Abstract
Background
Recent reports demonstrate that thoracoscopic lobectomy for lung cancer may be associated with lower rates of surgical upstaging. We queried a state-wide cancer registry for differences in upstaging rates and survival by surgical approach.
Methods
The Kentucky Cancer Registry (KCR) collects data, including centralized pathology reporting, on cancer patients treated statewide. We performed a retrospective review from 2010-2012 to examine clinical and pathologic stage. We assessed rates of upstaging and whether or not the surgical approach, thoracotomy (THOR) versus minimally invasive techniques (VATS), had an impact on final pathologic stage and survival.
Results
The KCR database from 2010 to 2012 contained information on 2830 lung cancer cases, 1964 having THOR and 500 having VATS resections. Preoperatively, 36.4% of THOR were clinically stage 1a vs. 47.4% % VATS (p=0.0002). Of these, final pathologic stage remained stage 1a in 30.5% of THOR and 38.0% of VATS (p=0.0002). The overall nodal upstaging rate for THOR was 9.9% and 4.8% for VATS (p=0.002). There was decreased nodal upstaging with VATS, independent of tumor size and extent of resection (OR 0.6, 95% CI 0.387-0.985, p=0.04). However there was improved survival with VATS compared with THOR (HR 0.733, 95% CI 0.592-0.907, p = 0.0042).
Conclusions
Consistent with other reports, we demonstrate a lower upstaging rate with VATS. Nevertheless, there is a survival advantage in VATS patients. Although selection bias may play a role in these observed differences, the improved quality of life measures associated with VATS, may explain survival improvement despite lower surgical upstaging.
Keywords: Lung cancer; diagnosis (incl staging, imaging, fiducials); Lung cancer surgery; Lymph nodes (mediastinal); Outcomes (incl mortality, morbidity, survival, etc.)
Introduction
The ideal surgical approach for the treatment of lung cancer is still a matter of debate.[1] There are clear advantages to thoracoscopy. Patients experience decreased pain, shorter length of stay, quicker return to work, and are more likely to adhere to adjuvant regimens when indicated.[2-7] However, several recent papers raise concerns that thoracoscopy results in lower rates of nodal upstaging when compared to open surgery.[8-10]
Surgical upstaging reflects the inherent limitations of clinical staging and the sensitivity and specificity of the tools which are used.[11] Additionally, it is possible that there may be disease progression during the course of workup which in some cases may be delayed for a variety of reasons.[12] Although surgical upstaging occasionally represents intraoperative findings of pleural invasion or multiple nodules (modification of T stage), intraoperative finding of lymph node involvement is the most common reason for upstaging.[8-10, 13]
Kentucky has the nation's highest incidence of and mortality from lung cancer. Age-adjusted annual incidence rates are in excess of 150/100,000. [14] With a population of 4.4 million and an area of 40,000 mi2 many patients have to travel significant distances to obtain care which is provided by two academic medical centers and a large number of busy community hospitals and cancer centers. The statewide Kentucky Cancer Registry (KCR) offers an opportunity to study cancer incidence, treatment and outcomes in this very diverse and “real world” population.
Therefore, we sought to utilize these data to evaluate the primary endpoint of nodal upstaging stratified by surgical approach and to explore possible effects on survival.
Patients and Methods
The University of Kentucky institutional review board approved this retrospective review of the cancer registry. The Kentucky Cancer Registry (KCR) was instituted in 1986, with mandatory reporting by all state hospitals in 1991. The KCR attained NCI/SEER designation in 2000. Today, all Kentucky acute-care hospitals, outpatient facilities, and other nonhospital facilities participate in the registry. A key component is the cancer patient data management system (CPDMS) which is a standardized reporting system. Statewide data is collated at KCR, and these data are used for research, and participation in the national registries. The data management system includes e-path which allows for near real-time reporting of pathology data from participating laboratories throughout the state to the centralized cancer registry.
In 2010, a stratification variable was introduced which allowed for comparison of surgical approach (thoracoscopy, robot-assisted, conversion, open). We chose to examine registry data between 2010 and 2012 to allow for at least two years of follow-up data for survival. The KCR database was linked to external data sources including Kentucky death certificates, the national death index, Social Security Administration, and Center for Medicaid services.
Cancer records were stratified by surgical approach. Nodal upstaging was defined as cN0 patients being found to have either pN1 and/or pN2 nodes.
Baseline characteristics included age, gender, clinical staging (c stage, cT, cN, cM and tumor size). Operative characteristics included surgical approach, extent of resection, pathologic data (p stage, pT, pN, pM), and histology. Measurements also included analysis of survival data and extent of resection categorized as anatomic (lobectomy, bilobectomy, pneumonectomy), extended anatomic (when additional structures such as chest wall, diaphragm, pericardium were involved), segmentectomy, and nonanatomic resections.
Descriptive statistics included mean and standard deviation for normally distributed continuous variables, median and interquartile range for non-normally distributed continuous variables, and counts and percentages for categorical variables. Univariate comparisons used Student's t-test, ANOVA, Chi-Square and Fisher's Exact as appropriate.
Logistic regression was used to measure the association between the occurrence of nodal upstaging and the other measured variables. Significant variables were entered into a final logistic model to estimate multivariable odds ratios that reflected the risk of nodal upstaging.
Survival analysis was initially conducted using the standard Kaplan-Meier method. This was stratified by stage of surgical approach. The individual association of each variable with survival was estimated using univariate Cox regression, and significant factors were entered into a final multivariable Cox regression model.
Analyses were performed in SAS 9.3 (SAS Institute, Cary NC).
Results
During the study period of 2010 to 2012, there were 2830 lung cancer resections performed in the state of Kentucky. Of these, 1964 (69%) were performed open. An additional 134 (5%) began as a minimally invasive approach and were converted. The remaining cases were completed either using conventional thoracoscopy (18%) or robot-assisted (8%).
Baseline characteristics are outlined in Table 1. Overall the cases were similarly distributed in terms of age, gender, race and smoking history. Patients who underwent open resections had slightly larger tumors (median 25 mm, versus 20 mm, p<0.001) when comparing open to minimally invasive patients.
Table 1. Demographics.
| Robot | VATS | MIS Convert | Open | Total | p-value | ||
|---|---|---|---|---|---|---|---|
| (N=232) | (N=500) | (N=134) | (N=1964) | (N=2830) | |||
| Sex | Male | 101 (43.5) | 224 (44.8) | 61 (63.5) | 1035 (52.7) | 1436 (50.7) | <0.001 |
| Female | 131 (56.5) | 276 (55.2) | 35 (36.5) | 929 (47.3) | 1394 (49.3) | ||
| Race | White | 219 (94.4) | 463 (92.6) | 90 (93.8) | 1862 (94.8) | 2672 (94.4) | 0.587 |
| Black | 12 (5.2) | 35 (7.0) | 6 (6.3) | 96 (4.9) | 149 (5.3) | ||
| Smoke | median pack years | 48.5 | 45.0 | 42.5 | 45.0 | 45.0 | 0.386 |
| q1-q3 | 30.0-68.5 | 30.0-60.0 | 22.5-67.5 | 30.0-64.0 | 30.0-63.0 | ||
| Age | median | 67.0 | 68.0 | 65.0 | 67.0 | 67.0 | 0.048 |
| q1-q3 | 61.0-73.0 | 61.0-74.5 | 58.0-72.0 | 59.0-73.0 | 60.0-73.0 | ||
| Tsize | median (mm) | 20.5 | 20.0 | 22.0 | 25.0 | 24.0 | <0.001 |
| q1-q3 | 15.0-32.0 | 14.0-32.0 | 15.0-33.0 | 17.0-40.0 | 15.0-37.0 | ||
| Laterality | Right | 121 (52.2) | 261 (52.2) | 53 (55.2) | 1118 (56.9) | 1579 (55.8) | 0.15 |
| left | 111 (47.8) | 234 (46.8) | 43 (44.8) | 838 (42.7) | 1238 (43.7) | ||
| Extent of | NonAnatomic | 38 (16.4) | 149 (31.2) | 12 (12.6) | 365 (18.8) | 566 (20.4) | <0.001 |
| Surgery | Segment | 7 (3.0) | 24 (5.0) | 3 (3.2) | 81 (4.2) | 115 (4.1) | |
| Anatomic | 186 (80.2) | 304 (63.6) | 78 (82.1) | 1437 (74.1) | 2040 (73.4) | ||
| ExtendedAnatomic | 1 (0.4) | 1 (0.2) | 2 (2.1) | 55 (2.8) | 59 (2.1) |
Operative approach was in the form of an anatomic resection in the majority of patients, however in the thoracoscopy group there were 31% (149) non-anatomic resections compared with 19% (365) in the open group. (Table 1)
The prevalence of nodal upstaging overall was 8.8%. This represented the finding of pN1 in 5.5% and pN2 in 3.3%. Nodal upstaging in the thoracoscopy group was only 4.8%, compared with 8.6% in the robot-assisted group, and 9.9% in the open group (p=0.002). (Table 2)
Table 2. Pathology and Staging.
| Robot | VATS | MIS Convert | Open | Total | p-value | ||
|---|---|---|---|---|---|---|---|
| (N=232) | (N=500) | (N=134) | (N=1964) | (N=2830) | |||
| Histology | Adeno | 139 (59.9) | 269 (53.8) | 52 (54.2) | 924 (47.0) | 1407 (49.7) | <0.001 |
| Squamous | 63 (27.2) | 158 (31.6) | 22 (22.9) | 754 (38.4) | 1009 (35.7) | ||
| Large | 10 (4.3) | 12 (2.4) | 1 (1.0) | 64 (3.3) | 90 (3.2) | ||
| OthNonSpec | 3 (1.3) | 17 (3.4) | 5 (5.2) | 60 (3.1) | 85 (3.0) | ||
| OthSpec | 17 (7.3) | 43 (8.6) | 16 (16.7) | 158 (8.0) | 234 (8.3) | ||
| Clinical | cStage 1 | 169 (72.8) | 309 (61.8) | 60 (62.5) | 1114 (56.7) | 1679 (59.3) | <0.001 |
| Stage | cStage 2 | 30 (12.9) | 37 (7.4) | 11 (11.5) | 294 (15.0) | 379 (13.4) | |
| cStage 3 | 13 (5.6) | 43 (8.6) | 7 (7.3) | 205 (10.4) | 270 (9.5) | ||
| Pathologic | pStage 1 | 146 (62.9) | 276 (55.2) | 48 (50.0) | 974 (49.6) | 1468 (51.9) | <0.001 |
| Stage | pStage 2 | 35 (15.1) | 62 (12.4) | 19 (19.8) | 391 (19.9) | 516 (18.2) | |
| pStage 3 | 22 (9.5) | 32 (6.4) | 11 (11.5) | 272 (13.8) | 340 (12.0) | ||
| pStage 4 | 3 (1.3) | 20 (4.0) | 3 (3.1) | 37 (1.9) | 64 (2.3) | ||
| Incomplete Data | 26 (11.2) | 110 (22.0) | 15 (15.6) | 290 (14.8) | 442 (15.6) | ||
| Upstaging | Nodal Upstage (overall) | 20 (8.6) | 24 (4.8) | 9 (9.4) | 195 (9.9) | 248 (8.8) | 0.002 |
| pN1 upstage | 12 (5.2) | 15 (3.0) | 6 (6.3) | 123 (6.3) | 156 (5.5) | ||
| pN2 upstage | 8 (3.4) | 9 (1.8) | 3 (3.1) | 72 (3.7) | 92 (3.3) |
As there were significantly more wedge resections in the VATS group, we performed an additional stratified subgroup analysis excluding wedge resections and extended anatomic resections (chest wall) and limiting to clinical stage 1 only. Thoracoscopy had a nodal upstaging rate of 9.1% compared with 13.9% for thoracotomy in clinical stage I (Table 5)
Table 5.
Nodal Upstaging Rates, Comparison. In this table we exclude those patients from our data who underwent wedge resections or extended anatomic resections and limit to clinical stage 1 only.
| Reference | Approach | n | cN0-pN1 | cN0-pN2 | Overall |
|---|---|---|---|---|---|
| Boffa 2012[9] | Thoracotomy | 7,137 | 9.3% | 5.0% | 14.3% |
| VATS | 4,494 | 6.7% | 4.9% | 11.6% | |
| Licht 2013[10] | Thoracotomy | 796 | 13.1% | 11.5% | 24.6% |
| VATS | 717 | 8.1% | 3.8% | 11.9% | |
| Merritt 2013[19] | Thoracotomy | 69 | 17.4% | 7.2% | 24.6% |
| Vats | 60 | 8.3% | 1.8% | 10.1% | |
| Wilson 2014[17] | Robotic | 302 | 6.6% | 4.3% | 10.9% |
| Current Study | Thoracotomy | 797 | 8.2% | 5.8% | 13.9% |
| VATS | 187 | 5.9% | 3.2% | 9.1% | |
| Robotic | 130 | 6.9% | 6.2% | 13.1% |
Multivariate logistic regression analysis confirmed the findings of the univariate and stratified analysis. Thoracoscopy was associated with decreased nodal upstaging, independent of tumor size and extent of resection (OR 0.6, 95% CI 0.387-0.985, p=0.04). Anatomic resection versus other approaches was also independently associated with nodal upstaging (OR 2.6, 95% CI 1.473-4.533, p=0.0009). (Table 3)
Table 3. Logistic regression: nodal upstaging.
Univariate regression estimates the unadjusted odds ratio (OR) between each variable and the outcome of nodal upstaging. Multivariate regression estimates adjusted odds ratios.
|
|
|
||||||
|---|---|---|---|---|---|---|---|
| Univar iate Logistic | Multiv ariate Logistic | ||||||
|
| |||||||
| Variable | OR | 95% CI | p value | OR | 95% CI | p value | |
|
| |||||||
| Age | per year | 0.991 | 0.997-1.004 | 0.1755 | |||
|
| |||||||
| Sex | male vs female | 0.909 | 0.690-1.197 | 0.4967 | |||
|
| |||||||
| Race | Other vs white | 0.908 | 0.491-1.677 | 0.7571 | |||
|
| |||||||
| Smoke | per pack year | 0.996 | 0.989-1.001 | 0.0824 | |||
|
| |||||||
| Laterality | right vs left | 1.025 | 0.776-1.353 | 0.8616 | |||
|
| |||||||
| Histology | Squamous | ref | |||||
| Adeno | 1.288 | 0.951-1.744 | 0.1025 | ||||
| Large | 0.979 | 0.408-2.349 | 0.9617 | ||||
|
| |||||||
| Approach | Open | ref | ref | ||||
| MIS Convert | 0.657 | 0.326-1.324 | 0.2494 | 0.625 | 0.309-1.264 | 0.1909 | |
| Robot | 0.821 | 0.498-1.353 | 0.4386 | 0.824 | 0.498-1.366 | 0.4533 | |
| VATS | 0.553 | 0.348-0.876 | 0.0117 | 0.619 | 0.387-0.985 | 0.0432 | |
|
| |||||||
| Extent | Nonanatomic | ref | ref | ||||
| Segment | 0.711 | 0.199-2.534 | 0.5987 | 0.646 | 0.189-2.414 | 0.5462 | |
| Anatomic | 2.842 | 1.629-4.959 | 0.0002 | 2.585 | 1.473-4.533 | 0.0009 | |
| Extended Anatomic | 2.313 | 0.792-6.754 | 0.1251 | 1.61 | 0.539-4.808 | 0.5462 | |
|
| |||||||
| Tumor Size | per cm | 1.009 | 1.004-1.013 | 0.0002 | 1.007 | 1.002-1.012 | 0.0045 |
Kaplan Meier stratified analysis demonstrated a survival advantage to thoracoscopy in pathologic stage 1 tumors. (Figure 1).
Figure 1. Overall survival for pathologic stage 1 and 2 lung cancer resections in KY performed in 2010 to 2012, stratified by operative approach.
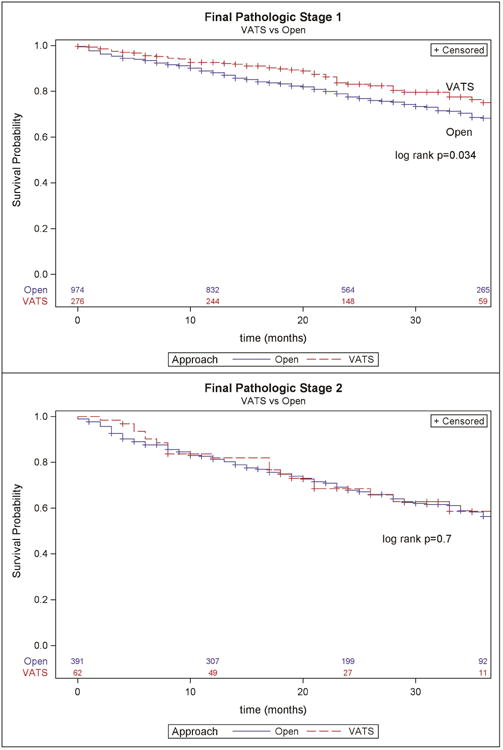
Multivariable Cox regression found that thoracoscopic approach was independently associated with improved survival (HR 0.733, 95% CI 0.592-0.907, p = 0.0042) while controlling for extent of resection, nodal upstaging, and pathologic stage. While nodal upstaging did not affect survival, anatomic resection was associated with improved survival compared with non-anatomic (HR 0.687, 95% CI 0.575-0.821, p=<.0001). (Table 4)
Table 4. Survival Analysis, Cox regression.
Univariate cox regression estimates the unadjusted hazard ratio (HR) for each variable while multivariable cox regression estimates adjusted hazard ratios.
|
|
|
||||||
|---|---|---|---|---|---|---|---|
| Univariate Cox Regres sion | Multiv ariate Cox Regr ession | ||||||
|
| |||||||
| Variable | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | per year | 1.025 | 1.018-1.032 | <0.0001 | 1.029 | 1.021-1.037 | <.0001 |
|
| |||||||
| Sex | male vs female | 1.586 | 1.380-1.824 | <0.0001 | 1.414 | 1.227-1.630 | <.0001 |
|
| |||||||
| Race | white vs black | 1.467 | 1.030-2.089 | 0.0336 | |||
|
| |||||||
| Smoke | per pack year | 1.003 | 1.001-1.004 | 0.0021 | |||
|
| |||||||
| Laterality | right vs left | 1.009 | 0.883-1.153 | 0.8966 | |||
|
| |||||||
| Histology | Squamous | ref | |||||
| Adeno | 0.791 | 0.683-0.917 | 0.0018 | ||||
| Large | 1.105 | 0.764-1.596 | 0.5967 | ||||
|
| |||||||
| Approach | Open | ref | ref | ||||
| MIS Convert | 0.882 | 0.643-1.210 | 0.6034 | 0.953 | 0.693-1.309 | 0.7625 | |
| Robot | 0.843 | 0.648-1.096 | 0.2021 | 0.968 | 0.743-1.261 | 0.8081 | |
| VATS | 0.732 | 0.594-0.902 | 0.0034 | 0.733 | 0.592-0.907 | 0.0042 | |
|
| |||||||
| Extent | Nonanatomic | ref | ref | ||||
| Segment | 0.793 | 0.555-1.133 | 0.2023 | 0.816 | 0.571-1.166 | 0.265 | |
| Anatomic | 0.746 | 0.628-0.886 | 0.0008 | 0.687 | 0.575-0.820 | <.0001 | |
| Extended Anatomic | 1.539 | 1.043-2.269 | 0.0298 | 1.147 | 0.769-1.71 | 0.5012 | |
|
| |||||||
| Nodal Upstaging | vs none | 1.487 | 1.217-1.817 | 0.0001 | |||
|
| |||||||
| Tumor Size | per cm | 1.007 | 1.005-1.008 | <0.0001 | |||
|
| |||||||
| pStage | I | ref | ref | ||||
| II | 1.52 | 1.279-1.807 | <0.0001 | 1.567 | 1.304-1.882 | <.0001 | |
| III | 2.73 | 2.297-3.245 | <0.0001 | 2.794 | 2.301-3.392 | <.0001 | |
| IV | 4.449 | 3.284-6.029 | <0.0001 | 4.53 | 3.315-6.191 | <.0001 | |
Comment
Nodal upstaging is a function of both preoperative clinical workup and the intraoperative detection of occult nodal disease.[9, 11, 13, 15] Our study finds that thoracoscopy is independently associated with lower rates of nodal upstaging. A retrospective study such as this cannot infer causality, and rather further highlights the association of decreased nodal upstaging which has been demonstrated in other studies.
There exist two scenarios: First, decreased rates of upstaging may not be reflective of surgical technique. The difference may represent selection bias as surgeon preference for thoracoscopy may favor smaller tumors which are more likely to indeed be node negative. We lack sufficient detail in the database to determine the clinical staging methods which have been employed, therefore it is possible that patients undergoing thoracoscopy receive a more detailed evaluation. The alternative hypothesis however, is that this may represent a technical issue with thoracoscopy. Accurate nodal evaluation during thoracoscopy is challenging, and commonly the mediastinal dissection is relegated to the end of the case and therefore may not receive the same attention to detail as during an open procedure. The clinical importance of nodal upstaging is the selection of patients for adjuvant therapy which may have an effect on survival.[3, 10, 15]
Positive mediastinal nodes are more likely to be found with larger tumors.[9] Our model for nodal upstaging demonstrated an association between increasing tumor size and upstaging. Although the overall distribution of tumor sizes was not clinically very different between our VATS and thoracotomy groups, the strength of association between tumor size and nodal upstaging was sufficient to be significant in the multivariable model. The benefits of thoracoscopy are less clear for larger tumors, and as such surgeons performing minimally invasive resection of larger and locally advanced tumors should be careful to be attentive to a detailed nodal dissection of these patients were upstaging is likely.
Overall, thoracoscopy is associated with improved survival in pathologic stage I patients. This has been demonstrated in other series.[10, 16] The survival advantage is found on multivariable analysis to be independent of pathologic stage, extent of resection, and histology. It has been shown that thoracoscopy is associated with lower morbidity in older patients and those with comorbidities.[4, 5] It is possible that some of the survival benefit is due to improved early postoperative survival. However, the Kaplan-Meier curves do continue to separate beyond the postoperative period, so this may point to unmeasured differences in the populations reflecting selection bias in our data.
Importantly, our data highlights the importance of an anatomic resection in cancer survivorship. Anatomic resection is independently associated with improved two year survival (HR 0.687, 95% CI 0.575-0.821, p=<.0001). Despite this, 30% of the resections in the thoracoscopy group in our series were non-anatomic resections. This practice pattern likely reflects the relative ease of performing a thoracoscopic wedge versus a lobectomy. Considering our data set is representative of widespread practice throughout the state of Kentucky today, these findings indicate that surgeons need to be cognizant of the benefits of an anatomic resection regardless of choice of surgical approach.
We chose to separate the conversion and robotic groups from vats and thoracotomy in our analysis. There are likely to be confounders in both of these cases which affect upstaging rates. These groups were independently analyzed. Although not a primary endpoint of our study, we noted that there were higher rates of nodal upstaging in the robotic group. This finding is in line with recent work which hypothesized that the ergonomic and visual advantages of the robot may facilitate a better lymph node dissection.[17] Likewise, upstaging was higher in the converted group, however reasons for conversion are not documented. In Kentucky, hilar dissection is made more challenging by the presence of histoplasmosis,[18] therefore we cannot be sure if the higher rate of nodal disease in this group is related to positive nodes creating a situation of needing to convert, versus histoplasmosis causing a conversion and thoracotomy subsequently finding more nodes.
Our study has significant limitations. First, this is a retrospective review of a cancer registry, with all of the inherent bias of a retrospective review and the limitations of registry-level data. Data validation is a problem which is increasingly recognized with database studies, and this was evident in some of the variables which we could not analyze, such as smoking history, due to significant missing data. Nevertheless, we are more confident in the pathology data, which was our primary endpoint of the study. Second, the outcome of nodal upstaging is a relatively rare occurrence with a small effect size. Therefore, our study may be underpowered to detect an effect on survival. As the database matures, this effect may become more pronounced. Third, comorbidity data are not available. Although the patients were matched based on gender and age, unmeasured comorbidities may cause a surgeon to choose one approach over another, or additionally may affect overall survival. While this effect may be limited in our two-year follow-up, it is an important consideration for which we cannot control. Finally, our follow-up data is limited to two years in this study.
Our study has important strengths in that, as a statewide registry, this represents a census of all surgically treated lung cancer patients in the underlying population. The variety of practice settings across the state of Kentucky may mean this sample is reflective of “real world” thoracic surgery in the United States today.
In summary, nodal upstaging is less likely to occur during thoracoscopy, and more likely to occur with larger tumors. Therefore surgeons must be meticulous with N1 dissection, and attentive to N2 stations during thoracoscopy. The known advantages to thoracoscopy may be responsible for the improved survival seen in the pathologic stage I cohort in this study. Whether or not thoracoscopy is appropriate for more advanced stages of disease, and whether the advantages persist remains a point of interest and warrants continued investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathisen DJ. Is video-assisted thoracoscopic lobectomy inferior to open lobectomy oncologically? Ann Thorac Surg. 2013;96(3):755–6. doi: 10.1016/j.athoracsur.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–22. doi: 10.1016/0003-4975(95)00537-u. discussion 622-3. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RP, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007;83(4):1245–9. doi: 10.1016/j.athoracsur.2006.12.029. discussion 1250. [DOI] [PubMed] [Google Scholar]
- 4.Cattaneo SM, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85(1):231–5. doi: 10.1016/j.athoracsur.2007.07.080. discussion 235-6. [DOI] [PubMed] [Google Scholar]
- 5.Berry MF, et al. Risk factors for morbidity after lobectomy for lung cancer in elderly patients. Ann Thorac Surg. 2009;88(4):1093–9. doi: 10.1016/j.athoracsur.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Paul S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(2):366–78. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Puri V, Meyers BF. Video-assisted thoracoscopic surgery lobectomy for lung cancer. Surg Oncol Clin N Am. 2013;22(1):27–38. v. doi: 10.1016/j.soc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico TA, et al. Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann Thorac Surg. 2011;92(1):226–31. doi: 10.1016/j.athoracsur.2011.03.134. discussion 231-2. [DOI] [PubMed] [Google Scholar]
- 9.Boffa DJ, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg. 2012;94(2):347–53. doi: 10.1016/j.athoracsur.2012.04.059. discussion 353. [DOI] [PubMed] [Google Scholar]
- 10.Licht PB, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. 2013;96(3):943–9. doi: 10.1016/j.athoracsur.2013.04.011. discussion 949-50. [DOI] [PubMed] [Google Scholar]
- 11.Silvestri GA, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 12.Chandra S, et al. Delays during the diagnostic evaluation and treatment of lung cancer. Asian Pac J Cancer Prev. 2009;10(3):453–6. [PubMed] [Google Scholar]
- 13.Fibla JJ, et al. Re-evaluation of the prognostic value of visceral pleura invasion in Stage IB non-small cell lung cancer using the prospective multicenter ACOSOG Z0030 trial data set. Lung Cancer. 2012;78(3):259–62. doi: 10.1016/j.lungcan.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Registry, K.C. Age-Adjusted Cancer Mortality Rates by in Kentucky. 2014 1 Nov 2014 [cited 2015 9 May 2015]; Cancer incidence and mortality data by geographic region. Cancer-rates.info is a contractual web service hosted by the Kentucky Cancer Registry at the Markey Cancer Center at the University of Kentucky http://www.kcr.uky.edu. Data are provided by participating population-based central cancer registries. Inquiries or questions related to data content should be directed to Kentucky Cancer Registry.]. Available from: http://cancer-rates.info/ky.
- 15.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136(1):260–71. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 16.Berry MF, et al. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg. 2014;98(1):197–202. doi: 10.1016/j.athoracsur.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson JL, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg. 2014;97(6):1901–6. doi: 10.1016/j.athoracsur.2014.01.064. discussion 1906-7. [DOI] [PubMed] [Google Scholar]
- 18.Myint T, et al. Temporal trends, clinical characteristics, and outcomes of histoplasmosis in a tertiary care center in Kentucky, 2000 to 2009. J Int Assoc Provid AIDS Care. 2014;13(2):100–5. doi: 10.1177/2325957413500535. [DOI] [PubMed] [Google Scholar]
- 19.Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg. 2013;96(4):1171–7. doi: 10.1016/j.athoracsur.2013.05.044. [DOI] [PubMed] [Google Scholar]


