Abstract
Purpose of review
To evaluate evidence that statins reduce cardiovascular risk in patients living with HIV
Recent findings
Moderate to high dose atorvastatin and rosuvastatin appear to reduce non-calcified coronary plaque volume and slow progression of carotid intima-media thickness in patients with treated HIV infection. Expected lipoprotein changes with statins on the background of modern ART are similar to the general population. In addition to lipids, the statin benefit may be mediated in part by improvements in vascular inflammation and levels of T-cell and monocyte activation. One concern is the potential for rosuvastatin to cause insulin resistance. Decisions to prescribe statins must be done in the context of global risk assessment, but traditional risk calculators such as the Framingham risk score or the ACC/AHA pooled-risk equations underestimate risk in this population. Furthermore, many patients with subclinical disease would not be recommended for statins according to the most recent ACC/AHA guidelines.
Summary
Statins are likely to improve cardiovascular outcomes for patients with HIV, but results of the first outcome study are not expected until 2020. In the meantime, clinicians should individualize statin prescriptions, and should consider using more potent statins (rosuvastatin, atorvastatin, and pitavastatin) when possible.
Keywords: statin, HIV, inflammation, lipids
Introduction
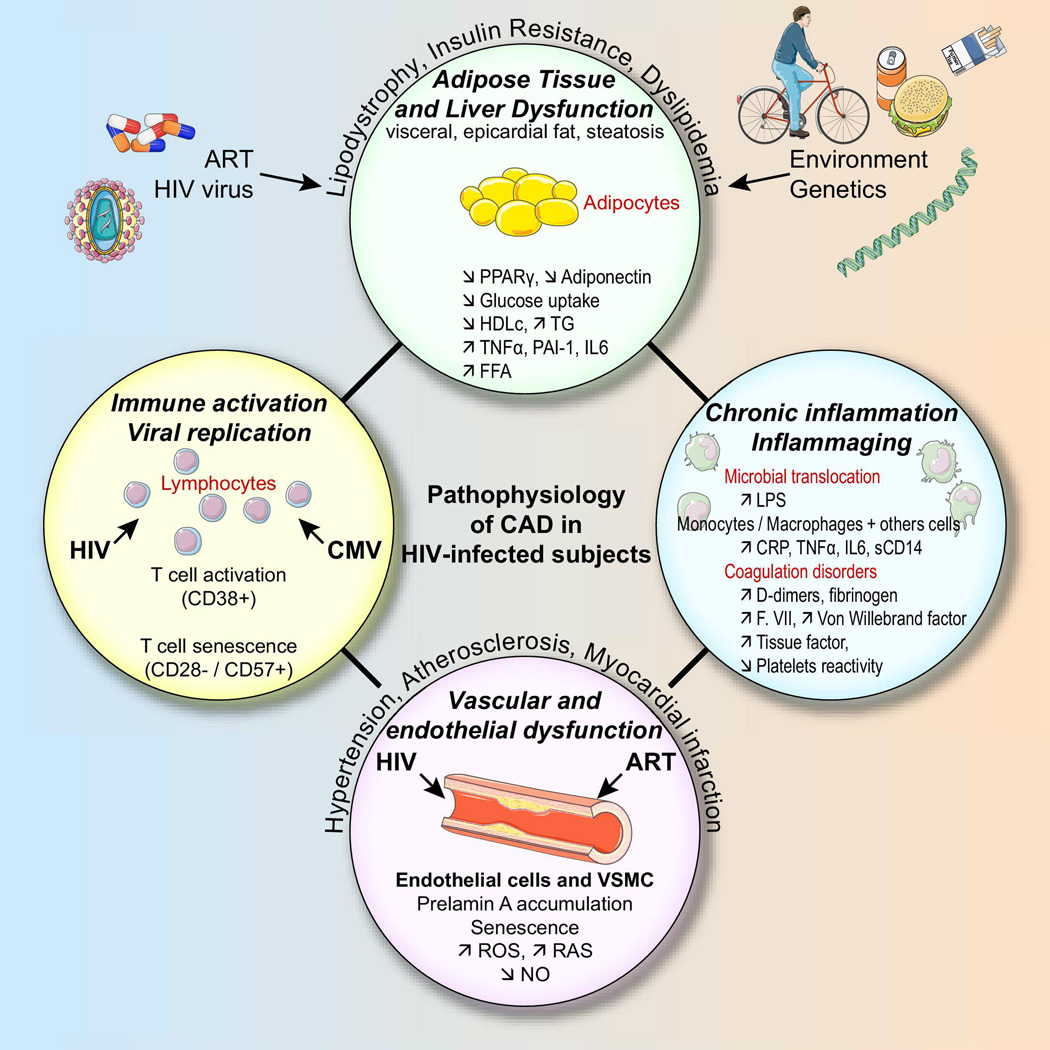
The cardiovascular complications of HIV/AIDS have evolved considerably since the early years of the epidemic. Pericarditis and dilated cardiomyopathy—common in the 1980’s and 90’s—are now rarely seen in the setting of effective antiretroviral therapy (ART). Yet despite effective viral suppression with ART, atherosclerotic cardiovascular disease (CVD) events such as myocardial infarction (MI) and ischemic stroke occur more commonly in HIV-infected patients compared to uninfected controls[1, 2]. Proposed mechanisms of this increased risk in HIV have been recently reviewed[3] and include metabolic dysfunction, direct vascular effects, immune activation, and chronic “inflammaging” (Figure 1). Initial epidemiologic studies from the early 2000’s associated antiretroviral drugs, particularly protease inhibitors, with risk of MI[4], but newer generations of these drugs have proven to be less toxic[5]. In light of data from the SMART study and others, the current consensus is that ART has a net cardiovascular benefit[6].
Figure. Proposed mechanisms of increased cardiovascular risk in chronic HIV infection.
Reproduced with permission from Boccara et al.[3]
How can we now further reduce the risk of cardiovascular disease in patients with chronic treated HIV infection? Recent attention has focused on statins (HMG-CoA reductase inhibitors) because of their proven benefit to reduce cardiovascular events in the general population, and because of their highly touted “pleiotropic” effects on inflammation and vascular health. Although initially developed to reduce LDL cholesterol, statins have been shown to dramatically reduce the relative risk of MI even in patients with relatively normal LDL levels[7]. Furthermore, the risk reduction is related to the magnitude of reduction in markers of chronic inflammation[8]. Despite the interest in this area, there are currently few data on the cardiovascular benefits of statins in an HIV-infected population.
In this paper, we review recently published studies of the cardiovascular benefits of statins in treated HIV infection and explore some of the potential mediators of this benefit. We also provide an update of current evidence and a framework for how clinicians might approach prescribing statins to patients living with HIV.
Statins to improve CVD outcomes
In general, for patients who have had a myocardial infarction or acute coronary syndrome (ACS), intensive statin therapy reduces the risk of future major adverse cardiovascular events including cardiovascular death, stroke, and all-cause mortality[9] and the benefit is greater for high vs. moderate intensity statin[10]. Therefore, guidelines recommend that all patients with known CVD (including patients with HIV) should be prescribed high-dose statin therapy if tolerated[11]. For primary prevention of cardiovascular events in patients without known CVD, statins also reduce relative risk of vascular events on the order of 20–40%, but the absolute risk reduction is much lower than for secondary prevention[12]. The most impressive results come from the JUPITER trial in subjects with low LDL cholesterol (<130mg/dL) but elevated high-sensitivity C-reactive protein (hsCRP >2.0mg/L)[7]. In this trial, 20mg of rosuvastatin was associated with a 44% [95%CI 31–54%] relative risk reduction in major cardiovascular events.
Trials to reduce cardiovascular events in patients with relatively normal cholesterol were preceded by many trials of statins to reduce surrogate outcomes such as progression of carotid intima media thickness (CIMT). In the METEOR trial, for example, individuals with low Framingham risk score with evidence of CIMT thickening assigned to 40mg of daily rosuvastatin had a lower rate of CIMT progression over 2 years compared to placebo[13].
The data in patients with chronic HIV infection are limited to observational studies or trials that have focused on surrogate outcomes like in METEOR. Retrospective observational studies have suggested dramatic benefits of statins on all-cause mortality. In the Johns Hopkins HIV Clinical Cohort, for example, statin use was associated with a relative hazard of 0.33 (95%CI 0.14–0.76) for mortality after multivariate adjustment for HIV factors and pre-ART cholesterol levels. In a large Danish cohort, statin use seemed to be associated with a similar adjusted mortality rate ratio [aMRR 0.34 (95%CI 0.11–1.04)] among individuals with at least one comorbidity in an analysis restricted to observation time without virologic failure[14]. In contrast, statin use was not associated with reduction in time to all non-AIDS-defining events in an analysis of 3601 subjects from the AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT) cohort[15]. Prospective observational data are limited to one study that followed 36 patients with hypercholesterolemia and CIMT thickening (CIMT ≥0.9mm) who were started on 10mg daily rosuvastatin as part of their clinical care[16]. After 24 months of treatment, the investigators reported significant mean (standard deviation) reductions in mean CIMT ranging from −0.21(0.12) mm at the right common carotid to −0.34(0.19) mm at the left internal carotid.
The first three clinical trials testing the effect of statins on markers of vascular disease were published a decade ago. All used a relatively weak statin (pravastatin 40mg) due to concerns about drug interactions and used brachial artery reactivity testing (i.e. endothelial function) as the outcome. The first was a placebo-controlled, double-blind, crossover trial of 20 patients that demonstrated a trend toward improved flow-mediated dilation (FMD) with pravastatin (0.7% +/− 0.6%, p=0.08)[17]. Another similarly designed trial was conducted among 29 mostly male subjects on protease inhibitor-based ART with total cholesterol levels >5 mmol/L (193mg/dL)[18]. Median (interquartile range) FMD increased from a baseline of 2.0 (1.5—2.6)% to 3.2 (1.9—4.1)% after 8 weeks of statin therapy. In contrast, no difference in FMD change was seen in another similarly sized placebo-controlled trial of pravastatin[19].
Recently, the results of two larger randomized, double-blind, placebo-controlled trials of more potent statins to improve other surrogate CVD outcomes were reported[20, 21]. Lo et al. randomized 40 HIV-infected subjects on ART to 20mg of atorvastatin titrated to 40mg after 3 months vs. placebo and followed them for 12 months[20]. All subjects had LDL <130mg/dL but also had both subclinical coronary atherosclerosis on coronary CT angiography and increased aortic inflammation by positron emission tomography (PET/CT). There was no change in the primary cardiovascular outcome of aortic target to background ratio (TBR) of fluorodeoxyglucose uptake measured by PET/CT; however, there were dramatic reductions in non-calcified plaque volume measured by coronary CT angiography [−19(−39 to +9)% vs. +20(−7 to +94)%, statin v. placebo, p=0.009] and reductions in the number of plaques with “vulnerable” or high-risk features [fewer low attenuation plaques (p=0.03) and fewer positively remodeled plaques (p=0.04)].
The SATURN-HIV trial was conducted in 147 patients on ART with low LDL cholesterol (<130mg/dL) and elevated markers of systemic inflammation (hsCRP >2.0mg/dL) OR T-cell activation (CD8+CD38+HLA-DR+ ≥19%). Subjects were randomized to rosuvastatin 10mg daily vs. placebo and followed for 96 weeks, and randomization was stratified by the use of protease inhibitor and presence of coronary calcifications. The primary outcome of mean CIMT progression was reduced in the statin arm [+0.003 (95%CI, −0.014 to +0.021) mm vs. +0.028 (+0.008 to 0.047) mm, statin vs. placebo, p=0.031][21].
Together, the two most recent trials suggest that there will be a benefit of the more potent statins to reduce cardiovascular events for HIV-infected patients who are otherwise at low risk—the mean 10-year Framingham Risk Score was <7.5% in Lo et al and <5% in SATURN-HIV; however, both studies enrolled mostly men (~80%) and required elevated markers of vascular or systemic inflammation as an entry criteria. The larger REPRIEVE trial will test whether statin therapy reduces cardiovascular events in a more generalizable population of patients on ART with 10-year ACC/AHA risk of <7.5%, but results are not anticipated until 2020. Completed and ongoing trials of statins with non-lipid cardiovascular endpoints are described in Table 1.
Table 1.
Randomized clinical trials of statins to improve vascular measures and clinical CVD outcomes in HIV-infected adults on ART
| (A) Completed | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Clinicaltrials.gov Identifier |
Year Completed |
Population** | N | Intervention | Duration | Surrogate CVD outcome |
Main Results | References |
| N/A | 2004 | On stable PI; total cholesterol >3.36mmol/L and either triglycerides >3.88mmol/L or HDL <1.07mmol/L | 20 | Pravastatin 40mg vs. Placebo | 8 weeks | FMD | Trend toward improved FMD with pravastatin (p=0.08) | Stein et al.[17] |
| N/A | 2006 | On stable PI; total cholesterol >5mmol/L (193mg/dL) | 29 | Pravastatin 40mg vs. Placebo | 8 weeks | FMD | Improved FMD in statin arm vs. no change in placebo. | Hurlimann et al.[18] |
| NCT00227500 | 2006 | Men >18yrs; Stable PI; Total cholesterol >6.5mmol/L (251mg/dL) | 33 | Pravastatin 40mg vs. Placebo | 12 weeks | FMD | No difference in FMD change | Mallon et al.[19] |
| NCT00965185 | 2014 | Stable ART; subclinical coronary athero by CTA AND aortic inflammation by PET; LDL 70–130mg/dL | 40 | Atorvastatin (20–40mg) vs. Placebo | 12 months | Coronary CTA plaque features; Aortic TBR* | Reduced non-calcified plaque volume and # of high risk plaques; no difference in aortic TBR change | Lo et al.[20] |
| NCT01218802 | 2014 | Stable ART; LDL <130mg/dL; hsCRP >2 or elevated CD 8+ T-cell activation | 147 | Rosuvastatin 10mg vs. Placebo | 96 weeks | CIMT; FMD; CAC | Reduced 48 and 96 week CIMT progression; no difference in CAC change | Longenecker et al.[21] |
| (B) Ongoing | ||||||
|---|---|---|---|---|---|---|
|
Clinicaltrials.gov Identifier |
Estimated Completion |
Population | N | Intervention | Duration | CVD outcome(s) |
| NCT01881971 | 2016 | Stable ART; COPD | 30 | Rosuvastatin vs. Placebo | 24 months | CIMT; FMD |
| NCT02081638 | 2017 | Elite Controller or ART >4 yrs | 80† | Open label Aspirin 81mg vs. Atorvastatin 40mg | 9 months | Carotid MRI |
| NCT01813357 | 2017 | Stable ART; FRS 10–15% | 102 | Rosuvastatin 20mg vs. Placebo | 24 months | CIMT; FMD |
| NCT02234492 | 2018 | >40yrs; ART >2 yrs; FRS 10–20%; LDL <4mmol/L (<155mg/dL); | 82 | Open label Rosuvastatin 10mg vs. usual medical care | 6 months | Coronary Flow Reserve‡; Vascular TBR* |
| NCT02344290 | 2020 | 40–75yrs; stable ART; <7.5% 10yr risk | 6500 | Pitavastatin 4mg vs. Placebo | 42–72 months | MACE; Coronary CTA substudy |
Measured using 18-fluorodeoxyglucose positron emission tomography (18-FDG PET/CT)
Population includes most important inclusion/exclusion criteria; full criteria available at clinicaltrials.gov or in the published manuscript
40 elite controllers; 40 treated progressors
Measured using myocardial contrast echocardiography
PI, protease inhibitor; CTA, computed tomography angiography; TBR, Target to background ratio; CIMT, carotid intima-media thickness; FMD, flow-mediated dilation of the brachial artery; CAC, coronary artery calcium; COPD, chronic obstructive pulmonary disease; FRS, Framingham Risk Score; MACE, major adverse cardiovascular events (CVD death, nonfatal myocardial infarction, unstable angina hospitalization, coronary or peripheral arterial revascularization, nonfatal stroke or transient ischemic attack, urgent peripheral arterial disease ischemic event)
Potential mediators of the statin effect
As shown in Figure 1, there are multiple proposed mechanisms by which HIV infection may increase the risk of cardiovascular disease: (1) metabolic dysfunction, (2) direct vascular effect, (3) immune activation, and (4) chronic inflammaging[3]. Because of their pleiotropic effects, statins are likely to improve cardiovascular outcomes through mediators in each of these categories. In this next section, we will explore recent data on some of these potential mediators.
Lipids and adiposity
The effects of statins on lipids in treated HIV infection have been recently summarized in a systematic review[22]. Eighteen studies were included in the review, although not all studies reported lipid changes. The authors conclude that atorvastatin, pravastatin, and rosuvastatin are all well-tolerated and efficacious at lowering LDL cholesterol. For example, in a trial that compared atorvastatin 10mg daily, rosuvastatin 10mg daily, and pravastatin 20mg daily in patients on protease inhibitor-based regimens, LDL was reduced by 20%, 25%, and 18%, respectively, after 1 year of therapy[23]. Another trial confirmed the efficacy of more potent statins, showing that rosuvastatin 10mg was nearly two-fold more effective at lowering LDL compared to pravastatin 40mg (−37% vs. −19%, respectively)[24]. Despite some disagreement among experts and observational data to suggest possible “statin resistance”[25, 26], the most recent clinical trials would suggest that the LDL lowering effect is similar to that seen in the general population; however, the lipid response often depends on the background ART use due to drug interactions.
Historically, HIV-infection was characterized by profound changes in body composition previously referred to as lipodystrophy (peripheral fat loss and/or central fat gain) in some patients. Although lipoatrophy is less commonly seen with current ART, the central fat accumulation continues to occur, regardless of ART regimen[27–29] Because HIV-lipodystrophy is associated with alterations in cardiometabolic risk factors, it is possible that improvements in body fat content and distribution may mediate some of the cardiovascular benefit of statins in HIV infection. In a large observational study of the ALLRT cohort, statin use was associated with 0.6cm greater 32-week change in hip circumference after multivariable adjustment[30]. The authors estimate this difference to correspond to a 0.22 kg difference in limb fat. A randomized trial of pravastatin resulted in a somewhat larger 0.53 kg increase in limb fat compared to placebo over 12 weeks in patients receiving PI-based ART[19]. In contrast, 2 other placebo-controlled randomized trials showed no benefit of pravastatin on peripheral fat gain in patients with clinical lipodystrophy[31, 32]. Recent data from SATURN-HIV similarly showed no effect of rosuvastatin 10mg on peripheral or central fat volumes by DEXA[33]. Expanding on this study, future analyses of the SATURN-HIV trial may shed light on ectopic fat changes after statin therapy[34].
Inflammation and immune activation
Statins have wide-reaching anti-inflammatory and immunomodulatory effects, including influencing both the innate and adaptive immune responses and affecting a variety of biomarkers of systemic inflammation and endothelial dysfunction that are important in CVD[35, 36]. These effects may be particularly beneficial in the HIV-infected population as heightened inflammation and immune activation are thought to play critical roles in CVD development. Studies published to date, however, are conflicting as to whether statin therapy significantly reduces levels of CRP and other inflammatory markers such as interleukins and adhesion molecules in this population[20, 37–41]. The discrepancies are most likely related to different statins used and/or differences among the study subjects.
The two randomized trials previously mentioned[20, 21] have both shown a reduction in lipoprotein-associated phospholipase A2 (Lp-PLA2), a vascular inflammation marker that predicts both primary and recurrent future coronary or cardiovascular events in the general population[42]. The implication of this reduction is unclear, however, as a large Lp-PLA2 inhibitor trial showed no benefit for time to first major CVD event in the general population[43].
There are also a few studies that have investigated changes in immune activation, exhaustion and/or T-cell function in ART-treated HIV-infected subjects[41, 44–47]. All of these studies showed decreases in some or all of the markers studied with atorvastatin and rosuvastatin, mainly T-cell and monocyte activation; however, the effect on soluble markers of inflammation has been more inconsistent. Further research is needed to determine the significance of these reductions on outcomes of CVD morbidity and death.
Other pleiotropic mediators
Chronic kidney disease is one of the strongest predictors of CVD events in the general population[48] and in patients with HIV[1]. Although statins reduce CVD events in the general population, they appear to have little effect on kidney function decline[49]. In SATURN-HIV, rosuvastatin was associated with improvements in the kidney biomarker cystatin C and creatinine-based estimates of glomerlular filtration rate[50].
Other analyses of secondary endpoints in SATURN-HIV suggest a beneficial effect on N-terminal pro-B-type natriuretic peptide[51], a biomarker of cardiac wall stress that predicts both vascular and heart failure events, but there was no effect on the expression of two well-known transcriptional mediators of vascular inflammation—Krüppel-like factors 2 and 4[52].
Future translational studies should further investigate statin mechanisms in the setting of chronic inflammatory diseases such as treated HIV. Placebo-controlled study designs will be possible in low-risk individuals at least until the results of the REPRIEVE study are known. Such mechanistic studies should also consider the value of HIV-uninfected persons as negative controls and persons with inflammatory polyarthropathies as positive controls.
Risks and side effects of statin therapy
Statins used at recommended doses in HIV-infected individuals have a relatively low risk of side effects comparable to the general population in most studies[24, 39, 53]. The most serious side effect is rhabdomyolysis[54], but this is rare. For example, over 96 weeks in SATURN-HIV, there were no cases of rhabdomyolysis and only 3 participants (2 statin and 1 placebo) discontinued study drug due to myalgias[55]. Additionally, there was no change in creatinine kinase and a trend toward increased lean body mass in the rosuvasatin arm. The implications of the increased diabetes risk with statins is concerning for the HIV population, however. A single study (SATURN-HIV) to date has investigated this side effect in HIV-infected subjects and showed a >50% increase in insulin resistance after only 48 weeks of rosuvastatin therapy[56].
Who should be prescribed statins?
Prescribing statins to reduce the risk of CVD in patients with HIV infection should always be done in the context of global cardiovascular risk assessment; however, few studies have described the performance of commonly available risk calculators for the HIV-infected population. Recent studies from North American cohorts suggest that these risk calculators have reasonable discrimination (c-statistics 0.65–0.75) in HIV-infected populations, but underestimate risk for MI[57, 58] and stroke[59] with observed:expected ratios of ~1.25[57, 58]. This underestimation of risk is most significant for patients who are categorized as low risk (<7.5% 10-year risk)[57]. Thus, HIV-specific calculators have been proposed. Friis-Moller et al. used the D:A:D cohort to construct a risk model that incorporates current use and duration of specific ART drugs[60]. In the derivation cohort, the prediction tool had better discrimination and calibration when compared to the Framingham Risk Score[60], but in other cohorts, the model performs similarly to Framingham and other risk calculators[57, 58]. Another model that may more accurately assess CVD risk in patients living with HIV is the Veterans Affairs Cohort Study (VACS) Index. Although extensively studied as a predictor of mortality[61], it is less well-validated as a CVD risk estimator[62].
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) issued joint heart disease and stroke prevention guidelines that focus on lifestyle, obesity, risk assessment, and treatment of cholesterol[11, 63]. Instead of recommending target LDL goals, the new cholesterol treatment guidelines identified four types of patients that would benefit from statin therapy: (1) high LDL >190mg/dL; (2) history of known CVD; (3) diabetes; and (4) 40–75 years old with a >7.5% 10-year risk of CVD. The guidelines were released together with a new calculator to predict risk that was created using pooled data from multiple cohort studies[63]. Although some have argued that the pooled cohort equations overestimate risk for some patients, there is emerging evidence that they underestimate risk in patients with HIV[57]. For example, although the new guidelines expand recommendations for statin use above what would have been recommended by the Adult Treatment Panel (ATP III) guideline, nearly 40% of HIV-infected patients who have a CVD event would not have been recommended to receive statin therapy prior to the event under either of these guidelines[64]. Similarly, the ACC/AHA guidelines recommend statins for a larger number of HIV-infected patients with high risk plaque features by coronary angiography compared to ATP III; however, statins would not be recommended by either guideline for nearly three-quarters of patients with high risk plaque features[65].
In 2015, the National Lipid Association issued recommendations for patient-centered management of dyslipidemia that re-affirmed the importance of treating to target cholesterol levels[66]. In Part 2 of the recommendations, expert panels were convened to create specific recommendations for at-risk populations. The expert panel concluded that HIV-infection should be considered a major risk factor for CVD, which can be used to determine absolute risk category and corresponding treatment goals[67]. These recommendations include an update on statin interactions with ART based on recent studies[68–70] and suggested that more potent statins such as rosuvastatin, atorvastatin, and pitavastatin are generally preferable to the less potent pravastatin. To further assist the clinician in navigating drug-drug interactions, we have compiled an overview in Table 2. The National Lipid Association has previously reviewed these interactions in more detail[67, 71]. Table 2 displays significant interactions with protease inhibitors including ritonavir, cobicistat, and non-nucleoside reverse transcriptase inhibitors. Except for cobicistat-boosted elvitegravir, there are no significant statin interactions with integrase inhibitors or entry inhibitors.
Table 2.
Antiretroviral drug interactions with commonly used statins
| PI | Cobicistat | NNRTI | |
|---|---|---|---|
| Atorvastatin | ↑↑ Do not exceed 20–40mg X with TPV/r | ↑↑ Do not exceed 20–40mg | ↓ EFV and ETR ↔ RPV |
| Rosuvastatin | ↑↑ LPV/r and ATV/r Do not exceed 10–20mg ↑ DRV/r | ↑ Do not exceed 10–20mg | ↔ |
| Pitavastatin | ↔ | ↔ | ↔ |
| Pravastatin | ↔ ↑ DRV/r | ↔ | ↔ ↓ EFV |
| Simvastatin | X | X | ↓ |
This table is compiled from multiple sources and is meant to be a simplified overview. For details, please refer to the following review articles [67, 71].
↑ = mild increase in statin concentration; ↑↑ = moderate increase; X = contraindicated due to strong interaction; ↓ = decrease; ↔ no to minimal interaction
PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; r, ritonavir; TPV, tipranavir; LPV, lopinavir; ATV, atazanavir; DRV, darunavir; EFV, efavirenz; ETR, etravirine; RPV, rilpivirine
Considering the relative merits of these guidelines and others (e.g. European Society of Cardiology), it is important for the clinician to take an individualized approach to statin prescriptions for patients living with HIV. For each patient, expected absolute risk reductions should be weighed against potential risks and side-effects. This discussion of risks and benefits should result in a mutual decision between patient and provider about whether to start a statin.
Conclusion
Recent studies have sparked new interest in the potential of statins to improve cardiovascular outcomes in patients living with HIV/AIDS. But are statins a magic bullet? Our opinion is that while clearly more study is needed to determine which patients will benefit, efforts must be made to increase use in patients for whom there is a clear indication. In 2009, less than 40% of HIV-infected veterans who met ATP III recommendations for lipid-lowering therapy actually received it [72]. With expanded recommendations under ACC/AHA guidelines, these numbers are likely to be even lower. Clinicians should strongly consider having a discussion about statins with patients in the context of how to live well with HIV in the modern treatment era.
Key Points.
Single-centered randomized clinical trials suggest that moderate to high dose atorvastatin and rosuvastatin reduce the burden of subclinical carotid and coronary atherosclerosis in patients with treated HIV infection, but the results of a multicenter cardiovascular outcomes study (REPRIEVE) are not expected until 2020.
In treated HIV, the cardiovascular benefit of statins may be mediated in part by reductions in atherogenic lipoproteins, but also by reductions in vascular inflammation and immune activation.
There may be a risk of insulin resistance and diabetes with certain statins, and this should be assessed further in future studies.
Statins should be prescribed in the context of global risk assessment, keeping in mind that commonly used risk calculators tend to underestimate risk in patients with treated HIV infection.
Clinicians should consider the more potent statins (atorvastatin, rosuvastatin, and pitavastatin) whenever possible, while being aware of modest drug-drug interactions.
Acknowledgements
None
Financial support and sponsorship: This work was supported in part by the National Institutes of Health (K23 HL123341 to CTL; K23 HD069199 to ARE; and R01 HD070490 and R01 NR012642 to GAM).
Conflicts of Interest: CTL has received research grants from Bristol-Myers Squibb and Medtronic Philanthropy. ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor for Gilead. GAM has served as a scientific advisor or speaker for Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Gilead Sciences, has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and has served as the DSMB Chair for a Pfizer-sponsored study.
References
- 1.Freiberg MS, Chang CC, Kuller LH, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA internal medicine. 2013:1–9. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sico JJ, Chang CC, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84:1933–1940. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: time for a better understanding. Journal of the American College of Cardiology. 2013;61:511–523. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 4.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. The New England journal of medicine. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 5.Monforte A, Reiss P, Ryom L, et al. Atazanavir is not associated with an increased risk of cardio or cerebrovascular disease events. AIDS. 2013;27:407–415. doi: 10.1097/QAD.0b013e32835b2ef1. [DOI] [PubMed] [Google Scholar]
- 6.Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Curr Opin HIV AIDS. 2014;9:54–62. doi: 10.1097/COH.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England journal of medicine. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 9.Hebert PR, Gaziano JM, Chan KS, et al. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA. 1997;278:313–321. [PubMed] [Google Scholar]
- 10.Mills EJ, O'Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40 000 patients. Eur Heart J. 2011;32:1409–1415. doi: 10.1093/eurheartj/ehr035. [DOI] [PubMed] [Google Scholar]
- 11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. These guidelines identified four types of patients that would benefit from statin therapy: (1) high LDL >190mg/dL; (2) history of known CVD; (3) diabetes; (4) 40–75 years old with a >7.5% 10-year risk of CVD. The guidelines have been somewhat controversial for removing treatment targets and for greatly expanding the number of patients recommended for therapy.
- 12.Cholesterol Treatment Trialists C. Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crouse JR, 3rd, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen LD, Kronborg G, Larsen CS, et al. Statin therapy and mortality in HIV-infected individuals; a Danish nationwide population-based cohort study. PLoS One. 2013;8:e52828. doi: 10.1371/journal.pone.0052828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overton ET, Kitch D, Benson CA, et al. Effect of statin therapy in reducing the risk of serious non-AIDS-defining events and nonaccidental death. Clin Infect Dis. 2013;56:1471–1479. doi: 10.1093/cid/cit053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calza L, Manfredi R, Colangeli V, et al. Two-year treatment with rosuvastatin reduces carotid intima-media thickness in HIV type 1-infected patients receiving highly active antiretroviral therapy with asymptomatic atherosclerosis and moderate cardiovascular risk. AIDS Res Hum Retroviruses. 2013;29:547–556. doi: 10.1089/aid.2012.0015. [DOI] [PubMed] [Google Scholar]
- 17.Stein JH, Merwood MA, Bellehumeur JL, et al. Effects of pravastatin on lipoproteins and endothelial function in patients receiving human immunodeficiency virus protease inhibitors. Am Heart J. 2004;147:E18. doi: 10.1016/j.ahj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Hurlimann D, Chenevard R, Ruschitzka F, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart. 2006;92:110–112. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallon PW, Miller J, Kovacic JC, et al. Effect of pravastatin on body composition and markers of cardiovascular disease in HIV-infected men--a randomized, placebo-controlled study. AIDS. 2006;20:1003–1010. doi: 10.1097/01.aids.0000222072.37749.5a. [DOI] [PubMed] [Google Scholar]
- 20. Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet HIV. 2015;2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0. In this small, single-center RCT, moderate dose atorvastatin reduced non-calcified coronary plaque volume over 12 months.
- 21. Longenecker CT, Jiang Y, Debanne SM, et al. Rosuvastatin Arrests Progression of Carotid Intima-Media Thickness in Treated HIV; 22nd Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA, USA. 2015. In this report of the primary outcome of SATURN-HIV, rosuvastatin 10mg daily slowed progression of carotid intima media thickness compared to placebo over 96 weeks.
- 22. Feinstein MJ, Achenbach CJ, Stone NJ, et al. A Systematic Review of the Usefulness of Statin Therapy in HIV-Infected Patients. Am J Cardiol. 2015 doi: 10.1016/j.amjcard.2015.03.025. This review summarizes 18 clinical trials of statins in persons living with HIV. In general, statins appear to be safe and effacacious in reducing levels of atherogenic lipoproteins. Data on subclinical or clinical cardiovascular disease outcomes are limited in this population.
- 23.Calza L, Manfredi R, Colangeli V, et al. Rosuvastatin, pravastatin, and atorvastatin for the treatment of hypercholesterolaemia in HIV-infected patients receiving protease inhibitors. Curr HIV Res. 2008;6:572–578. doi: 10.2174/157016208786501481. [DOI] [PubMed] [Google Scholar]
- 24.Aslangul E, Assoumou L, Bittar R, et al. Rosuvastatin versus pravastatin in dyslipidemic HIV-1-infected patients receiving protease inhibitors: a randomized trial. AIDS. 2010;24:77–83. doi: 10.1097/QAD.0b013e328331d2ab. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009;150:301–313. doi: 10.7326/0003-4819-150-5-200903030-00006. [DOI] [PubMed] [Google Scholar]
- 26.Johns KW, Bennett MT, Bondy GP. Are HIV positive patients resistant to statin therapy? Lipids Health Dis. 2007;6:27. doi: 10.1186/1476-511X-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McComsey GA, Kitch D, Sax PE, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:185–196. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srinivasa S, Grinspoon SK. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur J Endocrinol. 2014;170:R185–R202. doi: 10.1530/EJE-13-0967. [DOI] [PubMed] [Google Scholar]
- 29.McComsey GA, Moser C, Currier JS, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors; Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. 2015. [Google Scholar]
- 30.Brown TT, Smurzynski M, Wu K, et al. Statin therapy and changes in hip circumference among HIV-infected participants in the ALLRT Cohort. Antivir Ther. 2009;14:853–858. doi: 10.3851/1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macallan DC, Baldwin C, Mandalia S, et al. Treatment of altered body composition in HIV-associated lipodystrophy: comparison of rosiglitazone, pravastatin, and recombinant human growth hormone. HIV Clin Trials. 2008;9:254–268. doi: 10.1310/hct0904-254. [DOI] [PubMed] [Google Scholar]
- 32.Calmy A, Bloch M, Wand H, et al. No significant effect of uridine or pravastatin treatment for HIV lipoatrophy in men who have ceased thymidine analogue nucleoside reverse transcriptase inhibitor therapy: a randomized trial. HIV Med. 2010;11:493–501. doi: 10.1111/j.1468-1293.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 33. Erlandson KM, Jiang Y, Debanne SM, et al. Effects of randomized rosuvastatin compared with placebo on bone and body composition among HIV-infected adults. AIDS. 2015;29:175–182. doi: 10.1097/QAD.0000000000000526. Rosuvastatin did not have significant effects on fat distribution in the SATURN-HIV trial.
- 34.Longenecker CT, Jiang Y, Yun CH, et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int J Cardiol. 2013;168:4039–4045. doi: 10.1016/j.ijcard.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mira E, Manes S. Immunomodulatory and anti-inflammatory activities of statins. Endocr Metab Immune Disord Drug Targets. 2009;9:237–247. doi: 10.2174/187153009789044383. [DOI] [PubMed] [Google Scholar]
- 36.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 37.Aslangul E, Fellahi S, Assoumou LK, et al. High-sensitivity C-reactive protein levels fall during statin therapy in HIV-infected patients receiving ritonavir-boosted protease inhibitors. AIDS. 2011;25:1128–1131. doi: 10.1097/QAD.0b013e328346be29. [DOI] [PubMed] [Google Scholar]
- 38.Calza L, Vanino E, Salvadori C, et al. Tenofovir/emtricitabine/efavirenz plus rosuvastatin decrease serum levels of inflammatory markers more than antiretroviral drugs alone in antiretroviral therapy-naive HIV-infected patients. HIV Clin Trials. 2014;15:1–13. doi: 10.1310/hct1501-1. [DOI] [PubMed] [Google Scholar]
- 39. Eckard AR, Jiang Y, Debanne SM, et al. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209:1156–1164. doi: 10.1093/infdis/jiu012. As the first pre-specified interim analysis of the SATURN-HIV trial, this study showed that rosuvastatin 10mg daily reduced LpPLA2 levels--a marker of vascular inflammation--compared to placebo, although other soluble biomarkers of generalized inflammation were not significantly changed by statin therapy.
- 40.Fichtenbaum CJ, Evans SE, Aberg JA. High-sensitivity C-reactive protein levels do not decrease with the use of statins in all persons with HIV infection. AIDS. 2011;25:2053. doi: 10.1097/QAD.0b013e32834b9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Wit S, Delforge M, Necsoi CV, et al. Downregulation of CD38 activation markers by atorvastatin in HIV patients with undetectable viral load. AIDS. 2011;25:1332–1333. doi: 10.1097/QAD.0b013e328347c083. [DOI] [PubMed] [Google Scholar]
- 42.Lp PLASC, Thompson A, Gao P, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Investigators S. White HD, Held C, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 44. Nakanjako D, Ssinabulya I, Nabatanzi R, et al. Atorvastatin reduces T-cell activation and exhaustion among HIV-infected cART-treated suboptimal immune responders in Uganda: a randomised crossover placebo-controlled trial. Trop Med Int Health. 2015;20:380–390. doi: 10.1111/tmi.12442. nother recent small cross-over RCT in which atorvastatin reduced markers of CD4+ and CD8+ T-cell activation and exhaustion. Importantly, this study was done in an Ugandan population.
- 45. Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin Reduces Vascular Inflammation and T-cell and Monocyte Activation in HIV-Infected Subjects on Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. In addition to the 24-week analysis (REF 43) of the SATURN-HIV trial, this 48-week analysis provides insight into the longer term effects of statins on cellular markers of monocyte and T-cell activation. It also confirmed ~10% reductions in soluble CD14 and LpPLA2 along with a ~20% reduction in IP-10.
- 46.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–595. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Overton ET, Sterrett S, Westfall AO, et al. Effects of atorvastatin and pravastatin on immune activation and T-cell function in antiretroviral therapy-suppressed HIV-1-infected patients. AIDS. 2014;28:2627–2631. doi: 10.1097/QAD.0000000000000475. This retrospective study of 21 patients on ART suggested that atorvastatin improved markers of immune activation and exhaustion, but pravastatin did not. This study, along with other RCTs, reinforces the idea that potent statins may be more likely than weaker statins to achieve beneficial immune effects in chronic HIV infection.
- 48.Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U.S. Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2012;156:570–581. doi: 10.7326/0003-4819-156-8-201204170-00004. [DOI] [PubMed] [Google Scholar]
- 50. Longenecker CT, Hileman CO, Funderburg NT, et al. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: the SATURN-HIV trial. Clin Infect Dis. 2014;59:1148–1156. doi: 10.1093/cid/ciu523. This interim analysis of the SATURN-HIV trial suggests that persons living with HIV might also gain a renal protective benefit from statin therapy. If confirmed in longer term studies, a durable renal protective effect might also reduce the risk of cardiovascular events.
- 51. Dirajlal-Fargo S, Kinley B, Jiang Y, et al. Statin therapy decreases N-terminal pro-B-type natriuretic peptide in HIV: randomized placebo-controlled trial. AIDS. 2015;29:313–321. doi: 10.1097/QAD.0000000000000547. Rosuvastatin decreased NT-proBNP, a marker of ventricular wall stress that has also been associated with vascular events in the general population.
- 52. Hale A, Longenecker C, Jiang Y, et al. HIV Vasculopathy: Role of Mononuclear cell-associated Krüppel-like Factors 2 and 4. AIDS. 2015 doi: 10.1097/QAD.0000000000000756. In Press. Expression of transcription factors KLF 2 and 4 in peripheral blood mononuclear cells were related to markers of inflammation and subclinical vascular disease, but did not appear to be changed by 24 weeks of rosuvastatin.
- 53.Singh S, Willig JH, Mugavero MJ, et al. Comparative Effectiveness and Toxicity of Statins Among HIV-Infected Patients. Clin Infect Dis. 2011;52:387–395. doi: 10.1093/cid/ciq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 55.Erlandson K, Jiang Y, Debanne S, et al. Rosuvastatin has Minimal Impact on Bone, Fat, and Muscle among Antiretroviral Treated, HIV-Infected Adults: 96-week Results of SATURN-HIV. 2015 In Press. [Google Scholar]
- 56. Erlandson KM, Jiang Y, Debanne SM, et al. Rosuvastatin Worsens Insulin Resistance in HIV-Infected Adults on Antiretroviral Therapy. Clin Infect Dis. 2015 doi: 10.1093/cid/civ554. In the SATURN-HIV trial, rosuvastatin was associated with significant increases in insulin levels and insulin resistance over 96 weeks compared to placebo; however, there was no increase in the incidence of diabetes and no significant change in oral glucose tolerance testing. This study highlights the importance of carefully considering the risk/benefit of statins in patients at low global CVD risk.
- 57.Regan S, Meigs J, Grinspoon S, et al. Application of New ACC/AHA Cholesterol Guidelines to an HIV Clinical Care Cohort; Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. 2015. [Google Scholar]
- 58.Thompson-Paul A, Lichtenstein KA, Armon C, et al. Cardiovascular Disease Risk Prediction in the HIV Outpatient Study (HOPS); Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. 2015. [Google Scholar]
- 59.Mateen FJ, Post WS, Sacktor N, et al. Long-term predictive value of the Framingham Risk Score for Stroke in HIV-positive vs HIV-negative men. Neurology. 2013;81:2094–2102. doi: 10.1212/01.wnl.0000437296.97946.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 61.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Justice AC, Tate JP, Freiberg MS, et al. Reply to Chow et al. Clinical Infectious Diseases. 2012;55:751–752. [Google Scholar]
- 63. Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. This guideline document specifically addresses CVD risk assessment and the use of the new pooled cohort equations. Patients >40 years of age fall into a statin benefit group if the 10-year risk of atherosclerotic CVD is >7.5%.
- 64.Mosepele M, Regan S, Meigs J, et al. Application of New ACC/AHA Cholesterol Guidelines to an HIV Clinical Care Cohort; Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. 2015. [Google Scholar]
- 65. Zanni MV, Fitch KV, Feldpausch M, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS. 2014;28:2061–2070. doi: 10.1097/QAD.0000000000000360. Nearly three quarters of HIV-infected subjects with high risk coronary plaque features identified by cardiac CT would not be recommended to receive statin under the 2013 ACC/AHA guidelines. Together with REF #60, these studies highlight the need for alternative strategies to identify which HIV-infected patients will be likely to benefit from statins.
- 66.Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1 - executive summary. J Clin Lipidol. 2014;8:473–488. doi: 10.1016/j.jacl.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 67. Aberg JA, Fichtenbaum C, Gallant JE, et al. National Lipid Association Recommendations for HIV Infected Persons. Journal of the National Lipid Association. 2015 In press. The National Lipid Association has made recommendations for management of dyslipidemia that is an alternative to the ACC/AHA guidelines approach that includes treating to target lipoprotien levels. For Part II of the NLA recommendations, an expert panel was convened that recommended consideration of HIV-infection as a major risk factor in the algorithm for determining lipoprotein goals for individual patients.
- 68.Yu CY, Campbell SE, Sponseller CA, et al. Steady-state pharmacokinetics of darunavir/ritonavir and pitavastatin when co-administered to healthy adult volunteers. Clin Drug Investig. 2014;34:475–482. doi: 10.1007/s40261-014-0198-x. [DOI] [PubMed] [Google Scholar]
- 69.Malvestutto CD, Ma Q, Morse GD, et al. Lack of pharmacokinetic interactions between pitavastatin and efavirenz or darunavir/ritonavir. J Acquir Immune Defic Syndr. 2014;67:390–396. doi: 10.1097/QAI.0000000000000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Custodio JM, Wang H, Hao J, et al. Pharmacokinetics of cobicistat boosted-elvitegravir administered in combination with rosuvastatin. J Clin Pharmacol. 2014;54:649–656. doi: 10.1002/jcph.256. [DOI] [PubMed] [Google Scholar]
- 71.Kellick KA, Bottorff M, Toth PP. A clinician’s guide to statin drug-drug interactions. J Clin Lipidol. 2014;8:S30–S46. doi: 10.1016/j.jacl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 72.Freiberg MS, Leaf DA, Goulet JL, et al. The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid lowering medication. J Gen Intern Med. 2009;24:334–340. doi: 10.1007/s11606-008-0891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]