics suppresses PTEN expression (C), increases Akt phosphorylation (D) and VEGF expression (E) in HUVECs. Transfection of miR-130a mimics increases the levels of miR-130a (F), suppresses PTEN expression (G) and increases Akt phosphorylation (H) in H9C2 cardiomyoblasts . N=3-4 in each group. *p<0.05 compared with indicated group. #p<0.05 compared with other groups.
MiR-130a increases Akt phosphorylation, suppresses PTEN expression and enhances VEGF levels in endothelial cells
Activation of PI3K/Akt signaling has been demonstrated to stimulate endothelial cell proliferation and migration [26]. PTEN is a negative regulator for PI3K activity [3]. We examined whether miR-130a would suppress PTEN expression, resulting in activation of PI3K. We observed that miR-130a mimic transfection markedly decreased PTEN levels in HUVECs under normoxia condition (↓50.9%) or H/R condition (↓59.4%), when compared with respective controls (Figure 5C). We also examined whether miR-130a would induce Akt phosphorylation in HUVECs. As shown in Figure 5D, miR-130a mimic transfection significantly increased the levels of phosphorylated Akt by 148.8% in HUVECs that were cultured under normoxia condition and by 179.2% in HUVECs that were subjected to hypoxia followed by reoxygenation (H/R), when compared with respective controls. Transfection of miR-control or anti-miR-130a did not alter PTEN expression or the levels of Akt phosphorylation in HUVECs under normoxia or H/R condition. In addition, we examined the effect of miR-130a on VEGF expression in HUVECs under normoxia or H/E condition. We observed that H/R alone significantly induced VEGF expression by (↑78%) compared with normoxia control (Figure 5E). Transfection of miR-130a also significantly increased the levels of VEGF in both normoxia (↑84%) and H/R-treated cells (↑40%). Transfection of miR-control did not alter H/R-induced increases in expression of VEGF, while transfection of anti-miR-130a attenuated H/R-induced increases in the levels of VEGF.
We also examined whether miR-130a would target PTEN, leading to Akt phosphorylation in cardiomyoblasts, H9C2 cells. Figure 5F shows that the levels of miR-130a in H9C2 cells were significantly increased following transfection of miR-130a mimics. Increased miR-130a levels suppressed PTEN expression by 49% and enhanced the levels of phosphorylated Akt by 89% under normoxia condition, when compared with the LmiR-control (Figures 5G and H). After the cells were subjected to H/R, the levels of PTEN expression were lower (↓65%) and phospho-Akt were greater (↑178%) in miR-130a transfected group than in miR-control group (Figures 5G and H). Transfection of miR-control or anti-miR-130a did not alter PTEN expression and levels of Akt phosphorylation in H9C2 cells under normoxia or H/R condition.
Increased expression of miR-130a LmiR-130a suppresses PTEN expression and increases the levels of phosphorylated Akt in the myocardium
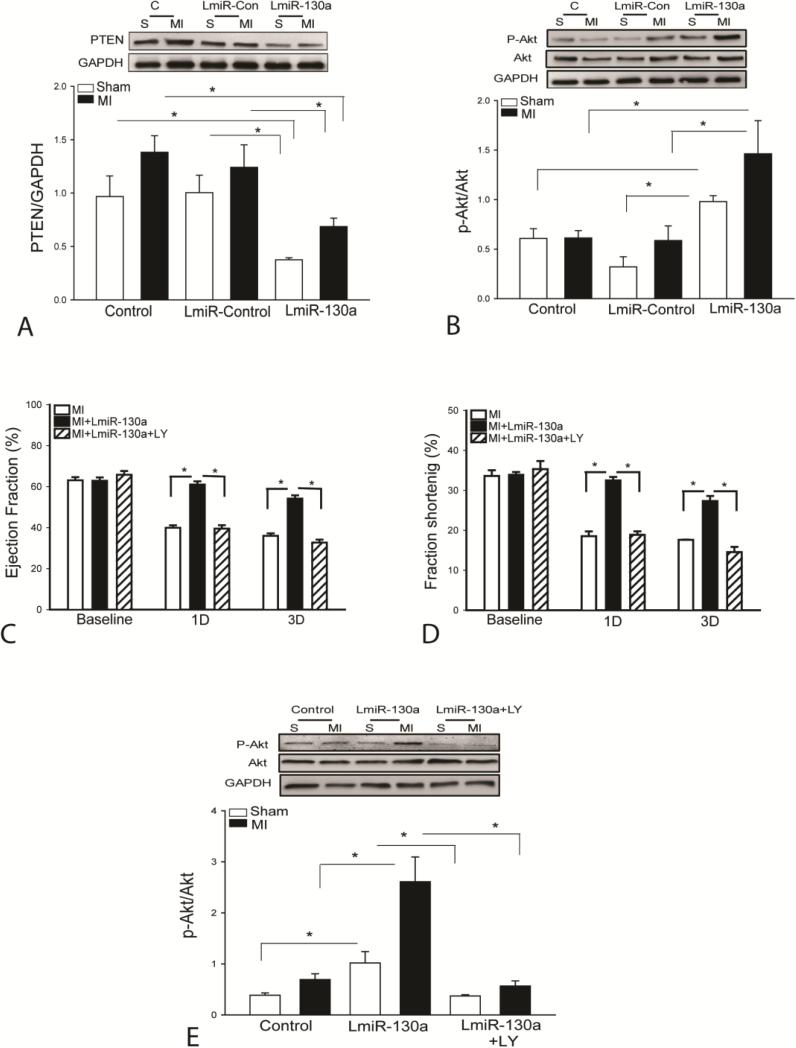
Activation of PI3K/Akt signaling has been shown to stimulate endothelial cell proliferation and angiogenesis [33]. We examined whether increased miR-130a expression by transfection of LmiR-130a would activate PI3K/Akt signaling in the myocardium following MI for three days. Figure 6A shows that increased miR-130a expression markedly decreased the levels of PTEN by 62.4% in sham control and by 44.6% in MI hearts, respectively, when compared with LmiR-control groups. LmiR-130a transfection also significantly increased Akt phosphorylation by 376% in sham control and by 147.5% in MI hearts compared with the respective LmiR-controls (Figure 6B). Transfection of LmiR-control did not alter the levels of PTEN expression and Akt phosphorylation (Figures 6A and B). The data suggests that increased expression of miR-130a activates PI3K/Akt activity via suppression of PTEN expression in the myocardium.
Figure 6.
PI3K inhibition abolished the cardioprotective effect of miR-130a following myocardial infarction. (A and B) Transfection of LmiR-130a suppresses PTEN expression and increases the levels of Akt phosphorylation in the presence and absence of MI. Three days after induction of MI, hearts were harvested for analysis of PTEN (A) and phosphorylated Akt (B) levels in the myocardium. N=4 in sham control group. N=6 in MI group. (C-E) PI3K inhibition by LY294004 administration abolished the attenuation of MI-induced cardiac dysfunction by LmiR-130a transfection. (C) EF% and (D) FS% values following MI. N=24 in untransfected group, N=11 in LmiR-Control group, and N=9 in LmiR-130a transfected group. (E) PI3K inhibition by LY294002 abolished LmiR-130a-induced increase in the levels of phosphorylated Akt. N=4-5 in sham control group. N=6 in MI group. * p<0.05 compared with indicated group.
PI3K inhibition abolishes the cardioprotective effect of miR-130a following MI
To determine whether activation of PI3K/Akt signaling plays a critical role in miR-130a-mediated protection against MI, we administered a PI3K specific inhibitor, LY294002 to LmiR-130a-transfected mice immediately after induction of MI. Cardiac function was measured by echocardiography on day 1 and day 3 after MI. Figures 6C and D show that LY294002 administration completely abolished the cardioprotective effect of LmiR-130a transfection. The EF% (Figure 6C) and FS% (Figure 6D) values in LY294002 treated MI hearts were significantly decreased on day 1 (35.2% and 39.6%) and on day 3 (41.5% and 45.1%) respectively, when compared with LmiR-130a transfected MI hearts. LY294002 administration also significantly prevented miR-130a-induced increases in the levels of Akt phosphorylation (Figure 6E). The data indicates that increased expression of miR-130a-induced protection after MI is mediated by a PI3K/Akt dependent mechanism.
Discussion
The present study demonstrates that miR-130a plays an important role in protection against myocardial ischemic injury. Specifically, we observed that increased expression of miR-130a by transfection of a lentivirus expressing miR-130a (LmiR-130a) into the myocardium significantly improved cardiac function and attenuated remodeling following myocardial infarction. The mechanisms are involved in activation of PI3K/Akt signaling via suppression of PTEN expression in the myocardium. PI3K inhibition by administration of LY294002 abolished the cardioprotective effect of LmiR-130a in the infarcted hearts. The data suggests that miR-130a serves a protective role in myocardial ischemic injury and that delivery of miR-130a by an appropriate vector may be a new therapeutic approach for heart attack patients.
We have previously reported that treatment of mice with Pam3CSK4 (TLR2 ligand) or CpG-ODN (TLR9 ligand) protects the myocardium from I/R injury through a PI3K/Akt dependent mechanism [5, 7]. We also reported that the TLR2 ligand, Pam3CSK4 increased the expression of myocardial miR-146a which suppresses TRAF6-mediated NF-κB activation[19]. Interestingly, we observed in the present study that treatment of mice with either Pam3CSK4 or CpG-ODN markedly increased the levels of miR-130a in the myocardium in the presence or absence of MI, indicating that miR-130a may play a protective role in myocardial ischemic injury via activation of PI3K/Akt signaling [5, 7]. Indeed, we observed that increased expression of miR-130a attenuates MI-induced cardiac dysfunction and remodeling. It is well known that angiogenesis is a critical step for the recovery of cardiac function and attenuation of remodeling after MI. To investigate whether miR-130a will induce the proliferation and migration of endothelial cells into wound area, we transfected human umbilical vein endothelial cells (HUVECs) with miR-130a mimics and examined the migration of HUVECs into scratch-induced wound area. We observed that increased miR-130a levels in the HUVECs stimulated the migration of HUVECs into scratch-induced wound area. In contrast, transfection of HUVECs with anti-miR-130a mimics markedly decreased the proliferation and migration of HUVECs into the wound area 24 h after scratch, when compared with untransfected HUVECs, indicating that miR-130a is required for endothelial cell migration into wound area.
Activation of PI3K/Akt signaling has been shown to play a critical role in the regulation of cell growth, proliferation, and survival [8] as well as angiogenesis [33]. Importantly, induction of angiogenesis has been reported to reduce the progression of MI, decreases cardiac fibrosis, improves cardiac function, and decreases the risk of cardiac rupture following MI [34], while activation of PI3K/Akt signaling has been shown to promote endothelial cell proliferation and migration [26]. We observed that transfection of miR-130a mimics significantly suppressed PTEN expression and enhanced the levels of phosphorylated Akt in HUVECs. Although we did not observe the alteration of PTEN levels following transfection of anti-miR-130a, the levels of Akt phosphorylation in HUVECs that were subjected to H/R were markedly reduced following anti-miR-130a transfection. The data indicates that miR-130a may stimulate angiogenesis through activation of PI3K/Akt signaling. Indeed, our in vivo data showed that increased expression of miR-130a in the myocardium significantly increased the numbers of small vessels in the ischemic area and VEGF expression, suggesting that miR-130a promoted angiogenesis in vivo after MI. At present, we do not understand the mechanism by which miR-130a stimulated VEGF expression in the myocardium following MI. Yun et al. reported that miR-130a suppressed the expression of the anti-angiogenic genes GAX and Homeobox A5 (HoxA5) in endothelial cells [18]. HoxA5 has been demonstrated to play a role in antiangiogenesis [32]. We observed that HoxA5 expression in the myocardium was markedly suppressed by LmiR-130a transfection in the presence and absence of MI. Collectively, our in vitro and in vivo data show that miR-130a suppresses PTEN expression, leading to activation of PI3K/Akt signaling. Therefore, it is possible that miR-130a stimulates angiogenesis by targeting the 3’-UTR of the antiangiogenic homeobox gene HoxA5[18], leading to VEGF expression and by suppressing PTEN expression, resulting in activation of PI3K/Akt signaling which promotes angiogenesis [33].
MI induces apoptosis in the myocardium of humans[35] and experimental animals [36]. Inhibition of myocardial apoptosis has been shown to attenuate cardiac dysfunction and remodeling after MI [37, 38]. We observed that transfection of LmiR-130a markedly decreased myocardial apoptosis in infarcted hearts, which positively correlated with improved cardiac function following MI. We also found that transfection of LmiR-130a suppresses MI-induced increases in Bax levels and increases the levels of Bcl2 in the myocardium. Bcl2 is an anti-apoptotic protein while Bax acts as an antagonist of anti-apoptotic Bcl2 [30], resulting in increases in mitochondrial membrane permeability and the release of cytochrome c[39]. It is unclear how miR-130a regulates myocardial apoptosis following myocardial infarction. However, activation of PI3K/Akt signaling has been shown to protect against apoptosis induced by ischemic injury [40]. It is possible, therefore, that inhibition of myocardial apoptosis by transfection of LmiR-130a is mediated through activation of PI3K/Akt signaling.
Our in vivo data confirmed that transfection of LmiR-130a significantly increased the levels of phosphorylated Akt by suppressing PTEN expression in the myocardium in the presence and absence of myocardial infarction. PTEN is a negative regulator for PI3K activity[3]. Suppression of PTEN expression has been reported to decrease myocardial ischemic injury via activation of PI3K/Akt signaling[8]. We demonstrated in the present study that increased expression of miR-130a suppressed PTEN expression in the myocardium in the presence or absence of MI. Similarly, in vitro transfection of endothelial cells or cardiomyoblasts H9C2 cells with miR-130a mimics suppressed PTEN expression in the presence or absence of H/R. Suppression of PTEN expression resulted in increases in the levels of phosphorylated Akt, indicating activation of PI3K/Akt signaling, which positively correlated with improved cardiac function, increased microvascular density in the myocardium, and attenuated remodeling following MI. To further confirm that activation of PI3K/Akt signaling by LmiR-130a transfection plays a critical role in the protection against myocardial ischemic injury, we treated LmiR-130a transfected mice with a PI3K specific inhibitor, LY294002, immediately after induction of MI and measured cardiac function following MI. We observed that PI3K inhibition by LY294002 completely abolished LmiR-130a induced improvement of cardiac function after myocardial infarction.
In summary, we demonstrated that miR-130a serves a protective role in attenuation of cardiac dysfunction and remodeling after MI. The mechanisms involved suppression of PTEN expression, resulting in activation of PI3K/Akt signaling.
Highlight.
Increased expression of miR-130a significantly attenuates myocardial infarction-induced cardiac dysfunction, decreases myocardial infarct size, reduces fibrosis, and promotes angiogenesis.
MiR-130a activates PI3K/Akt signaling by suppression of PTEN expression.
PI3K inhibition abolished miR-130a-induced attenuation of cardiac dysfunction after MI.
MicroRNA-130a could be a novel approach for the treatment of heart attack patients.
Acknowledgments
None
Sources of Funding:
This work was supported, in part, by National Institutes of Health grants HL071837 (CL), GM083016 (CL, DLW), GM53522 (DLW), GM093878 (RLK), and C06RR0306551.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
None.
Reference List
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart Disease and Stroke Statistics -- 2011 Update. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiedler J, Thum T. MicroRNAs in Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2013;33:201–5. doi: 10.1161/ATVBAHA.112.300137. [DOI] [PubMed] [Google Scholar]
- 3.Shiojima I, Walsh K. Role of Akt Signaling in Vascular Homeostasis and Angiogenesis. Circ Res. 2002;90:1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 4.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–60. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 5.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Shioi T, et al. TLR2 ligands induce cardioprotection against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, et al. Lipopolysaccharide-induced myocardial protection against ischemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovascr Res. 2008;78:546–53. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Ren D, Ha T, Liu L, Wang X, Kalbfleisch J, et al. CpG-ODN, the TLR9 agonist, attenuates myocardial ischemia/reperfusion injury: Involving activation of PI3K/Akt signaling. Biochim Biophys Acta. 2013;1832:96–104. doi: 10.1016/j.bbadis.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oudit GY, Sun H, Benoit-Gilles K, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–71. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Keyes KT, Xu J, Long B, Zhang C, Hu Z, Ye Y. Pharmacological inhibition of PTEN limits myocardial infarct size and improves left ventricular function postinfarction. Am J Physiol Heart Circ Physiol. 2010;298:H1198–H1208. doi: 10.1152/ajpheart.00915.2009. [DOI] [PubMed] [Google Scholar]
- 10.Schroen B, Heymans S. MicroRNAs and Beyond: The Heart Reveals Its Treasures. Hypertension. 2009;54:1189–94. doi: 10.1161/HYPERTENSIONAHA.109.133942. [DOI] [PubMed] [Google Scholar]
- 11.van Rooij E, Marshall WS, Olson EN. Toward MicroRNA-Based Therapeutics for Heart Disease: The Sense in Antisense. Circ Res. 2008;103:919–28. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science. 2009;324:1710–3. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 13.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–66. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardial injury. Circulation. 2010;122:1308–18. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. The Journal of Clinical Investigation. 2007;117:2369–76. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Li N, Wang H, Jia X, Wang X, Luo J. Altered microRNA expression in cisplatin-resistant ovarian cancer cells and upregulation of miR-130a associated with MDR1/P-glycoprotein-mediated drug resistance. Oncol Rep. 2012;28:592–600. doi: 10.3892/or.2012.1823. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–26. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Ha T, Liu L, Zou J, Zhang X, Kalbfleisch J, et al. Increased expression of microRNA-164a decreases myocardial ischemia/reperfusion injury. Cardiovasc Res. 2013;97:432–42. doi: 10.1093/cvr/cvs356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Ha T, Zou J, Ren D, Liu L, Zhang X, et al. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–95. doi: 10.1093/cvr/cvu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C, Ren D, Wang X, Ha T, Liu L, Lee EJ, et al. Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochim Biophys Acta. 2014;1842:22–31. doi: 10.1016/j.bbadis.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, et al. Protection against Myocardial Ischemia/Reperfusion Injury in TLR4 Deficient Mice is Mediated through a Phosphoinositide 3-Kinase Dependent Mechanism. J Immunol. 2007;178:7317–24. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Ha T, Gao X, Kelley J, Williams DL, Browder IW, et al. NK-kB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1712–H1720. doi: 10.1152/ajpheart.00124.2004. [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Ha T, Zhang X, Liu L, Wang X, Kelley J, et al. Toll-like receptor 3 plays a central role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2012;40:2390–9. doi: 10.1097/CCM.0b013e3182535aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mono-nuclear cell implantation. Lancet. 2003;361:47–9. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 26.Goetze S, Bungenstock A, Czupalla C, Eilers F, Stawowy P, Kintscher U, et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension. 2002;40:748–54. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: niR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–9. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acuta myocardial infarction and heart failure: Role of apoptosis. Int J Biochem Cell Biol. 2006;38:1834–40. doi: 10.1016/j.biocel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Herrmann JL, Beham AW, Sarkiss M, Chiao PJ, Rands MT, Bruckheimer EM, et al. Bcl-2 suppresses apoptosis resulting from disruption of the NF-kappa B survival pathway. Exp Cell Res. 1997;237:101–9. doi: 10.1006/excr.1997.3737. [DOI] [PubMed] [Google Scholar]
- 31.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–63. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphat Res Biol. 2005;3:240–52. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- 33.Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Laan AM, Piek JJ, van Royen N. Targeting antiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol. 2009;6:515–23. doi: 10.1038/nrcardio.2009.103. [DOI] [PubMed] [Google Scholar]
- 35.Olivetti G, Abbi R, Quaini F, Krajewski S, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–41. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 36.Abbate A, Morales C, De Falco M, Fedele V, Biondi Zaccai GG, Santini D, et al. Ischemia and apoptosis in an animal model of permanent infarct-related artery occlusion. Int J Cardiol. 2007;121:109–11. doi: 10.1016/j.ijcard.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 37.Chandrashekhar Y, Sen S, Anway R, Shuros A, Anand I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol. 2004;43:295–301. doi: 10.1016/j.jacc.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Hochhauser E, Cheporko Y, Yasovich N, Pinchas L, Offen D, Barhum Y, et al. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–20. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- 39.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;285:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 40.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–5. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]