Abstract
Purpose of review
Human immunodeficiency virus (HIV) is now managed as a chronic disease. Non-infectious pulmonary conditions have replaced infection as the biggest threat to lung health, particularly as HIV cohorts age, but there is no consensus on how best to maintain long-term lung health. We review the epidemiology and pathogenesis of chronic obstructive pulmonary disease (COPD), pulmonary arterial hypertension (PAH) and lung cancer in HIV-seropositive individuals.
Recent Findings
Diagnoses of COPD are now up to 50% more prevalent in HIV-seropositive individuals than HIV-uninfected controls, and prospective pulmonary function studies find significant impairment in 7%–>50% of HIV-seropositive individuals. The prevalence of HIV-PAH is 0.2%–0.5%, and lung cancer is 2–3 times more prevalent in HIV-seropositive individuals. Although host factors such as age and smoking have a role, HIV is an independent contributor to the pathogenesis of COPD, PAH and lung cancer. Chronic inflammation, immune senescence, oxidative stress and direct effects of viral proteins are all potential pathogenetic mechanisms. Despite their prevalence, non-infectious lung diseases remain under-recognized and evidence for effective screening strategies in HIV-seropositive individuals is limited.
Summary
COPD, PAH and lung cancer are a growing threat to lung health in the HAART era necessitating early recognition.
Keywords: HIV, chronic obstructive pulmonary disease, pulmonary arterial hypertension, lung cancer
Introduction
In countries with well-resourced health care systems, and increasingly in low and middle-income countries (LMIC), human immunodeficiency virus (HIV) infection has become a chronic illness. The focus of care has shifted from management of AIDS-associated illnesses to maintaining long-term health through optimal disease control and the prevention of secondary, non-AIDS complications. Primary prevention of cardiovascular disease, for example, is now a routine activity in the HIV clinic. This practice followed epidemiological studies showing the increased risk of coronary artery disease with HIV and guidelines that supported primary prophylaxis (1, 2). Neurocognitive, bone and renal health are similarly monitored. By contrast, long-term respiratory health receives less attention in the clinic, yet there is significant increased risk of non-AIDS lung disease in HIV-seropositive individuals (3–8). This review focuses on the most prevalent of these, namely chronic obstructive pulmonary disease (COPD), pulmonary arterial hypertension (PAH) and lung cancer, discusses current thinking on how HIV contributes to their pathogenesis and sets out what evidence exists to guide the promotion of long-term lung health in the HIV clinic.
COPD in HIV
It had become apparent by the 1990s that HIV was associated with both impaired diffusion capacity and COPD (9). Both case-control and prospective longitudinal studies in largely therapy-naïve HIV-seropositive cohorts showed reduced forced expiratory volume (FEV1), impaired diffusing capacity for carbon monoxide (DLCO) and emphysema on computed tomography (CT) scan of the thorax, even when matched for smoking, sex and age (9–11). Self-reported COPD was almost 3 times more common in HIV-seropositive individuals (12). Either or both COPD and impaired DLCO were associated with lower CD4 counts, pulmonary and non-pulmonary AIDS-associated conditions and non-HIV factors; principally age, smoking and injecting drug use (IDU) (10, 13–15).
The association has persisted into the highly active antiretroviral therapy (HAART) era. A 47% increased risk for COPD was seen in HIV-seropositive individuals of the prospective Veterans Aging Cohort (VACs), even after adjustment for known COPD risk factors (16). The same group have since reported data from the VACs and the 1999 Large Health Survey of Veteran Enrollees (LHS) and found the prevalence of COPD was 15% higher in HIV with a higher incidence rate ratio in individuals <50 years old (1.17 (95% confidence interval 1.11–1.24) and younger non-smokers (1.25 (1.08–1.45)) compared with seronegative controls (5). Most recently, COPD has been identified as an increasing cause of death in the San Francisco HIV/AIDS registry (17). Incident COPD diagnoses have again been reported as higher in those with HIV in the Multicenter AIDS Cohort Study (MACS), although incident COPD was not higher in HIV-seropositive individuals in the Women’s Interagency HIV Study (WIHS) cohort, possibly reflecting a diagnostic bias away from investigating chronic respiratory illness (18). This study also suggests that HIV clinicians might be under-diagnosing obstructive lung disease given that those with HIV report more cough, dyspnea and wheeze than HIV-seronegative individuals, but are not more likely to have had pulmonary function testing (4, 18).
These studies relied on diagnostic codes or self-report which may miss undiagnosed cases or incorrectly categorize lung disease. A more precise estimate of COPD prevalence and rates can be drawn from prospective clinic-based cohort-studies, typically involving 100–300 individuals who undergo pulmonary function testing (PFT). These studies find Global Initiative for Chronic Obstructive Lung Disease (GOLD)-defined COPD (FEV1/FVC <70% or <lower limit of normal (LLN) and FEV1<80% predicted) in 7% to 23% of HIV-seropositive individuals, 2 to 5-fold that of HIV-seronegative controls or reference populations, even in never-smokers (3, 4, 19–21). Impaired DLCO is even more prominent; 30–64% of HIV-seropositive individuals record DLCO below predicted values (22–24). The prevalence of DLCO impairment is significantly greater than in HIV-seronegative controls and while higher in smokers, it is also raised in HIV-seropositive non-smokers (22, 23).
HIV in the pathogenesis of COPD
Several lines of evidence suggest that HIV is an independent contributor to COPD. Airflow obstruction and DLCO impairment continue to be associated with prior history of pulmonary infection (e.g. Pneumocystis pneumonia), smoking exposure, injecting drug use (IDU) and increasing age, as in the pre-HAART era (3, 19) (Figure 1). New work confirms that disease is more pronounced in older HIV-seropositive individuals (over 40 years) who smoke more, showing 17–25% prevalence of COPD, diffusion impairment in more than a third and CT-confirmed emphysema in 26–37% (20, 21, 25, 26). Yet HIV, despite HAART, appears to remain as a driver; impairments in DLCO and airflow continue to progress over time in those receiving HAART (23, 27) and among IDU with established COPD, HIV is independently associated with increased acute exacerbations (28). Also new among the literature are studies from LMIC, where cohorts are typically younger with low rates of smoking providing further insight into the contribution of HIV, age and smoking to COPD. In Cameroon, COPD was 2.85 times more common in HIV-seropositive individuals and 90% were GOLD stage 2 or 3, yet the mean age was 42.6 and more than 80% were never-smokers (29). COPD was, however, associated with moderate to heavy biomass exposure and prior pulmonary TB. This prevalence is similar to the 7.8% prevalence among a similar population of African participants of the Pulmonary Substudy of the Strategic Timing of AntiRetroviral Treatment (START) trial where 50% of COPD was in never-smokers (30).
Figure 1.
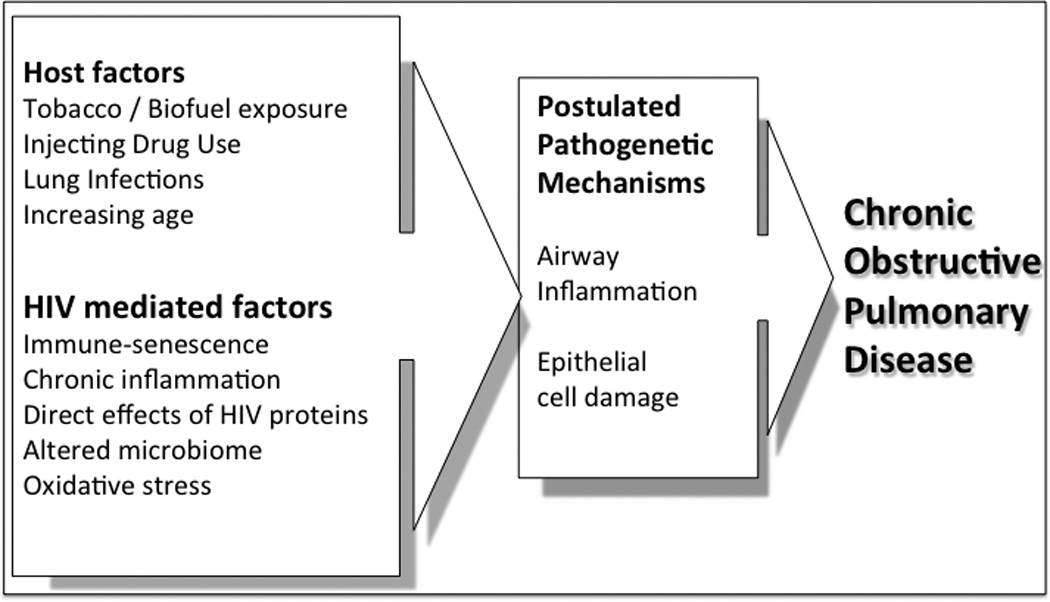
The potential contribution of host factors and HIV to the development of COPD in HIV-seropositive individuals
The potential role of HIV as an independent factor in COPD development (figure 1) is also supported by associations between COPD and measures of HIV severity. Recent studies still find a correlation between lower CD4 and worse FEV1 (20, 21, 31), DLCO (22) or emphysema scores on CT (32), similar to associations reported in the pre-HAART era (13–15). Meanwhile, undetectable viral load and the use of HAART are both protective factors (22, 27).
It is possible that the immune activation that persists with HAART is implicated in COPD pathogenesis. The observation by Diaz et al. that emphysema in HIV was associated with increased numbers of cytotoxic T cells in the lung (10) has been followed by new associations between PFT abnormalities and measures of immune activation including CD8 T cell activation, CD4 T cell death receptor expression and levels of IL-6, sCD14, D-Dimer and IL-8 in the BAL, sputum or blood (31, 33–35). The importance of HIV-induced inflammation in parenchymal damage is supported by a study in which smokers and never-smokers were analyzed separately; impaired DLCO was found in the never-smokers and, in this group, it correlated with airway inflammation but not FEV1/FVC or CT measures of emphysema as seen in the smokers (26). The dysregulated immune phenotypes of HIV and COPD share some common CD8 T cell features (36, 37) and have both been described as having features of accelerated immune senescence (5, 38), a hypothesis reinforced by the recent findings that reduced leukocyte telomere length is associated with PFT abnormalities and CT emphysema scores in HAART-treated HIV-seropositive cohorts (35, 39).
It is well documented that HIV is associated with oxidative stress (reviewed in (40)) and oxidant/antioxidant imbalance in the lung is a likely mechanism of airway damage; HIV, and specifically gp120 and tat, cause oxidative stress and alveolar epithelial barrier dysfunction in the lungs of animal models (41, 42) while the antioxidant glutathione (GSH) is reduced in both the plasma (43, 44) and the lungs (45, 46) of HIV seropositive individuals and may not return to normal with ART (47). Meanwhile, there is a compensatory GSH response in more emphysematous upper lobes of HIV-seropositive smokers and non-smokers (48). Oxidative stress also contributes to the pathogenesis of COPD; for instance GSH is reduced and linked to impaired nuclear factor (erythroid-derived 2)-like (NRF)-2 responses (reviewed in (49)), which are also documented in HIV (40). Confirming the relevance of oxidative stress, antioxidant N-acetylcysteine has been demonstrated to reduce COPD exacerbations (50).
The HIV virus and its secreted proteins may also act directly to cause lung damage. gp120 can induce mucus production from and cause damage to bronchial epithelial cells (51, 52), and greater levels of HIV RNA have been detected in more emphysematous regions of the lung (53). This finding is not solely relevant in the HAART naïve lung, as new evidence from HAART-treated macaques and virally-suppressed individuals indicates that the alveolar space may be a distinct compartment with persistent viral replication (54–57).
Finally, new data from the US Lung HIV Microbiome Program demonstrate that those with HIV and COPD have overrepresentation of fungal species (58), while there is an altered lung microbiome in HIV in general, in one series with increased T. whipplei, although the microbiome may be restored with HAART (59, 60). It is at least plausible that the lung microbiome could have a role, as it is well-established that bacterial microbiota in the lung are altered in COPD. The exact mechanism by which the microbiome contributes to disease progression is not yet known (61).
Pulmonary Arterial Hypertension in HIV
The prevalence of pulmonary arterial hypertension in HIV (HIV-PAH) is reported at 0.2% to 0.5% (6, 7, 62); however, prospective screening studies that use transthoracic echo to measure pulmonary artery pressure (PAP), rather than right heart catheterization (RHC), tend to report higher prevalence (63–66). PAH is defined as an elevated mean (m) PAP on RHC whereas echo estimates systolic (s) PAP as a surrogate for mPAP. sPAP is derived from measurements of the tricuspid valve regurgitant jet velocity, which may either under- or overestimate true mPAP (67). Furthermore, age- and weight-based reference values must be used as sPAP increases with age and body mass index. For example, 6% of individuals aged over 50 have sPAP>40mmHG (68). Finally, left ventricular diastolic dysfunction, which can alter sPAP and is common in HIV, must be accounted for (69). Nevertheless, even on RHC studies, the prevalence is still some hundred-fold that of the normal population (70), and the VACs database study that relied on much less sensitive diagnostic coding of HIV-PAH still reported a significantly increased prevalence of 0.2% and incident rate ratio of 1.57(5).
These studies span the current and pre-HAART eras and, overall, show no clear change in HIV-PAH prevalence. In fact, two studies have found that HAART use correlates with HIV-PAH prevalence (64, 66). However, HIV-PAH has also been associated with higher HIV viral load (VL) and lower CD4 cell counts (33, 65); and longitudinal follow-up of pressure gradients and 6-minute walk tests in HIV-PAH shows that progression can be slowed by HAART (71, 72). Additionally, both HAART and PAH-specific therapy are associated with better outcomes in HIV-PAH patients (73, 74).
Pathogenesis of HIV-PAH
PAH prevalence in HIV-seropositive individuals is likely to be increased, in part by the co-occurrence of other PAH risk factors such as venous thromboembolic disease, IDU and hepatitis C virus (HCV) infection (75) (figure 2). HIV does appear to be an independent risk factor for PAH, and simian immunodeficiency virus (SIV)-infected macaque studies demonstrate a high frequency of PAH development (76). HIV-PAH shares the histopathological features of hypertrophy and proliferation of the arterial wall and plexiform lesions with idiopathic PAH, despite not directly infecting pulmonary endothelial cells (77), which has led to suggestions that the two have common pathogenic mechanisms, albeit with different triggers (78). Chief among these are the HIV proteins; Nef may contribute to pulmonary vascular remodeling in a primate model and is found in human endothelial cells (79, 80). Distinct nef polymorphisms are associated with HIV-PAH, although the causal relationship cannot be determined (81). HIV tat suppresses bone morphogenic protein receptor 2 (BMPR-2), implicated in idiopathic PAH. Tat can induce oxidative stress in pulmonary endothelial cells and has recently been found to act synergistically with cocaine to induce reactive oxygen species (ROS)-mediated endothelial damage (82–85). Finally, gp120 can cause apoptosis and oxidative stress in pulmonary endothelial cells (77, 86), with the latter also resulting in HIF-1α-mediated upregulation of platelet derived growth factor, implicated in PAH (84). Further evidence of the importance of gp120, and perhaps a potential therapeutic approach, come from a new study that prevented gp120-induced pulmonary artery smooth muscle cell hypertrophy by blocking chemokine receptor 5 (CCR5) engagement using Maraviroc (ViiV Healthcare, Middlesex, UK) in a mouse model of hypoxia-induced PAH (87). gp120 also increases secretion of another important PAH factor, endothelin-1 (88). Endothelin-1 levels are higher in HIV-seropositive individuals and correlate with HIV-PAH severity (89).
Figure 2.
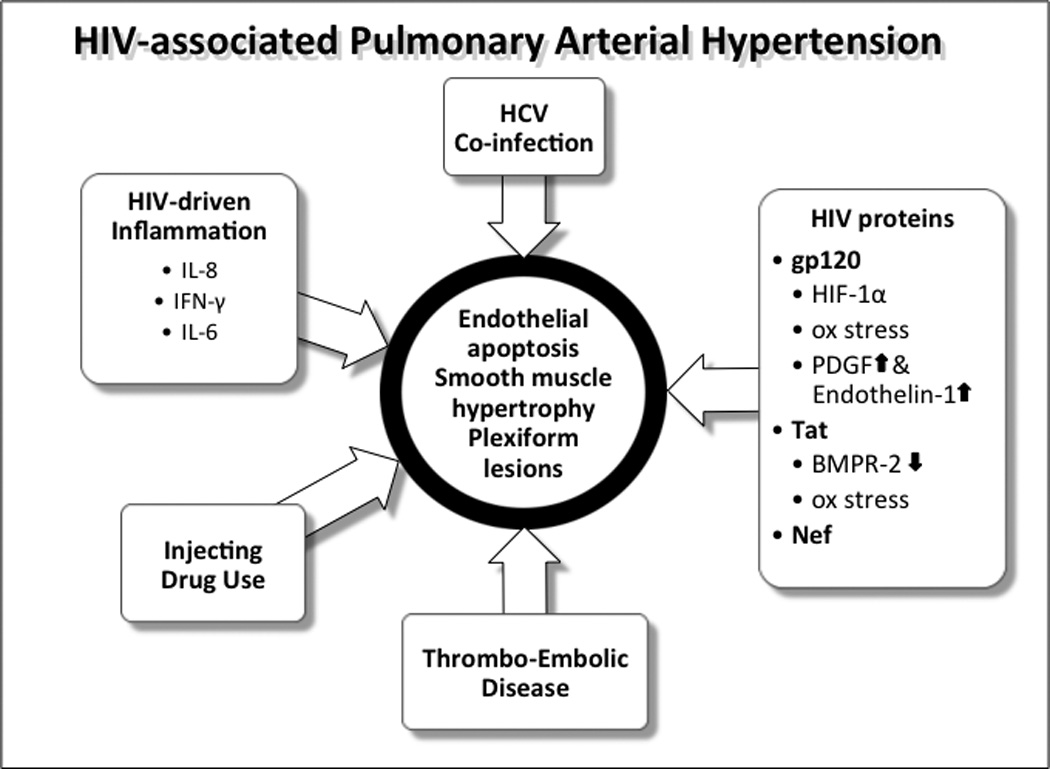
The role of viral and other factors in HIV-PAH pathogenesis
IL – interleukin, IFN – interferon, HIF – hypoxia inducible factor, PDGF – platelet derived growth factor, BMPR - bone morphogenic protein receptor, HCV – hepatitis C virus
Reports of associations between markers of systemic and pulmonary inflammation with HIV-PAH severity, including interleukin (IL)-8, interferon (IFN)-γ and, most recently, IL-6 and the endogenous inhibitor of endothelial nitric oxide synthase asymmetric dimethylarginine (ADMA)(33, 90), support the hypothesis that HIV-PAH is also driven by inflammation, even during HAART (91).
Lung cancer in HIV
Lung cancer, a non-AIDS defining cancer (NADC), is 2–3 times more common (8) and carries a higher mortality in HIV-seropositive individuals than in the general population (92–94). This higher mortality may result from lung cancer presenting at a more advanced stage in HIV-seropositive individuals (95), rather than due to any difference in the subtypes, which are broadly similar to the general population (96). As the HAART era continues, the number of HIV-seropositive individuals with lung cancer is growing in proportion. In France, it is now the leading cause of death in HIV (97).
The pathogenesis of lung cancer in HIV
Smoking is more common in HIV-seropositive individuals and continues to be the major contributing factor to lung cancer in this population (98, 99). Recent data emphasize that smoking is central to the risk of non-virological cancers (100, 101). However, lower CD4 and higher VL are also relevant to cancer mortality and the association with HIV is independent after adjustment for other factors (96, 99). Additionally, Sigel et al. have reported that CD4 counts < 500 cells/mm3 and reduced CD4/CD8 ratios were associated with increased lung cancer risk (102). While the pathogenic mechanism linking HIV to lung cancer is not understood, these latter studies implicate immunosuppression, similar to its role in the pathogenesis of other malignancies in HIV (103, 104). HIV-associated inflammation is also likely to be relevant, as markers of inflammation (IL-6, D-dimer and C-reactive protein) have been linked to both infection-related and non-infection-related cancers in HIV (105) and more specifically to lung cancer (106). Finally, inflammation may also underlie the contribution of recurrent bacterial pneumonia to an increased risk of lung cancer in HIV (107).
Screening for Lung Disease in HIV
Given the disparity between COPD diagnoses and rates of respiratory symptoms or PFT abnormalities in prospective studies, the current approach is inadequate to identify COPD in the HIV clinic (4, 18). Whether this lack of detection is due to poor physician awareness is not clear, but one barrier may be that screening often requires referral and evaluation elsewhere (108). The use of readily-available screening tools like peak flow, with or without symptom questionnaires, may be an approach that identifies those who need spirometry (109), but there remains a lack of prospective trials to guide an evidence-based approach to COPD screening in the HIV clinic.
Screening for HIV-PAH is more difficult. Prevalence is lower than COPD and symptoms of fatigue, dyspnea and cough may initially be absent (64). Echo-based screening may both over and under-diagnose PAH depending on sPAP cut-offs used (110, 111). These observations lead some to conclude screening asymptomatic HIV-seropositive patients for PAH should not be performed (69). The invasiveness and availability of RHC limit its use as a screening tool; however, growth differentiation factor (GDF)-15 and N-terminal pro-B-type natriuretic peptide (NT-proBNP) in serum are strongly associated with increased sPAP (63, 111), so could have potential as biomarkers in a screening protocol.
Radiographically, the appearance of lung cancer in HIV is similar to that of HIV-seronegative individuals (112) and given the success of lung cancer screening trials in high-risk groups (113), the use of CT screening has been investigated in HIV. Due to the presence of other lung pathology in HIV-seropositive individuals, such as scarring from previous infection (25), there might be unacceptably high rates of false positive scans resulting in significant over-investigation. This concern is borne out by Hulbert et al. (114) who found pulmonary nodules in more than 20% of participants, but detected only a single lung cancer in 678 person-years’ screening of a high-smoking HIV cohort. They concluded that screening might not be worthwhile except in older age groups. In contrast, the Examinations of HIV Associated Lung Emphysema (EXHALE) study investigators found no difference in rates of abnormal CT screening scans compared with HIV-seronegative individuals, albeit only in those with a CD4 > 200 cells/mm3 (115). Initial reports from the French HIV CHEST study, targeting HIV-seropositive individuals over 40 years of age with significant recent smoking history, suggest that using low dose CT thorax for early lung cancer diagnosis and nodule follow-up may be feasible in HIV-seropositive smokers (116). Currently, lung cancer screening of HIV-seropositive populations with CT is not recommended beyond the guidelines for the general population (117).
Conclusions
Non-infectious pulmonary complications are more common in HIV-seropositive individuals, and their prevalence and severity will likely increase as the HIV-seropositive population ages. To maintain lung health in long-standing HIV, these complications have to be prevented, recognized and managed. Starting all individuals with HIV on HAART, as now advocated by guidelines (118), and promoting smoking cessation will undoubtedly aid prevention. However, the evidence base for effective strategies to identify COPD, PAH and lung cancer in HIV clinics is weak. Furthermore, the risk factors for each condition may be different, and there is much still to learn about the underlying mechanisms.
An individualized, patient-centered approach should be taken. HIV clinicians must be alert to the early symptoms of respiratory disease, particularly exertional breathlessness, and have a low threshold (and tenacious insistence) for organizing basic spirometry and DLCO measurement. Echocardiography and CT of the thorax are readily available and can detect emphysema, HIV-PAH and lung cancers, but may lack specificity for the latter two. In the absence of HIV-specific evidence, the screening for these conditions should follow guidelines for HIV-seronegative individuals.
Key Points.
Chronic obstructive pulmonary disease (COPD), pulmonary arterial hypertension (PAH) and lung cancer are all more common in HIV-seropositive individuals than in the general population in the era of highly active antiretroviral therapy (HAART).
Chronic HIV-associated lung diseases are driven by the virus itself, non-HIV risk factors that are more prevalent in HIV-seropositive individuals such as smoking and injecting drug use (IDU) and ageing of the HIV-seropositive population.
Clinicians caring for HIV-seropositive patients should be alert to these conditions, but current evidence does not indicate that screening and diagnostic approaches should differ from the general population.
Acknowledgements
Dr. Paul Collini: None declared
Financial support and sponsorship: None Declared
Alison Morris: None declared
Financial support and sponsorship: NIH: K24 HL123342, P01 HL103455, R01 HL125409, R01HL120398
Footnotes
Dr. Paul Collini. Conflicts of interest: None Declared
Alison Morris. Conflicts of interest: None Declared
Contributor Information
Paul Collini, Dr., Infectious Diseases, Department of Infection and Immunity, University of Sheffield Medical School, Beech Hill Road, Sheffield S10 2JF, UK, Tel +44 (0)114-226 1113, Fax +44 (0)114-226 8898, p.collini@sheffield.ac.uk.
Alison Morris, Medicine, Department of Medicine, University of Pittsburgh, 628 NW Montefiore University Hospital, 3459 Fifth Avenue, Pittsburgh, PA 15213, United States, Tel 412-624-8209, morrisa@upmc.edu.
References
- 1.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1–e34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 3.George MP, Kannass M, Huang L, et al. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4(7):e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madeddu G, Fois AG, Calia GM, et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection. 2013;41(2):347–353. doi: 10.1007/s15010-012-0330-x. [DOI] [PubMed] [Google Scholar]
- 5.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 7.Opravil M, Sereni D. Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS. 2008;22(Suppl 3):S35–S40. doi: 10.1097/01.aids.0000327514.60879.47. [DOI] [PubMed] [Google Scholar]
- 8.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52(5):611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz PT, Clanton TL, Pacht ER. Emphysema-like pulmonary disease associated with human immunodeficiency virus infection. Ann Intern Med. 1992;116(2):124–128. doi: 10.7326/0003-4819-116-2-124. [DOI] [PubMed] [Google Scholar]
- 10.Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132(5):369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 11.Morris AM, Huang L, Bacchetti P, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. The Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 2000;162(2 Pt 1):612–616. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- 12.Gingo MR, Balasubramani GK, Kingsley L, et al. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS One. 2013;8(3):e58812. doi: 10.1371/journal.pone.0058812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen MJ, Lou Y, Kvale PA, et al. Pulmonary function tests in HIV-infected patients without AIDS. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 1995;152(2):738–745. doi: 10.1164/ajrccm.152.2.7633736. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell DM, Miller RF. AIDS and the lung: update 1992. 2. Recent developments in the management of the pulmonary complications of HIV disease. Thorax. 1992;47(5):381–390. doi: 10.1136/thx.47.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz PT, King MA, Pacht ER, et al. The pathophysiology of pulmonary diffusion impairment in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;160(1):272–277. doi: 10.1164/ajrccm.160.1.9812089. [DOI] [PubMed] [Google Scholar]
- 16.Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130(5):1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 17.Schwarcz SK, Vu A, Hsu LC, Hessol NA. Changes in causes of death among persons with AIDS: San Francisco, California, 1996–2011. AIDS Patient Care STDS. 2014;28(10):517–523. doi: 10.1089/apc.2014.0079. [DOI] [PubMed] [Google Scholar]
- 18. Gingo MR, Balasubramani GK, Rice TB, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med. 2014;14:75. doi: 10.1186/1471-2466-14-75. A prospective case-control study of >3500 participants in the HAART era which found respiratory symptoms to be significantly more common in HIV-seropositive individuals. Despite this, there was no difference in the rates of pulmonary function testing compared with seronegative controls, suggesting that diagnoses of COPD might be being missed.
- 19.Hirani A, Cavallazzi R, Vasu T, et al. Prevalence of obstructive lung disease in HIV population: a cross sectional study. Respir Med. 2011;105(11):1655–1661. doi: 10.1016/j.rmed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Samperiz G, Guerrero D, Lopez M, et al. Prevalence of and risk factors for pulmonary abnormalities in HIV-infected patients treated with antiretroviral therapy. HIV Med. 2014;15(6):321–329. doi: 10.1111/hiv.12117. [DOI] [PubMed] [Google Scholar]
- 21. Makinson A, Hayot M, Eymard-Duvernay S, et al. High prevalence of undiagnosed COPD in a cohort of HIV-infected smokers. Eur Respir J. 2015;45(3):828–831. doi: 10.1183/09031936.00154914. The most recent of several prospective HAART era studies using PFTs and CT scans of the thorax reporting increased prevalence of COPD in HIV.
- 22.Crothers K, McGinnis K, Kleerup E, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64(3):271–278. doi: 10.1097/QAI.0b013e3182a9215a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristoffersen US, Lebech AM, Mortensen J, et al. Changes in lung function of HIV-infected patients: a 4.5-year follow-up study. Clin Physiol Funct Imaging. 2012;32(4):288–295. doi: 10.1111/j.1475-097X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 24.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182(6):790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen E, Wittman C, Gingo M, et al. Chest computed tomography findings in HIV-infected individuals in the era of antiretroviral therapy. PLoS One. 2014;9(11):e112237. doi: 10.1371/journal.pone.0112237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gingo MR, He J, Wittman C, et al. Contributors to diffusion impairment in HIV-infected persons. Eur Respir J. 2014;43(1):195–203. doi: 10.1183/09031936.00157712. A cross-sectional analysis of DLCO and CT of the thorax in HIV-seropositive individuals in the HAART era which observed that diffusion impairment exists even in never-smokers supporting an independent role for HIV in the pathogenesis of COPD.
- 27.Drummond MB, Kirk GD, Astemborski J, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67(4):309–314. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lambert AA, Kirk GD, Astemborski J, et al. HIV Infection Is Associated With Increased Risk for Acute Exacerbation of COPD. J Acquir Immune Defic Syndr. 2015;69(1):68–74. doi: 10.1097/QAI.0000000000000552. A longitudinal HAART era study of injecting drug users who smoke and have COPD that found HIV infection was independently associated with increased risk for acute exacerbations.
- 29. Pefura-Yone EW, Fodjeu G, Kengne AP, et al. Prevalence and determinants of chronic obstructive pulmonary disease in HIV infected patients in an African country with low level of tobacco smoking. Respir Med. 2015;109(2):247–254. doi: 10.1016/j.rmed.2014.12.003. One of the first cross-sectional PFT studies to demonstrate increased prevalence of COPD in a low-smoking, LMIC setting.
- 30.Kunisaki KM, Niewoehner DE, Collins G, et al. Pulmonary function in an international sample of HIV-positive, treatment-naive adults with CD4 counts >500 cells/muL: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):119–128. doi: 10.1111/hiv.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung JM, Liu JC, Mtambo A, et al. The determinants of poor respiratory health status in adults living with human immunodeficiency virus infection. AIDS Patient Care STDS. 2014;28(5):240–247. doi: 10.1089/apc.2013.0373. [DOI] [PubMed] [Google Scholar]
- 32.Attia EF, Akgun KM, Wongtrakool C, et al. Increased risk of radiographic emphysema in HIV is associated with elevated soluble CD14 and nadir CD4. Chest. 2014;146(6):1543–1553. doi: 10.1378/chest.14-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris A, Gingo MR, George MP, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26(6):731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popescu I, Drummond MB, Gama L, et al. Activation-induced cell death drives profound lung CD4(+) T-cell depletion in HIV-associated chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(7):744–755. doi: 10.1164/rccm.201407-1226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzpatrick ME, Singh V, Bertolet M, et al. Relationships of pulmonary function, inflammation, and T-cell activation and senescence in an HIV-infected cohort. AIDS. 2014;28(17):2505–2515. doi: 10.1097/QAD.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 37.Hodge G, Nairn J, Holmes M, et al. Increased intracellular T helper 1 proinflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelial T cells of COPD subjects. Clin Exp Immunol. 2007;150(1):22–29. doi: 10.1111/j.1365-2249.2007.03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(7):573–580. doi: 10.1513/pats.200904-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu JC, Leung JM, Ngan DA, et al. Absolute leukocyte telomere length in HIV-infected and uninfected individuals: evidence of accelerated cell senescence in HIV-associated chronic obstructive pulmonary disease. PLoS One. 2015;10(4):e0124426. doi: 10.1371/journal.pone.0124426. A study that contributes to understanding the pathogenetic mechanisms of HIV in COPD, reporting an association between airflow obstruction and a marker of immune-senescence in HIV-seropositive individuals.
- 40.Porter KM, Sutliff RL. HIV-1, reactive oxygen species, and vascular complications. Free Radic Biol Med. 2012;53(1):143–159. doi: 10.1016/j.freeradbiomed.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota-Gomez A, Flores AC, Ling XF, et al. HIV-1 Tat increases oxidant burden in the lungs of transgenic mice. Free Radic Biol Med. 2011;51(9):1697–1707. doi: 10.1016/j.freeradbiomed.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lassiter C, Fan X, Joshi PC, et al. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther. 2009;6:1. doi: 10.1186/1742-6405-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanchu A, Rana SV, Pallikkuth S, Sachdeva RK. Short communication: oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS Res Hum Retroviruses. 2009;25(12):1307–1311. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- 44.Buhl R, Jaffe HA, Holroyd KJ, et al. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989;2(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- 45.Pacht ER, Diaz P, Clanton T, et al. Alveolar fluid glutathione decreases in asymptomatic HIV-seropositive subjects over time. Chest. 1997;112(3):785–788. doi: 10.1378/chest.112.3.785. [DOI] [PubMed] [Google Scholar]
- 46.Cribbs SK, Guidot DM, Martin GS, et al. Anti-retroviral therapy is associated with decreased alveolar glutathione levels even in healthy HIV-infected individuals. PLoS One. 2014;9(2):e88630. doi: 10.1371/journal.pone.0088630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aukrust P, Muller F, Svardal AM, et al. Disturbed glutathione metabolism and decreased antioxidant levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy--potential immunomodulatory effects of antioxidants. J Infect Dis. 2003;188(2):232–238. doi: 10.1086/376459. [DOI] [PubMed] [Google Scholar]
- 48.Diaz PT, Wewers MD, King M, et al. Regional differences in emphysema scores and BAL glutathione levels in HIV-infected individuals. Chest. 2004;126(5):1439–1442. doi: 10.1378/chest.126.5.1439. [DOI] [PubMed] [Google Scholar]
- 49.Boutten A, Goven D, Artaud-Macari E, et al. NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease. Trends Mol Med. 2011;17(7):363–371. doi: 10.1016/j.molmed.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2(3):187–194. doi: 10.1016/S2213-2600(13)70286-8. [DOI] [PubMed] [Google Scholar]
- 51.Gundavarapu S, Mishra NC, Singh SP, et al. HIV gp120 induces mucus formation in human bronchial epithelial cells through CXCR4/alpha7-nicotinic acetylcholine receptors. PLoS One. 2013;8(10):e77160. doi: 10.1371/journal.pone.0077160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan X, Joshi PC, Koval M, Guidot DM. Chronic alcohol ingestion exacerbates lung epithelial barrier dysfunction in HIV-1 transgenic rats. Alcohol Clin Exp Res. 2011;35(10):1866–1875. doi: 10.1111/j.1530-0277.2011.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yearsley MM, Diaz PT, Knoell D, Nuovo GJ. Correlation of HIV-1 detection and histology in AIDS-associated emphysema. Diagn Mol Pathol. 2005;14(1):48–52. doi: 10.1097/01.pas.0000142168.72253.11. [DOI] [PubMed] [Google Scholar]
- 54.Santangelo PJ, Rogers KA, Zurla C, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods. 2015;12(5):427–432. doi: 10.1038/nmeth.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cribbs SK, Lennox J, Caliendo AM, et al. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Res Hum Retroviruses. 2015;31(1):64–70. doi: 10.1089/aid.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buzon MJ, Codoner FM, Frost SD, et al. Deep molecular characterization of HIV-1 dynamics under suppressive HAART. PLoS Pathog. 2011;7(10):e1002314. doi: 10.1371/journal.ppat.1002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horiike M, Iwami S, Kodama M, et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423(2):107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 58.Cui L, Lucht L, Tipton L, et al. Topographic diversity of the respiratory tract mycobiome and alteration in HIV and lung disease. Am J Respir Crit Care Med. 2015;191(8):932–942. doi: 10.1164/rccm.201409-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lozupone C, Cota-Gomez A, Palmer BE, et al. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med. 2013;187(10):1110–1117. doi: 10.1164/rccm.201211-2145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beck JM, Schloss PD, Venkataraman A, et al. Multi-center Comparison of Lung and Oral Microbiomes of HIV-infected and HIV-uninfected Individuals. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201501-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sze MA, Hogg JC, Sin DD. Bacterial microbiome of lungs in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:229–238. doi: 10.2147/COPD.S38932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Speich R, Jenni R, Opravil M, et al. Primary pulmonary hypertension in HIV infection. Chest. 1991;100(5):1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 63. Secemsky EA, Scherzer R, Nitta E, et al. Novel Biomarkers of Cardiac Stress, Cardiovascular Dysfunction, and Outcomes in HIV-Infected Individuals. JACC Heart Fail. 2015;3(8):591–599. doi: 10.1016/j.jchf.2015.03.007. The most recent study to demonstrate increased prevalence of echo-defined PAH in a large HIV-seropositive cohort, which also finds associations between two serum biomarkers and PAH.
- 64.Reinsch N, Buhr C, Krings P, et al. Effect of gender and highly active antiretroviral therapy on HIV-related pulmonary arterial hypertension: results of the HIV-HEART Study. HIV Med. 2008;9(7):550–556. doi: 10.1111/j.1468-1293.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 65.Quezada M, Martin-Carbonero L, Soriano V, et al. Prevalence and risk factors associated with pulmonary hypertension in HIV-infected patients on regular follow-up. AIDS. 2012;26(11):1387–1392. doi: 10.1097/QAD.0b013e328354f5a1. [DOI] [PubMed] [Google Scholar]
- 66.Isasti G, Moreno T, Perez I, et al. High prevalence of pulmonary arterial hypertension in a cohort of asymptomatic HIV-infected patients. AIDS Res Hum Retroviruses. 2013;29(2):231–234. doi: 10.1089/AID.2012.0166. [DOI] [PubMed] [Google Scholar]
- 67.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104(23):2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 69.ten Freyhaus H, Vogel D, Lehmann C, et al. Echocardiographic screening for pulmonary arterial hypertension in HIV-positive patients. Infection. 2014;42(4):737–741. doi: 10.1007/s15010-014-0610-8. [DOI] [PubMed] [Google Scholar]
- 70.Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30(1):104–109. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 71.Opravil M, Pechere M, Speich R, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med. 1997;155(3):990–995. doi: 10.1164/ajrccm.155.3.9117037. [DOI] [PubMed] [Google Scholar]
- 72.Degano B, Guillaume M, Savale L, et al. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24(1):67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 73.Cicalini S, Chinello P, Grilli E, Petrosillo N. Treatment and outcome of pulmonary arterial hypertension in HIV-infected patients: a review of the literature. Curr HIV Res. 2009;7(6):589–596. doi: 10.2174/157016209789973583. [DOI] [PubMed] [Google Scholar]
- 74.Araujo I, Enjuanes-Grau C, Lopez-Guarch CJ, et al. Pulmonary arterial hypertension related to human immunodeficiency virus infection: A case series. World J Cardiol. 2014;6(6):495–501. doi: 10.4330/wjc.v6.i6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sangal RB, Taylor LE, Gillani F, et al. Risk of echocardiographic pulmonary hypertension in individuals with human immunodeficiency virus-hepatitis C virus coinfection. Ann Am Thorac Soc. 2014;11(10):1553–1559. doi: 10.1513/AnnalsATS.201405-225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.George MP, Champion HC, Simon M, et al. Physiologic changes in a nonhuman primate model of HIV-associated pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;48(3):374–381. doi: 10.1165/rcmb.2011-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanmogne GD, Kennedy RC, Grammas P. Analysis of human lung endothelial cells for susceptibility to HIV type 1 infection, coreceptor expression, and cytotoxicity of gp120 protein. AIDS Res Hum Retroviruses. 2001;17(1):45–53. doi: 10.1089/088922201750056771. [DOI] [PubMed] [Google Scholar]
- 78.Correale M, Palmiotti GA, Lo Storto MM, et al. HIV-associated pulmonary arterial hypertension: from bedside to the future. Eur J Clin Invest. 2015;45(5):515–528. doi: 10.1111/eci.12427. [DOI] [PubMed] [Google Scholar]
- 79.Marecki JC, Cool CD, Parr JE, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174(4):437–445. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marecki J, Cool C, Voelkel N, et al. Evidence for vascular remodeling in the lungs of macaques infected with simian immunodeficiency virus/HIV NEF recombinant virus. Chest. 2005;128(6 Suppl):621S–622S. doi: 10.1378/chest.128.6_suppl.621S-a. [DOI] [PubMed] [Google Scholar]
- 81.Almodovar S, Knight R, Allshouse AA, et al. Human Immunodeficiency Virus nef signature sequences are associated with pulmonary hypertension. AIDS Res Hum Retroviruses. 2012;28(6):607–618. doi: 10.1089/aid.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caldwell RL, Gadipatti R, Lane KB, Shepherd VL. HIV-1 TAT represses transcription of the bone morphogenic protein receptor-2 in U937 monocytic cells. J Leukoc Biol. 2006;79(1):192–201. doi: 10.1189/jlb.0405194. [DOI] [PubMed] [Google Scholar]
- 83.Dalvi P, O'Brien-Ladner A, Dhillon NK. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: implications for HIV-related pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2013;33(11):2585–2595. doi: 10.1161/ATVBAHA.113.302054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mermis J, Gu H, Xue B, et al. Hypoxia-inducible factor-1 alpha/platelet derived growth factor axis in HIV-associated pulmonary vascular remodeling. Respir Res. 2011;12:103. doi: 10.1186/1465-9921-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dalvi P, Wang K, Mermis J, et al. HIV-1/cocaine induced oxidative stress disrupts tight junction protein-1 in human pulmonary microvascular endothelial cells: role of Ras/ERK1/2 pathway. PLoS One. 2014;9(1):e85246. doi: 10.1371/journal.pone.0085246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Green LA, Yi R, Petrusca D, et al. HIV envelope protein gp120-induced apoptosis in lung microvascular endothelial cells by concerted upregulation of EMAP II and its receptor, CXCR3. Am J Physiol Lung Cell Mol Physiol. 2014;306(4):L372–L382. doi: 10.1152/ajplung.00193.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amsellem V, Lipskaia L, Abid S, et al. CCR5 as a treatment target in pulmonary arterial hypertension. Circulation. 2014;130(11):880–891. doi: 10.1161/CIRCULATIONAHA.114.010757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun. 2005;333(4):1107–1115. doi: 10.1016/j.bbrc.2005.05.198. [DOI] [PubMed] [Google Scholar]
- 89.Feijoo MQ, Toro R, Lopez Vazquez de la Torre M, et al. Relationship between endothelin-1 levels and pulmonary arterial hypertension in HIV-infected patients. AIDS. 2014;28(18):2693–2699. doi: 10.1097/QAD.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 90.Parikh RV, Scherzer R, Nitta EM, et al. Increased levels of asymmetric dimethylarginine are associated with pulmonary arterial hypertension in HIV infection. AIDS. 2014;28(4):511–519. doi: 10.1097/QAD.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tcherakian C, Couderc LJ, Humbert M, et al. Inflammatory mechanisms in HIV-associated pulmonary arterial hypertension. Semin Respir Crit Care Med. 2013;34(5):645–653. doi: 10.1055/s-0033-1356489. [DOI] [PubMed] [Google Scholar]
- 92.Worm SW, Bower M, Reiss P, et al. Non-AIDS defining cancers in the D:A:D Study--time trends and predictors of survival: a cohort study. BMC Infect Dis. 2013;13:471. doi: 10.1186/1471-2334-13-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol. 2015;33(21):2376–2383. doi: 10.1200/JCO.2014.59.5967. HAART era population prevalence study showing increased risk of lung cancer in HIV-seropositive individuals
- 94. Marcus JL, Chao C, Leyden WA, et al. Survival among HIV-Infected and HIV-Uninfected Individuals with Common Non-AIDS-Defining Cancers. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1167–1173. doi: 10.1158/1055-9965.EPI-14-1079. A HAART era study demonstrating inferior 5-year survival from lung cancer in HIV-seropositive individuals.
- 95. Shiels MS, Copeland G, Goodman MT, et al. Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer. 2015;121(12):2063–2071. doi: 10.1002/cncr.29324. A HAART era US cancer registry study demonstrating that lung cancer presents at more advanced stage in HIV-seropositive individuals.
- 96.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26(8):1017–1025. doi: 10.1097/QAD.0b013e328352d1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vandenhende MA, Roussillon C, Henard S, et al. Cancer-Related Causes of Death among HIV-Infected Patients in France in 2010: Evolution since 2000. PLoS One. 2015;10(6):e0129550. doi: 10.1371/journal.pone.0129550. Longitudinal study of the change in incidence of cancer-related deaths showing that lung cancer has grown to be the single biggest cause of death in HIV-seropositive individuals in France.
- 98.Kirk GD, Merlo CA. Lung HIVS. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 2011;8(3):326–332. doi: 10.1513/pats.201009-061WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patel P, Armon C, Chmiel JS, et al. Factors associated with cancer incidence and with all-cause mortality after cancer diagnosis among human immunodeficiency virus-infected persons during the combination antiretroviral therapy era. Open Forum Infect Dis. 2014;1(1):ofu012. doi: 10.1093/ofid/ofu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Helleberg M, Gerstoft J, Afzal S, et al. Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS. 2014;28(10):1499–1508. doi: 10.1097/QAD.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 101.Althoff K, Gange S, Jacobson L, et al. Smoking Outweighs HIV-Related Risk Factors for Non–AIDS-Defining Cancers. Seattle: CROI; 2015. [Google Scholar]
- 102.Sigel K, Crothers K, Gordon K, et al. CD4 Measures as Predictors of Lung Cancer Risk and Prognosis in HIV Infection. Seattle: CROI; 2015. [Google Scholar]
- 103.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116(22):5306–5315. doi: 10.1002/cncr.25311. [DOI] [PubMed] [Google Scholar]
- 104.Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 105.Borges AH, Silverberg MJ, Wentworth D, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS. 2013;27(9):1433–1441. doi: 10.1097/QAD.0b013e32835f6b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8(4):605–615. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 107.Shebl FM, Engels EA, Goedert JJ, Chaturvedi AK. Pulmonary infections and risk of lung cancer among persons with AIDS. J Acquir Immune Defic Syndr. 2010;55(3):375–379. doi: 10.1097/QAI.0b013e3181eef4f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sheth AN, Moore RD, Gebo KA. Provision of general and HIV-specific health maintenance in middle aged and older patients in an urban HIV clinic. AIDS Patient Care STDS. 2006;20(5):318–325. doi: 10.1089/apc.2006.20.318. [DOI] [PubMed] [Google Scholar]
- 109.Shirley DK, Kaner RJ, Glesby MJ. Screening for Chronic Obstructive Pulmonary Disease (COPD) in an Urban HIV Clinic: A Pilot Study. AIDS Patient Care STDS. 2015;29(5):232–239. doi: 10.1089/apc.2014.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Selby VN, Scherzer R, Barnett CF, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS. 2012;26(15):1967–1969. doi: 10.1097/QAD.0b013e3283579653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schwarze-Zander C, Pabst S, Hammerstingl C, et al. Pulmonary hypertension in HIV infection: a prospective echocardiographic study. HIV Med. 2015 doi: 10.1111/hiv.12261. [DOI] [PubMed] [Google Scholar]
- 112.Fishman JE, Schwartz DS, Sais GJ, et al. Bronchogenic carcinoma in HIV-positive patients: findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1995;164(1):57–61. doi: 10.2214/ajr.164.1.7998569. [DOI] [PubMed] [Google Scholar]
- 113.National Lung Screening Trial Research, T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hulbert A, Hooker CM, Keruly JC, et al. Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol. 2014;9(6):752–759. doi: 10.1097/JTO.0000000000000161. The first trial using CT of the thorax to screen for lung cancer in high-risk HIV-seropositive individuals. It only identified one new cancer, yet found many individuals with non-cancerous pulmonary nodules.
- 115.Sigel K, Wisnivesky J, Shahrir S, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS. 2014;28(7):1007–1014. doi: 10.1097/QAD.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Makinson A, Eymard-Duvernay S, Raffi F, et al. High Frequency of Early Lung Cancer Diagnosis With Chest CT in HIV-Infected Smokers. Seattle: CROI; 2015. [Google Scholar]
- 117.Bower M, Palfreeman A, Alfa-Wali M, et al. British HIV Association guidelines for HIV-associated malignancies 2014. HIV Med. 2014;15(Suppl 2):1–92. doi: 10.1111/hiv.12136. [DOI] [PubMed] [Google Scholar]
- 118.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2015. [cited 2015 13 August]. Panel_on_Antiretroviral_Guidelines_for_Adults_and_Adolescents. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.. [Google Scholar]


