Abstract
In the infarcted myocardium, necrotic cardiomyocytes activate innate immune pathways, stimulating pro-inflammatory signaling cascades. Although inflammation plays an important role in clearance of the infarct from dead cells and matrix debris, repair of the infarcted heart requires timely activation of signals that negatively regulate the innate immune response, limiting inflammatory injury. We have previously demonstrated that Interleukin receptor-associated kinase (IRAK)-M, a member of the IRAK family that suppresses toll-like receptor/interleukin-1 signaling, is upregulated in the infarcted heart in both macrophages and fibroblasts, and restrains pro-inflammatory activation attenuating adverse remodeling. Although IRAK-M is known to suppress inflammatory activation of macrophages, its role in fibroblasts remains unknown. Our current investigation examines the effects of IRAK-M on fibroblast phenotype and function. In vitro, IRAK-M null cardiac fibroblasts have impaired capacity to contract free-floating collagen pads. IRAK-M loss reduces transforming growth factor (TGF)-β-mediated α-smooth muscle actin (α-SMA) expression. IRAK-M deficient cardiac fibroblasts exhibit a modest reduction in TGF-β-stimulated Smad activation and increased expression of the α-SMA repressor, Y-box binding protein (YB)-1. In a model of non-reperfused myocardial infarction, IRAK-M absence does not affect collagen content and myofibroblast density in the infarcted and remodeling myocardium, but increases YB-1 levels and is associated with attenuated α-SMA expression in isolated infarct myofibroblasts. Our findings suggest that, in addition to its role in restraining inflammation following reperfused infarction, IRAK-M may also contribute to myofibroblast conversion.
Keywords: myofibroblast, myocardial infarction, α-smooth muscle actin, interleukin receptor-associated kinase (IRAK)-M, innate immunity, transforming growth factor (TGF)-β
INTRODUCTION
The adult mammalian heart has negligible regenerative capacity. Repair of the infarcted myocardium is dependent on an inflammatory reaction that clears the wound from dead cells and matrix debris, and sets the stage for recruitment and activation of reparative cardiac fibroblasts that deposit extracellular matrix proteins maintaining the structural integrity of the ventricle [1, 2]. During the first few hours after coronary occlusion, necrotic cardiomyocytes release alarmins, danger-associated molecular signals that activate Toll-like receptor (TLR) and interleukin (IL)-1 signaling in cardiomyocytes, fibroblasts and immune cells, triggering pro-inflammatory cascades [3],[4],[5],[6],[7],[8]. TLR/IL-1 stimulation activates the Nuclear Factor (NF)-κB system [9], inducing expression of chemokines and adhesion molecules in endothelial cells. Sensing of chemokines bound on the endothelial surface by circulating leukocytes triggers adhesive interactions, ultimately resulting in extravasation of neutrophils and pro-inflammatory monocytes in the infarcted region. Although inflammation is important to clear the wound from dead cells, effective cardiac repair is dependent on timely suppression of inflammatory signaling and resolution of the leukocyte infiltrate. Inhibition of inflammatory pathways following infarction is an active process that requires secretion of anti-inflammatory mediators, and activation of intracellular signaling pathways that downmodulate the innate immune response [10],[11],[12]. Extensive experimental evidence suggests that defects in molecular signals responsible for negative regulation of inflammation extend injury and causes adverse remodeling following myocardial infarction [2],[13],[14].
Engagement of TLR by danger signals leads to recruitment of one of the Toll/IL-1 receptor (TIR) domain-containing adaptor proteins. Of these proteins, myeloid differentiation primary-response gene 88 (MyD88) is required for all TLR signaling pathways (except TLR3) and mediates leukocyte infiltration in the infarcted heart [15]. Members of the Interleukin receptor-associated (IRAK) family of intracellular kinases function are critically involved in regulation of TLR/IL-1 signaling cascades downstream of the TIR adaptors [16]. Genetic loss-of-function approaches demonstrated that IRAK-1, IRAK-2 and IRAK-4 stimulate distinct pro-inflammatory signaling pathways in response to TLR and IL-1 activation [17],[18],[19]. In the ischemic myocardium, activation of IRAK-1 and IRAK-4 signaling cascades has been implicated in extension of injury [20], [21]. In contrast, to the pro-inflammatory actions of the other IRAKs, IRAK-M serves as a negative regulator of the innate immune response [22],[23],[24]. We have previously demonstrated that IRAK-M is markedly upregulated in the infarcted myocardium and restrains monocyte-driven inflammation [12]. Using a mouse model of myocardial ischemia/reperfusion, we found that timely induction of IRAK-M is critical for suppression of inflammation, and protects the infarcted heart from adverse remodeling without affecting the size of the infarct. Although traditional concepts suggest that IRAK-M is a macrophage-specific product [25], our studies suggested that it is also expressed in activated cardiac fibroblasts upon stimulation with growth factors [12]. However, the role of IRAK-M in fibroblast biology remains unknown.
Our current study demonstrates a novel role for IRAK-M in regulation of myofibroblast conversion. We found that loss of IRAK-M in fibroblasts attenuates TGF-β-induced α-smooth muscle actin (α-SMA) expression, resulting in impaired capacity to contract collagen gels. Defective function of IRAK-M deficient fibroblasts is associated with increased expression of Y-box binding protein (YB)-1, a potent repressor of α-SMA synthesis. Our findings suggest that in addition to its role in suppression of the innate immune response, IRAK-M may also contribute to fibroblast activation, serving as a molecular switch from inflammation to fibrosis.
METHODS
Animals
For all in vivo and in vitro experiments, we used male and female IRAK-M null mice in a C57BL6 background [22] and corresponding C57BL6 controls (provided by Jackson labs, Bar Harbor ME) [12]. All protocols were approved by the institutional animal care and use committee at Albert Einstein College of Medicine.
Isolation and stimulation of mouse cardiac fibroblasts
Fibroblasts were isolated from normal WT and IRAK-M null mouse hearts as previously described and cultured in Corning T150mm × 25mm dishes [26],[27],[28]. Cells were serum-starved at passage 2 for 24h and subsequently stimulated with 10 ng/ml of Transforming Growth Factor (TGF)-β (R&D Systems, Minneapolis MN) for 1h, 4h and 24h. At the end of stimulation total protein was extracted using RIPA lysis buffer (Pierce). Protein samples were used for assessment of Smad2 phosphorylation. In additional experiments, cardiac fibroblasts from WT and IRAK-M null mice were stimulated in the presence and absence of TGF-β (10 ng/ml) for 72h and protein was harvested to assess expression of α-SMA and type III collagen.
Collagen pad contraction assay
To assess the effects of IRAK-M on the contractile properties of fibroblasts, free floating collagen pads were populated with cardiac fibroblasts from WT and IRAK-M null, as previously described [29]. Following polymerization, fibroblast-populated collagen pads were incubated in triplicates (WT and IRAKM null; n=9) in the presence or absence of fetal bovine serum (FBS 1%) and TGF-β1 (10 ng/ml). After 24h, the pictures of the plates were taken in a flatbed scanner, and the area of each pad was measured using Image Pro software.
Mouse model of non-reperfused myocardial infarction
Both male and female 2–4 month-old WT and IRAK-M KO mice underwent coronary occlusion protocols as previously described [12]. Mice were anesthetized by isoflurane inhalation (isoflurane 2–3% vol/vol). Non-reperfused myocardial infarction was induced using an open chest model of permanent left coronary artery ligation. After 7 days of permanent coronary occlusion, the chest was opened, the heart was immediately excised, snap frozen in liquid nitrogen, and stored at −80°C for protein extraction. In additional experiments hearts were used for isolation of cardiac fibroblasts from the infarcted heart.
Echocardiography
Short axis M-mode echocardiography was performed prior to instrumentation and before the end of each experiment (after 7 days of permanent coronary occlusion, WT n=13, KO n=10) using the Vevo 2100 system (VisualSonics, Toronto ON), as previously described [12]. The following parameters were measured as indicators of function and remodeling: left ventricular end-diastolic diameter (LVEDD), left ventricular end-diastolic volume (LVEDV), ejection fraction, and end-diastolic left ventricular anterior wall thickness (LVAWt(d)).
Histopathology and morphometric analysis
Collagen pads populated with WT or IRAK-M KO cardiac fibroblasts for 24h were fixed in formalin and embedded in paraffin for histologic analysis. Infarcted hearts were also fixed in formalin, embedded in paraffin and sectioned systematically from base to apex as previously described [30]. Histological sections from fibroblast-populated pads were stained with sirius red, and counterstained with hematoxylin, as previously described [29], to identify the fibroblasts and to quantitate their density and cell area. For assessment of cell density 10 random low power fields were used for each pad. 50 fibroblasts from each pad were assessed for quantitation of cell area. In order to quantitatively assess collagen content in the infarcted heart, histological sections were stained for sirius red at three different levels, base, mid-myocardium and apex. Fifteen fields from the infarcted segments of each mouse were used to assess the collagen-stained area. Ten fields from the remote remodeling myocardium were used to quantitatively assess the collagen content in the non-infarcted region for each animal.
Immunofluorescence
Myofibroblasts were harvested from infarcted hearts as previously described [26]. Cells were transferred to 4-chamber polystyrene-treated tissue culture glass slides (BD falcon™, Fisher Scientific, Pittsburgh PA), were allowed to attach overnight and were fixed for 10 min in a 3.7% solution of paraformaldehyde (Sigma, St Louis MO). Cells were then washed twice with PBS and stained with FITC-labeled anti–α-SMA (Sigma). Nuclei were stained with DAPI (Invitrogen). Myofibroblasts in infarcted hearts were identified, as spindle-shaped cells located outside the vascular media with α-SMA immunofluorescence using the anti-α-SMA antibody and an Alexa-Fluor 594-labeled secondary antibody (Molecular Probes). Sections were counterstained with DAPI. Myofibroblast density was quantitatively assessed in infarcted myocardium by scanning fifteen fields for each mouse at three different levels (basal, mid and apical). Myofibroblast density in the non-infarcted myocardium was assessed by counting cell numbers in ten fields from remote remodeling myocardial segments.
Protein extraction and western blotting
Protein was extracted from hearts and from cultured cardiac fibroblasts using standard protocols. Western blotting was performed using antibodies to αSMA, type 3 collagen α1 (Santa Cruz Biotechnology, Dallas TX), pSmad2 (Cell Signaling, Danvers MA), and YB-1 (Bioss, Woburn MA).
RNA extraction and qPCR
Control and TGF-β-stimulated fibroblasts were used for RNA extraction after 24h of stimulation. Isolated total RNA from mouse hearts was reverse transcribed to cDNA using the iScript™ cDNA synthesis kit (Bio-Rad) following the manufacturer’s guidelines. Quantitative PCR was performed using the SsoFast™ EvaGreen® Supermix (Bio-Rad) method on the CFX384™ Real-Time PCR Detection System (Bio-Rad). Primers were synthesized by Integrated DNA Technologies. The following sets of primers were used in the study: collagen I forward 5′-GAGGTATGCTTGATCTGTA-3′, reverse 5′-CTGAGTTTGGTGATACGTA-3′, α-SMA forward 5′-ATCAGCGCCTCCAGTTCCTT-3′, α-SMA reverse 5′-TCTCACCTAACAGAAACACAA-3′, GAPDH forward 5′-AACGACCCCTTCATTGACCT-3′, GAPDH reverse 5′-CACCAGTAGACTCCACGACA-3′. The housekeeping gene GAPDH was used as internal control. The qPCR procedure was repeated three times in independent runs; gene expression levels were calculated using the ΔΔCT method.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using GraphPad version 6 (GraphPad Software Inc., LaJolla, CA, USA) using unpaired, 2-tailed Student’s t test using Welch’s correction for unequal variances and 1-way ANOVA with Tukey’s multiple comparison test. Results were considered statistically significant at P<0.05.
RESULTS
1. IRAK-M null fibroblasts exhibit impaired ability to contract collagen pads
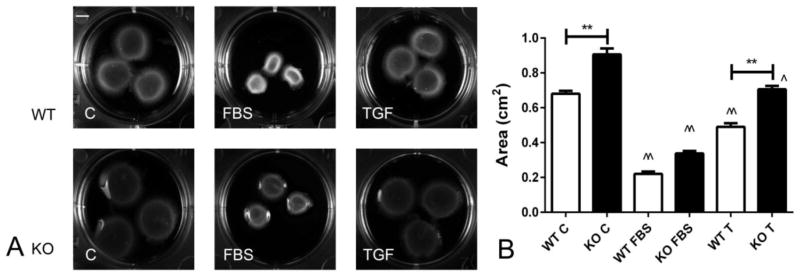
We have previously demonstrated that IRAK-M is expressed in myofibroblasts infiltrating the infarcted myocardium and that in vitro, IRAK-M expression is upregulated in growth factor-stimulated cardiac fibroblasts [12]. In order to explore the role of IRAK-M in regulating fibroblast function, we studied the effects of IRAK-M loss on contraction of fibroblast-populated collagen pads. When compared with WT cardiac fibroblasts, IRAK-M null cells exhibited reduced capacity to contract collagen pads (Fig 1A, B). TGF-β1 stimulation enhanced pad contraction in both WT and IRAK-M KO cells; however, IRAK-M absence was associated with impaired pad contraction by TGF-β stimulated cells. In contrast, pad contraction by FBS-stimulated cells was comparable between WT and IRAK-M KO cells (Figure 1).
Figure 1.
IRAK-M KO cardiac fibroblasts exhibit impaired capacity to contract collagen pads. A: Representative collagen pads populated with WT or IRAK-M KO fibroblasts, in the presence or absence of fetal bovine serum (FBS) and TGF-β1 (TGF). B: FBS and TGF-β1 increased fibroblast-mediated pad contraction (^p<0.05, ^^<0.01 vs. control). When compared with WT cells, IRAK-M null cells had reduced capacity to contract gels at baseline and after stimulation with TGF-β1 (**p<0.01 vs. WT). (n=10–12 per group).
2. IRAK-M loss does not affect cell density in fibroblast-populated collagen pads, but is associated with increased cell size
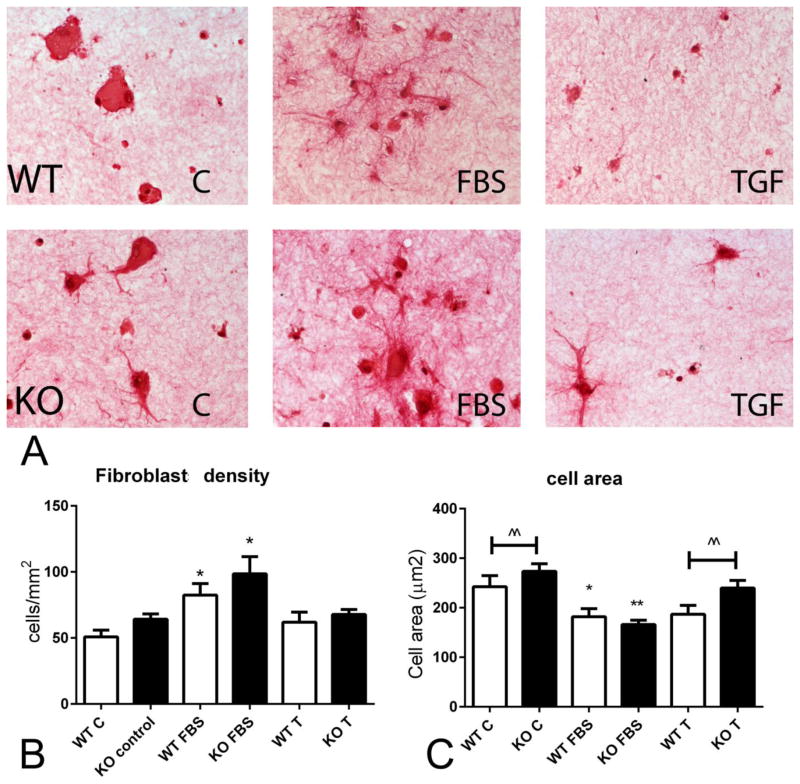
We hypothesized that impaired contraction of collagen pads populated with IRAK-M KO cells may be due to effects of IRAK-M on cell proliferation and/or survival that may affect the number of cells in the pad. We used sirius red staining to identify and quantitate pad fibroblasts (Figure 2A). FBS stimulation increased WT and KO fibroblast density in collagen pads; in contrast TGF-β1 stimulation had no significant effect. IRAK-M loss did not significantly affect the density of fibroblasts in the pad (Figure 2B). IRAK-M absence was associated with a significant increase in cell size, evidenced by a higher mean cross-sectional area. FBS stimulation decreased cell size in both WT and IRAK-M KO cells (Figure 2C).
Figure 2.
Impaired contractile activity of IRAK-M KO fibroblasts is not due to a reduction in cell density. A. Representative images of sirius-red stained collagen pads populated with WT or IRAK-M KO cardiac fibroblasts (arrows), in the presence or absence of FBS and TGF-β1 (T). B. FBS stimulation increased fibroblast density in the pad (*p<0.05 vs. corresponding control). IRAK-M loss did not affect fibroblast density. TGF-β stimulation did not affect fibroblast density in WT or IRAK-M KO cells. C. FBS stimulation decreased cell area in both WT and IRAK-M KO fibroblasts (*p<0.05, **p<0.01 vs. corresponding control). IRAK-M KO cells had a significantly higher area at baseline and after stimulation with TGF-β1 (^^p<0.01 vs. WT). (for cell density, n=4–6 pads/group; for cell area, n=200–300 cells/group).
3. IRAK-M loss is associated with impaired TGF-β-induced myofibroblast transdifferentiation
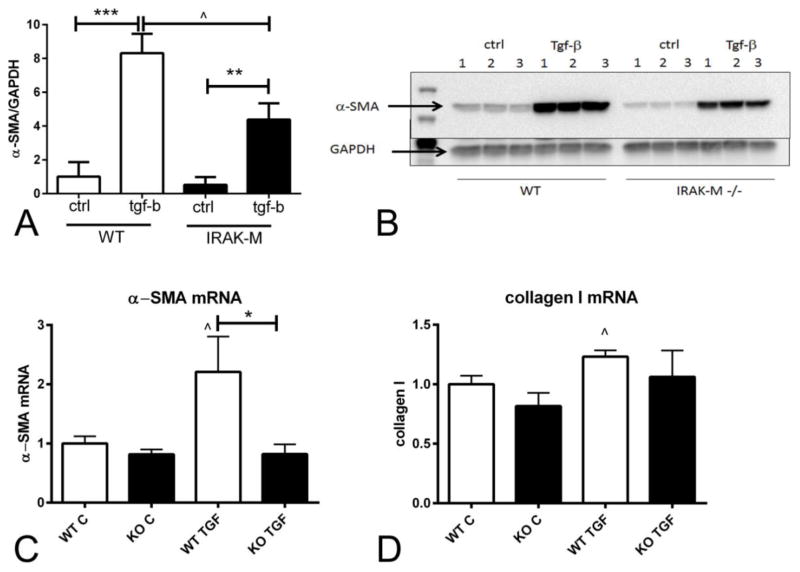
Because myofibroblasts are critically involved in wound contraction, we examined whether the impaired capacity of IRAK-M null cells to contract collagen pads is associated with defective myofibroblast transdifferentiation. In cells cultured in plates, TGF-β1 stimulation for 72h markedly increased α-SMA protein expression in both WT and IRAK-M KO cells. However, when compared with WT cells, IRAK-M KO cardiac fibroblasts exhibited significantly lower levels of α-SMA expression (Figure 3A–B).
Figure 3.
IRAK-M absence is associated with attenuated TGF-β-mediated α-SMA mRNA and protein upregulation. A–B: Western blotting experiments showed that after 72h of TGF-β1 stimulation, WT and IRAK-M KO cardiac fibroblasts exhibited markedly increased α-SMA expression (**p<0.01, ***p<0.001 vs. corresponding control). When compared with WT cells, IRAK-M KO cardiac fibroblasts had significantly lower levels of α-SMA expression after stimulation with TGF-β1 (n=5/group). C–D: qPCR showed that IRAK-M loss attenuated TGF-β1-mediated a-SMA upregulation after 24 h of stimulation. C: TGF-β1 induced a 2.2-fold increase in α-SMA mRNA levels in WT cells (^p<0.05 vs. WT C). After stimulation with TGF-β1, IRAK-M KO cells had significantly lower α-SMA mRNA expression than corresponding WT cells. D: TGF-β1 induced a modest, but statistically significant increase in type 1 collagen mRNA expression in WT cells (^p<0.05 vs. control). IRAK-M loss did not affect baseline or TGF-β-stimulated collagen transcription (n=6/group).
4. IRAK-M loss is associated with attenuated TGF-β-induced α-SMA mRNA and collagen 1 synthesis
Next, we examined whether IRAK-M loss affects α-SMA and collagen synthesis by activated cardiac fibroblasts. After 24h of stimulation, TGF-β induced a >2-fold increase in α-SMA mRNA expression in cardiac fibroblasts and a statistically significant 25% increase in collagen 1 mRNA transcription (Figure 3C–D). IRAK-M absence abrogated the effects of TGF-β stimulation on α-SMA mRNA levels. Moreover, the effects of TGF-β stimulation on collagen 1 mRNA levels by IRAK-M null cells were not statistically significant.
5. IRAK-M loss modestly attenuates TGF-β-mediated Smad phosphorylation
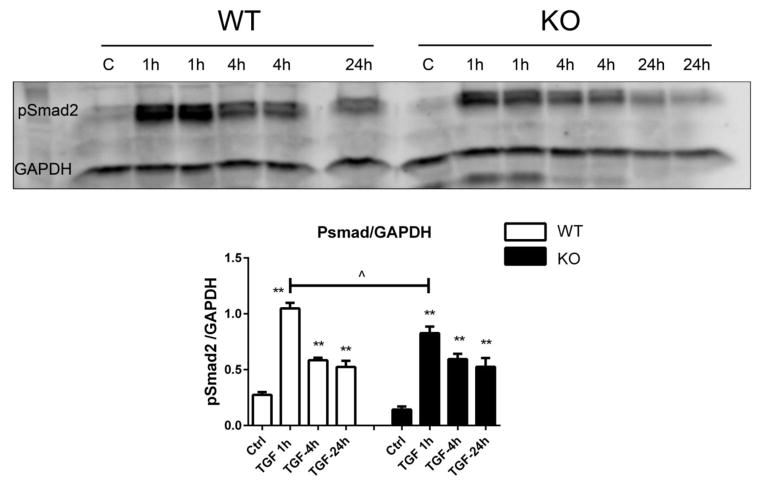
Reduced α-SMA synthesis in IRAK-M null cells (Figure 3) suggests that IRAK-M promotes TGF-β-mediated myofibroblast transdifferentiation. In order to examine the mechanism responsible for the effects of IRAK-M, we examined whether IRAK-M loss affects TGF-β-induced Smad activation. TGF-β1 increased Smad2 phosphorylation in both WT and IRAK-M KO cells after 1h, 4h or 24h of stimulation. IRAK-M null cells exhibited modestly, but significantly, attenuated peak p-Smad2 expression after 1h of stimulation (Figure 4). In contrast, after 4–24h of stimulation p-Smad2 levels were comparable between WT and IRAK-M KO cells.
Figure 4.
Effects of IRAK-M loss on TGF-β-mediated Smad2 phosphorylation. Western blotting for pSmad2 showed that TGF-β1 activates Smad signaling in both WT and IRAK-M KO cardiac fibroblasts after 1–24h of stimulation (**p<0.01 vs. corresponding control). When compared with WT cells, IRAK-M KO cells exhibited a modest, but significant attenuation of peak pSmad2 expression after 1h of stimulation (^p<0.05 vs. WT) (n=4–6/group).
6. IRAK-M loss is associated with increased expression of the α-SMA repressor YB-1
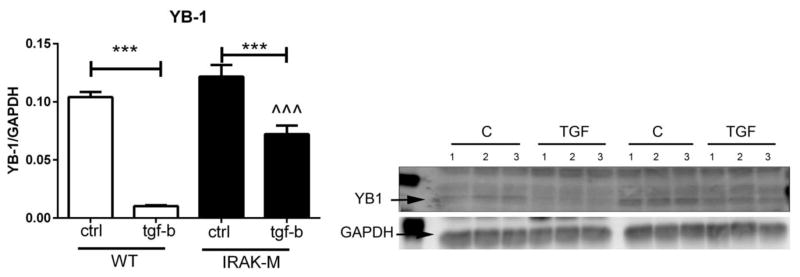
Expression of the potent transcriptional repressor YB-1 negatively regulates α-SMA expression in myofibroblasts [31]. Accordingly, we examined whether reduced α-SMA expression in IRAK-M null cells was due to increased expression of the repressor YB-1. Baseline YB-1 expression was comparable between WT and IRAK-M KO cells. TGF-β1 stimulation markedly suppressed YB-1 expression. TGF-β1-mediated suppression of YB-1 was attenuated in IRAK-M null cells (Figure 5).
Figure 5.
IRAK-M loss is associated with attenuated downregulation of YB-1 expression upon stimulation with TGF-β1. TGF-β1 stimulation decreased YB-1 expression in both WT and IRAK-M KO cardiac fibroblasts (***p<0.001 vs. control). After TGF-β stimulation, IRAK-M KO cells had significantly higher YB-1 levels than WT cells (^^^p<0.001 vs. WT) (n=3/group).
7. In a model of non-reperfused myocardial infarction, IRAK-M loss does not affect cardiac dysfunction and collagen deposition
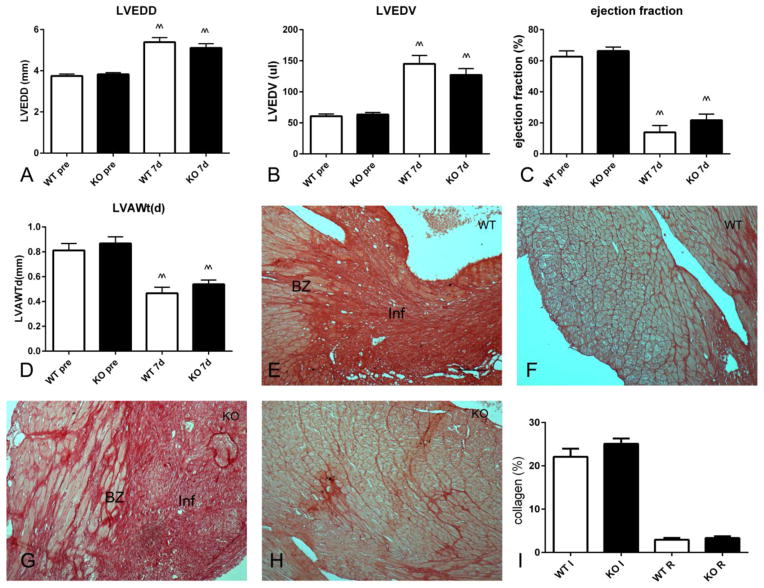
We have previously demonstrated that IRAK-M absence is associated with accentuated dilative remodeling following reperfused myocardial infarction [12]. The effects of IRAK-M loss are associated with increased inflammation and a pro-inflammatory macrophage phenotype [12]. When compared with reperfused infarcts, non-reperfused infarcts exhibit markedly attenuated and delayed inflammatory response [32]. Echocardiographic analysis showed that, in a model of non-reperfused myocardial infarction, IRAK-M loss did not affect chamber dimensions (Figure 6A–B) and ejection fraction (Figure 6C) after 7 days of permanent coronary occlusion. Left ventricular anterior wall thickness was also comparable between groups (Figure 6D). Quantitative analysis of collagen staining (Figure 6I) using Sirius red showed that WT (Figure 6E, F) and IRAK-M KO mice (Figure 6G, H) had comparable collagen content in the infarcted (Figure 6E, G) and in the remote remodeling myocardium (Figure 6F, H).
Figure 6.
Effects of IRAK-M loss on cardiac remodeling, dysfunction and fibrosis in a mouse model of permanent coronary occlusion. A–D. Echocardiographic analysis demonstrated that both WT and IRAK-M KO mice exhibited markedly increased left ventricular end-diastolic diameter (A, LVEDD) and left ventricular end-diastolic volume (B, LVEDV) after 7 days of permanent coronary occlusion (^^p<0.01 vs. corresponding baseline values). Moreover, non-reperfused infarction markedly reduced ejection fraction (C) and decreased end-diastolic left ventricular anterior wall thickness (LVAWt(d) (^^p<0.01 vs pre). IRAK-M loss had no significant effect on dilative remodeling, systolic dysfunction and anterior wall thinning (n=10–13/group). E–I: IRAK-M absence did not affect collagen content in the infarcted and remote remodeling myocardium. Sirius red staining was used to label collagen in infarcted and remote remodeling segments of WT (E, F) and IRAK-M KO (G, H) infarcts. IRAK-M loss did not affect collagen content in the infarcted and remote remodeling myocardium (n=8–11/group) (Inf, infarction; BZ, border zone, scalebar=60μm).
8. IRAK-M absence does not affect myofibroblast infiltration in non-reperfused myocardial infarction, but is associated with increased YB-1 expression, and reduced α-SMA immunoreactivity in isolated infarct myofibroblasts
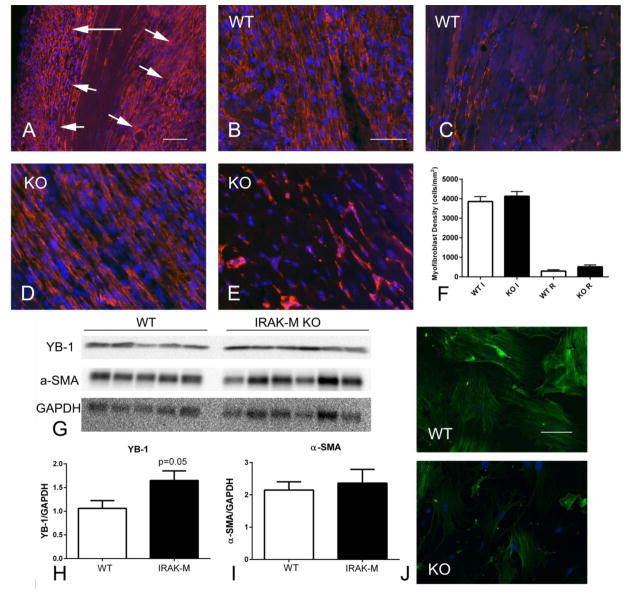
In the model of non-reperfused myocardial infarction, both WT and IRAK-M KO animals exhibited intense infiltration of the border zone with α-SMA+ myofibroblasts (Figure 7A, B). IRAK-M loss did not affect myofibroblast density in the infarcted, or in the remote remodeling myocardium (Figure 7C–G). In order to examine whether IRAK-M loss in fibroblasts is associated with increased YB-1 expression in vivo, we compared YB-1 levels between WT and IRAK-M KO infarcts (Figure 7A, B). After 7 days of coronary occlusion, IRAK-M KO infarcts had significantly lower YB-1 expression than corresponding WT infarcts (Figure 7B). In contrast, α-SMA expression levels were not significantly different between groups (Figure 7A, C). Because α-SMA is expressed by both vascular smooth muscle cells and myofibroblasts, we harvested myofibroblasts from non-reperfused infarcts and stained the cells for α-SMA. Cells harvested from IRAK-M KO infarcts exhibited reduced α-SMA immunofluorescence in comparison to cells derived from WT infarcts (Figure 7D).
Figure 7.
IRAK-M loss does not affect myofibroblast density in non-reperfused myocardial infarction, but is associated with increased YB-1 expression and reduced α-SMA expression in infarct myofibroblasts. A–F. α-SMA immunofluorescence identifies myofibroblasts infiltrating the border zone of the infarcted heart after 7 days of permanent coronary occlusion (A, arrows) (scalebar=80μm. Representative images show identification of α-SMA-expressing myofibroblasts in the infarcted (B, D) and non-infarcted remodeling myocardium (C, E), in WT (B, C) and IRAK-M KO infarcts (D, E; scalebar=50μm). Quantitative analysis showed that myofibroblast density was comparable between WT and IRAK-M infarcts (n=8–11/group) (F). G–H Western blotting showed that IRAK-M KO animals had higher levels of YB-1 in the infarcted myocardium than WT animals after 7 days of coronary occlusion. I. α-SMA levels in the infarcted myocardium (reflecting expression by both smooth muscle cells and myofibroblasts) were comparable between groups. J. Immunofluorescent staining showed that myofibroblasts harvested from KO infarcts had attenuated α–SMA expression when compared to WT cells (n=5/group, scalebar=40μm).
DISCUSSION
Our study reports for the first time that, in addition to its established roles in suppression of innate immune signaling and in modulation of macrophage phenotype, IRAK-M is also implicated in myofibroblast activation. The effects of IRAK-M in fibroblasts may be mediated through suppression of YB-1, a repressor of α-SMA transcription. Thus, IRAK-M may serve as a molecular “switch” responsible for the transition from inflammation to fibrosis, by restraining pro-inflammatory signaling in macrophages, while activating a fibrogenic/contractile phenotype in infarct myofibroblasts,
The role of IRAK-M in inflammation and immunity
Both in vivo studies and in vitro experiments have demonstrated that IRAK-M is implicated in negative regulation of immune responses by exerting critical actions on macrophages and dendritic cells [33]. In a model of infectious lung injury, IRAK-M loss was associated with increased mortality due to unrestrained inflammatory activation [34]. In experimental models of murine lupus [35] and type 1 diabetes [36], IRAK-M loss exacerbated autoimmune tissue injury. IRAK-M upregulation is an important mechanism responsible for the broad range anti-inflammatory actions of glucocorticoids [37]. The molecular signals mediating the anti-inflammatory actions of IRAK-M in macrophages remain poorly understood. It has been suggested that IRAK-M may prevent dissociation of IRAK-1 and IRAK-4 from their complex with the adaptor protein MyD88, thus preventing formation of IRAK-1/TNF receptor associated factor 6 (TRAF6) complexes and inhibiting downstream NF-κB and activator protein (AP)-1 signaling [22]. In vitro studies in macrophages have suggested additional mechanisms that may mediate the anti-inflammatory actions of IRAK-M. IRAK-M may act by stabilizing mitogen-activated protein kinase phosphatase (MKP)-1, thus attenuating p38 mitogen-activated protein kinase (MAPK) activation and repressing MAPK-driven pro-inflammatory actions [38]. Experiments in bone marrow-derived macrophages suggested that IRAK-M may also mediate the mitogen-activated protein kinase kinase kinase 3 (MEKK3)-dependent “second wave” of NF-κB activation that results in production of inhibitory molecules, while negatively regulating translation of pro-inflammatory cytokines and chemokines [39].
IRAK-M in tissue fibrosis
Information on the role of IRAK-M in tissue fibrosis is extremely limited. In a model of renal injury, progressive fibrosis was associated with IRAK-M upregulation [40]. A recently published investigation demonstrated that IRAK-M may play a critical role in mediating fibrosis in a model of bleomycin-induced lung injury [41]. IRAK-M null mice exhibited attenuated pulmonary fibrosis following bleomycin administration. The fibrogenic actions of IRAK-M were presumed due to activation of a pro-fibrotic macrophage phenotype, associated with expression of IL-13 and subsequent fibroblast stimulation.
Our experiments suggest that, in addition to its role in activation of fibrogenic macrophages, IRAK-M may exert direct actions on cardiac fibroblasts. We have previously demonstrated that IRAK-M is expressed in a subset of cardiac fibroblasts infiltrating the healing infarct; in vitro, IRAK-M is induced in cardiac fibroblasts upon stimulation with cytokines and growth factors [12]. Our current study suggests that IRAK-M plays a direct role in myofibroblast conversion and activation. IRAK-M null fibroblasts exhibited impaired ability to contract free-floating collagen pads upon stimulation with TGF-β (Figure 1) and had attenuated TGF-β-induced α-SMA expression (Figure 3). The effects of IRAK-M loss on cardiac fibroblasts were associated with impaired suppression of YB-1, a potent repressor of α-SMA transcription [31]. Our findings suggest for the first time that IRAK-M expression may directly stimulate myofibroblast conversion by suppressing YB-1 levels, thus releasing α-SMA synthesis. Considering the limited information on the mechanisms regulating YB-1 expression [42], the basis for the effects of IRAK-M loss on YB-1 levels remains unclear. Pro-inflammatory cytokines (such as IL-1β) are upregulated in the absence of IRAK-M, and are known to induce YB-1 transcription and activation [43]. Moreover, MAPKs have been implicated in regulation and activation of YB-1 [44]. In the absence of IRAK-M, accentuation of p38 MAPK signaling [38] may enhance YB-1 expression, repressing α-SMA.
The role of IRAK-M in the infarcted myocardium: from inflammation to fibrosis
We have previously demonstrated that in reperfused myocardial infarcts, IRAK-M expression in macrophages and fibroblasts serves as a critical inhibitory signal that restrains the post-infarction inflammatory response and protects the myocardium from dilative remodeling [12]. Cardiomyocyte necrosis releases danger signals that activate innate immune signaling pathways, triggering an intense inflammatory reaction [45]. Release of IL-1 and engagement of Toll-like Receptors (TLRs) initiates signaling responses that lead to phosphorylation of IRAK-1 and IRAK-4, and trigger activation of the NF-κB system. However, repair of the infarcted heart is dependent on timely suppression of pro-inflammatory signaling and activation of a reparative program. Repression of pro-inflammatory mediators is not a passive process, but requires activation of STOP signals that suppress inflammatory activity. In the healing infarct, repression of pro-inflammatory signaling by regulatory macrophages is temporally linked with activation of fibroblasts that acquire a myofibroblast phenotype and deposit large amounts of extracellular matrix proteins [1],[46],[47].
Our current study demonstrates that, in contrast to its critical role in preventing adverse remodeling in reperfused infarcts, IRAK-M does not significantly affect remodeling in non-reperfused infarction. The contrasting findings may reflect the consequences of the marked accentuation of pro-inflammatory activation induced by reperfusion [32],[48]. In the pro-inflammatory environment of the reperfused infarct, IRAK-M may function as a key inhibitory signal that protects the heart from cytokine-mediated injury. IRAK-M expression in macrophages plays an important role in negative regulation of the post-infarction inflammatory reaction, suppressing cytokine and chemokine expression [12]. In the absence of IRAK-M, unrestrained inflammation results in accentuated matrix metalloproteinase expression worsening adverse ventricular remodeling [12]. In contrast, in non-reperfused infarcts, levels of cytokines and chemokines are much lower, and the anti-inflammatory effects of IRAK-M may be much less important.
What is the in vivo significance of the effects of IRAK-M on myofibroblast transdifferentiation? Although in vitro IRAK-M absence impairs fibroblast-mediated collagen pad contraction and inhibits myofibroblast transdifferentiation, the in vivo consequences of IRAK-M loss on the reparative process are less impressive. IRAK-M absence does not significantly affect collagen content and myofibroblast density in the infarcted and remodeling myocardium, but is associated with increased YB-1 levels in the infarct and with attenuated α-SMA content in isolated infarct myofibroblasts. The absence of significant in vivo effects may suggest a limited role for IRAK-M in myofibroblast conversion in the complex environment of the infarct, where a wide range of mediators co-operate to activate cardiac fibroblasts. The findings could also reflect competing actions of IRAK-M in macrophages and in myofibroblasts, differentially regulating the reparative response. Cell-specific loss-of-function approaches are needed to dissect the cell biological basis for the effects of IRAK-M.
Conclusions
In healing myocardial infarcts, expression of IRAK-M by macrophages and fibroblasts marks the end of the inflammatory phase of healing and the transition to fibroblast-driven repair. In reperfused infarcts, IRAK-M functions as a key negative regulator of the innate immune response, preventing excessive inflammatory signaling. In cardiac fibroblasts, IRAK-M enhances fibroblast-mediated matrix contraction and facilitates myofibroblast conversion, suppressing expression of the α-SMA repressor, YB-1. These effects of IRAK-M may reflect a link between termination of innate immune signaling and activation of a reparative program.
HIGHLIGHTS.
Interleukin receptor-associated kinase (IRAK)-M loss is associated with impaired fibroblast-mediated contraction in collagen pads.
IRAK-M absence is associated with impaired transforming growth factor (TGF)-β-induced α-smooth muscle actin expression (α-SMA) in cardiac fibroblasts.
IRAK-M loss is associated with a modest attenuation of TGF–β-mediated Smad signaling in cardiac fibroblasts.
Impaired myofibroblast transdifferentiation in the absence of IRAK-M is associated with increased expression of the transcriptional repressor Y-box binding protein (YB)-1.
In non-reperfused infarction, IRAK-M absence does not affect myofibroblast density, but is associated with increased YB-1 levels and attenuated α-SMA expression in infarct myofibroblasts.
Acknowledgments
SOURCES OF FUNDING: Supported by NIH grants R01 HL76246 and R01 HL85440. Dr Shinde is supported by an American Heart Association Founders’ affiliate post-doctoral grant.
Footnotes
DISCLOSURES: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arslan F, Smeets MB, O’Neill LA, Keogh B, McGuirk P, Timmers L, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 4.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–64. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 5.Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–83. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 6.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Lavine KJ, Epelman S, Evans SA, Weinheimer CJ, Barger PM, et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.115.001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30:240–5. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–32. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 10.Lorchner H, Poling J, Gajawada P, Hou Y, Polyakova V, Kostin S, et al. Myocardial healing requires Reg3beta-dependent accumulation of macrophages in the ischemic heart. Nat Med. 2015;21:353–62. doi: 10.1038/nm.3816. [DOI] [PubMed] [Google Scholar]
- 11.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–8. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, et al. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2598–608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, et al. The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2012;32:2206–13. doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Zhao H, Xu X, Buys ES, Raher MJ, Bopassa JC, et al. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol. 2008;295:H1311–H8. doi: 10.1152/ajpheart.00119.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling. Biochem Pharmacol. 2010;80:1981–91. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Kanakaraj P, Schafer PH, Cavender DE, Wu Y, Ngo K, Grealish PF, et al. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med. 1998;187:2073–9. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–91. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–6. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, et al. Myocardial Ischemia Activates an Injurious Innate Immune Signaling via Cardiac Heat Shock Protein 60 and Toll-like Receptor 4. J Biol Chem. 2011;286:31308–19. doi: 10.1074/jbc.M111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maekawa Y, Mizue N, Chan A, Shi Y, Liu Y, Dawood S, et al. Survival and cardiac remodeling after myocardial infarction are critically dependent on the host innate immune interleukin-1 receptor-associated kinase-4 signaling: a regulator of bone marrow-derived dendritic cells. Circulation. 2009;120:1401–14. doi: 10.1161/CIRCULATIONAHA.109.865956. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 23.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–7. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–42. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403–10. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 26.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, et al. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J Immunol. 2013;191:4838–48. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-Quesada C, Lu B, et al. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res. 2014;103:217–27. doi: 10.1093/cvr/cvu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, et al. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–83. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, et al. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013;61:555–70. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang A, Liu X, Cogan JG, Fuerst MD, Polikandriotis JA, Kelm RJ, Jr, et al. YB-1 coordinates vascular smooth muscle alpha-actin gene activation by transforming growth factor beta1 and thrombin during differentiation of human pulmonary myofibroblasts. Mol Biol Cell. 2005;16:4931–40. doi: 10.1091/mbc.E05-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kukielka GL, Smith CW, LaRosa GJ, Manning AM, Mendoza LH, Daly TJ, et al. Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest. 1995;95:89–103. doi: 10.1172/JCI117680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiu J, Czinn SJ, Kobayashi KS, Sun Y, Blanchard TG. IRAK-M expression limits dendritic cell activation and proinflammatory cytokine production in response to Helicobacter pylori. PLoS One. 2013;8:e66914. doi: 10.1371/journal.pone.0066914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seki M, Kohno S, Newstead MW, Zeng X, Bhan U, Lukacs NW, et al. Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia. J Immunol. 2010;184:1410–8. doi: 10.4049/jimmunol.0901709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lech M, Kantner C, Kulkarni OP, Ryu M, Vlasova E, Heesemann J, et al. Interleukin-1 receptor-associated kinase-M suppresses systemic lupus erythematosus. Ann Rheum Dis. 2011;70:2207–17. doi: 10.1136/ard.2011.155515. [DOI] [PubMed] [Google Scholar]
- 36.Tan Q, Majewska-Szczepanik M, Zhang X, Szczepanik M, Zhou Z, Wong FS, et al. IRAK-M deficiency promotes the development of type 1 diabetes in NOD mice. Diabetes. 2014;63:2761–75. doi: 10.2337/db13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyata M, Lee JY, Susuki-Miyata S, Wang WY, Xu H, Kai H, et al. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nature communications. 2015;6:6062. doi: 10.1038/ncomms7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su J, Xie Q, Wilson I, Li L. Differential regulation and role of interleukin-1 receptor associated kinase-M in innate immunity signaling. Cell Signal. 2007;19:1596–601. doi: 10.1016/j.cellsig.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou H, Yu M, Fukuda K, Im J, Yao P, Cui W, et al. IRAK-M mediates Toll-like receptor/IL-1R-induced NFkappaB activation and cytokine production. EMBO J. 2013;32:583–96. doi: 10.1038/emboj.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunthner R, Kumar VR, Lorenz G, Anders HJ, Lech M. Pattern-recognition receptor signaling regulator mRNA expression in humans and mice, and in transient inflammation or progressive fibrosis. Int J Mol Sci. 2013;14:18124–47. doi: 10.3390/ijms140918124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballinger MN, Newstead MW, Zeng X, Bhan U, Mo XM, Kunkel SL, et al. IRAK-M Promotes Alternative Macrophage Activation and Fibroproliferation in Bleomycin-Induced Lung Injury. J Immunol. 2015;194:1894–904. doi: 10.4049/jimmunol.1402377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB-1 protein: functions and regulation. Wiley Interdiscip Rev RNA. 2014;5:95–110. doi: 10.1002/wrna.1200. [DOI] [PubMed] [Google Scholar]
- 43.Mertens PR, Martin IV, Frye BC, Rauen T, Strauch S, Pabst M, et al. Rat Mrp2 gene expression is regulated by an interleukin-1beta-stimulated biphasic response with enhanced transcription and subcellular shuttling of YB-1. Eur J Cell Biol. 2012;91:533–41. doi: 10.1016/j.ejcb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Sinnberg T, Sauer B, Holm P, Spangler B, Kuphal S, Bosserhoff A, et al. MAPK and PI3K/AKT mediated YB-1 activation promotes melanoma cell proliferation which is counteracted by an autoregulatory loop. Exp Dermatol. 2012;21:265–70. doi: 10.1111/j.1600-0625.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- 45.Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, et al. Cutting Edge: IL-1alpha Is a Crucial Danger Signal Triggering Acute Myocardial Inflammation during Myocardial Infarction. J Immunol. 2015;194:499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 47.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–38. [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar AG, Ballantyne CM, Michael LH, Kukielka GL, Youker KA, Lindsey ML, et al. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation. 1997;95:693–700. doi: 10.1161/01.cir.95.3.693. [DOI] [PubMed] [Google Scholar]