Abstract
Objective
To examine the trends in use and safety of ovarian conservation in young women with early-stage endometrial cancer undergoing hysterectomy.
Methods
We conducted a population-based analysis. The National Cancer Database (NCDB) was used to identify women <50 years of age with stage I endometrioid adenocarcinoma of the endometrium who underwent hysterectomy from 1998–2012. Patients were stratified based on whether they underwent oophorectomy or had ovarian conservation. Multivariable models were used to examine predictors of ovarian conservation and the association between ovarian conservation and survival.
Results
The cohort of 15,648 women included 1121 (7.2%) who had ovarian conservation and 14,527 (92.8%) who underwent oophorectomy. The rate of ovarian conservation was relatively stable from 6.9% (95% CI, 4.9–9.7%) in 1998 to 7.1% (95% CI, 5.8–8.7%) in 2012 (P=0.91). Ovarian conservation was more commonly performed in younger women, black women, those with low grade and earlier stage tumors, and in women treated at community hospitals. In a multivariable model, ovarian conservation was not independently associated with survival (HR=0.94; 95% CI, 0.65–1.37). Similarly, in a Kaplan-Meier analysis, there was no association between ovarian conservation and survival (P=0.19).
Conclusion
Ovarian conservation does not adversely affect survival for women with early stage endometrial cancer. Despite the oncologic safety of ovarian conservation, the majority of young women with endometrial cancer still undergo oophorectomy at the time surgery.
Introduction
Treatment for most women with localized endometrial cancer begins with hysterectomy in combination with bilateral salpingo-oophorectomy and possibly lymph node evaluation.1 In young women, hysterectomy results in loss of fertility, while oophorectomy induces surgical menopause with the sequelae of estrogen deprivation.2 This is particularly important as the outcome of early-stage endometrial cancer is excellent and most young women will be cured of the disease.
The potential benefits of ovarian preservation in the general population have now been well established.3–8 A decision analysis of women undergoing hysterectomy for benign indications suggested that the benefits of ovarian conservation outweighed the risks until age 65.4 Follow-up from the Nurses Health Study found that oophorectomy was associated with increased mortality in women <50 years of age who never used estrogen therapy.7 Similarly, a large cohort study reported that oophorectomy before age 45 years was associated with an increased risk of cardiovascular and all-cause mortality.5,6
Despite the potential benefits of ovarian conservation, preservation of the ovaries in women undergoing hysterectomy for benign indications is highly variable.9,10 For premenopausal women with endometrial cancer, the decision to perform oophorectomy at the time of hysterectomy is further complicated by the potential of the ovaries to harbor occult metastatic disease and provide estrogenic stimulation. To date, observational studies have suggested that ovarian conservation is safe in young women with early-stage endometrial cancer.11–17
Given the potential benefits of ovarian preservation in young women with endometrial cancer, we conducted a population-based analysis to examine the trends and oncologic safety of ovarian conservation in women <50 years of age.
Materials and Methods
Patient level data from the National Cancer Data Base (NCDB) was used for analysis. NCDB is a nationwide registry developed by the American College of Surgeons and American Cancer Society.18,19 We utilized the Participant Use Files from the NCDB. NCDB records all patients with newly diagnosed invasive tumors from over 1500 Commission on Cancer (CoC) affiliated hospitals from throughout the United States. The database includes information on patient demographics, tumor characteristics, treatment data, staging, and follow-up and survival.18,19 Data are abstracted by trained cancer registrars, are audited regularly and have been utilized in a large number of outcomes studies.18 The data did not contain patient identifiers and was deemed exempt by the Columbia University Institutional Review Board.
We selected women <50 years of age with stage I endometrioid adenocarcinomas of the endometrium. Tumors were classified as stage IA (tumor confined to the endometrium or <50% of the myometrium), IB (tumor with >50% myoinvasion) endometrioid or stage INOS if the depth of myoinvasion was not available. Patients with cervical involvement of spread beyond the uterus were excluded (stages II–IV). The cohort was limited to women who underwent hysterectomy between 1998 and 2012 with exclusion of patients who received preoperative radiotherapy. Patients were stratified based on performance of oophorectomy into two groups: ovarian conservation vs. oophorectomy.
Demographic data analyzed included age (<30, 30–34, 35–39, 40–44, 45–49 years), race (white, black, other or unknown), and insurance status (commercial, Medicare, Medicaid, uninsured, other and unknown. Comorbidity was estimated using the Deyo classification of the Charlson comorbidity score (0, 1, ≥2).20,21 Tumor grade (1, 2, 3, unknown) was noted for each patient. Hospital characteristics analyzed included region and location (metropolitan, urban, rural). Hospitals were classified as academic/research cancer centers or community cancer centers based on the ACS CoC criteria.19
Frequency distributions between categorical variables were compared using χ2 tests. Trends in ovarian conservation over time were analyzed using the Cochran-Armitage Trend Test. Rates of ovarian conservation are reported descriptively stratified by age, stage, and tumor grade with 95% confidence intervals.
The associations between the clinical and demographic characteristics and ovarian conservation were examined using multivariable random effects log-linear models with Poisson distribution to account for hospital-level clustering of patients. These models included all clinically relevant demographic, clinical, and oncologic variables. Results are reported as risk ratios (RR) with 95% confidence intervals (CI).
All-cause mortality was estimated as the number of months from the date of diagnosis until death from any cause. Patients alive at last follow-up were censored. Random effects Cox proportional hazards models that account for hospital clustering were developed to estimate the association between ovarian conservation and overall survival, while adjusting for other clinical, demographic, and tumor characteristics. Kaplan-Meier curves were developed to compare survival between women who underwent oophorectomy and those who had ovarian conservation. The log-rank test was used to compare the survival curves. All hypothesis tests were two-sided. A P-value of <0.05 was considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
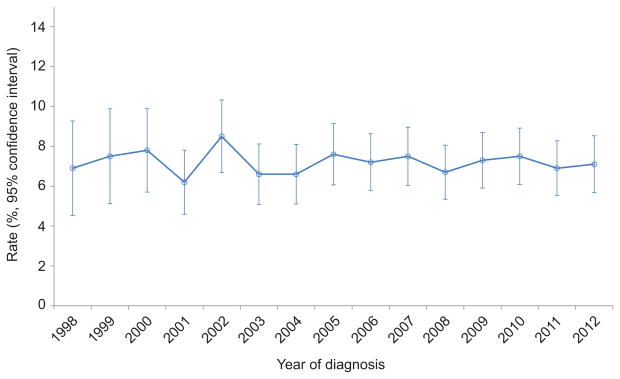
A total of 15,648 women <50 years of age with stage I endometrial cancer were identified (Table 1). The cohort included 1121 (7.2%) women who had ovarian conservation and 14,527 (92.8%) women who underwent oophorectomy. The median follow-up time in the ovarian conservation group was 61.9 months (IQR, 32.5–92.0) and 61.0 months (IQR, 33.1–95.1) in the oophorectomy group. The rate of ovarian conservation was 6.9% (95% CI, 4.9–9.7%) in 1998 rose to a peak of 8.5% (95% CI, 6.8–10.5%) in 2002 and then declined slightly and remained relatively stable at 7.1% (95% CI, 5.8–8.7%) in 2012 (Figure 1) (P=0.93.
Table 1.
Clinical and demographic characteristics of the cohort stratified by performance of oophorectomy
| Ovarian Conservation | Oophorectomy | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | (%) | N | (%) | P-value | Multivariable Risk Ratio (95% CI) | |
| 1121 | (7.2) | 14,527 | (92.8) | |||
| Year of diagnosis | 0.91 | |||||
| 1998 | 31 | (6.9) | 417 | (93.1) | Referent | |
| 1999 | 36 | (7.5) | 443 | (92.5) | 1.08 (0.67–1.75) | |
| 2000 | 49 | (7.8) | 581 | (92.2) | 1.12 (0.71–1.76) | |
| 2001 | 54 | (6.2) | 818 | (93.8) | 0.94 (0.60–1.46) | |
| 2002 | 76 | (8.5) | 823 | (91.6) | 1.21 (0.80–1.85) | |
| 2003 | 68 | (6.6) | 967 | (93.4) | 0.98 (0.64–1.50) | |
| 2004 | 71 | (6.6) | 1,003 | (93.4) | 0.98 (0.64–1.50) | |
| 2005 | 88 | (7.6) | 1,065 | (92.4) | 1.14 (0.76–1.73) | |
| 2006 | 90 | (7.2) | 1,167 | (92.8) | 1.08 (0.72–1.64) | |
| 2007 | 94 | (7.5) | 1,163 | (92.5) | 1.13 (0.75–1.70) | |
| 2008 | 87 | (6.7) | 1,219 | (93.3) | 1.01 (0.67–1.53) | |
| 2009 | 98 | (7.3) | 1,245 | (92.7) | 1.11 (0.74–1.67) | |
| 2010 | 100 | (7.5) | 1,238 | (92.5) | 1.14 (0.76–1.71) | |
| 2011 | 90 | (6.9) | 1,215 | (93.1) | 1.05 (0.69–1.59) | |
| 2012 | 89 | (7.1) | 1,163 | (92.9) | 1.07 (0.71–1.62) | |
| Age (years) | <0.0001 | |||||
| 45–49 | 357 | (4.7) | 7,191 | (95.3) | Referent | |
| 40–44 | 314 | (7.4) | 3,941 | (92.6) | 1.52 (1.30–1.77)** | |
| 35–39 | 241 | (10.4) | 2,070 | (89.6) | 2.19 (1.85–2.58)** | |
| 30–34 | 134 | (12.1) | 972 | (87.9) | 2.54 (2.07–3.10)** | |
| <30 | 75 | (17.5) | 353 | (82.5) | 3.64 (2.82–4.69)** | |
| Race | 0.001 | |||||
| White | 914 | (6.9) | 12,312 | (93.1) | Referent | |
| Black | 112 | (10.0) | 1,013 | (90.0) | 1.36 (1.11–1.67)* | |
| Other/unknown | 95 | (7.3) | 1,202 | (92.7) | 1.01 (0.81–1.26) | |
| Insurance status | 0.85 | |||||
| Commercial | 870 | (7.2) | 11,302 | (92.9) | Referent | |
| Medicare | 53 | (6.8) | 727 | (93.2) | 0.95 (0.72–1.26) | |
| Medicaid | 89 | (7.2) | 1,150 | (92.8) | 0.89 (0.71–1.12) | |
| Uninsured | 69 | (7.0) | 920 | (93.0) | 0.87 (0.68–1.13) | |
| Other | 12 | (7.5) | 148 | (92.5) | 0.98 (0.55–1.76) | |
| Unknown | 28 | (9.1) | 280 | (90.9) | 1.23 (0.83–1.81) | |
| Comorbidity | 0.001 | |||||
| 0 | 729 | (7.6) | 8,892 | (92.4) | -- | |
| 1 | 129 | (5.6) | 2,179 | (94.4) | -- | |
| ≥2 | 17 | (4.4) | 374 | (95.7) | -- | |
| Unknown | 246 | (7.4) | 3,082 | (92.6) | -- | |
| Region | 0.0003 | |||||
| New England | 90 | (9.1) | 895 | (90.9) | Referent | |
| Middle Atlantic | 133 | (5.8) | 2,170 | (94.2) | 0.65 (0.48–0.88)* | |
| South Atlantic | 234 | (7.8) | 2,763 | (92.2) | 0.77 (0.59–1.02) | |
| East North Central | 205 | (6.3) | 3,040 | (93.7) | 0.66 (0.50–0.87)* | |
| East South Central | 67 | (6.7) | 931 | (93.3) | 0.71 (0.50–1.02) | |
| West North Central | 68 | (5.9) | 1,090 | (94.1) | 0.64 (0.45–0.91) | |
| West South Central | 102 | (8.0) | 1,181 | (92.1) | 0.80 (0.58–1.10) | |
| Mountain | 70 | (9.4) | 679 | (90.7) | 1.02 (0.72–1.45) | |
| Pacific | 152 | (7.9) | 1,778 | (92.1) | 0.79 (0.59–1.06) | |
| Metropolitan location | 0.14 | |||||
| Metropolitan | 914 | (7.3) | 11,556 | (92.7) | Referent | |
| Urban | 148 | (6.4) | 2,184 | (93.7) | 0.89 (0.74–1.06) | |
| Rural | 12 | (4.8) | 238 | (95.2) | 0.68 (0.38–1.22) | |
| Unknown | 47 | (7.9) | 549 | (92.1) | 0.97 (0.72–1.32) | |
| Hospital type | <0.0001 | |||||
| Academic | 442 | (6.3) | 6,556 | (93.7) | Referent | |
| Comprehensive community cancer program | 563 | (7.4) | 7,025 | (92.6) | 1.74 (1.40–2.17)** | |
| Community cancer program | 114 | (11.2) | 908 | (88.9) | 1.19 (1.03–1.37)* | |
| Other1 | - | - | - | - | ||
| Grade | <0.0001 | |||||
| 1 | 790 | (8.4) | 8,627 | (91.6) | Referent | |
| 2 | 205 | (4.9) | 3,955 | (95.1) | 0.61 (0.52–0.71)** | |
| 3 | 52 | (5.2) | 940 | (94.8) | 0.70 (0.53–0.93)* | |
| Unknown | 74 | (6.9) | 1,005 | (93.1) | 0.85 (0.67–1.08) | |
| Stage | 0.001 | |||||
| IA | 997 | (7.2) | 12945 | (92.8) | Referent | |
| IB | 67 | (5.7) | 1105 | (94.3) | 0.76 (0.58–0.99)* | |
| INOS | 57 | (10.7) | 477 | (89.3) | 0.65 (0.45–0.93)* | |
Due to co-linearity, we were not able to fit comorbidity and year of diagnosis in the same model.
Data suppressed due to small sample size within the categories.
P<0.05
P<0.0001
Figure 1.
Rate of ovarian conservation for stage I endometrial cancer (P=.93).
Among women with stage IA neoplasms, ovarian conservation decreased with increasing grade from 8.3% (95% CI, 7.7–8.9%) for grade 1 neoplasms, to 5.0% (95% CI, 4.3–5.7%) for grade 2 tumors, and 4.7% (95% CI, 3.4–6.5%) for those with grade 3 carcinomas (Table 2). For each grade, ovarian conservation decreased with advancing age. For example, for women <30 years of age with stage IA, grade 1 tumors, ovarian conservation was utilized in 20.3% (95% CI, 15.7–25.9%) of women and decreased incrementally with age to 5.3% (95% CI, 4.6–6.0) in those age 45–49 years. Likewise, for stage IA, grad 3 tumors, ovarian conservation was used in 7.1% (95% CI, 1.3–31.5%) of those <30 years, 8.3% (95% CI, 4.1–16.2%) of women aged 35–39 years, and 3.9% (95% CI, 2.5–6.2%) of those age 45–49 years.
Table 2.
Rate of ovarian conservation stratified by stage and grade (%).
| Grade 1 | Grade 2 | Grade 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N* | (95% CI) | N* | (95% CI) | N* | (95% CI) | |
| Stage IA | ||||||
| All patients | 8615 | 8.3% (7.7–8.9%) | 3627 | 5.0% (4.3–5.7%) | 761 | 4.7% (3.4–6.5%) |
| <30 years | 236 | 20.3% (15.7–25.9%) | 96 | 12.5% (7.3–20.6%) | 14 | 7.1% (1.3–31.5) |
| 30–34 years | 630 | 14.3% (11.8–17.2%) | 25 | 8.8% (5.9–12.9%) | 40 | 7.5% (2.6–19.9%) |
| 35–39 years | 1296 | 12.2% (10.5–14.1%) | 556 | 7.2% (5.3–9.6%) | 84 | 8.3% (4.1–16.2%) |
| 40–44 years | 2400 | 8.6% (7.6–9.8%) | 940 | 4.4% (3.2–5.9%) | 1784 | 4.2% (2.2–8.1%) |
| 45–49 years | 4053 | 5.3% (4.6–6.0%) | 1784 | 3.7% (2.9–4.7%) | 434 | 3.9% (2.5–6.2%) |
Total patients (ovarian conservation and oophorectomy).
Ovarian conservation was more common in younger women; compared to women age 45–49 years, the risk ratio for ovarian conservation was 3.64 (95% CI, 2.82–4.69) in women <30 years of age and 2.54 (95% CI, 2.07–3.10) in those age 30–34 years (Table 1). Black women were more likely than white women to have ovarian conservation (RR=1.36; 95% CI, 1.11–1.67). Compared to patients treated at academic centers, women who underwent surgery at community centers were more likely to have ovarian conservation. Compared to women with stage IA neoplasms, those with IB neoplasms were less likely to have ovarian conservation (RR=0.76; 95% CI, 0.58–0.99). Similarly, ovarian conservation decreased with increasing tumor grade.
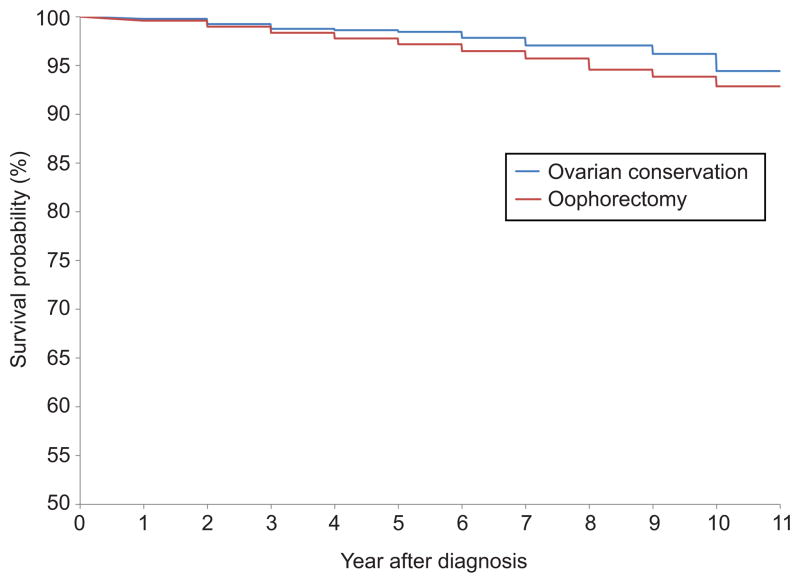
In a multivariable model, ovarian conservation was not independently associated with survival (HR=0.94; 95% CI, 0.65–1.37) (Table 3). Survival decreased with more advanced stage, higher tumor grade, and older age. Similarly, in a Kaplan-Meier analysis, there was no association between ovarian conservation and survival (P=0.19) (Figure 2, Table 4). Similar findings were noted when the analysis was limited to women with stage IA patients (data for stage IB not displayed given small number of women who had ovarian conservation).
Table 3.
Multivariable models of predictors of mortality.
| Hazard ratio for mortality (95% CI) | |
|---|---|
| Oophorectomy | |
| Oophorectomy | Referent |
| Ovarian conservation | 0.94 (0.65–1.37) |
| Year of diagnosis | |
| 1998 | Referent |
| 1999 | 1.05 (0.67–1.67) |
| 2000 | 1.26 (0.81–1.95) |
| 2001 | 1.27 (0.83–1.94) |
| 2002 | 0.91 (0.57–1.43) |
| 2003 | 0.76 (0.48–1.22) |
| 2004 | 1.05 (0.67–1.65) |
| 2005 | 1.04 (0.66–1.64) |
| 2006 | 1.00 (0.62–1.59) |
| 2007 | 0.99 (0.60–1.63) |
| 2008 | 0.68 (0.39–1.20) |
| 2009 | 0.86 (0.49–1.50) |
| 2010 | 0.61 (0.31–1.21) |
| 2011 | 0.78 (0.36–1.68) |
| 2012 | -- |
| Age (years) | |
| 45–49 | Referent |
| 40–44 | 0.76 (0.52–1.09) |
| 35–39 | 0.56 (0.42–0.75)* |
| 30–34 | 0.75 (0.61–0.93)* |
| <30 | 0.48 (0.23–0.97)* |
| Race | |
| White | Referent |
| Black | 1.17 (0.86–1.59) |
| Other/unknown | 0.94 (0.66–1.34) |
| Insurance status | |
| Commercial | Referent |
| Medicare | 3.78 (2.88–4.97)** |
| Medicaid | 2.85 (2.19–3.69)** |
| Uninsured | 1.88 (1.34–2.65)* |
| Other | 1.04 (0.38–2.83) |
| Unknown | 2.28 (1.48–3.51)* |
| Comorbidity | |
| 0 | -- |
| 1 | -- |
| ≥2 | -- |
| Region | |
| New England | Referent |
| Middle Atlantic | 0.90 (0.58–1.41) |
| South Atlantic | 1.46 (0.97–2.18 |
| East North Central | 1.06 (0.70–1.61) |
| East South Central | 1.30 (0.79–2.15) |
| West North Central | 0.95 (0.56–1.61) |
| West South Central | 1.38 (0.85–2.22) |
| Mountain | 1.18 (0.68–2.05) |
| Pacific | 1.18 (0.77–1.82) |
| Metropolitan location | |
| Metropolitan | Referent |
| Urban | 1.10 (0.86–1.40) |
| Rural | 1.63 (0.93–2.86) |
| Unknown | 1.90 (1.30–2.78)* |
| Hospital type | |
| Academic | Referent |
| Comprehensive community cancer program | 0.86 (0.61–1.23) |
| Community cancer program | 0.97 (0.80–1.18) |
| Other | 1.10 (0.13–9.36) |
| Grade | |
| 1 | Referent |
| 2 | 1.54 (1.26–1.89)** |
| 3 | 2.76 (2.12–3.59)** |
| Unknown | 2.05 (1.45–2.90)** |
| Stage | |
| IA | Referent |
| IB | 2.09 (1.64–2.67)** |
| INOS | 1.01 (0.58–1.76) |
P<0.05
P<0.0001
Figure 2.
Kaplan-Meier analysis of survival stratified by performance of oophorectomy (P=.15).
Table 4.
Follow-up time for women in the cohort based on Kaplan-Meier analysis.
| Follow-up Time | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | 4-year | 5-year | 6-year | 7-year | 8-year | 9-year | 10-year | 11-year | 12-year | 13-year | 14-year | 15-year | 16-year | |
| Ovarian conservation | ||||||||||||||||
| Women at risk | 982 | 910 | 825 | 720 | 621 | 508 | 396 | 311 | 227 | 180 | 121 | 77 | 50 | 23 | 9 | 3 |
| Dead | 2 | 5 | 3 | 1 | 1 | 3 | 3 | 0 | 2 | 3 | 0 | 1 | 2 | 2 | 0 | 0 |
| Censored | 70 | 80 | 102 | 98 | 112 | 109 | 82 | 84 | 45 | 56 | 44 | 26 | 25 | 12 | 6 | 3 |
| Cumulative deaths | 2 | 7 | 10 | 11 | 12 | 15 | 18 | 18 | 20 | 23 | 23 | 24 | 26 | 28 | 28 | 28 |
| Oophorectomy | ||||||||||||||||
| Women at risk | 12,906 | 11,999 | 10,784 | 9,327 | 8,010 | 6,593 | 5,198 | 4,069 | 3,156 | 2,352 | 1,691 | 1,117 | 669 | 347 | 138 | 33 |
| Dead | 51 | 72 | 64 | 51 | 46 | 41 | 35 | 45 | 22 | 22 | 20 | 9 | 5 | 7 | 1 | 0 |
| Censored | 856 | 1,143 | 1,393 | 1,266 | 1,371 | 1,354 | 1,094 | 868 | 782 | 639 | 554 | 439 | 317 | 202 | 104 | 33 |
| Cumulative deaths | 51 | 123 | 187 | 238 | 284 | 325 | 360 | 405 | 427 | 449 | 469 | 478 | 483 | 490 | 491 | 491 |
Discussion
Our findings suggest that ovarian conservation is safe for women with early stage endometrial cancer. Despite the oncologic safety of ovarian conservation, the majority of young women with endometrial cancer still undergo oophorectomy at the time of surgery. Age, stage, and tumor grade are important factors associated with the decision to offer ovarian conservation.
There is a growing body of literature supporting the oncologic safety of ovarian conservation in young women with endometrial cancer.11–17 In a cohort of women <45 years of age derived from the Surveillance, Epidemiology, and End Results database, ovarian conservation did not negatively affect survival.13 Similarly, the Korean Gynecologic Oncology group has demonstrated that among women with stage I–II endometrial cancer, ovarian conservation has no effect on either recurrence rates or survival.14 Our current analysis included over 1100 women who had ovarian preservation and in accord with prior work, found that conservation of the ovaries for women with stage I tumors did not influence survival.
The reticence to consider ovarian preservation for premenopausal women with endometrial cancer stems from a number of theoretic concerns.22 Perhaps most importantly, the ovaries may be the site of spread of metastatic endometrial cancer or harbor a concurrent primary ovarian tumor. One analysis of 102 women with endometrial cancer found coexisting ovarian tumors in 25% of women. The majority of the neoplasms (88%) were synchronous primary tumors while the remaining 12% were thought to be metastases.23 More recent studies have reported a lower rate of ovarian involvement and suggested that the majority of women with ovarian disease have grossly visible ovarian lesions or extrauterine disease.11,12
In addition to the possibility of occult metastatic disease, there is concern that ovarian-derived estrogen may stimulate occult endometrial cancer cells. However, studies to date have not found an increase in recurrence rates with ovarian preservation. A study of 495 women with endometrial cancer noted a recurrence rate of 2.3% in patients who had ovarian preservation compared to 2.5% after salpingo-oophorectomy.14 Likewise, among women who have undergone surgery for endometrial cancer, exogenous hormonal replacement therapy has not been shown to increase recurrence risk or alter prognosis. A prospective study of hormone replacement therapy undertaken by the Gynecologic Oncology Group found no increased risk with exogenous estrogen administration.24
While our study benefits from inclusion of a large cohort of young women with endometrial cancer, we acknowledge a number of limitations. First, we lack data on prior surgical history and cannot exclude the possibility that some women had undergone oophorectomy previously. However, given the young age of the women included, it is unlikely that many women would have undergone bilateral oophorectomy prior to the index procedure (hysterectomy). Second, as with any observational study, a number of unmeasured confounders may have influenced the allocation of treatment. We lack data on family history, the presence of inherited genetic abnormalities such as Lynch syndrome, body mass index, and the gross appearance of the ovaries at the time of operation. All of these factors likely affect the choice to perform oophorectomy, and our findings should be interpreted in the context of these and other factors that influence the risk of ovarian neoplasms. Third, while our study included a large number of women, there were a relatively small number of patients with deep myoinvasion and high tumor grade.
Despite multiple observational studies, we noted that the rate of ovarian conservation has changed little over the last decade. There are likely a number of factors contributing to the slow dissemination of ovarian conservation including patient and provider perceptions of increased risk, lack of awareness of the available data, and hesitancy to change established practices.25,26 However, despite the recognized limitations of observational data, it is unlikely that a randomized controlled trial of oophorectomy versus ovarian conservation would ever be performed. For women undergoing hysterectomy for benign indications, ovarian conservation has now been suggested as a quality metric.10
In the context of prior work, our data suggests that ovarian preservation is a reasonable option in some young women with endometrial cancer. For premenopausal women, the risks of long-term estrogen deprivation may outweigh the oncologic benefits of oophorectomy. Individualized risk assessment may help both patients and providers weigh the risks and benefits of oophorectomy. As the prognosis is excellent for the majority of women with early stage endometrial cancer, treatment paradigms that focus on long-term health benefits and well-being are clearly needed.
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 2.Lobo RA, Davis SR, De Villiers TJ, et al. Prevention of diseases after menopause. Climacteric. 2014;17:540–56. doi: 10.3109/13697137.2014.933411. [DOI] [PubMed] [Google Scholar]
- 3.Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009;113:1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219–26. doi: 10.1097/01.AOG.0000167394.38215.56. [DOI] [PubMed] [Google Scholar]
- 5.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–8. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 7.Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709–16. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera CM, Grossardt BR, Rhodes DJ, Rocca WA. Increased mortality for neurological and mental diseases following early bilateral oophorectomy. Neuroepidemiology. 2009;33:32–40. doi: 10.1159/000211951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obstet Gynecol. 2013;121:717–26. doi: 10.1097/AOG.0b013e3182887a47. [DOI] [PubMed] [Google Scholar]
- 10.Karp NE, Fenner DE, Burgunder-Zdravkovski L, Morgan DM. Removal of normal ovaries in women under age 51 at the time of hysterectomy. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 11.Lee TS, Jung JY, Kim JW, et al. Feasibility of ovarian preservation in patients with early stage endometrial carcinoma. Gynecol Oncol. 2007;104:52–7. doi: 10.1016/j.ygyno.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Pan Z, Wang X, Zhang X, Chen X, Xie X. Retrospective analysis on coexisting ovarian cancer in 976 patients with clinical stage I endometrial carcinoma. J Obstet Gynaecol Res. 2011;37:352–8. doi: 10.1111/j.1447-0756.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol. 2009;27:1214–9. doi: 10.1200/JCO.2008.19.8150. [DOI] [PubMed] [Google Scholar]
- 14.Lee TS, Lee JY, Kim JW, et al. Outcomes of ovarian preservation in a cohort of premenopausal women with early-stage endometrial cancer: a Korean Gynecologic Oncology Group study. Gynecol Oncol. 2013;131:289–93. doi: 10.1016/j.ygyno.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Sun C, Chen G, Yang Z, et al. Safety of ovarian preservation in young patients with early-stage endometrial cancer: a retrospective study and meta-analysis. Fertil Steril. 2013;100:782–7. doi: 10.1016/j.fertnstert.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Koskas M, Bendifallah S, Luton D, Darai E, Rouzier R. Safety of uterine and/or ovarian preservation in young women with grade 1 intramucous endometrial adenocarcinoma: a comparison of survival according to the extent of surgery. Fertil Steril. 2012;98:1229–35. doi: 10.1016/j.fertnstert.2012.07.1142. [DOI] [PubMed] [Google Scholar]
- 17.Kinjyo Y, Kudaka W, Ooyama T, Inamine M, Nagai Y, Aoki Y. Ovarian preservation in young women with endometrial cancer of endometrioid histology. Acta Obstet Gynecol Scand. 2015;94:430–4. doi: 10.1111/aogs.12588. [DOI] [PubMed] [Google Scholar]
- 18.The National Cancer Data Base. at https://www.facs.org/qualityprograms/cancer/ncdb.
- 19.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Wright JD. Take ‘em or leave’ em: management of the ovaries in young women with endometrial cancer. Gynecol Oncol. 2013;131:287–8. doi: 10.1016/j.ygyno.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Walsh C, Holschneider C, Hoang Y, Tieu K, Karlan B, Cass I. Coexisting ovarian malignancy in young women with endometrial cancer. Obstet Gynecol. 2005;106:693–9. doi: 10.1097/01.AOG.0000172423.64995.6f. [DOI] [PubMed] [Google Scholar]
- 24.Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:587–92. doi: 10.1200/JCO.2005.02.8464. [DOI] [PubMed] [Google Scholar]
- 25.Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health. 2012;102:1274–81. doi: 10.2105/AJPH.2012.300755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenfant C. Shattuck lecture--clinical research to clinical practice--lost in translation? N Engl J Med. 2003;349:868–74. doi: 10.1056/NEJMsa035507. [DOI] [PubMed] [Google Scholar]